Abstract
Although the differentiation of CD4+T cells is widely studied, the mechanisms of antigen-presenting cell-dependent T-cell modulation are unclear. Here, we investigate the role of dendritic cell (DC)-dependent T-cell differentiation in autoimmune and antifungal inflammation and find that mammalian sterile 20-like kinase 1 (MST1) signalling from DCs negatively regulates IL-17 producing-CD4+T helper cell (Th17) differentiation. MST1 deficiency in DCs increases IL-17 production by CD4+T cells, whereas ectopic MST1 expression in DCs inhibits it. Notably, MST1-mediated DC-dependent Th17 differentiation regulates experimental autoimmune encephalomyelitis and antifungal immunity. Mechanistically, MST1-deficient DCs promote IL-6 secretion and regulate the activation of IL-6 receptor α/β and STAT3 in CD4+T cells in the course of inducing Th17 differentiation. Activation of the p38 MAPK signal is responsible for IL-6 production in MST1-deficient DCs. Thus, our results define the DC MST1–p38MAPK signalling pathway in directing Th17 differentiation.
The differentiation of Th17 cells is central to infection and autoimmunity. Here, the authors show that expression of MST1 by dendritic cells limits IL-6 production and thereby controls Th17 differentiation in immunity to fungal infection and experimental autoimmune encephalomyelitis.
CD4+T cells are an essential component of the adaptive immune system and regulate immune responses to foreign antigens1,2,3,4,5,6. The activation and differentiation of CD4+T cells are regulated by the three main signalling components of the T-cell receptor (TCR) (signal 1), co-stimulatory molecules (signal 2) and cytokine receptors (signal 3)4,5,6,7. These signals depend on the regulatory role of innate immune cells. In the presence of cytokines produced by innate immune cells, naive CD4+T cells differentiate into helper T-cell subsets with distinct functions and cytokine profiles. These include interferon-γ (IFNγ)-producing type 1 helper T (Th1) cells, which are essential for immunity to intracellular microorganisms, IL-4-producing Th2 cells, which protect against parasites and extracellular pathogens4, and Th17 cells that produce IL-17A, IL-17F, IL-21 and IL-22 and protect against bacterial and fungal infections at mucosal surfaces8.
Dendritic cells (DCs) are professional antigen-presenting cells (APC) that bridge innate and adaptive immunity. In addition to presenting antigens and modulating cell surface co-stimulatory molecules, DC-derived cytokines and chemokines can be proinflammatory or anti-inflammatory, and can engage distinct T-cell differentiation programs9. For example, the binding of the proinflammatory cytokine IL-6 to a complex of the IL-6 receptor α (IL-6Rα, also known as CD126) and IL-6Rβ (CD130; signal transducing receptor gp130) activates the transcription activator STAT3, resulting in differentiation of naive CD4+T cells into Th17 cells by inducing the lineage-specific transcription factor RORγt10,11,12,13,14,15. Studies from our lab and others have shown that innate signalling in DCs mediated by G protein-coupled receptor S1P1 (refs 16, 17), sirtuin 1 (ref. 18), mitogen-activated protein kinase (MAPKs)19,20 and Wnt-β-catenin21 has a critical role in shaping adaptive immune responses by directing naive CD4+T-cell differentiation. How the differentiation of CD4+T cells is modulated and regulated by innate immune signals in DCs remains to be understood.
Mammalian sterile 20-like kinase 1 (MST1) is mammalian class II germinal center protein kinase, also known as serine/threonine kinase 4 and kinase responsive to stress 2 (refs 22, 23). MST1 has been implicated in regulating the cell cycle and apoptosis in various species24,25,26,27,28,29. MST1 is also involved in regulating adaptive immune cell function30,31. MST1-deficient mice accumulate mature lymphocytes in the thymus and have low numbers of naive T cells in the peripheral lymphoid organs due to a dysregulation of chemotaxis and apoptosis32,33,34. MST1 controls the development and function of regulatory T (Treg) cells through modulation of Foxo1/Foxo3 stability in autoimmune disease35. In addition, MST1 regulates the activation of T cells by phosphorylating the cell cycle inhibitory proteins MOBKL1A and MOBKL1B36. Furthermore, MST1 is important for optimal reactive oxygen species (ROS) production and bactericidal activity of phagocytes because it promotes the activation of the small GTPase Rac as well as mitochondrial trafficking and juxtaposition to the phagosome through the assembly of a TRAF6–ECSIT complex37. However, whether MST1 is involved in bridging the innate immune signal to the adaptive immune response is not clear.
Here, we show that MST1 has a critical role in directing the T-cell lineage fate by producing DC-derived cytokines, which link innate and adaptive immune modulation. Through a p38MAPK–MK2/MSK1–CREB dependent signalling pathway, MST1 is required for IL-6 production by DCs as well as for the expression of IL-6Rα/β and phosphorylation of STAT3 in responding T cells, resulting in specific lineage engagement of Th17 cells in experimental autoimmune encephalomyelitis (EAE) and fungal infection-induced inflammation.
Results
Deficiency of MST1 in DCs does not alter DC homoeostasis
To investigate the role of MST1 in the immune system, we purified many types of mouse immune cells including macrophages (CD11b+F4/80+ cells), DCs (CD11c+MHCII+F4/80−Ly6G−NK1.1−CD19−TCR− cells), neutrophils (CD11b+ Ly6G+ cells), CD4+T cells (CD4+TCR+ cells) and CD8+T cells (CD8+TCR+ cells) as described previously18 and analysed MST1 expression. This showed that MST1 is highly expressed in DC cells (Supplementary Fig. 1A). To study the role of MST1 in DCs, we generated CD11c+ cell-specific MST1-deficient mice by crossing Mst1flox/flox with Cd11c-Cre (referred to as Mst1ΔDC hereafter). As a result, a significant reduction of MST1 mRNA and protein was observed in DCs, but not in macrophages or neutrophils (Supplementary Fig. 1B,C), indicating that Mst1ΔDC could be used for studies of DC function.
Next, we examined the composition of DC subsets in vivo. MST1 deletion in DCs showed a comparable percentage and CD11c+ cell number, as well as CD11b+CD11c+, and CD8α+CD11c+ cell subpopulations in the spleen compared with wild type (WT) (Supplementary Fig. 2A–C). MST1 deletion in DCs did not alter the percentages of other immune cells including macrophages, neutrophils, B cells and T cells (Supplementary Fig. 2D,E). Furthermore, the co-stimulatory molecule expressions displayed similar changes including those of MHCII, CD80, CD86, CD40 and CD54 in DC cells, even during LPS stimulation or on antigen processing (Supplementary Fig. 3). Moreover, DC MST1 deficiency does not affect the apoptosis or proliferation of DC cells (Supplementary Fig. 4). Altogether, these data show the deletion of MST1 in DCs does not alter DC homoeostasis.
