Abstract
Current optogenetic methodology enables precise inhibition or excitation of neural circuits, spanning timescales as needed from the acute (milliseconds) to the chronic (many days or more), for experimental modulation of network activity and animal behavior. Such broad temporal versatility, unique to optogenetic control, is particularly powerful when combined with brain activity measurements that span both acute and chronic timescales as well. This enables, for instance, the study of adaptive circuit dynamics across the intact brain, and tuning interventions to match activity patterns naturally observed during behavior in the same individual. Although the impact of this approach has been greater on basic research than on clinical translation, it is natural to ask if specific neural circuit activity patterns discovered to be involved in controlling adaptive or maladaptive behaviors could become targets for treatment of neuropsychiatric diseases. Here we consider the landscape of such ideas related to therapeutic targeting of circuit dynamics, taking note of developments not only in optical but also in ultrasonic, magnetic, and thermal methods. We note the recent emergence of first-in-kind optogenetically-guided clinical outcomes, as well as opportunities related to the integration of interventions and readouts spanning diverse circuit-physiology, molecular, and behavioral modalities.
INTRODUCTION
Over the past half-century, electrical, genetic, and pharmacological interventions have been developed and applied to obtain causal insights into the functional significance of nervous system activity. Discoveries ranging from the delineation of critical periods in the developing brain, to the characterization of perceptual and memory processes in the adult brain, have emerged from studies using these diverse interventions as basic neuroscience tools in laboratory animals. Meanwhile in the clinical realm, pharmacological interventions for neuropsychiatric disease states have greatly increased in number over the same time period (though actual therapeutic impact and conceptual insight have not kept pace with the proliferation in medication options). Recent years have also witnessed the emergence of new electrical interventions in the clinical setting—supplementing the small toolkit that was long largely limited to electroconvulsive therapy (ECT, used to treat certain psychiatric diseases such as major depression by eliciting brainwide seizure activity). Newer electromagnetic therapies such as transcranial magnetic stimulation (TMS, currently approved for psychiatric clinical use in major depression) and deep brain stimulation (DBS, currently approved for Parkinson’s disease and other neurological conditions) are targeted more focally than ECT, but also (thus far) tend to be less effective in psychiatric disease.
With these newer electromagnetic stimulation modalities, one brain region is targeted. For example, currently-approved use of TMS for depression involves repetitive focal stimulation of left dorsolateral prefrontal cortex with parameters chosen to have the best chance of increasing activity in this directly-targeted region, and DBS for Parkinson’s disease involves high-frequency current pulses typically delivered to the subthalamic nucleus with the goal of decreasing activity in the directly-targeted region. In contrast, what properties might define a circuit target, or more precisely a cell type-resolved circuit-dynamical target? Among the intriguing possibilities, one could imagine temporally-precise tuning of the activity of a brain cell population, defined by cell body origin and axonal termination target, to resolve the most debilitating symptom domain of a patient’s affective disorder. Another example might involve detecting in real time pathological shifts in activity balance between cell types or among several brain-spanning circuits, followed by appropriate cellular-resolution compensation to terminate the incipient pathological state. In laboratory animal subjects, such cellular-level control over local and global neural circuit activity dynamics is now commonplace; indeed, over the last ten years, the development of genetically-encoded optical tools has led to many examples in which such population-level fast circuit dynamical processes have been identified and shown to control physiological and disease-related behavior (Deisseroth, 2015).
While these cell-type and circuit element-specific observational and interventional tools have illuminated clinical questions in animal models of disease, direct therapeutic application of these methods to the human brain has not yet occurred. Such direct translation would require gene delivery to targeted human brain cells to produce light-responsive proteins, as well as light delivery deep into opaque and photon-scattering human brain tissue. Emerging non-optical modalities (Figure 1A–F; including magnetic, thermal, ultrasonic, and chemical components) similarly require gene delivery, and may extend depth penetration of control in direct clinical applications compared with optical methods, but typically at the expense of spatial or temporal precision. Shared features unify this broad field; for example, engineering challenges are common to all approaches, since energy delivery of any kind (in electrical, optical, or other forms) will require hardware-based brain interfaces designed to be minimally-invasive, biocompatible, stable over chronic clinical timescales, cost-effective, and energy-efficient. And regardless of modality, therapeutic control of neural activity may be best realized in closed-loop configurations (wherein observations of endogenous activity can be used to inform interventional activity manipulations in real-time; Grosenick et al., 2015). Such challenges are currently driving interdisciplinary innovation in both device-hardware engineering and computational methods.
Figure 1. Technologies for targeting specified regions & circuits.
