Abstract
Background
Most studies assessing the predictors of recurrent IgA nephropathy in the renal allograft have focused on post-transplant features. Identifying high risk pre-transplant features of IgA nephropathy is useful for counseling patients and may help tailor immunosuppression post-transplant.
Methods
We investigated the pre-transplant clinical and biopsy features of 62 patients with IgAN who received transplants at Columbia University Medical Center from 2001 to 2012 and compared the characteristics and outcomes of patients with IgAN recurrence to those without recurrence. The primary outcome was time to recurrent IgAN. Secondary outcomes were a composite of doubling of creatinine or allograft failure, and recurrent IgAN as a cause of allograft dysfunction.
Results
Of the 62 patients, 14 had recurrent IgAN in the allograft. Mean time to recurrence was 2.75 years. Those with recurrent disease were younger at time of native kidney biopsy (29 years vs. 41 years, P < 0.0009). Black race and Hispanic ethnicity composed a higher proportion of the recurrent disease group. On multivariable analysis, significant predictors of recurrent IgAN included age at diagnosis (HR 0.911, 95% CI 0.85 to 0.98), burden of crescents on native biopsy (HR 1.21 per 10% increase in crescents, 95% CI 1.00 to 1.47), and allograft rejection (HR 3.59, 95% CI 1.10 to 11.7)
Conclusions
Features of native IgAN can help predict the risk of recurrent disease in the renal allograft. In particular, immunologically active disease represented by earlier age of onset and greater burden of crescents on native biopsy is more likely to recur post-transplant.
Keywords: Crescentic glomerulonephritis, glomerulonephritis, graft function, IgA nephropathy, Kidney transplantation
Introduction
IgA nephropathy (IgAN) is a major contributor to the worldwide burden of end-stage renal disease (ESRD)1. Approximately 30 – 50% of patients with IgAN will progress to ESRD over 30 years2,3. Compared to the general ESRD population, IgAN patients are substantially more likely to be transplanted, receive transplants at a younger age, and have lower post-transplant mortality4–6. However, long term allograft survival is no better for IgAN patients compared to those with other causes of ESRD7, and this is at least partly due to disease recurrence.
IgAN may recur in the renal allograft in 9 – 61% of patients8. Recurrent disease was thought to have little impact on graft outcomes9. However, recent retrospective studies with longer duration of follow-up suggest that recurrent disease contributes substantially to allograft damage6,10,11. Risk factors for recurrence include presence of specific HLA genotypes, zero mismatch kidneys, high serum IgA levels, choice of induction therapy, choice of maintenance immunosuppression, and duration of follow-up post-transplant12–16 Post-transplant crescentic IgAN confers poorer allograft outcomes compared to post-transplant recurrence of non-crescentic IgAN17,18. Most studies assessing the predictors of recurrent IgAN have focused on post-transplant clinical features. Improving clinicians’ ability to estimate risk of recurrence prior to transplant would be useful, particularly for counseling patients and designing management strategies.
Here we investigated the pre-transplant clinical and biopsy features of patients with IgAN who received renal transplants at Columbia University Medical Center from 2001 to 2012 and compare the characteristics and graft outcomes of patients with IgAN recurrence to those without recurrent disease.
Methods
Subjects
During the period from January 2001 through December 2012, 2,256 adult (≥18 years of age) patients with unique medical record numbers underwent renal transplantation at Columbia University Medical Center (CUMC), including 150 with ESRD resulting from biopsy-documented IgAN. Only patients whose native kidney biopsies were read or reviewed at CUMC were included in this study. Secondary IgAN and SLE were excluded by history and clinical features. One patient had a history of Henoch-Schonlein Purpura and IgA nephropathy on biopsy. Clinical and pathology data for 62 of the 150 patients was retrospectively reviewed. Patients were stratified into two groups, IgAN recurrence and no-IgAN recurrence for comparison of risk factors.
Histopathological evaluation
All native biopsies were processed by standard techniques for light microscopy, immunofluorescence (IF), and electron microscopy (EM). Each biopsy was independently re-reviewed and scored by a single pathologist (P.R.). The diagnosis of IgAN was based on the presence of dominant or codominant staining for IgA by IF, with confirmatory EM findings of electron dense deposits in the majority of cases. Each biopsy was graded according to the Oxford classification (“MEST”), including scores for mesangial hypercelluarity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubulointerstitial fibrosis (T)19. Crescents were categorized as cellular, fibrocellular, or fibrous. Burden of crescents was calculated for each biopsy as the percentage of non-sclerotic glomeruli with cellular or fibrocellular crescents among all open glomeruli. Purely fibrous crescents were excluded from the numerator due to the lack of consensus in identifying fibrous crescents, and globally sclerotic glomeruli were excluded from the denominator.
