Abstract
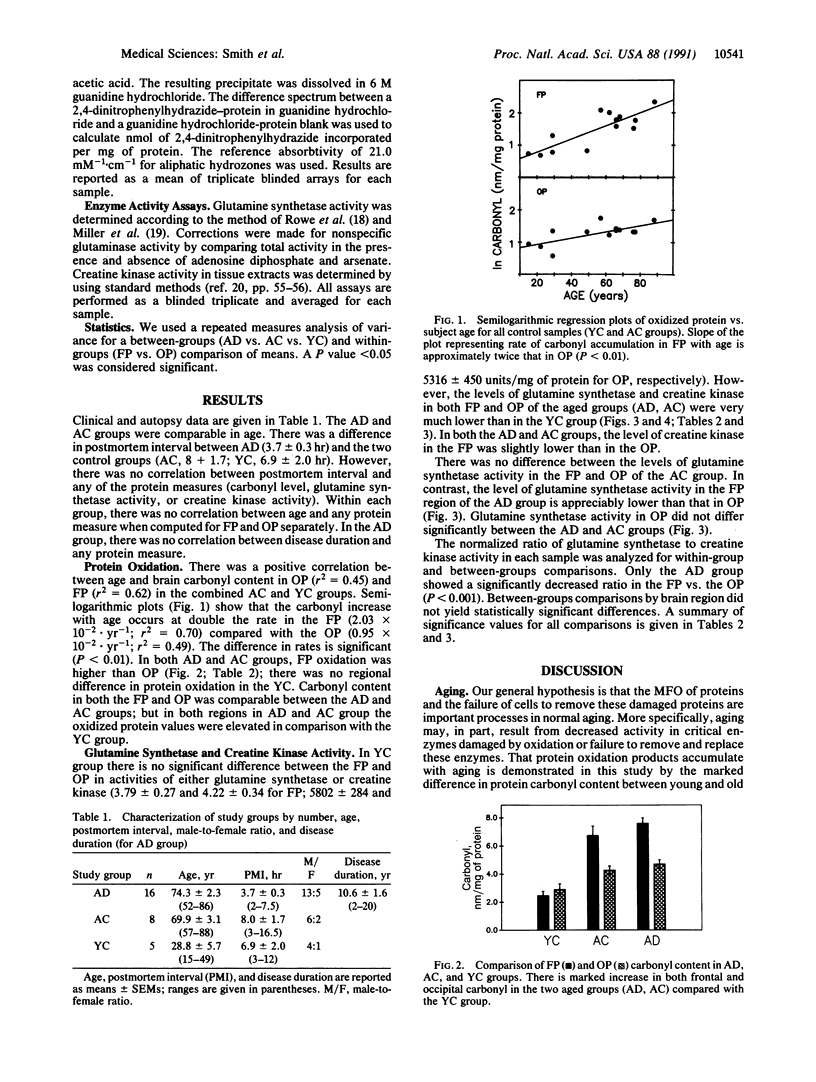
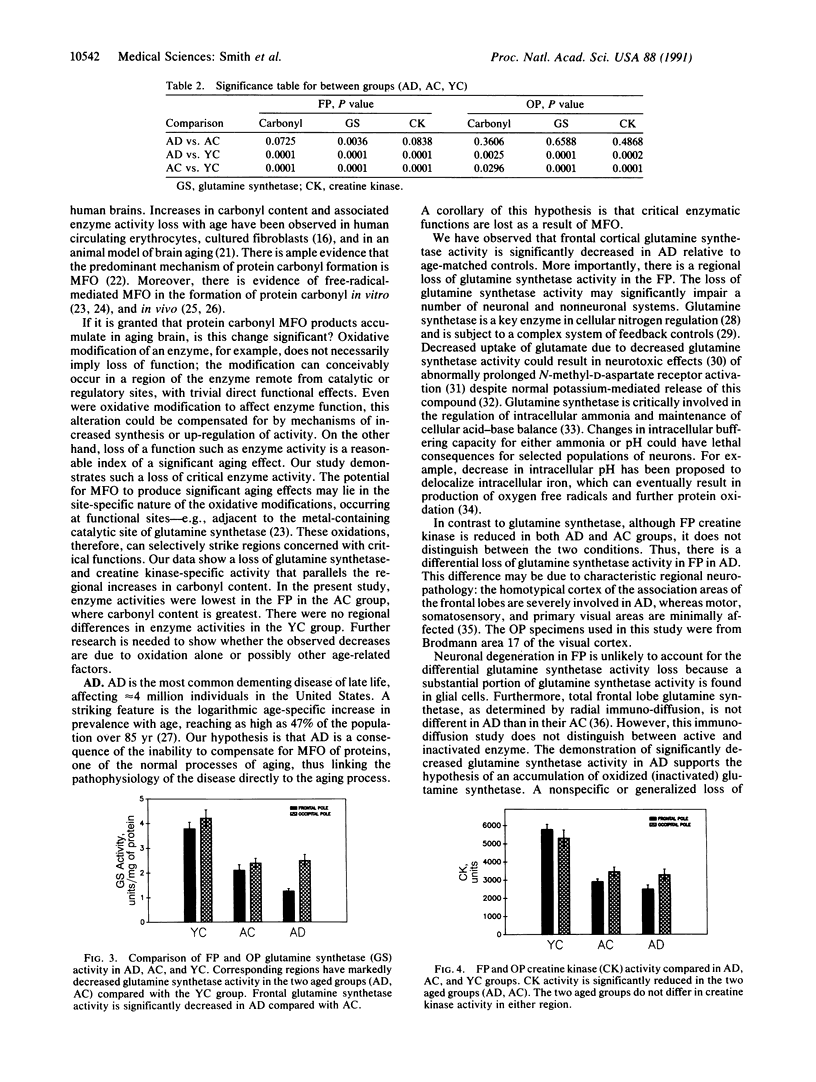
The relationship between Alzheimer disease (AD) and aging is not currently known. In this study, postmortem frontal- and occipital-pole brain samples were obtained from 16 subjects with AD, 8 age-matched controls, and 5 young controls. These samples were analyzed both for protein oxidation products (carbonyl) and the activities of two enzymes vulnerable to mixed-function oxidation, glutamine synthetase and creatine kinase. Glutamine synthetase is more sensitive to mixed-function oxidation than creatine kinase. Carbonyl content rises exponentially with age, at double the rate in the frontal pole compared with the occipital pole. Compared with young controls, both aged groups (AD and age-matched controls) have increased carbonyl content and decreased glutamine synthetase and creatine kinase activities, which are more marked in the frontal than occipital pole in all instances. We conclude that protein oxidation products accumulate in the brain and that oxidation-vulnerable enzyme activities decrease with aging in the same regional pattern (frontal more affected than occipital). However, only glutamine synthetase activity distinguishes AD from age-matched controls: Because glutamine synthetase activity is differentially reduced in the frontal pole in AD, we suggest that AD may represent a specific brain vulnerability to age-related oxidation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A., Vlassara H., Brownlee M. Role of nonenzymatic glycosylation in atherogenesis. J Cell Biochem. 1986;30(2):111–120. doi: 10.1002/jcb.240300203. [DOI] [PubMed] [Google Scholar]
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989 Mar 24;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Funkenstein H. H., Albert M. S., Scherr P. A., Cook N. R., Chown M. J., Hebert L. E., Hennekens C. H., Taylor J. O. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- Floyd R. A., Carney J. M. Age influence on oxidative events during brain ischemia/reperfusion. Arch Gerontol Geriatr. 