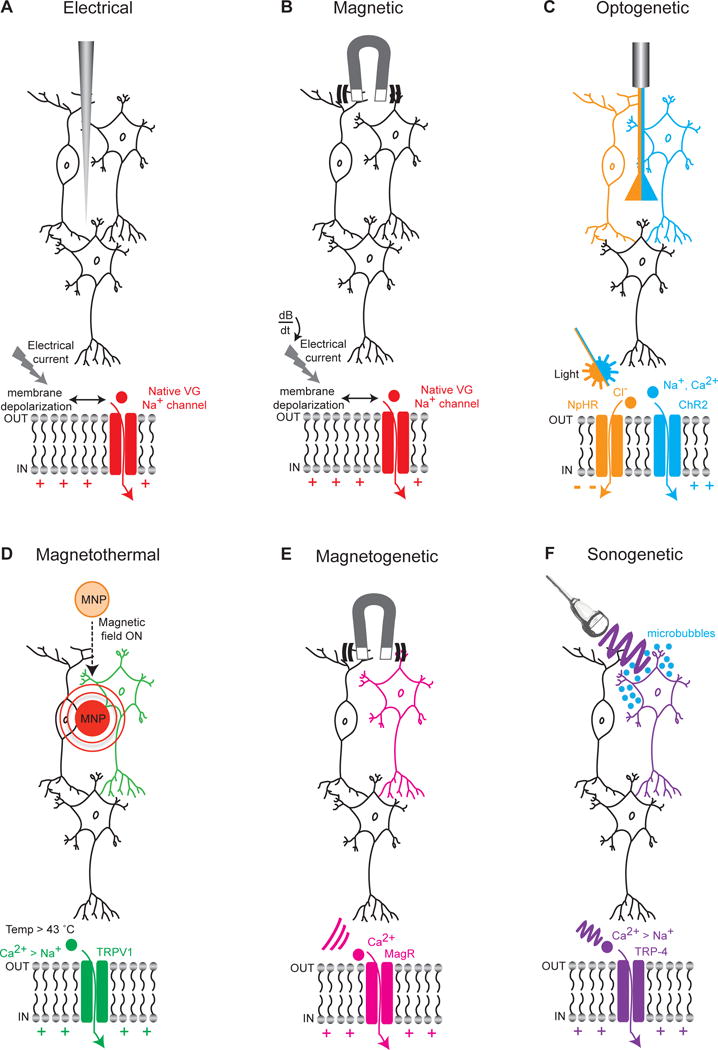
(A) Extracellular electrical stimulation of neurons through application of current; with regional targeting capability but without neuron-type specificity, capacitance currents are driven that lead to membrane depolarization, opening of native voltage-gated sodium channels, and further depolarization of the membrane with spike firing. (B) Stimulation of neurons through application of a transcranial magnetic field; again with regional targeting capability but without cell-type specificity, rapidly-changing magnetic fields (dB/dt) induce electrical currents in tissue and spike firing as in (A). (C) Cell type-specific optical stimulation or inhibition of neurons expressing light-sensitive excitatory cation channels (for example the channelrhodopsin ChR2) or inhibitory pumps (for example the halorhodopsin NpHR) that are expressed in a cell-type specific manner (here depicted by NpHR expressed specifically in the orange cell and ChR2 specifically in the blue cell). (D) Magnetothermal activation of neurons through application of a magnetic field targeted toward magnetic nanoparticles (MNP) that can transduce magnetic into thermal energy capable of opening heat sensitive (TRPV1) depolarizing channels, which can be expressed in a cell-type specific manner (here depicted as the green neuron). Generalizable AAV-based cell-type targeting strategies as developed for optogenetics may also be used to target TRP channels. (E) Application of a magnetic field that can open magnetically-sensitive channels (MagR) allowing the influx of Ca2+ with the potential for cell cell type-specific activation (here depicted as the pink neuron). (F) Ultrasonic activation of neurons through application of low pressure ultrasound waves that are transduced by gas-filled microbubbles into mechanical energy sufficient to open mechanosensitive (TRP-4) channels, which might also be expressed in a cell-type specific manner (here depicted as the purple neuron).
Framed by these active clinical and preclinical scientific challenges, here we consider the present state (and future possibilities) of targeting circuits. While simply-defined locations in the brain currently already serve as clinical targets for electrical intervention, the precise targeting of cellular-resolution circuit-dynamics can be routinely only carried out in animals, and with optical methods. A key emerging theme is that approaches spanning the novel and the traditional (e.g. optogenetically-guided electromagnetic or combined electromagnetic/pharmacological interventions, without even introducing genes or devices) may be especially powerful. We highlight ongoing opportunities and challenges relating to the development of such integrated formulations, for clinical or basic science application, and in central or peripheral nervous system disorders.
Optical control: opsins, hardware, and software for large brain volumes
Microbe-derived genetically-encoded optically-activated conductance regulators such as halorhodopsin (HR), channelrhodopsin (ChR), bacteriorhodopsin (BR), and their many variants, have been developed into a diverse palette of tools for single-component and precisely-timed control of targeted cell populations in freely-moving animals (Deisseroth, 2015). This experimental approach is termed optogenetics (Figure 1C), for which recent developments in opsin engineering are particularly relevant to large-brained animal subjects in general, and potentially to human and non-human primates. The development of excitatory ChR derivatives that flux cations in response to redder light than the initially-identified blue-responsive ChRs (redshifted variants include VChR1, C1V1, ReaChR, Chrimson and bReaChES; Zhang et al., 2008; Yizhar et al., 2011; Lin et al., 2014; Klapoetke et al., 2014; Grosenick et al., 2015; Rajasethupathy et al., 2015) extends optical stimulation to moderately greater depths in large brains due to reduced scattering of red-shifted light (Yizhar et al., 2011). Far more extensive volume recruitment may be obtained by increasing operational light sensitivity (Mattis et al., 2011) via prolonging the opsin deactivation time (Berndt et al., 2008; Yizhar et al., 2011); among many other applications, this step-function opsin (SFO) class of excitatory channelrhodopsin with orders-of-magnitude greater light-sensitivity at the cellular level has recently been found to be useful for studying brainwide circuit-dynamical underpinnings of anhedonia and abnormal social behavior, both representing major psychiatric symptom domains (Ferenczi et al., 2016; Yizhar et al., 2011). The greatest utility of the fast red light-activated ChRs, rather than for depth penetration, instead may be for enhanced compatibility with optical readouts such as blue light-activated genetically encoded Ca2+ indicators (Akerboom et al., 2013, Chen et al., 2013, Inoue et al., 2015; Deisseroth & Schnitzer 2013). For example, such all-optical combinations (Rajasethupathy et al., 2015; Grosenick et al., 2015; Packer et al., 2015; Rickgauer et al., 2014; Kim et al., 2016) may be useful for closed-loop configurations in basic or clinically-inspired applications, wherein observed neural activity is fed back to modify manipulations of neural activity in real time (reviewed in Grosenick et al., 2015).