Outcomes and definitions
The primary outcome was time to recurrence of IgAN. The two secondary outcomes were 1) Composite of doubling of serum creatinine (compared to post-transplant nadir) or allograft failure, and 2) Recurrent IgAN as a cause of allograft dysfunction (determined by the treating clinician with concurrence by the pathologist). We chose recurrence as the primary outcome because this can be diagnosed on biopsy and analyzed in an objective time-to-event fashion.
Pre-transplant variables collected included demographic data (age, gender, ethnicity), clinical presentation at time of biopsy, time from first biopsy to ESRD (defined as initiation of dialysis or transplantation), and duration of dialysis. Annualized loss of eGFR may have been a more meaningful measure of pre-transplant disease activity20, however creatinine at time of biopsy was not consistently documented. Post-transplantation data included allograft type (living vs. deceased donor), induction and maintenance immunosuppression, biopsy proven allograft rejection and grade, BK viremia and/or nephropathy, occurrence of proteinuria, nephrotic syndrome (defined as proteinuria > 3.5g per gram of creatinine, hypoalbuminemia, and clinical documentation), hypertension (defined as BP > 140/90 mmHg or need for antihypertensives to achieve a BP of < 140/90mmHg), graft loss (defined as return to dialysis or re-transplantation) and mortality.
Statistical Analysis
Descriptive statistics for continuous variables are presented as the mean ± standard deviation (SD) if parametric or median and interquartile range (IQR) if non-parametric. For categorical variables, data are presented as count with percentage. Inter-group comparisons were made by Student’s T test for normally distributed continuous variables, the Mann-Whitney U test for nonparametric continuous variables, and Fisher’s exact test for categorical variables. Univariable and multivariable Cox proportional hazards models were created for the time-dependent outcomes of recurrent IgAN and the composite of allograft failure or doubling creatinine. Logistic regression models were created for the outcome of recurrent IgAN as a cause of allograft dysfunction. All P-values were two-tailed and values less than 0.05 were considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical Characteristics Pre-transplant
We reviewed the charts and original native kidney biopsies of 62 IgAN patients who received a transplant at our center and whose native biopsies had been read at our center. Of these 62 patients, 14 had recurrent IgAN in the allograft. Baseline characteristics of the cohort, including demographics and biopsy features from the native diagnostic biopsy, are presented in Table 1. Those who experienced recurrent disease were significantly younger at time of native kidney biopsy than those who did not have recurrence (29 years vs. 41 years, P < 0.0009). Black race and Hispanic ethnicity composed a higher proportion of the recurrent disease group than the no-recurrence group. Progression from native kidney biopsy to ESRD was significantly more rapid in those who developed post-transplant IgAN recurrence versus those who did not recur (median 808 days vs. 1325 days, P = 0.02).
Table 1.
Baseline Characteristics Prior to Renal Transplantation
| Entire cohort N=62 |
Recurrent IgAN post-transplant N=14 |
No recurrence N=48 |
P | |
|---|---|---|---|---|
| Male sex | 40 (65%) | 11 (79%) | 29 (60%) | 0.34 |
| Race/Ethnicity | 0.003 | |||
| White | 35 (56%) | 6 (43%) | 29 (60%) | |
| Asian | 12 (19%) | 1 (7%) | 11 (23%) | |
| Latino | 11 (18%) | 3 (21%) | 8 (17%) | |
| Black | 4 (6%) | 4 (29%) | 0 | |
| Age at diagnosis, y | 38.1 ± 12.2 | 28.9 ± 9.4 | 40.8 ± 11.7 | 0.0009 |
| Time from diagnosis to ESRD, days | 1107 (512 to 2110) | 808 (100 to 1245) | 1325 (596 to 2218) | 0.02 |
| Time on dialysis, days* | 299 (138 to 464) | 827 (120 to 1069) | 402 (138 to 432) | 0.65 |
| Glomeruli on biopsy, n | 14 (9 to 19) | 17 (13 to 23) | 12 (7.5 to 19) | 0.11 |
| Globally sclerotic glomeruli, % | 50 (33 to 63) | 33 (29 to 50) | 55 (33 to 67) | 0.1 |
| Segmentally sclerotic glomeruli, % | 13 (0 to 21) | 7.7 (0 to 24) | 13 (0 to 21) | 0.82 |
| M1 | 62 (100%) | 14 (100%) | 48 (100%) | >0.99 |
| E1 | 31 (50%) | 10 (71%) | 21 (44%) | 0.13 |
| S1 | 43 (69%) | 9 (64%) | 34 (71%) | 0.74 |
| T0/1/2 | 9/26/27 | 2/8/4 | 7/18/23 | 0.38 |
| Interstitial fibrosis/tubular atrophy, % | 50 (40 to 70) | 50 (40 to 65) | 50 (37 to 70) | 0.38 |
| Fibrinoid Necrosis, any | 4 (6.6%) | 2 (15.4%) | 2 (4.2%) | 0.2 |
| Proportion of open glomeruli with cellular/fibrocellular crescents, % | 11.4 (0 to 25) | 28 (20 to 42) | 0 (0 to 19) | 0.0002 |
| Foot process effacement, % | 50 (30 to 75) | 70 (30 to 80) | 50 (30 to 70) | 0.16 |
Values are presented as Number (%), Mean ± SD, or Median (IQR)
33 patients received dialysis prior to transplant, 9 had recurrent IgAN and 24 did not.