DC MST1-deficient mice exhibited altered Th17 cell responses
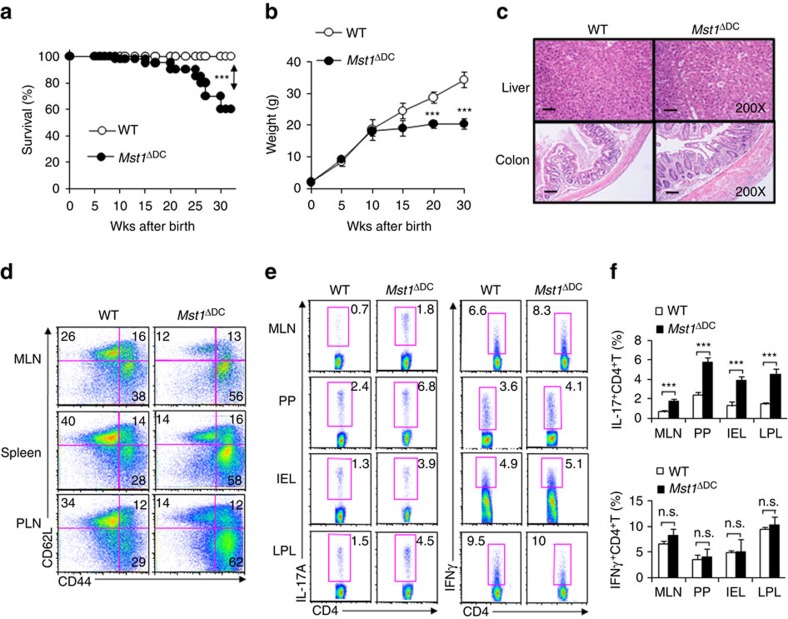
While Mst1ΔDC mice displayed no gross abnormalities within 6–8 weeks of birth, these mice started spontaneously dying 15 weeks after birth (Fig. 1a). In addition, Mst1ΔDC mice displayed more weight loss (Fig. 1b) compared with WT control. Pathological analysis revealed a typical severely destructive inflammation in the liver and colon of Mst1ΔDC mice (Fig. 1c). Finally, Mst1ΔDC mice displayed a more pronounced autoimmune phenotype (CD4+TCR+CD44highCD62Llow cells) in the mesenteric lymph nodes (MLNs), spleen and peripheral lymph nodes (PLNs) (Fig. 1d and Supplementary Fig. 4C). Importantly, the deficiency of MST1 in DCs significantly promotes the percentage of Th17 but not Th1, Th2 and Foxp3+CD4+T cells (Treg cells) in MLNs, peyer's patch (PPs), intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) (Fig. 1e,f and Supplementary Fig. 5). Next, we created Mst1ΔDC→WT and WT→WT complete chimeras and Mst1ΔDC; WT mixed chimeras (1:1) to determine whether MST1 deficiency in DCs would lead to these alterations. Mst1ΔDC→WT complete chimera mice resulted in earlier death and weight loss, like the phenotype in the Mst1ΔDC mice, but the Mst1ΔDC-WT mixed chimeras' mice were largely normal (Supplementary Fig. 6). Therefore, we concluded that MST1 deficiency in DCs did lead to altered Th17 cell responses and autoimmune phenotypes.
Figure 1. DC MST1 deficiency leads to spontaneous diseases.
(a) Kaplan–Meier plots of WT and Mst1ΔDC mouse survival after birth are shown (n=20). (b) Body weights of the WT and Mst1ΔDC mice after birth are summarized (n=20). (c) H&E staining of hepatic and intestinal tissues from sex- and age-matched WT and Mst1ΔDC mice at 20 weeks after birth is presented. Scale bars, 100 μm. (d) DC MST1 deficiency leads to more CD44highCD62Llow cells among CD4+T cells than WT control mice at 20 weeks after birth. Gating strategies for determining the percentage of CD4+TCR+CD44highCD62Llow cells are showed in Supplementary Fig. 4C. Intracellular IL-17A and IFN-γ expression of CD4+T cells isolated from MLN, PP, IEL and LPL in WT and Mst1ΔDC mice with a typical figure displayed (e) and the frequencies of positive cells summarized (f). Data are representative of three to four independent experiments (mean±s.d.; n=4). ***P<0.001, compared with the indicated groups. n.s., not significant. P-values were determined using Student's t-tests.
DC MST1 deletion enhances Th17 differentiation in EAE
We observed the effects of MST1 deficiency in DCs in EAE, a typical Th1 and Th17-dependent autoimmune disease model38,39,40,41, to determine whether MST1 functions in innate DC cells to regulate adaptive immunity. After immunization with a myelin oligodendrocyte glycoprotein peptide comprised of amino acids 35–55 (called simply ‘MOG' here), Mst1ΔDC displayed much greater disease progression (Fig. 2a). Flow cytometry analysis showed that the central nervous system (CNS) of Mst1ΔDC mice had significantly more inflammatory cell infiltration into the spinal cord, including CD4+T cells and CD11b+ myeloid cells (Supplementary Fig. 7). Thus, MST1 signalling in DCs was required for protection against autoimmune diseases of the CNS.
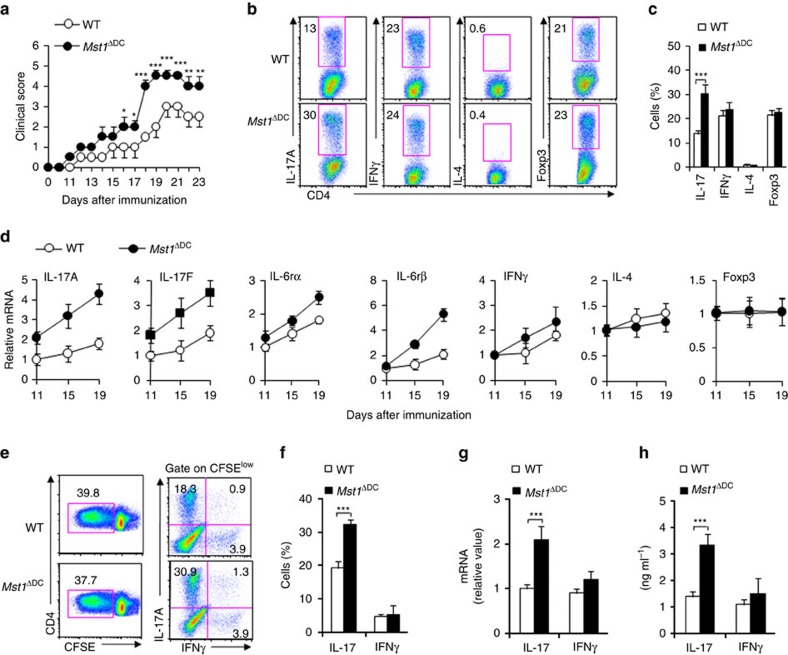
Figure 2. DC MST1 inhibits Th17 differentiation in EAE.