A major challenge for real-time circuit-dynamical intervention in large-brained organisms has been achieving inhibition over wide brain areas, since for many years the only microbial opsins suitable for inhibition were pumps (HR and BR derivatives) rather than channels, which while broadly useful in laboratory animals, tend to be less operationally light-sensitive than channels because only one ion is transported per photon by pumping (instead of the hundreds that can be moved across the membrane by channel mechanisms; reviewed in Deisseroth, 2015). Moreover pumps cannot be converted into the SFO form to achieve the highest levels of operational light sensitivity. However, single-component inhibitory channels have at long last been engineered and discovered over the past two years, beginning with the structure-guided development of light-sensitive chloride channels (Berndt et al., 2014; Wietek et al., 2014), which were made significantly more potent, light-sensitive, and functional for behavioral control with further engineering including via application of the SFO mutations; Berndt et al., 2008, 2014, 2015). This initial discovery was followed by identification of naturally-occurring chloride channels (Govorunova et al., 2015) which share ion-conduction pathway and selectivity properties of the engineered chloride channels (Berndt and Deisseroth, 2015; Berndt et al., 2015), and separately by the engineering of a light-activated potassium channel, also inhibitory in neural systems (Cosentino et al., 2015). In the latter study, the authors fused a small viral potassium channel to the photosensitive LOV2-Jα domain of the plant blue-light receptor, with several additional modifications to reduce dark activity; the resulting single-component hyperpolarizing tool (BLINK1) was capable of modulating escape behavior in transparent zebrafish larvae. Although together these studies show promise for basic science, it remains to be demonstrated that the light sensitivity of these inhibitory channels will functionally suffice for behavioral control in primate brains.
An independent strategy to improve large-volume recruitment (and enable new kinds of cell –type targeting) has been development of specialized optical hardware to bypass or overcome scattering, building on the basic initial design of the laser diode-coupled fiberoptic methods for delivering light to deep brain structures in freely-moving mammals (Aravanis et al., 2007; Adamantidis et al., 2007). Many fibers can be employed if needed in freely-moving animals (Kim et al., 2016), and the thin, flexible structure of the now widely-used fiberoptic interface (2–3x smaller in diameter than 1.4 mm clinical DBS electrodes) plays several useful and clinically-relevant roles. For instance, these fiberoptic interfaces allow targeting of cells by virtue of their projection anatomy (a crucial capability for human and nonhuman primates; reviewed in Deisseroth, 2014), and additionally enable not just delivery but also collection of light from deep-brain populations and projections during behavior for activity-guided and closed-loop control (Gunaydin et al., 2014; Grosenick et al., 2015; Lerner et al., 2015; Kim et al., 2016; Zalocusky et al., 2016)– while also separating from the tissue the major heat-generating elements of the device, thus avoiding local damage as well as direct nonspecific excitation of the neural tissue.
While direct implantation of LEDs poses such a risk for local heating (except when opsins such as SFOs are used, that are bistable or extremely light sensitive), new kinds of LED engineering may offer other capabilities (Montgomery et al., 2015). For example, ultrathin (50×50×6 μm) inorganic gallium nitride LEDs can be directly injected into the brain and controlled remotely by a wireless receiver mounted above the skull (0.7 g; Jeong et al., 2015). This provides neural control with minimal hindrance to the subject and the potential for substantial scale-up. In addition to the miniaturized nature of this device, other advantages stem from the fact that the LED strips can be printed in many different configurations (e.g. horizontal configurations might be more ideal for targeting brain regions that cover large rostro-caudal extents such as the cingulate gyrus or the hippocampus). Though implanted LEDs by themselves (unlike the fiberoptic interface) cannot deliver feedback information regarding local neural activity, other kinds of flexibility may be enabled. For example by binding the LED strips with microfluidic channels that can simultaneously release pharmacologic agents on demand, or incorporate other flexible devices that can detect or actuate electrical, mechanical, or thermal information simultaneously (Canales et al., 2015).
Beyond optical actuation: magnetic, thermal, and ultrasonic
While existing optical methods are well-suited for use in animal subjects, clinical application of optical control and readout methods will be limited by the size and scattering-related opacity of the human brain. Guided by the optogenetic principle of targeting single-component energy receptors for cellular-resolution control, non-optical methods for modulating neural activity are therefore being explored. For instance, cell type-targeted control of neural activity could be accomplished by transducing a magnetic signal with targeted receptors analogous to microbial opsins; magnetic fields can penetrate more deeply than light into tissue. In one such approach, genetically-encoded ferritin fused to heat-sensitive TRPV1 channels was found to transduce magnetic energy into thermal and/or mechanical energy sufficient to open the channel and allow Ca2+ entry in mouse pancreatic cells (Stanley et al., 2014; Figure 1D) and in neurons in vitro and in vivo (Wheeler et al., in press; Friedman in press paper). This approach, while novel and worthy of exploration, faces difficulties for immediate brain application due to potential nonspecific local heating of tissue and slow kinetics of activation and deactivation (exceeding temporal dynamics of neural activity signals by orders of magnitude).
A subsequent study proposed that injection of iron oxide nanoparticles (22 nm in diameter) with efficient heat dissipation could result in potent transduction of magnetic energy into thermal energy sufficient to activate TRPV1 channels on neurons, and achieve Ca2+ entry on the seconds timescale (Chen et al., 2015; Figure 1D). The investigators were able to show reliable magnetic field-induced spiking of neurons (<1 Hz) in primary hippocampal neurons as well as in deep brain tissue. CNS neurons remained responsive for up to a month after iron oxide particle injection, though limited dispersion of the particles from the deep site of injection remained an issue. The investigators also developed a field stimulus protocol that allowed cyclical heating (reaching TRPV1 channel opening thresholds) and cooling (back to 37 degrees) in vivo within a single stimulus pulse (5s) to avoid prolonged exposure to noxious heat. While this step is noteworthy, 5 s is still extremely slow on the neuronal signaling timescale, and the extent to which even this step would be feasible clinically is unclear. Challenges for the future thus include increasing temporal precision (into the millisecond domain, perhaps via nanoparticles with greater specific power), reducing temperature requirements by engineering channels with lower activation thresholds while ensuring lack of activity at rest, and crucially increasing the dispersion of nanoparticles within tissue. A related line of research has explored the use of naturally-occurring bacterial magnetoreceptors to transduce magnetic signals into local genetically-targetable neural actuation (Long et al., 2015; Qin et al., 2015; Figure 1E); these magnetothermal responses have potential utility relevant to the twin challenges of tissue heating and limited nanoparticle dispersion discussed above, but nevertheless remain considerably slower than optogenetic responses, and a magnetic apparatus remains to be developed that is compatible with free mammalian behavior as in optogenetics.