1991 Mar-Jun;12(2-3):155–177. doi: 10.1016/0167-4943(91)90025-l. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Free radicals in aging. Mol Cell Biochem. 1988 Dec;84(2):155–161. doi: 10.1007/BF00421050. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Knowler J. T. Role of ribonuclease and ribonuclease inhibitor activities in Alzheimer's disease. J Neurochem. 1989 Nov;53(5):1341–1344. doi: 10.1111/j.1471-4159.1989.tb08523.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen O. S., Brooksbank B. W., Balázs R. Neuronal plasticity and astrocytic reaction in Down syndrome and Alzheimer disease. J Neurol Sci. 1990 Aug;98(1):63–79. doi: 10.1016/0022-510x(90)90182-m. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H., Favaron M., Guidotti A., Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989 Jul;36(1):106–112. [PubMed] [Google Scholar]
- Mantle D., Perry E. K. Comparison of aminopeptidase, dipeptidyl aminopeptidase and tripeptidyl aminopeptidase activities in brain tissue from normal and Alzheimer's disease cases. J Neurol Sci. 1990 Aug;98(1):13–20. doi: 10.1016/0022-510x(90)90178-p. [DOI] [PubMed] [Google Scholar]
- Maschhoff K., White C. L., 3rd, Jennings L. W., Morrison-Bogorad M. R. Ribonuclease activities and distribution in Alzheimer's and control brains. J Neurochem. 1989 Apr;52(4):1071–1078. doi: 10.1111/j.1471-4159.1989.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Hackenberg R., Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C. N., Ahn B. W., Moerman E. J., Goldstein S., Stadtman E. R. Age-related changes in oxidized proteins. J Biol Chem. 1987 Apr 25;262(12):5488–5491. [PubMed] [Google Scholar]
- Oliver C. N., Starke-Reed P. E., Stadtman E. R., Liu G. J., Carney J. M., Floyd R. A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W. D., Jr, Filley C. M., Parks J. K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990 Aug;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy M. E., Dalton A. J., Markovic V. D., McLachlan D. R., Hummel J. T., Rusk A. C., Andrews D. F. Red cell superoxide dismutase, glutathione peroxidase and catalase in Down syndrome patients with and without manifestations of Alzheimer disease. Am J Med Genet. 