(a) EAE disease course in WT and Mst1ΔDC mice. Intercellular staining of IL-17+ cells, IFNγ+ cells, IL-4+ cells (after stimulation with PMA and ionomycin) and Foxp3+ cells among CD4+T cells from the spinal cord of WT or Mst1ΔDC mice were determined with flow cytometry method (FCM) on day 19 after MOG immunization. A typical figure is shown (b) and the data summarized (c). (d) CD4+T cells isolated from spinal cord of Mst1ΔDC mice on the indicated day after MOG immunization along with mRNA expression of the indicated gene (levels in WT groups were set to 1). (e,f) CD4+T cells isolated from dLN of MOG-immunized WT or Mst1ΔDC mice were labelled with CFSE and stimulated with MOG for 5 days. Intercellular staining of IL-17 and IFNγ in CFSElow cells was determined with FCM. The typical figure is shown in e and data summarized (f). (g,h) CD4+T cells isolated from the dLN of MOG-immunized WT or Mst1ΔDC mice, and ex vivo stimulation with anti-CD3 (1 μg ml−1) for 24 h and mRNA expression (g) or cytokine secretion (h) of the indicated gene were analysed using qPCR (levels in the WT groups were set to 1) or ELISA. Data are representative of three to four independent experiments (mean±s.d.; n=4–6). *P<0.05 and ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
Moreover, CNS-infiltrating T cells from Mst1ΔDC mice had more IL-17+ cells, but normal levels of IFNγ+ cells, IL-4+ cells and Foxp3+ cells among the CNS-infiltrating CD4+T cells (Fig. 2b,c). Consistent with this, MST1 deficiency in DC resulted in significantly higher and sustained expression of IL-17A, IL-17F, IL-6Rα, IL-6Rβ (gp130), but the expression of IFNγ, IL-4 and Foxp3 were similar in the CNS-infiltrating T cells compared with WT DC control (Fig. 2d).
To determine whether an alteration in Th17 differentiation is responsible for the more severe EAE in the Mst1ΔDC mice, we observed the T-cell responses in the draining lymph node (dLN) at the preclinical stage of EAE on day 7 after immunization. MST1 deficiency in DCs does not alter T-cell proliferation (Fig. 2e and Supplementary Fig. 8), but significantly enhances the IL-17+ cell percentage, IL-17 mRNA expression and IL-17 secretion compared with WT mice, whereas the IFNγ+ cell percentage and expression are similar in the two group mice immunized with MOG (Fig. 2e–h). These data suggest MST1 deficiency plays a critical role in promoting Th17 differentiation in vivo, thus contributing to EAE.
DC MST1 precipitates antifungal Th17 cell responses
Next we challenged Mst1ΔDC mice with Candida albicans, a fungal infection model known to induce a strong Th17 cell response. Nine days after infection, Mst1ΔDC mice displayed significantly attenuated pathological kidney injuries, less fungal survival in the kidney and higher serum IL-17 production, but not IFNγ production, indicating that MST1 signalling is required for precipitating the fungal infection in DCs (Fig. 3a–c).
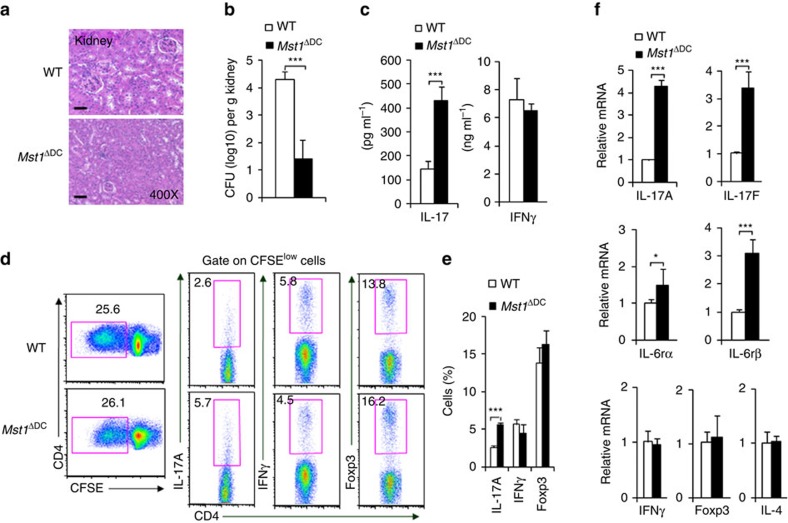
Figure 3. DC MST1 inhibits Th17 differentiation in anti-fungi infection.
Mice were infected with 105 C. albicans SC5314 by i.v. injection. After 9 days, kidneys were collected and a photo of the H&E staining of pathological kidney injuries is shown (Scale bars, 100 μm) (a) and the fungal burden in the kidneys expressed as CFU per g (b). (c) Serum cytokine level of IL-17 and IFNγ in infected mice. (d,e) CD4+T cells isolated from the spleen of the WT or Mst1ΔDC mice were labelled with CFSE and stimulated with heat-killed C. albicans for 3 days. Intracellular staining of IL-17 and IFNγ in CFSElow cells was determined with FCM. A typical figure is shown (d) and the data summarized (e). (f) CD4+T cells isolated from the spleen of infected WT or Mst1ΔDC mice, and ex vivo stimulation was performed with anti-CD3 (1 μg ml−1) for 24 h, then mRNA expression of the indicated gene was analysed using qPCR (Levels in the WT groups were set to 1). Data are representative of three to four independent experiments (mean±s.d.; n=4–8). *P<0.05 and ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
Whether the Th17 response is related to antifungal immunity in Mst1ΔDC mice, we investigated the T-cell response of splenocytes on day 9 after infection. MST1 deficiency in the DC did not alter the T-cell proliferation (Fig. 3d,e), but did significantly enhance the IL-17+ cell percentage compared with the WT mice, whereas the percentages of IFNγ+ cells, IL-4+ cells and Foxp3+ cells were similar in the two groups of infected mice. Consistent with this, the mRNA expression of IL-17A, IL-17F, IL-6Rα/β, but not IFNγ, IL-4 or Foxp3 were significantly enhanced (Fig. 3f). Thus, these data collectively suggest MST1 deficiency in DCs results in enhanced Th17 cell responses against fungal infection.
DC MST1 directs antigen-specific Th17 differentiation
We first observed the endogenous CD4+T cells response following antigen immunization to investigate how DC MST1 directs antigen-specific T-cell differentiation. The T cells from Mst1ΔDC mice produce higher amounts of IL-17, but not IFNγ (Fig. 4a). Furthermore, we used adoptive transfer experiments to observe the T cells responses induced by MST1-deficient DCs. We transferred naive CD4+T cells (CD44lowCD62Lhigh; Thy1.1) from 2D2 mice (TCR specific for MOG peptide) into WT and Mst1ΔDC mice, and then immunized the mice with MOG in complete Freund's adjuvant (CFA). The donor cells were analysed on day 9 after immunization. Donor T cells from the Mst1ΔDC recipients exhibited a similar proliferation level compared with the WT recipients (Fig. 4b). However, donor T cells from Mst1ΔDC recipients displayed more IL-17+ cells, IL-17 secretion and mRNA expression compared with the WT recipients (Fig. 4c,d and Supplementary Fig. 9). The percentages of IFNγ+ cells and IFNγ levels were similar in the two groups.