Finally, a clinically inspired approach has built upon transcranial pulsed ultrasound to enable non-invasive and potentially-localized stimulation of modified neurons (although not yet in a genetically targeted manner) presumably operating through activation of mechanosensitive channels and involving cavitation forces (Figure F). Using this modality, a series of studies have demonstrated ultrasound-mediated stimulation of neural activity sufficient to elicit action potentials and synaptic transmission in vitro (Kraiche et al., 2008, Tyler et al., 2008, Menz et al., 2013), and in vivo in mice (motor cortex: Tufail et al., 2010, King et al., 2013; hippocampus: Tufail et al., 2010), without significant elevation in brain temperature (< 0.01°C). Average response latencies (~50–150 ms), while better than with the magnetothermal approaches, remain considerably slower than with typical optogenetic experiments (< 5 ms). There is also limited spatial resolution (spanning several mm3), though this could be improved at higher ultrasound frequencies (at the cost of impaired skull penetration), or through sculpting of the ultrasound beam and use of multiple transducers for further focusing.
Recent attempts to translate these findings to humans have led to demonstrated use of focused ultrasound to modulate the activity of human primary somatosensory cortex, with associated shifts in sensory discrimination (Legon et al., 2014, Lee et al., 2015). A useful feature of ultrasonic modulation is ready compatibility with existing MRI methods, potentially enabling closed-loop diagnostic and therapeutic circuit targeting. Indirect means for genetic targeting may be possible in a limited sense, for example by titrating stimulus properties so that cell populations expressing higher levels of mechanosensitivity preferentially respond. Direct genetic targeting is also theoretically possible via overexpression of mechanosensitive channels as deep tissue energy receptors, analogous to optogenetic or magnetogenetic approaches. Indeed an attempt to transduce sound waves into genetically-targeted neuronal activation has recently been reported (Ibsen et al., 2015), with similar caveats to magnetothermal approaches but representing another energy modality that could find relevance in the research or clinical setting. While much remains to be understood about the mechanisms and optimal stimulation parameters of ultrasound-mediated brain stimulation, progress in this area (Yoo et al., 2011, King et al., 2013, Moore et al., 2015) suggests that ultrasound may become a useful modality of neural stimulation.
Neuropsychiatric diseases with potential circuit-dynamical targets
When considering interventional therapeutics that extend beyond simply targeting a brain region, a key initial step will be to identify the most causally-relevant circuit dynamical property to be targeted for a specific symptom. In general such information is simply not known for neuropsychiatric disease states, though testable possibilities abound. These possibilities include alteration of the time-varying traffic along specific long-range projections or the dynamic connection strength between specific brain regions, modulation of cellular excitation/inhibition balance or coherence in activity oscillations between brain regions, and recruitment or suppression of brainwide activity patterns such as specific resting-state or task-associated networks. Given the ubiquity of the unknowns however, the most immediate clinically-relevant importance of circuit-dynamics interrogation technology remains basic, foundational identification of causal principles in animal models. Here we highlight a few recent specific examples of diseases in which related circuit-targeting progress has been made, and in which circuit-dynamical ideas are influencing the design and delivery of effective brain stimulation protocols.
While lesion of the subthalamic nucleus as a regional target can offer temporary therapeutic benefit for a subset of parkinsonian symptoms in some patients, point sources for intervention such as fiberoptics for optogenetic control (Gradinaru et al., 2009) or electrodes for DBS (Whitmer et al., 2012) appear to be most effective if their initial direct target is afferent white matter tracts—an especially potent focal way to modulate the large downstream structure as well as to exert retrograde effects back at the cell bodies of tract origin. Consistent with this afferent-tract targeting principle, recent DBS protocols in humans have further revealed potential therapeutic value for supporting memory function by targeting structures afferent to the hippocampus, such as the fornix (Fontaine et al., 2013) or the entorhinal cortex (Suthana et al., 2012), but not attempting to target the entire elongate hippocampus directly itself. In fact, at this time, a large-scale randomized clinical trial (NCT01608061), of DBS in the fornix, is underway to assess safety and efficacy at 1 year for memory improvement in Alzheimer’s patients.
Deep brain stimulation for psychiatric disorders, including for treatment-resistant depression and obsessive compulsive disorder, has recently witnessed a rapidly-broadening potential application domain, in which targeting of afferent white matter tracts again may be most important (Mayberg et al., 2005; Ressler and Mayberg, 2007; reviewed in Deisseroth, 2014). Corresponding larger-scale clinical trials are ongoing, but in all of these studies, challenges are expected as many questions remain unanswered regarding the most suitable site for clinical efficacy, spurious consequences of electrical stimulation which unlike optogenetic methods can recruit off-target cells or passing fibers unrelated to the target population or region, and the likely need for individualized targeting aligned to patient-specific wiring (for example, as assessed by MRI tractography) and to the specific dynamical context of the patient (for example, as assessed by open-loop or closed-loop detection of native activity patterns which might span spatially segregated circuits and extended timescales).
Circuit targeting in the widespread and debilitating disease of drug abuse, using not optogenetics itself but rather optogenetically-guided treatment, has recently reached the clinic. DBS had earlier been considered for the treatment of certain forms of drug addiction, with much attention focused on the nucleus accumbens (NAc; a brain structure closely associated with reward and abused drug action) and on the medial prefrontal cortex (mPFC)-NAc projection. While high-frequency DBS delivered to NAc has in some cases been found to ameliorate drug abuse-related symptoms acutely, the mechanism remained largely unknown and symptoms typically reappeared once stimulation was stopped. Triggering long-term depression (LTD)-type synaptic plasticity in this projection (and hence lasting behavioral benefit) was robust with optogenetic low-frequency stimulation (LFS) in mice, but more challenging with electrical LFS, thus frustrating efforts to define a simple clinically-relevant electrical stimulation therapy. But a recent study reported that this discrepancy between optogenetic and electrical effects might be attributable to the additional recruitment (only with electrical stimulation) of an off-target pathway recruiting dopamine type 1 (D1) receptors which was inhibiting LTD; consistent with this hypothesis, electrical low-frequency DBS together with the application of D1 antagonists to cocaine-adapted mice was able to provide long-term restoration of normal behavior (Figure 2E; Creed et al., 2015).