Histopathological Characteristics
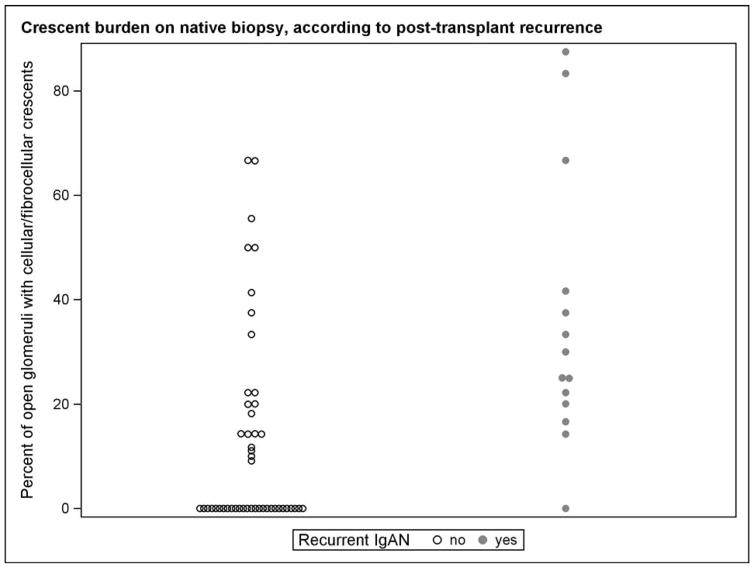
There were no differences in MEST classification, percent interstitial fibrosis and tubular atrophy, nor foot process effacement on native kidney biopsy in those who had post-transplant IgAN recurrence versus no recurrence (Table 1). All 62 patients were positive for the Oxford “M” classifier of mesangial hypercellularity. Of the 62 native biopsies, 47% had <10% crescents, 29% had 10–29% crescents, 11% had 30–49% crescents, and 13% had ≥50% crescents. Of those who had recurrent IgAN, the median percentage of open glomeruli containing cellular or fibrocellular crescents on native biopsy was 28% compared to 0% in those without recurrent disease (P = 0.0002). The distribution of crescents is shown in Figure 1. Among those with recurrent IgAN, 13/14 (93%) had at least 10% crescents on their native biopsies, whereas for those who did not recur only 20/48 (42%) had at least 10% crescents on their native biopsies (P = 0.007).
Figure 1.
Percentage of open glomeruli with cellular or fibrocellular crescents among patients with post-transplant recurrent IgAN (closed circles) and those without recurrent IgAN (open circles).
Clinical Characteristics Post-transplant
Characteristics of the kidney transplants and post-transplant course are summarized in Table 2. Age at transplant was significantly lower in those who had recurrent IgAN compared to those who had no recurrence (mean 32.5 years vs. 45.7 years, P < 0.01). Exposure to immunosuppression was similar between the groups. Rejection (either cellular or humoral), calcineurin toxicity (as documented in notes or pathology), and BK viremia was no different between the two groups. However, nadir creatinine (1.6 vs. 1.17, P = 0.005) and creatinine at last follow-up (2.01 vs. 1.21, P = 0.001) were significantly higher in those with recurrent IgA nephropathy.
Table 2.