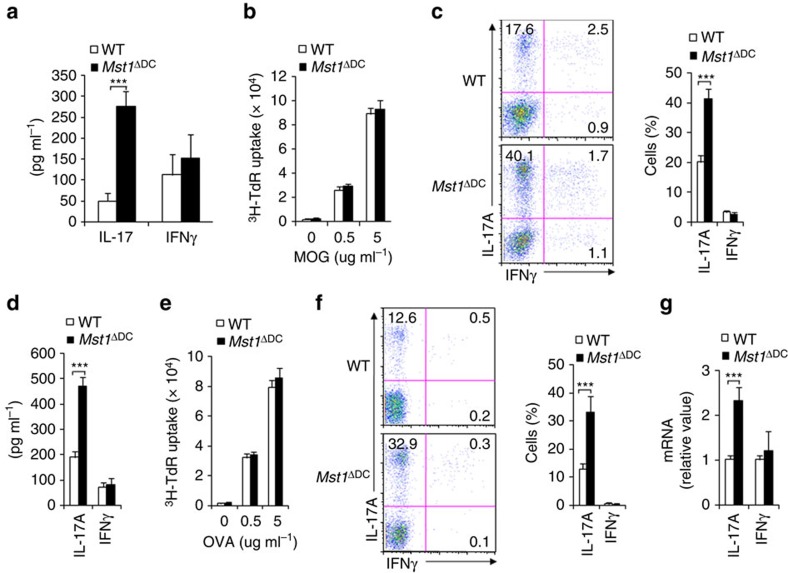
Figure 4. DC MST1 signalling directs Th17 differentiation in vivo.
(a) WT or Mst1ΔDC mice were immunized with OVA+CFA for 7–8 days. The T cells were isolated from the dLN and restimulated ex vivo for 3 days with OVA (5 μg ml−1) in the presence of irradiated splenocytes, and the secretion of indicated cytokines were analysed by ELISA. (b–d) Naive MOG-transgenic 2D2 T cells (Thy1.1+) were transferred into WT or Mst1ΔDC mice and immunized with MOG+CFA for 7–8 days. (b) Proliferation of dLN cells was determined with 3H-TdR incorporation. (c) Expression levels of IL-17A and IFNγ in donor cells in dLN. Right, proportion of IL-17A and IFNγ in donor cells. (d) Secretion of IL-17 and IFNγ in donor cells stimulated with MOG for 72 h. (e,f) Naive OT-II T cells (Thy1.1+) were transferred into WT or Mst1ΔDC mice and immunized with OVA+CFA for 7–8 days. (e) Proliferation of dLN cells was determined with 3H-TdR incorporation. (f) Expressions of IL-17A and IFNγ in donor cells in the dLN. Right, proportion of IL-17A and IFNγ in donor cells. (g) mRNA expression of IL-17 and IFNγ in donor cells stimulated with OVA for 72 h. Levels in WT control groups were set to 1. Data are representative of three to four independent experiments (mean±s.d.; n=3–6). ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
Next, we extended MOG antigen-specific experiments to other antigens to observe the T-cell responses. We used the ovalbumin amino acids 323–339 (called simply ‘OVA' here) antigen to perform this experiment. Naive CD4+T cells (as described above) from OT-II mice (TCR specific for OVA) had been previously transferred into the WT and Mst1ΔDC recipient mice immunized with OVA in CFA. The donor cells were analysed on day 9 after immunization. Donor T cells from the Mst1ΔDC recipients exhibited similar proliferation activity compared with that of the WT recipients (Fig. 4e). However, donor OT-II T cells from Mst1ΔDC recipients displayed enhanced Th17 differentiation compared with the WT-recipient mice (Fig. 4f,g). Collectively, these results suggest that MST1 deficiency in DCs promotes antigen-specific Th17 cell differentiation in vivo.
MST1 is required for DC-dependent Th17 differentiation
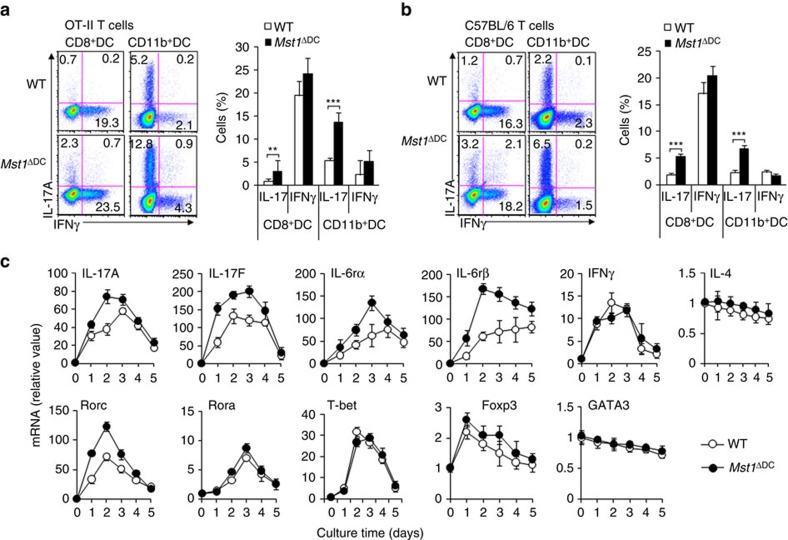
Next, we applied an in vitro coculture system to investigate the role of splenic DC to antigen-specific T cells, including purified antigen specific T cells and splenic DCs and the stimuli LPS and antigen. 2D2T cells and/or OT-II T cells stimulated by Mst1ΔDC DCs in the presence of antigen displayed similar proliferation activity compared with that of the WT DC (Supplementary Fig. 10A,B). The expression levels of IL-2, CD25 and CD69 were also similar in T cells co-cultured with the two kinds of DCs in vitro, suggesting the MST1-deficient in DC doesn't alter the T-cell activities (Supplementary Fig. 10C). However, we observed a higher level of IL-17+ and mRNA expression in OT-II T cells co-cultured with Mst1ΔDC splenic DC, even with a variety of Mst1ΔDC DC subsets (CD11b+DCs and CD8+DCs), than in T cells co-cultured with WT DCs (Fig. 5a and Supplementary Fig. 10D,E). Furthermore, polyclonal T cells activated by the anti-CD3 and MST1-deficient DC subsets displayed similar alterations in Th17 and Th1 differentiation (Fig. 5b). Moreover, the Th17-assoicated cytokine IL-17A/F, cytokine receptor IL-6Rα/β and gene encoding the transcriptional factor RORγt (Rorc) all displayed higher and more sustained upregulation in the T cells co-cultured with Mst1ΔDC DCs co-cultured with WT DCs (Fig. 5c). The other T-cell subtype-associated cytokines and transcriptional factors were found to be similar in the two kinds of T cells co-cultured with DCs (Fig. 5c). These data suggest that MST1 is required in DCs for the induction of Th17 cell differentiation.
Figure 5. DC MST1 signalling instructs Th17 differentiation in vitro.