Figure 2. Disease -related circuit-targeting demonstrations.
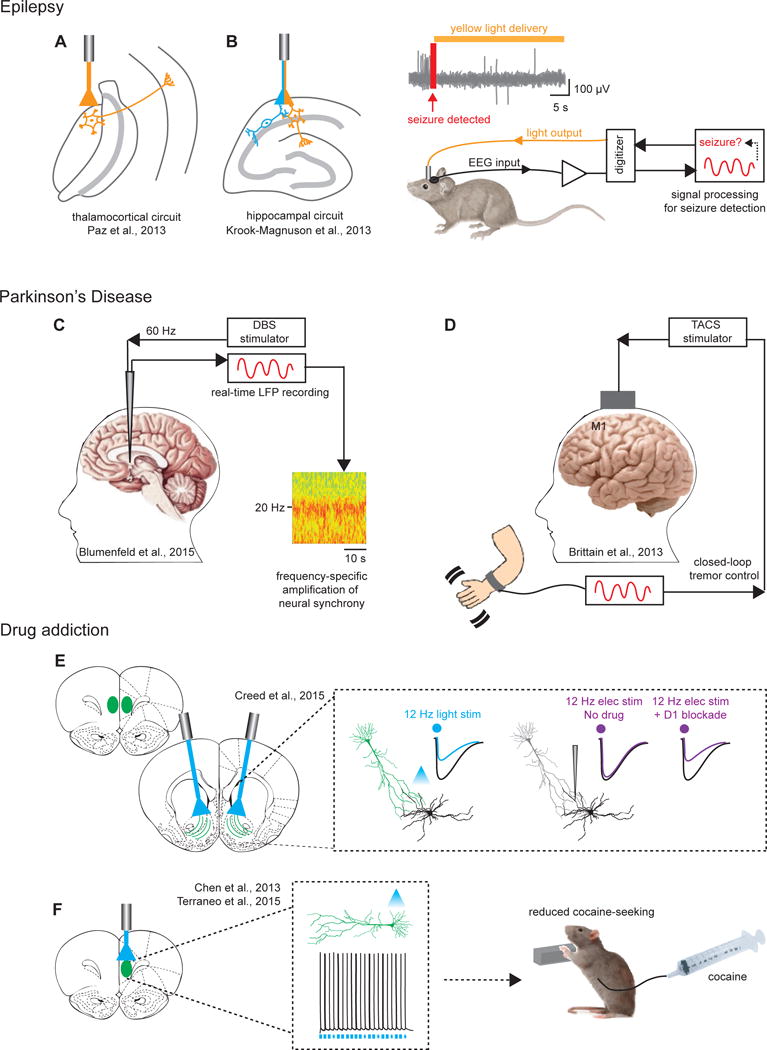
(A) Targeting thalamocortical neurons and (B) hippocampal neurons with closed-loop strategies to cause real-time interruption of EEG- and behaviorally-defined seizures. Right panel in (B): Depiction of closed-loop setup wherein EEG inputs are used to detect seizure onset, following which real-time inhibition can be administered through optogenetic manipulation. (C) Low frequency DBS stimulation (60Hz), in contrast to traditional high frequency stimulation (130 Hz), results in significant amplification of subthalamic neural synchrony and alpha/beta band power thought to underlie improved gait symptoms in Parkinson’s. (D) Use of an alternative non-invasive approach termed transcranial alternating current stimulation (TACS) is depicted for stimulation of motor cortex; guided by real-time readout of cortical oscillations linked to tremor, TACS was found to result in significant cancellation of the resting state tremor. (E) 12 Hz optical stimulation was reported in this study to produce robust LTD, whereas 12 Hz electrical stimulation did not. The discrepancy for these investigators was attributed to off-target recruitment of D1 receptors by electrical stimulation which impeded LTD; a combination of low frequency DBS together with D1 antagonists provided reliable LTD and restoration of behavior in cocaine seeking animals. (F) Optogenetic prelimbic cortical excitation significantly prevented cocaine-seeking in rats (by compensating for hypoactivity observed in cocaine-seeking rats), pointing to the prefrontal cortex as a promising therapeutic target for compulsive drug use.
An earlier paper addressing a similar question (Chen et al., 2013) came to a different optogenetically-inspired treatment concept, which has now led to reported therapeutic clinical benefit. In a rat model of compulsive drug seeking, prolonged cocaine self-administration decreased intrinsic excitability of deep-layer mPFC pyramidal neurons, which was especially pronounced in compulsive drug-seeking animals; compensating for this hypoactivity of these projection neurons with in vivo optogenetic mPFC stimulation prevented compulsive cocaine seeking, and the authors suggested this could represent “a promising therapy for treating compulsive drug use” (Figure 2F; Chen et al., 2013). Recent pilot clinical studies using TMS as the clinical interventional tool now indeed suggest that stimulation of the dorsolateral prefrontal cortex reduces cocaine use in human cocaine abusers (Terraneo et al., 2016; Ferenczi and Deisseroth, 2016) and curbs cue-induced craving in human heroin addicts (Shen et al., 2016). Potential causal brainwide mechanisms for this effect have been described (Ferenczi et al, 2016), discussed in more detail below.