Transplant and post-transplant characteristics
| Entire cohort N=62 |
Recurrent IgAN post-transplant N=14 |
No recurrence N=48 |
P | |
|---|---|---|---|---|
| Age at Transplant, y | 42.7 ± 13.0 | 32.5 ± 9.9 | 45.7 ± 12.4 | 0.0006 |
| Living donor, % | 46 (74%) | 12 (86%) | 34 (71%) | 0.32 |
| Preemptive, % | 29 (47%) | 5 (36%) | 24 (50%) | 0.38 |
| Thymoglobulin Induction | 49 (86%) | 8 (73%) | 41 (89%) | 0.17 |
| Standard maintenance therapy* | 48 (77%) | 10 (71%) | 38 (79%) | 0.72 |
| Corticosteroids | 12 (19%) | 4 (29%) | 8 (17%) | 0.44 |
| ACEI and/or ARB use | 26 (42%) | 8 (57%) | 18 (38%) | 0.23 |
| Hypertension | 41 (66%) | 9 (64%) | 32 (67%) | >0.99 |
| Diabetes mellitus | 12 (29%) | 2 (14%) | 10 (21%) | 0.72 |
| Any allograft biopsy | 47 (76%) | 14 (100%) | 33 (69%) | 0.01 |
| Rejection, any | 17 (27%) | 6 (43%) | 11 (23%) | 0.18 |
| Rejection, cellular | 15 (24%) | 6 (43%) | 9 (19%) | 0.08 |
| Rejection, humoral | 5 (8.0%) | 1 (7.1%) | 4 (8.3%) | >0.99 |
| BKV | 13 (21%) | 3 (21%) | 10 (21%) | >0.99 |
| Calcineurin inhibitor toxicity | 8 (13%) | 1 (7.1%) | 7 (15%) | 0.67 |
| Nadir serum Cr, mg/dl | 1.23 (1.00 to 1.50) | 1.60 (1.40 to 2.00) | 1.17 (0.98 to 1.40) | 0.005 |
| Serum Cr at IgAN Recurrence, mg/dl | . | 2.20 (1.75 to 2.74) | . | NA |
| Proteinuria at IgAN Recurrence, mg/gCr | . | 590 (319 to 977) | . | NA |
| Microhematuria at Recurrence | . | 7 (78%) | . | NA |
| Serum Creatinine at last followup, mg/dl (N=51) | 1.30 (1.09 to 1.65) | 2.01 (1.55 to 2.69) | 1.21 (1.01 to 1.55) | 0.001 |
| Proteinuria at last followup, mg/gCr (N=40) | 114 (100 to 367) | 541 (187 to 871) | 100 (100 to 252) | 0.06 |
| Microhematuria at last followup (N=31) | 8 (26%) | 3 (60%) | 5 (19%) | 0.09 |
| Allograft failure | 11 (18%) | 6 (43%) | 5 (10%) | 0.01 |
| Doubled serum creatinine | 11 (28%) | 7 (50%) | 4 (8.3%) | 0.001 |
| Composite outcome | 12 (29%) | 7 (50%) | 5 (10%) | 0.003 |
| Time from transplant to event or censoring, days | 1763 (1154 to 2961) | 1487 (1112 to 2566) | 1901 (1209 to 2978) | 0.38 |
Values are presented as Number (%), Mean ± SD, or Median (IQR). Cr, creatinine.
Defined as calcineurin inhibitor plus mycophenolate mofetil or mycophenolic acid.
Recurrence and treatment
Mean time to diagnosis of recurrent IgAN on allograft biopsy was 2.75 years after transplant (median 1.25 years). At the time of recurrence the median proteinuria was 590 mg/g creatinine and the median serum creatinine was 2.2 g/dL. Of the 14 recurrences, nine did not receive specific therapy beyond standard transplant immunosuppression, two patients received high-dose corticosteroid therapy alone, two patients received corticosteroid therapy and then rituximab therapy for a second recurrence, and one patient received corticosteroid therapy plus rituximab therapy for a second recurrence plus ACTH for a third recurrence.
Primary Outcome
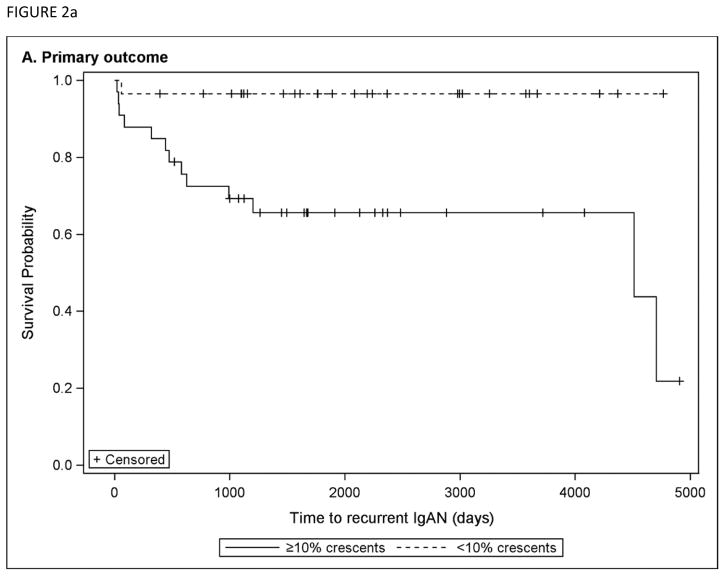
Univariable hazard ratios for the primary outcome of time to recurrent IgAN after transplant are presented in Table 3. By univariable analyses, significant predictors of recurrent IgAN in the allograft included age at diagnosis, black race, burden of crescents on native biopsy, and a diagnosis of allograft rejection. There was a suggestion that a longer time from diagnosis to ESRD was associated with a lower hazard of recurrent IgAN, but this did not reach statistical significance (HR 0.79, 95% CI 0.61 – 1.03, per year). In sensitivity analyses, a greater amount of crescents remained significantly associated with shorter time to recurrence when examined by tertile or as a binary variable. Figure 2a shows Kaplan-Meier curves for time to recurrent IgAN for those with <10% crescents vs. ≥10% crescents on native biopsy.