(a) Naive CD4+T cells sorted from OT-II mice stimulated with antigen and LPS-pulsed CD8+DCs or CD11b+DCs from WT or Mst1ΔDC mice for 5 days. Intercellular staining of IL-17 and IFNγ in T cells. Right, proportion of IL-17 and IFNγ in T cells. (b) Naive CD4+T cells sorted from C57BL/6 mice stimulated with anti-CD3 (2 μg ml−1) and LPS-pulsed CD8+DCs or CD11b+DCs from WT or Mst1ΔDC mice for 5 days. Intercellular staining of IL-17 and IFNγ in T cells was determined with FCM and a typical figure is shown. Right, proportion of IL-17 and IFNγ in T cells. (c) Naive T cells from C57BL/6 mice were co-cultured with WT or Mst1ΔDC splenic DCs for 5 days in the presence of anti-CD3 and LPS. mRNA expression of the indicated genes in T cells were determined with qPCR. Levels in the WT control groups were set to 1. Data are representative of three to four independent experiments (mean±s.d.; n=4–6). **P<0.01 and ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
To investigate whether DC MST1 is sufficient for deciding the fate of Th17 cells, we ectopically expressed MST1 with a retrovirus and sorted the positive green fluorescent protein (GFP) DCs. We co-cultured them with OT-II cells and observed the T-cell response as described above. Modest MST1 overexpression (MST1-RV) resulted in a lower IL-17+ percentage of T cells and IL-17 mRNA expression, but not IFNγ+ percentage or IFNγ mRNA expression compared with the control group (RV; Supplementary Fig. 11). These data suggest DC MST1 signalling is sufficient for inducing Th17 cell differentiation. Taken together, these data collectively suggest MST1 signalling in DCs, acting as a negatively regulator, directly inhibits Th17 cell differentiation.
DC MST1 directs Th17 differentiation through IL-6
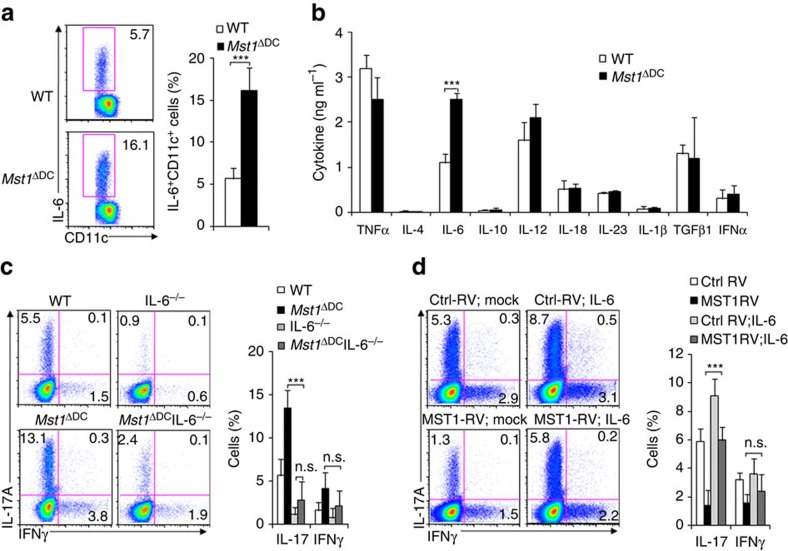
Next, we analysed the DC-derived cytokine levels, including TNF, IL-4, IL-6, IL-10, IL-12, IL-18, IL-23, IL-1β, TGFβ and IFNα. MST1 deficiency resulted in significant higher IL-6+ cells among the DCs compared with WT DCs after LPS stimulation (Fig. 6a). Also, similar alterations of IL-6 secretion and mRNA expression were observed in the DCs after LPS stimulation. However, the secretion and mRNA expression of other cytokines were comparable in the Mst1ΔDC and WT DCs after LPS stimulation in vitro (Fig. 6b and Supplementary Fig. 12). In contrast, modest expression of MST1 in DCs resulted in significantly lower IL-6 production compared with control, but the IL-23, IL-1β and TGFβ1 were similar in the two groups of DCs (Supplementary Fig. 13). Together, these data suggest IL-6 is likely to be critical for the Th17 cell differentiation induced by Mst1ΔDC DCs.
Figure 6. MST1 controls DC IL-6 production during Th17 differentiation.
(a) Intracellular staining of IL-6 in WT or Mst1ΔDC splenic DCs 5 h after LPS stimulation in vitro. (Right) Proportion of IL-6 among the CD11c+ cells. The supernatant was collected and cytokine secretions determined with ELISA (b). (c,d) CD4+T cells from the indicated mice stimulated with WT or Mst1ΔDC splenic DCs for 5 days. Intercellular staining of IL-17 and IFNγ in T cells was determined with FCM and a typical figure is shown. Right, proportion of IL-17 and IFNγ in T cells. Data are representative of three to four independent experiments (mean±s.d.; n=4–5). ***P<0.001, compared with the indicated groups. n.s., not significant. P-values were determined using Student's t-tests.
We applied a DC-T-cell co-culture system to determine whether MST1 signalling in DCs regulates Th17 differentiation through IL-6. In this system, we observed that Mst1ΔDCIL-6−/− DCs almost completely reversed the impact of Mst1ΔDC on Th17 differentiation (Fig. 6c). Consistently, we also were able to reverse the impact of MST1-RV on Th17 cell differentiation in our co-culture system by adding IL-6 (Fig. 6d). Altogether, these data suggest that IL-6 production in Mst1ΔDC DCs as well as MST1-RV DCs contributes to Th17 cell differentiation.
To investigate the wide availability of DC-directed T-cell responses, we isolated DCs from the spinal cord and intestine and found that MST1 deficiency does not alter the composition of DCs, it dose significantly promote the secretion of IL-6 and direct Th17 cell differentiation (Supplementary Figs 14 and 15).
DC MST1 controls the signalling of IL-6R/p-STAT3 of T cells
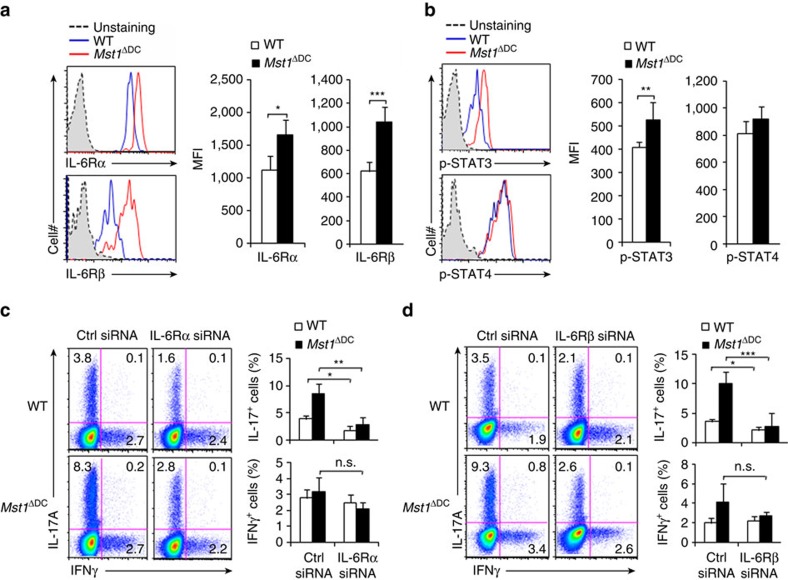
T-cell polarizing cytokines often induce the expression of their corresponding cytokine receptor on T cells, resulting in robust programing of cell fate determination1,4. This led us to investigate the expression of the cytokine receptor of IL-6 in T cells activated by WT and Mst1ΔDC DCs. We applied a DC-T-cell co-culture system (as described above) to investigate this. Mst1ΔDC DCs induced significantly higher expression of IL-6Rα and IL-6Rβ in T cells compared with WT DCs (Fig. 7a). The intercellular cytokine signalling in T cells also exhibited significant alteration. Mst1ΔDC DCs induced higher phosphorylation of STAT3 compared with WT DCs in a DC-T-cell co-culture system. However, the phosphorylation of STAT4 was similar in the T cells co-cultured with Mst1ΔDC DCs and WT DCs (Fig. 7b). These data suggest IL-6R-p-STAT3 signalling is probably involved in the modulation of Th17 cell differentiation that is induced by DC MST1.