1990 Apr;35(4):459–467. doi: 10.1002/ajmg.1320350403. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Yong V. W., Bergeron C., Hansen S., Jones K. Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer's disease. Ann Neurol. 1987 Apr;21(4):331–336. doi: 10.1002/ana.410210403. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Park R., Chock P. B., Stadtman E. R. Allosteric regulation of monocyclic interconvertible enzyme cascade systems: use of Escherichia coli glutamine synthetase as an experimental model. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3138–3142. doi: 10.1073/pnas.75.7.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J., Roseman J. E., Oliver C. N., Levine R. L., Stadtman E. R. Covalent modification of proteins by mixed-function oxidation: recognition by intracellular proteases. Prog Clin Biol Res. 1985;180:317–328. [PubMed] [Google Scholar]
- Southorn P. A., Powis G. Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc. 1988 Apr;63(4):390–408. doi: 10.1016/s0025-6196(12)64862-9. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990 Jul 10;29(27):6323–6331. doi: 10.1021/bi00479a001. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Wittenberger M. E. Inactivation of Escherichia coli glutamine synthetase by xanthine oxidase, nicotinate hydroxylase, horseradish peroxidase, or glucose oxidase: effects of ferredoxin, putidaredoxin, and menadione. Arch Biochem Biophys. 1985 Jun;239(2):379–387. doi: 10.1016/0003-9861(85)90703-9. [DOI] [PubMed] [Google Scholar]
- Yuan P. M., Talent J. M., Gracy R. W. Molecular basis for the accumulation of acidic isozymes of triosephosphate isomerase on aging. Mech Ageing Dev. 1981 Oct;17(2):151–162. doi: 10.1016/0047-6374(81)90081-6. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Thienhaus O. J., Bosmann H. B. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res. 1989 Jan 2;476(1):160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]