Table 3.
Univariable cox proportional hazards for time from transplant to diagnosis of recurrent IgAN
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Baseline characteristics | |||
| Age at diagnosis (per year) | 0.91 | 0.85 – 0.97 | 0.003 |
| Male sex | 2.56 | 0.70 – 9.35 | 0.15 |
| Black race (vs. other) | 7.14 | 2.09 – 24.4 | 0.002 |
| Time from diagnosis to ESRD (per year) | 0.79 | 0.61 – 1.03 | 0.09 |
| Native biopsy characteristics | |||
| Proportion of open glomeruli with cellular/fibrocellular crescents (per 10% increase) | 1.32 | 1.10 – 1.57 | 0.003 |
| E1 | 2.61 | 0.81 – 8.35 | 0.11 |
| S1 | 0.72 | 0.24 – 2.16 | 0.56 |
| T1 (vs T0) | 1.18 | 0.25 – 5.62 | 0.84 |
| T2 (vs T0) | 0.49 | 0.085 – 2.77 | 0.42 |
| IgG deposition (vs none) | 0.91 | 0.24 – 3.43 | 0.89 |
| Transplant characteristics | |||
| Preemptive transplant | 0.55 | 0.18 – 1.65 | 0.29 |
| Living donor | 1.75 | 0.38 – 8.00 | 0.47 |
| Rejection, any | 3.26 | 1.05 – 10.2 | 0.04 |
| Thymoglobulin Induction (vs. other) | 0.58 | 0.12 – 2.75 | 0.49 |
| Standard maintenance therapy (vs. other) | 0.94 | 0.28 – 3.16 | 0.92 |
| Corticosteroid use | 2.03 | 0.62 – 6.63 | 0.24 |
| ACEI and/or ARB use post-transplant | 1.95 | 0.68 – 5.63 | 0.22 |
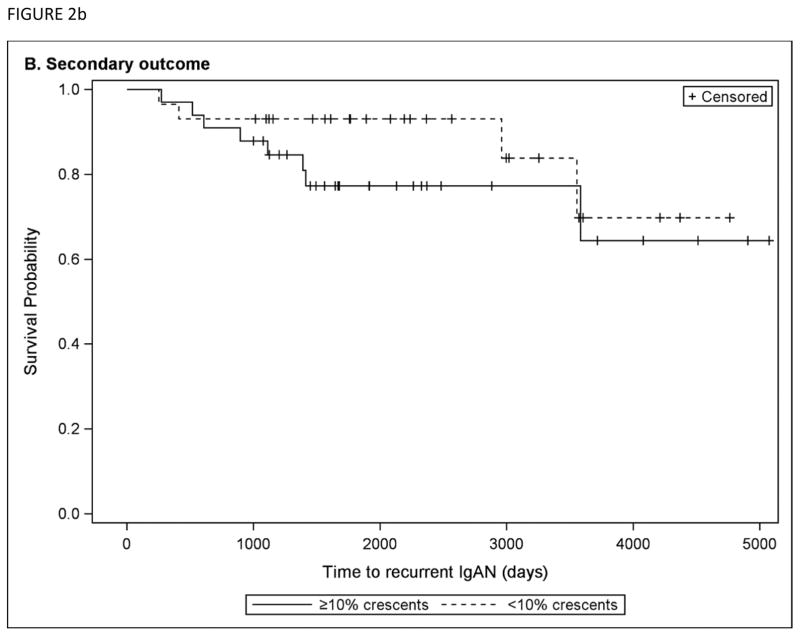
Figure 2.
Kaplan-Meier curves are shown comparing subjects with <10% crescents vs. ≥10% crescents on native biopsy, for a) the primary outcome of time to recurrent IgAN post-transplant and b) the secondary composite outcome of doubling creatinine post-transplant or allograft failure.
On multivariable analysis, significant predictors of recurrent IgAN included age at diagnosis (HR 0.911, 95% CI 0.85 to 0.98), burden of crescents on native biopsy (HR 1.21 per 10% increase in crescents, 95% CI 1.00 to 1.47), and allograft rejection (HR 3.59, 95% CI 1.10 to 11.7), after adjusting for sex, ethnicity, time to ESRD, oxford “E” lesion on native biopsy, steroid use, and angiotensin-converting-enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) use post-transplant.