Figure 7. T-cell IL-6R-p-STAT3 is responsible for DC MST1 signalling.
(a) Expression of IL-6Rα and IL-6Rβ (gp130) in T cells co-cultured with WT or Mst1ΔDC splenic DCs for 3 days. Right, the mean fluorescent intensity (MFI) is summarized. (b) Intercellular staining of p-STAT3 and p-STAT4 in T cells co-cultured with WT or Mst1ΔDC splenic DCs for 3 days. Right, MFI of the indicated protein is summarized. (c,d) Sorted CD4+ T cells were transfected with control or IL-6Rα (c) and IL-6Rβ (d) siRNA vector and stimulated with WT or Mst1ΔDC splenic DCs for 5 days. The intercellular staining of IL-17 and IFNγ in T cells performed with FCM and a typical figure shown. Right, the proportion of IL-17 and IFNγ in T cells. Data are representative of three to four independent experiments (mean±s.d.; n=4). *P<0.05, **P<0.01 and ***P<0.001, compared with the indicated groups. n.s., not significant. P-values were determined using Student's t-tests.
To examine whether IL-6R signliang is necessary for the T-cell differentiation induced by DC MST1, we applied an siRNA approach to knockdown of IL-6Rα and IL-6Rβ expression in OT-II T cells (Supplementary Fig. 16A,C), which were subsequently co-cultured with WT or Mst1ΔDC DCs in the DC-T-cell co-culture system as described above. Knockdown of IL-6Rα or IL-6Rβ consistently reversed the enhanced Th17 cell differentiation and phosphorylation of STAT3 by Mst1ΔDC DCs, but Th1 differentiation was unchanged (Fig. 7c,d and Supplementary Fig. 16B,D). Thus, IL-6R-p-STAT3 signalling in T cells is required for the Th17 cell differentiation induced by DC MST1.
DC MST1 controls IL-6 production through p38MAPK
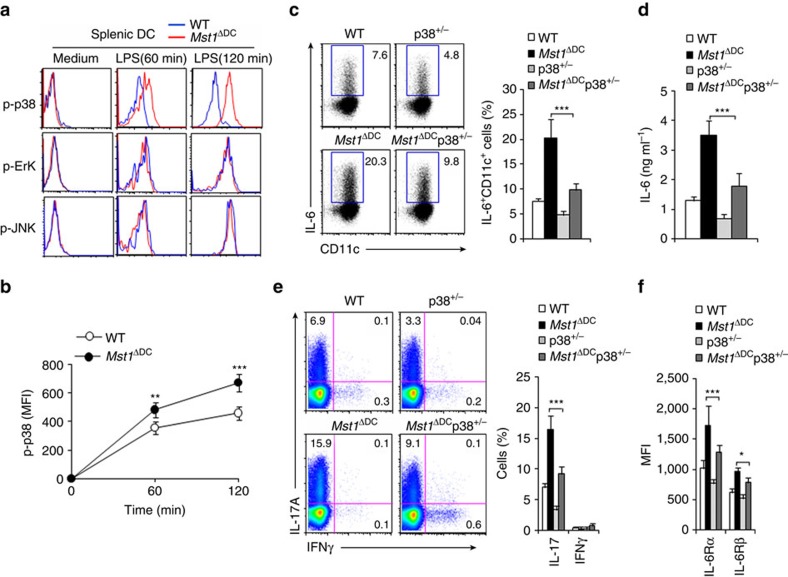
How does MST1 control cytokine production in DCs in directing T-cell differentiation? We assessed the activation of LPS downstream pathways, including ErK, c-jun-NH2-kinase (JNK), NF-kB, p38MAPK and AKT-mTOR by flow cytometry or immunoblot analysis to elucidate the mechanisms that mediate MST1 function in splenic DCs stimulated by LPS. As expected, LPS activated all the pathways in the WT DCs. In contrast, Mst1ΔDC DCs activated the ErK, JNK, NF-kB and AKT-mTOR pathways like the WT DCs did, but a stronger and more sustained phosphorylation of p38MAPK (Fig. 8a,b and Supplementary Fig. 17). Moreover, modest MST1 overexpression (MST1-RV) resulted in lower phosphorylation of p38MAPK than control (RV; Supplementary Fig. 18). Thus, MST1 is associated with p38MAPK.
Figure 8. MST1 regulates IL-6 production through p38MAPK.
(a) Intercellular staining of the indicated proteins in splenic DCs following LPS stimulation in vitro. Expression of the phosphorylation of p38MAPK (MFI) among CD11c+cells is summarized in b. (c,d) Intracellular staining of IL-6 in DC isolated from the indicated mice 4 h after the i.p. injection of 10 mg kg−1 LPS (c). Right, the proportion of IL-6+ cells in DCs is summarized. The serum IL-6 level in the indicated mice (d). (e) Intracellular staining of IL-17 and IFNγ in T cells co-cultured with splenic DCs isolated from indicated mice was determined with FCM. Right, proportion of IL-17 and IFNγ in CD4+T cells is summarized. (f) Expression of the indicated gene in T cells was determined with FCM and the MFI summarized. Data are representative of four independent experiments (mean±s.d.; n=3–5). *P<0.05, **P<0.01 and ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
We pretreated WT and Mst1ΔDC DCs with U0126 (an inhibitor of ErK) and SB203580 (an inhibitor of p38MAPK), respectively, followed by LPS stimulation. SB203580, but not U0126 significantly reversed the IL-6 production and IL-6 mRNA expression (Supplementary Fig. 19A,B) by blocking p38MAPK as indicated (Supplementary Fig. 19C). Furthermore, we used Mst1ΔDCp38+/− double knockout mice in this investigation (Supplementary Fig. 19D). Interestingly, the increased IL-6 expression and production in Mst1ΔDC DCs were significantly reversed in Mst1ΔDCp38+/− double knockout DCs (Fig. 8c,d). The enhanced Th17 differentiation as well as IL-6Rα and IL-6Rβ expression observed in T cells co-cultured with Mst1ΔDC DCs were also significantly reversed in T cells co-cultured with Mst1ΔDCp38+/− double knockout DCs (Fig. 8e,f). Therefore, these data suggest the p38MAPK pathway is responsible for the IL-6 production during DC MST1-dependent Th17 cell differentiation.