Some of the most promising optogenetic preclinical explorations relevant to neuropsychiatric disease have been in the field of epilepsy. Closed-loop studies in mouse models of temporal lobe epilepsy have shown that seizure onset can be detected in real time and abolished by closed loop optogenetic inhibition of thalamocortical neurons (Figure 2A) or granule cells in dentate gyrus (Figure 2B; Paz et al., 2013, Krook-Magnuson et al., 2013), or by closed-loop optogenetic excitation of midline cerebellar parvalbumin neurons (Krook-Magnuson et al., 2014). Closed-loop modulation of brain circuits (regardless of intervention modality) may be especially well-suited for paroxysmal conditions like epilepsy, although even chronic conditions such as depression could in principle benefit from closed-loop control as the field attains deeper knowledge of the underlying causal dynamics and plasticity of affective brain states.
Indeed, many types of clinical circuit-targeting might also leverage detection of local circuit activity patterns. For example, while many patients with Parkinson’s Disease have benefited from high-frequency DBS stimulatiom, low-frequency 60 Hz stimulation (as compared with the more classic >100 Hz stimulation) may be well-suited for amplifying resting state neural synchrony in the STN with concomitant improvements in gait and speech but not tremor (Figure 2C; Blumenfeld et al., 2015). On the other hand, high-frequency stimulation is classically associated with alleviation of symptoms of tremor but not necessarily gait or speech. This dichotomy therefore opens the door to the intriguing possibility of circuit-dynamical “knock-in” and “knock-out” of patient-specific resting-state synchrony bands for highly precise symptom alleviation (Blumenfeld et al., 2015). Other opportunities in Parkinson’s include minimally-invasive and closed-loop strategies using transcranial ultrasound (TUS) and transcranial alternating current stimulation (TACS) for the treatment of tremor. Recent pilot work used a closed-loop strategy to deliver TACS over M1 during tremor to induce phase cancellation of tremor rhythm, with intriguing results (Figure 2D; Brittain et al., 2013).
Beyond the brain
In addition to the domain of neuropsychiatric disease, there is emerging interest in circuit targeting in oncology, in part for deeper understanding of the cancer micro-environment. For example, neoortical neuronal activity has recently been found to promote the growth of malignant gliomas in a circuit-specific manner through activity-regulated secretion of specific growth factors (Venkatesh et al, 2015). And beyond the central nervous system, peripheral nerve circuit activity has been shown to contribute to the progression of prostate (Magnon et al, 2013), gastric (Zhao et al, 2014), pancreatic (Stopcyzynski et al, 2014) and skin (Peterson et al, 2015) cancers.
With the advent of genetically-resolved, bidirectional optogenetic modulation of peripheral nerves (e.g. Towne et al., 2013, Iyer et al., 2014) opportunities exist for enhanced understanding and treatment of other diseases originating outside the brain, including chronic pain and autoimmunity. Yet as in the CNS, a primary goal may simply be fundamental research rather than direct clinical application; any resulting optogenetically-guided clinical intervention need not be optogenetic itself, and basic-science optogenetic investigations in animals may illuminate circuit-targeting therapeutic concepts employing diverse electrical, optical magnetic, or pharmacological modalities in the peripheral and autonomic as well as central nervous systems.
Outlook for the next decade
Some of the most important growth into the future for fast cellular-resolution circuit-dynamical intervention will actually be in the readout domain. Readout quality is crucial for circuit targeting, in particular for 1) obtaining a particular known magnitude of desired response in the intended circuit elements, 2) aligning the timing of a desired response in the intended circuit elements to a naturally occurring activity pattern, or to other events, 3) closed-loop control to account in real-time for shifting brain state/response properties and to test models of system function, and 4) tracking brainwide relationships and activity patterns, since the desired circuit response may involve both local and global components.
In addressing the first two issues, matching desired magnitude (Kim et al., 2016) and timing (Zalocusky et al., 2016) of activity in deep-brain targeted circuit elements corresponding to patterns naturally observed in behavior has been recently achieved, a long-sought goal in optogenetics. Kim et al. (2016) developed frame-projected independent-fiber photometry (FIP) to precisely match the magnitude of optogenetically-evoked activity to that of naturally-occurring activity, in genetically-targeted circuitry deep in the brain of the same freely-behaving mouse. Zalocusky et al (2016) integrated fiber photometry and optogenetics to match the precise timing of a naturally-occurring activity signal within D2 receptor-expressing nucleus accumbens neurons of freely-moving rats. Regarding the third issue, closed-loop optogenetic control has taken major steps forward since 2009 with the advent of increasingly precise fluorescent, electrical, and behavioral system readouts as described above and recently reviewed (Emiliani et al., 2015; Grosenick et al., 2015); such closed-loop systems may become part of advanced clinical interventions relevant to neuropsychiatric disease states.
Fourth and finally, real-time brainwide activity readout in the setting of optogenetic control has been possible since 2010 (Lee et al., 2010), and recently Ferenczi et al (2016) used this clinically-relevant global readout (optogenetic fMRI, or ofMRI) to identify and quantify brainwide indirect consequences of successful precise direct targeting of a small and discrete cortex region, with resulting insights into the adaptive and maladaptive regulation of hedonic behaviors in the very same experimental subjects. Focal and subtle modulations were found to change the state of the entire brain, including the manner in which diverse distant brain regions interacted with each other (Ferenczi et al., 2016), revealing potent mechanisms by which natural processes such as neuromodulation or attention might be able to give rise to brain state changes with behavioral significance. Importantly, the temporal flexibility of optogenetic methods allows these effects to be elicited and studied on any acute or chronic timescale from milliseconds to many days or more (Goshen et al., 2011; Yizhar et al., 2011; Ferenczi et al., 2016) while maintaining precision of direct control over the same correctly-targeted circuit elements.