Secondary Outcomes
For the composite secondary outcome of doubling of serum creatinine post-transplant or allograft failure, significant predictors in univariable Cox analyses included allograft rejection (HR 6.50, 95% CI 1.97 to 21.4), recurrent IgAN (HR 4.94, 95% CI 1.56 to 15.6), and nadir serum creatinine (HR 2.49 per each 0.5 g/dL increase, 95% CI 1.48 to 4.18). On multivariable analysis, the only significant predictors of the composite outcome were allograft rejection (HR 4.76, 95% CI 1.40 to 16.1) and nadir serum creatinine (HR 2.36 per each 0.5 g/dL increase, 95% CI 1.29 to 4.33), whereas recurrent IgAN was no longer a significant predictor. Burden of crescents did not significantly predict the composite outcome (univariable P = 0.36). Figure 2b shows Kaplan-Meier curves for time to the composite outcome are shown for the group with <10% crescents vs. ≥10% crescents on native biopsy.
For the secondary outcome of recurrent IgAN as a cause of allograft dysfunction, univariable logistic regression showed significant associations with age at diagnosis (OR 0.89 per year, 95% CI 0.82 to 0.97), burden of crescents on native biopsy (OR 1.52 per 10% increase in crescents, 95% CI 1.13 to 2.02), and the presence of the Oxford “E” lesion on native biopsy (OR 12.3, 95% CI 1.45 to 104. There was a suggestion of a protective effect with the use of rabbit antithymocyte globulin induction, albeit not reaching statistical significance (OR 0.19, 95%CI 0.03 to 1.04, P = 0.06). Variables that were not significantly associated with recurrent IgAN as a cause of allograft dysfunction included sex, race/ethnicity, preemptive transplant, living donor transplant, any rejection, type of maintenance immunosuppression, use of corticosteroids, or post-transplant ACEI or ARB use. In a multivariable model, age at diagnosis (OR 0.89 per year, 95%CI 0.81 to 0.98) and burden of glomeruli on native biopsy (OR 1.55, 95% CI 1.07 to 2.25) remained significantly associated with the risk of recurrent IgAN causing allograft dysfunction, after adjusting for age, sex, and ethnicity.
Discussion
We retrospectively analyzed a cohort of patients with IgAN who had native biopsies reviewed and underwent transplantation at Columbia University Medical Center. We compared the pre-transplant clinical and biopsy features of patients with recurrent IgAN to those who had no recurrence. We found that patients who developed recurrent IgAN were younger and had a more rapid progression to ESRD from their native disease. On multivariable analysis, predictors of recurrent IgAN were younger age, burden of crescents in the native kidney biopsy, and allograft rejection. Recurrent IgAN was itself a predictor of doubling of serum creatinine and graft failure.
Though the rate of recurrence of IgAN is high (9 – 61% in the literature; 22.5% in this study), the rate of graft loss due to IgAN is thought to be low (3–4%)8,21. Moroni et al11 analyzed the post-transplant outcomes of 190 patients with IgAN transplanted between 1981 and 2010. The rate of IgAN recurrence was 37.2%. Of these patients, about half experienced allograft loss and about half of the graft losses were due to recurrent IgAN. In univariate analysis, predictors of IgA recurrence included year of transplant, younger age of recipient, maintenance immunosuppression without mycophenolate, and use of less than three immunosuppressive agents. IgAN was an independent predictor of worse graft outcomes compared to non-diabetic controls. Choy et al10 examined the post-transplant outcomes of 75 patients with IgAN transplanted between 1984 and 2001 and found recurrent disease in 19% of patients. Of these patients, roughly a third had graft failure of which 60% were due to IgAN. Patients with IgAN had superior outcomes to controls without IgAN up until 12 years post-transplant when patients with IgAN had poorer graft outcomes. Similar to our study, Choy et al did not find donor type (living vs. deceased) and immunosuppression to be significant predictors of recurrence.
Clayton et al14 found recurrent IgAN as the cause of graft loss in 12.6% of patients transplanted for IgAN. Interestingly they also found that patients who were corticosteroid-free at one year were more likely to have graft loss from IgAN than patients who received corticosteroids. Our study did not find that long term (>1 year) use of corticosteroids reduced the risk of recurrence. We found a suggestion that antithymocyte globulin induction was associated with a longer time to recurrence compared to other types of induction (mostly basiliximab in this cohort). While this hazard ratio did not reach statistical significance, the effect size was clinically important, and in line with the findings of Berthoux et al22 who also found a protective effect with antithymocyte globulin.
Our study found that a greater burden of crescents on the native biopsy was associated with a shorter time to recurrence of IgAN post-transplant, and was also associated with a greater likelihood that the recurrent IgAN was pathogenic. We did not find that the burden of native crescents affected the composite outcome of doubling serum creatinine or allograft failure, which appeared to be largely driven by nadir creatinine (likely a proxy for allograft quality) and subsequent rejection. Our findings support the hypothesis that crescentic IgAN represents a more severe and aggressive form of disease, and thus the pathophysiologic process leading to severe disease in the native kidney may lead to more aggressive recurrent disease in the transplanted kidney. In native IgAN, higher grades of proteinuria, hypertension, and impaired kidney function are predictors of poor clinical outcomes23. However, we did not find these variables to be of significant value in predicting post-transplant recurrence. Thus, we hypothesize that crescents may be more indicative of immunologic disease activity, and thus recurrence risk, while these other factors may be confounded by disease chronicity.