We determined the activation of MAPK-activated protein kinase 2 (MK2) and mitogen and stress-activated protein kinase 1 (MSK1), two p38 targets42,43 to investigate the signalling and transcriptioanl pathways involved in IL-6 production. Significantly unregulation of Mst1ΔDC DC cell activation was observed compared with WT control cells (Supplementary Fig. 20A). Furthermore, cAMP response-element-binding protein (CREB)44, which is important for IL-6 expression, was consistently upregulated in Mst1ΔDC cells, whereas the phosphorylation of transcription factor C/EBPβ, c-Fos and IkBα unaltered (Supplementary Fig. 20A). Consistent with this, modest overexpression of MST1 in DCs resulted in lower and less inactivation of the phosphorylation of MK2, MSK1 and CREB (Supplementary Fig. 20B). Thus, MST1 is assoicated with the p38MAPK–MK2/MSK1–CREB signalling axis. Importantly, MK2/MSK1/CREB siRNA efficiently silenced their expression in DC cells and consistently reversed the IL-6 production and Th17 cell differentiation in Mst1ΔDC DCs to a large extent (Supplementary Fig. 20C–K). These data collectively a p38–MK2/MSK1–CREB signalling axis for the regulation of IL-6 production in DCs for Th17 cell differentiation.
Knockdown of MST1 in human DCs directs Th17 differentiation
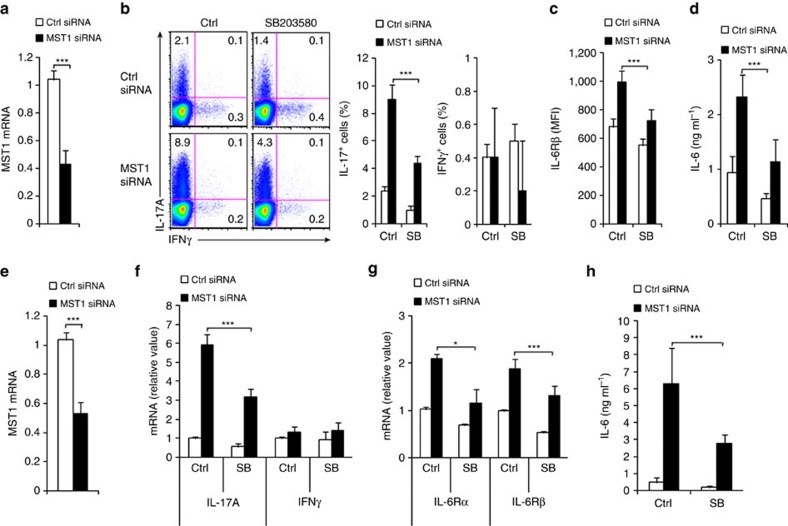
Next, we sought to knockdown of MST1 with an siRNA approach targeting MST1 in both mouse and human DCs to see if this would recapitulate our findings in the genetic targeting MST1. MST1 siRNA efficiently knocked down the expression of MST1 in mouse DC cells, promoted the IL-6 secretion of DCs and upregulated the percentage of IL-17+ cells and the level of IL-6R expression in T cells with a DC-T coculture system (as described above). Moreover, blocking the p38MAPK pathway almost completely reverse the change (Fig. 9a–d). The siRNA experiment was then extended to a human DC-T-cell coculture system in which the DCs were derived from human peripheral blood monocytes and the T cells were isolated from human cord blood. Knockdown of MST1 in human DCs largely recapitulated the alteration in mouse DCs, including IL-6 production in DCs and IL-17A+ and IFNγ+ cell percentage as well as IL-6R expression in T cells (Fig. 9e–h). Thus, the data show that indicated MST1 mediates an evolutionarily conserved signalling pathway in both mouse and human DCs.
Figure 9. Mouse and human DC MST1 modulates T-cell differentiation.
Mouse (a) or human (e) DCs transfected with MST1-specfic or control siRNA and mRNA of indicated gene were determined with qPCR. Levels in the control groups were set to 1. Mouse DCs (b–d) or human DCs (f–h) pulsed with LPS (100 ng ml−1) were co-cultured with mouse or human T cells, respectively. The expression of IL-17 and IFNγ in T cells was determined with FCM (b) or qPCR (f); MFI of the indicated gene in T cells (c) and mRNA of the indicated genes in T cells (g); the levels of IL-6 in the indicated supernatant (d,h) were determined. Data are representative of three independent experiments (mean±s.d.; n=4–6). *P<0.05 and ***P<0.001, compared with the indicated groups. P-values were determined using Student's t-tests.
Discussion
A genetic approach was employed to selectively delete MST1 in DC cells and results showed that loss of MST1 in DCs causes massive inflammation in multiple tissues in old-aged mice and promotes Th17 cell differentiation in autoimmune or fungal infection inflammation. Conditional deletion of MST1 in mouse DCs or the use of siRNA in mouse or human DCs increased the IL-17 production from CD4+T cells, whereas ectopic MST1 expression in DCs inhibited IL-17 production of CD4+T cells. MST1-deficient DCs produced excess amount of IL-6, upregulated IL-6Rα/β expression and phosphorylated STAT3 in responding CD4+T cells and contributed to Th17 differentiation. In addition, we showed that MST1-deficient DCs exhibited an increased phosphorylation of p38MAPK–MK2/MSK1–CREB, which is responsible for IL-6 production in MST1-deficient DCs. Our study thus indicates MST1–p38MAPK–MK2/MSK1–CREB to be the critical signalling pathway in DCs for controlling IL-6 production and IL-6R-STAT3 expression in CD4+T cells in directing Th17 cell differentiation (Supplementary Fig. 21).
The kinase MST1 is the mammalian homologue of the Drosophila melanogaster kinase Hippo, which inhibits cell proliferation and promotes apoptosis during development45,46,47,48,49,50,51,52,53,54,55,56,57,58,59. MST1 deficiency in humans results in a complex, combined immunodeficiency syndrome with recurrent bacterial and viral infections, lymphopenia and variable neutropenia60,61. In mice, MST1 is an important regulator of the adhesion, migration, proliferation and apoptosis of cells31,32,33. In adaptive immune cells, MST1 is critical for T-cell migration, activation and function34,35. In innate immune cells, MST1 is important for the optimal ROS production and bactericidal activity of phagocytes by promoting activation of the small GTPase Rac and also mitochondrial trafficking and juxtaposition to the phagosome through the assembly of a TRAF6–ECSIT complex37. However, the interplay between adaptive immune cells and innate immune cells remains poorly understood. The present study shows that an integrated MST1–p38MAPK–MK2/MSK1–CREB signalling axis in DCs directs the generation of the Th17 subset in both autoimmune and fungal infection inflammation. Whereas MST1 is not involved in regulating antigen presentation of DCs, the MST1–p38MAPK–MK2/MSK1–CREB axis in DCs instructs Th17 cell differentiation through its modulation of the DC-derived T-cell polarizing cytokine IL-6. The altered IL-6Rα/IL-6Rβ expression and downstream STAT3 signalling in responding T cells further confer a robust DC-T-cell cross-talk, directing the programming of specific Th17 cell differentiation.