Ongoing success with optogenetically-guided therapeutic interventions (e.g. Terraneo et al., 2016; Shen et al., 2016; Ferenczi and Deisseroth, 2016) will encourage further innovative uses of circuit knowledge. However, as with any other therapy, a circuit-targeting treatment may not be applicable to every patient suffering from a particular symptom domain or disorder. Personalized circuit diagnostics, through the use of biomarkers in the form of structural or functional readouts, will be required for providing individual safety, efficacy, and treatment decisions. Benefits of circuit targeting may be further complemented by molecular diagnostics and therapeutics as well; indeed, circuit-guided identification of molecular, transcriptional, or genomic/epigenomic changes that are causal to observed circuit dynamics and behavior will become readily addressable with pharmacological or nucleic acid intervention (e.g. siRNAs, CRISPR-Cas9). Such molecular circuit-targeting strategies may emerge from advanced cellular-resolution intact-tissue analyses in the laboratory. For example, once a projection or population activity pattern is identified as causally important for a specific symptom or behavior, high-content structural (Dodt et al., 2007; Erturk et al., 2012; Hama et al., 2015; Kuwajima et al., 2013), molecular (Chung et al., 2013, Murray et al., 2015; Renier et al., 2014; Susaki et al., 2014; Yang et al., 2014), and transcriptional (Sylwestrak et al., 2016) information may be registered at cellular resolution to the same intact volume on which activity dynamics were recorded and controlled to provide molecular handles for causal circuit elements.
Important molecular information relevant to circuit targeting will also emerge from clinical genetics, as the coming decade is certain to witness ongoing identification of genes linked to neuropsychiatric disease phenotypes. This accelerating genetic datastream will be powerfully enhanced by understanding of the circuits involved in causal processes leading from genes to behavior. Bidirectional information flow (using circuit-dynamical knowledge for mechanistic insight into influences of genes and molecules on behavior, and using genetics knowledge and tools to guide interventions and diagnostics relevant to causal circuit deficits), may well serve the ultimate goal of linking patient genotype to disease phenotype via circuit dynamics. On long timescales, as engineered interfaces and multimodal energy-delivery technologies improve in targetability, efficacy, and safety, concomitant improvements in the design of clinical trials will increasingly need to emphasize improved recruitment of patients with shared circuit etiology (e.g. through the use of biomarker sets that jointly represent circuit genetics and physiology), as well as improved design and alignment of treatment arm, placebo arm, and endpoint nature and timing, in order to ensure that the intended circuit targets are in fact engaged with maximal potency and specificity.
Diverse circuit-targeting interventional strategies (using tissue-penetrating energy delivery and cellular-resolution energy-receptor/transducer targeting, as with optogenetics; Fig 1) will also continue to evolve, with refinements likely to include improvements in temporal resolution, spatial resolution, sensitivity, local heating, and other issues. In their eventual mature forms, diverse energy-delivery modalities will have the potential to complement optogenetics in providing a broad palette of flexible, and multiplexed, neural circuit control tools that may also help guide and implement clinical interventions. But immediate or near-future opportunities for targeting clinically-relevant circuits, based on principled predictions from animal studies, already include 1) developing concomitant pharmacological (Fig. 2E) or behavioral-stimulus interventions to be used alongside, and to potentially improve effects of, currently-available brain interventions; 2) developing and leveraging potential long-term plasticity effects of available stimulation modalities for more lasting benefit; 3) improving patient-specific localization of interventions with individualized anatomy and open-loop activity mapping; and 4) implementing on-line data acquisition and analysis pipelines to enable closed-loop interventions. And as with so many fields of medicine, for neuropsychiatric diseases the most crucial process (now and likely for many decades to come) will be the ongoing basic science discovery of key principles in the laboratory.
Acknowledgments
We thank the Deisseroth lab for discussions and Lief Fenno for helpful comments on the manuscript. PR is an Ellison Fellow of the Life Sciences Foundation, EF is a Gerald J Lieberman fellow, and KD is supported by the DARPA Neuro-FAST program; NIMH; NIDA; NSF; the Simons Foundation; and the Gatsby Foundation. K.D. is a founder and Scientific Advisory Board member for Circuit Therapeutics, a startup company seeking to develop optogenetic strategies for controlling chronic pain and other nervous system disease states. All tools and reagents described above generated by the authors are freely available upon request (optogenetics.org).
References
- Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A, Zhang F, Aravanis A, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis A, Wang LP, Zhang F, Meltzer L, Mogri M, Schneider MB, Deisseroth K. An optical neural interface. Journal of Neural Engineering. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nature Neuroscience. 2008;12:229–34. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Deisseroth K. Expanding the optogenetics toolkit. Science. 2015;349:590–591. doi: 10.1126/science.aac7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, Iyer S, Pak S, Ährlund-Richter S, Delp SL, Malenka RC, Josselyn S, Carlen M, Hegemann P, Deisseroth K. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 2015 Dec 22; doi: 10.1073/pnas.1523341113. 2015. pii: 20152334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld Z, Velisar A, Miller Koop M, Hill BC, Shreve LA, Quinn EJ, Kilbane C, Yu H, Henderson JM, Brontë-Stewart H. Sixty Hertz. Neurostimulation Amplifies Subthalamic Neural Synchrony in Parkinson’s Disease. PLOS ONE. 2015;10:e0121067. doi: 10.1371/journal.pone.0121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015;33:277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–1480. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–7. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, Zuccolini P, Petersen J, Beltrame M, Van Etten JL, Christie JM, Thiel G, Moroni A. Engineering of a light-gated potassium channel. Science. 2015;348:707–710. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Luscher C. Redefining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Circuit dynamics of adaptive and maladaptive behaviour. Nature. 2014;505:309–317. doi: 10.1038/nature12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: ten years of microbial opsins in neuroscience. Nature Neuroscience. 2015;18:1213–25. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Schnitzer MJ. Engineering approaches to illuminating brain structure and dynamics. Neuron. 2013;80:568–77. doi: 10.1016/j.neuron.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Leischner U, Schierloh A, Jährling N, Mauch CP, Deininger K, Deussing JM, Eder M, Zieglgänsberger W, Becker K. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Meth. 2007;4:331–336. doi: 10.1038/nmeth1036. [DOI] [PubMed] [Google Scholar]
- Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt H-U. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nature Protocols. 2012;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- De Hemptinne C, Swann N, Ostrem J, Ryapolova-Webb E, San Luciano M, Galifianakis N, Starr P. Therapeutic deep brain stimulation reduces cortical phase amplitude coupling in Parkinson’s disease. Nat Neuro. 2015;18:779–86. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JW, Huss D, Voss T, et al. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. The New England Journal of Medicine. 2013 doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- Emiliani V, Cohen AE, Deisseroth K, Hausser M. All-optical interrogation of neural circuits. J Neurosci. 2015;5:13917–26. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi E, Deisseroth K. Illuminating next-generation brain therapies. Nature Neuroscience. 2016 doi: 10.1038/nn.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351(2016):aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine D, Deudon A, Lemaire JJ, Razzouk M, Viau P, Darcourt J, Robert P. J Alzheimers Dis. 2013;34:315–23. doi: 10.3233/JAD-121579. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–89. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349:647–50. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Marshel J, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015;86:106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, Kaneko T, Akagi T, Saito T, Saido T, et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 2015:1–14. doi: 10.1038/nn.4107. [DOI] [PubMed] [Google Scholar]
- Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Comm. 2015;6:8264. doi: 10.1038/ncomms9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnology. 2014;32:274–8. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Takeuchi A, Horigane S, Ohkura M, Gengyo-Ando K, Fujii H, Kamijo S, Takemoto-Kimura S, Kano M, Nakai J, et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat Methods. 2015;12:64–70. doi: 10.1038/nmeth.3185. [DOI] [PubMed] [Google Scholar]
- Jeong JW, McCall JG, Shin G, Zhang Y, Hasani R, Kim M, Li S, Sim JY, Jang KI, Shi Y, Hong DY, Liu Y, Schmitz GP, Xia L, He Z, Gamble P, Ray WZ, Huang Y, Bruchas MR, Rogers JA. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell. 2015;162:662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiche ML, Phillips WB, Jackson N, Muthuswamy J. Ultrasound induced increase in excitability of single neurons. Proc IEEE Eng Med Biol Soc. 2008:4246–4249. doi: 10.1109/IEMBS.2008.4650147. [DOI] [PubMed] [Google Scholar]
- Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, Davidson TJ, Inoue M, Bito H, Deisseroth K. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nature Methods. 2016 doi: 10.1038/nmeth.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol. 2013;39:312–31. doi: 10.1016/j.ultrasmedbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. Eneuro. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 2013;140:1364–1368. doi: 10.1242/dev.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim D, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–92. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim H, Jung Y, Song IU, Chung YA, Yoo SS. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci Rep. 2015;5:8743. doi: 10.1038/srep08743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17:322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, Deisseroth K. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162:635–47. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Ye J, Zhao D, Zhang SJ. Magnetogenetics: remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor. Sci Bull. 2015 doi: 10.1007/s11434-015-0902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O, Deisseroth K. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nature Methods. 2011;9:159–72. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Menz MD, Oralkan O, Khuri-Yakub PT, Baccus SA. Precise neural stimulation in the retina using focused ultrasound. J Neurosci. 2013;33:4550–60. doi: 10.1523/JNEUROSCI.3521-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KL, Yeh AJ, Ho JS, Grosenick L, Tsao V, Ferenczi EA, Iyer SM, Tanabe Y, Deisseroth K, Delp SL, Poon ASY. Nature Methods. 2015 doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ME, Loft JM, Clegern WC, Wisor JP. Manipulating neuronal activity in the mouse brain with ultrasound: A comparison with optogenetic activation of the cerebral cortex. Neurosci Lett. 2015;604:183–187. doi: 10.1016/j.neulet.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim SY, Choi H, Park YG, Park JY, Hubbert A, McCue M, Vassallo S, Bakh N, Frosch MP, Wedeen VJ, Seung S, Chung K. Cell. 2015;163:1500–1514. doi: 10.1016/j.cell.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Russell LE, Dalgleish HW, Häusser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat Methods. 2015;12:140–6. doi: 10.1038/nmeth.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong S. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Yin H, Yang C, Dou Y, Liu Z, Zhang P, Yu H, Huang Y, Feng J, Hao J, Hao J, Deng L, Yan X, Dong X, Zhao Z, Jiang T, Wang HW, Luo S-J, Xie C. A magnetic protein biocompass. Nat Mat. 2015 doi: 10.1038/nmat4484. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel J, Kim C, Ferenczi E, Lee S, Berndt A, Ramakrishnan C, Jaffe A, Lo M, Liston C, Deisseroth K. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526:653–9. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer JP, Deisseroth K, Tank DW. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat Neurosci. 2014;17:1816–24. doi: 10.1038/nn.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, He H, Yuan T-F. 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol Psychiatry. doi: 10.1016/j.biopsych.2016.02.006. In press ( http://dx.doi.org/10.1016/j.biopsych.2016.02.006) [DOI] [PubMed]
- Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med. 2014;21:92–98. doi: 10.1038/nm.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM, Davis BM. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1718–20. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157:726–739. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I. N Engl J Med. 2012;366:502–10. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, Rajasethupathy P, Wright MA, Jaffe A, Deisseroth K. Multiplexed intact-tissue transcriptional analysis at cellular resolution. Cell. 2016;164:792–804. doi: 10.1016/j.cell.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur Neuropsychopharmacology. doi: 10.1016/j.euroneuro.2015.11.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic Control of Targeted Peripheral Axons in Freely Moving Animals. PLoS ONE. 2013;8:e72691. doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Helms Tillery SI, Tyler WJ. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson E, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:1–14. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, Gaykema RP, Spano AJ, Beenhakker MP, Kucenas S, Patel MK, Deppmann CD, Guler AD. Genetically targeted magnetic control of the nervous system. Nat Neurosci. doi: 10.1038/nn.4265. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158:945–958. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, McDannold NJ, Pascual-Leone A, Jolesz FA. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky KA, Ramakrishnan C, Lerner T, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R neurons signal past unfavorable outcomes during decision-making and control risky choice. Nature. 2016 doi: 10.1038/nature17400. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen C, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon M, Kaneko K, Fox JG, Wang T, Chen D. Denervation suppresses gastric tumorigenesis. Sci Trans Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda S, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–3. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]