Clinically, patients with crescentic IgAN have a higher incidence of hypertension and nephrotic syndrome24,25. In IgAN, crescents are associated with decreased renal survival, with an inverse correlation between the number of crescents and duration of renal survival26,27. Lv et al found that in crescentic IgAN, defined as >50% crescents, progression to ESRD was as high as 70% at five years28. Though the prognostic value of crescents was not systematically assessed in the Oxford cohort29 due to low prevalence, other retrospective studies that have sought to investigate the prognostic value of crescents when added to the MEST score show conflicting results27,30,31. Moreover, the percent of crescents that define a biopsy as crescentic IgAN has been variably defined, ranging from any crescents to 50% glomerular involvement27,30,32. The largest systematic review and meta-analysis to examine the Oxford Classification found that the presence of any crescent had independent prognostic importance, and the authors proposed adding a “C” lesion (for any crescent) to the classification33. Our study supports the association of crescents with adverse outcomes, and further suggests a dose effect where a greater percentage of crescentic glomeruli is associated with higher risks of pathogenic recurrence.
There are several limitations of our study besides its retrospective design and single-center design. The sample size is small with only 62 patients as we designed the study to include only those who had their native biopsies reviewed at Columbia. A larger study including multiple centers would have sacrificed the ability to examine histology and other variables in detail. Because protocol biopsies were not done routinely, subclinical recurrent IgAN cannot be excluded in the 31% of patients with “no recurrence” who had not undergone renal allograft biopsy. Similarly, all subjects with recurrent IgAN had a for-cause biopsy for allogfraft dysfunction, and thus this group was likely enriched with rejection events. Our finding of an association between allograft rejection and time to recurrent IgAN was likely due to a confounding association. We found that Hispanic ethnicity and Black race were more prevalent in the recurrent IgAN group, and that black race predicted recurrent IgAN on univariable analysis, but this was based on small numbers (only 4 black patients in the study, all of whom recurred.) Other studies have produced conflicting data on whether black race portends worse prognosis in IgAN34,35. A strength of the study is that all patients received transplants after 2001 which makes for a more standardized induction and maintenance immunosuppression regimen compared to previous studies that included patients from prior eras.
Our study adds to the growing literature examining the impact of recurrent IgAN on long-term allograft outcomes. Importantly, we have found that younger age at diagnosis and crescentic disease in the native kidney are strongly associated with earlier post-transplant IgAN recurrence. This may be helpful in counseling IgAN patients on their expectations prior to transplant, on selecting post-transplant immunosuppressive regimens, and/or in designing strategies for managing recurrent disease. In addition to histologic and clinical predictors of recurrence, future prospective studies may include measurements of novel biomarkers of disease activity to predict recurrent IgAN36. Continuing to hone our predictive ability is important given our findings and those of others that suggest recurrent IgAN is associated with poorer allograft outcomes.
Acknowledgments
Dr. Bomback is supported by National Institutes of Health-National Institute on Minority Health and Health Disparities grant R01-MD009223
Footnotes
Conflict of Interest Statement: The results presented in this paper have not been published previously and are not being considered for publication elsewhere.
Disclosures: None
Financial Disclosures: Dr. Bomback is supported by National Institutes of Health-National Institute on Minority Health and Health Disparities grant R01-MD009223
References
- 1.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89:209–215. doi: 10.1016/0002-9343(90)90300-3. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 3.Moriyama T, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PloS one. 2014;9:e91756. doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shaughnessy MM, Montez-Rath ME, Lafayette RA, Winkelmayer WC. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol. 2015;10:1170–1178. doi: 10.2215/CJN.11261114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saran R, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67:A7–8. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyld ML, Chadban SJ. Recurrent IgA Nephropathy After Kidney Transplantation. Transplantation. 2016 doi: 10.1097/TP.0000000000001093. [DOI] [PubMed] [Google Scholar]
- 7.Andresdottir MB, Haasnoot GW, Doxiadis II, Persijn GG, Claas FH. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: a retrospective analysis of registry data. Transplantation. 2005;80:1012–1018. doi: 10.1097/01.tp.0000179150.84803.56. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C, Glassock RJ. Posttransplant Recurrence of Primary Glomerulonephritis. Clinical Journal of the American Society of Nephrology. 2010;5:2363–2372. doi: 10.2215/cjn.06720810. [DOI] [PubMed] [Google Scholar]
- 9.Berger J. Recurrence of IgA nephropathy in renal allografts. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1988;12:371–372. doi: 10.1016/s0272-6386(88)80027-1. S0272638688001362 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Choy BY, Chan TM, Lo SK, Lo WK, Lai KN. Renal transplantation in patients with primary immunoglobulin A nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:2399–2404. doi: 10.1093/ndt/gfg373. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:1305–1314. doi: 10.1093/ndt/gfs472. [DOI] [PubMed] [Google Scholar]
- 12.Andresdottir MB, Haasnoot GW, Persijn GG, Claas FH. HLA-B8, DR3: a new risk factor for graft failure after renal transplantation in patients with underlying immunoglobulin A nephropathy. Clinical transplantation. 2009;23:660–665. doi: 10.1111/j.1399-0012.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang AY, et al. Recurrent IgA nephropathy in renal transplant allografts. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;38:588–596. doi: 10.1053/ajkd.2001.26885. [DOI] [PubMed] [Google Scholar]
- 14.Clayton P, McDonald S, Chadban S. Steroids and recurrent IgA nephropathy after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1645–1649. doi: 10.1111/j.1600-6143.2011.03667.x. [DOI] [PubMed] [Google Scholar]
- 15.Odum J, et al. Recurrent mesangial IgA nephritis following renal transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1994;9:309–312. [PubMed] [Google Scholar]
- 16.Bachman U, et al. The clinical course of IgA-nephropathy and Henoch-Schonlein purpura following renal transplantation. Transplantation. 1986;42:511–515. doi: 10.1097/00007890-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Jeong HJ, et al. Glomerular crescents are responsible for chronic graft dysfunction in post-transplant IgA nephropathy. Pathology international. 2004;54:837–842. doi: 10.1111/j.1440-1827.2004.01751.x. [DOI] [PubMed] [Google Scholar]
- 18.Kowalewska J, et al. IgA nephropathy with crescents in kidney transplant recipients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;45:167–175. doi: 10.1053/j.ajkd.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Cattran DC, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 20.Bjorneklett R, et al. Pre-transplant course and risk of kidney transplant failure in IgA nephropathy patients. Clin Transplant. 2011;25:E356–365. doi: 10.1111/j.1399-0012.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- 21.Ponticelli C, et al. Kidney transplantation in patients with IgA mesangial glomerulonephritis1. Kidney Int. 2001;60:1948–1954. doi: 10.1046/j.1523-1755.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 22.Berthoux F, et al. Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation. 2008;85:1505–1507. doi: 10.1097/TP.0b013e3181705ad4. [DOI] [PubMed] [Google Scholar]
- 23.D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Seminars in nephrology. 2004;24:179–196. doi: 10.1016/j.semnephrol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Bitencourt-Dias C, Bahiense-Oliveira M, Saldanha LB, Barros RT, Woronik V. Comparative study of IgA nephropathy with and without crescents. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica … [et al.] 2004;37:1373–1377. doi: 10.1590/s0100-879x2004000900012. /S0100-879X2004000900011. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, et al. Predicting progression of IgA nephropathy: new clinical progression risk score. PloS one. 2012;7:e38904. doi: 10.1371/journal.pone.0038904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe T, et al. Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol. 1986;25:37–41. [PubMed] [Google Scholar]
- 27.Lee MJ, et al. Clinical implication of crescentic lesions in immunoglobulin A nephropathy. Nephrology Dialysis Transplantation. 2014;29:356–364. doi: 10.1093/ndt/gft398. [DOI] [PubMed] [Google Scholar]
- 28.Lv J, et al. Prediction of Outcomes in Crescentic IgA Nephropathy in a Multicenter Cohort Study. Journal of the American Society of Nephrology. 2013;24:2118–2125. doi: 10.1681/asn.2012101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Oxford classification of IgA nephropathy: pathology definitions, correlations and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 30.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation Study of Oxford Classification of IgA Nephropathy: The Significance of Extracapillary Proliferation. Clinical Journal of the American Society of Nephrology. 2011;6:2806–2813. doi: 10.2215/cjn.02890311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi S-F, et al. Pathologic Predictors of Renal Outcome and Therapeutic Efficacy in IgA Nephropathy: Validation of the Oxford Classification. Clinical Journal of the American Society of Nephrology. 2011;6:2175–2184. doi: 10.2215/cjn.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:1321–1329. doi: 10.1093/ndt/gfg081. [DOI] [PubMed] [Google Scholar]
- 33.Lv J, et al. Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:891–899. doi: 10.1053/j.ajkd.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo AH, et al. Predictors of outcome for severe IgA Nephropathy in a multi-ethnic U.S. cohort. Clin Nephrol. 2015;84:145–155. doi: 10.5414/CN108556. [DOI] [PubMed] [Google Scholar]
- 35.Hall YN, Fuentes EF, Chertow GM, Olson JL. Race/ethnicity and disease severity in IgA nephropathy. BMC nephrology. 2004;5:10. doi: 10.1186/1471-2369-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berthelot L, et al. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int. 2015;88:815–822. doi: 10.1038/ki.2015.158. [DOI] [PubMed] [Google Scholar]