DC-derived signals control adaptive immunity at multiple checkpoints that dictate the activation and differentiation of T-cell-mediated immune responses, but very few innate immune pathways have been shown to regulate Th17 responses62,63. Although some pathways have shown to be involved in the modulation of T cells, such as dectin-Syk-CARD9 signalling64, MKP-1 signalling19, Wnt-β-catenin signalling21 and sirtuin1-HIF1α metabolism signalling18, the essential innate cell-dependent Th17 modulation pathway still remains unclear. Previous studies have shown that p38 acts in a T-cell-intrinsic manner for Th17 differentiation, as IL-17 expression is diminished after pharmacological inhibition of p38 or dominant-negative p38 over-expression65,66. Other studies also showed that p38 is not responsible for T-cell-intrinsic modulation of Th17 differentiation20. It is reported that there is a selective role for p38 in DC-mediated Th17 differentiation but not in T-cell-intrinsic Th17 differentiation20,67. In accord with the latter studies, we found that DC MST1 deficiency significantly upregulated the activation of the p38MAPK–MK2/MSK1–CREB signal axis and contributed to the excess amount secretion of the T-cell polarizing cytokine IL-6 in directing Th17 cell differentiation.
It is intriguing that the germ line knockout of MST1 mice results in normal development, while the DC-specific MST1-deficient mice die as early as 15 weeks after birth. Similarly, the bone marrow chimeras (Mst1ΔDC→WT) mice also display an early death and weight loss, confirming the cell specificity of MST1 deficiency. Additional pathological and immunological analyses have revealed that the early death and weight loss in DC-specific MST1-deficient mice were due to a heightened Th17-dependent autoimmunity. We believe that the phenotypic discrepancy of the MST1 germ line and DC-specific deficiency are likely due to one of the following reasons. One possibility is that the germ line knockout of MST1 may lead to an adaptive and compensatory rewiring of downstream signalling pathways during early development, likely before the development of the DC lineage. On the other hand, such adaptive and compensatory responses are less likely to occur when MST1 is deleted in mature and terminally differentiated DC (Cd11c-Cre plays the major role at this stage). An alternative possibility is that MST1 deficiency in other immune compartments, likely in T cells, may result in an opposite T-cell response and counteract MST1 deficiency in DCs that elicits the T-cell response. A detailed analysis of DCs and other immune compartments in MST1 germ line knockout will be required to test these ideas.
In summary, it is shown that a targeting of MST1 in DCs promotes IL-6 along with the expression of IL-6Rα/β and STAT3, thereby contributing to the programming Th17 cell differentiation in the inflammation that arises in both autoimmunity and fungal infection. Thus, our results define the essential nature of the MST1–p38MAPK–MK2/MSK1–CREB pathway in DCs for inducing Th17 cell differentiation, with implications for targeting DCs as an approach to treatment of disorders of the immune system and immune-associated diseases.
Methods
Mice
All animal experiments were performed with the approval of the Animal Ethics Committees of Beijing Normal University and Fudan University. 2D2 TCR-transgenic mice, IL-6−/− and p38+/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). OT-II TCR-transgenic mice were obtained from Center of Model Animal Research at Nanjing University (Nanjing, China). CD45.1 and Thy1.1 mice were obtained from the Beijing University Experimental Animal Center (Beijing, China). C57BL/6 Mst1flox/flox and Cd11c-Cre mice were described previously18,32. C57BL/6 mice were obtained from the Beijing Weitonglihua Experimental Animal Center (Beijing, China) and Fudan University Experimental Animal Center (Shanghai, China). All mice had been backcrossed to the C57BL/6 background for at least eight generations and were used at an age of 6–12 week, age and sex matched male or female mice unless otherwise noted in the figure legend. WT control mice were of the same genetic background and, where relevant, included Cre+ mice to account for the effects of Cre (no adverse effects due to Cre expression itself were observed in vitro or in vivo).
Mouse EAE induction and CNS lymphocyte isolation
Mice were immunized s.c. with 100 μl of emulsified IFA supplemented with 500 μg Mycobacterium tuberculosis H37Ra (DIFCO) and 100 μg of MOG35–55, and received an i.p. injection of 200 ng pertussis toxin (List Biological Laboratories) at the time of immunization as well as 48 h later. The mice were observed daily for clinical signs and scored38. Mice were scored for clinical signs of disease on a daily basis using the following scores: 0=normal behaviour, 1=distal limp tail, 1.5=complete limp tail, 2=disturbed righting reflex, 3=ataxia, 4=early paralysis (hind legs), 5=full paralysis and 6=moribund or death. For the ex vivo recall response, mice were immunized as above and 7–8 days later, draining LN cells were stimulated with the cognate peptide for 2–3 days for proliferation, RNA and cytokine secretion assays, or were labelled with CFSE (Invitrogen, Carlsbad, CA, USA) and stimulated with antigen for 5 days followed by intracellular staining. T-cell proliferation was determined by pulsing the cells with [3H] thymidine at a dose of 1 Ci per well in a 96-well plate for the final 12–16 h of culture. Cytokine secretion was determined using Elisa (R&D system).
CNS leukocyte isolation was performed40. Mice were perfused with 25 ml 2 mM EDTA in PBS to remove blood from internal organs. The spinal columns were dissected, cut open and the intact spinal cord separated carefully from the vertebrae. The spinal cord was cut into small pieces and placed in 2 ml digestion solution containing 10 mg ml−1 Collagenase D (Roche) in HBSS. Digestion was performed for 45 min at 37 °C with brief vortexing every 15 min. At the end of digestion, the solution was mixed thoroughly and passed through a 40 μm cell strainer. The cells were washed once in PBS, placed in 6 ml of 38% Percoll solution and pelleted for 20 min at 2,000 r.p.m. Pellets were resuspended in buffer or medium for subsequent analysis.
Mouse fungal infection
For fungal infection, mice were injected intravenously with 2 × 105 live C. albicans yeast. After 9 days, mice were killed for analysis. Infected kidney samples were fixed in 4% paraformaldehyde, embedded in paraffin and stained with H&E. Splenocytes were harvested and stimulated with 106 per ml heat-killed C. albicans for 48 h for intracellular staining or RNA isolation.
Cell isolation from gut-associated lymphatic tissues
The isolation of lamina propia (LP) lymphocytes was performed16. The small intestine (SI) and large intestine (LI) were removed, opened longitudinally and cut into pieces. After vigorous shaking in HBSS containing EDTA, the supernatant containing epithelial cells and IELs was discarded. The remaining intestinal pieces were digested with Collagenase D (Worthington) and pelleted. The pellet was resuspended and placed in a Percoll gradient, and after centrifugation, the interface containing the LP lymphocytes was collected for further analysis.
Cell adoptive transfer
Naive T cells 2 × 106 (CD4+CD62LhighCD44lowCD25−) from OT-II or 2D2 TCR-transgenic mice were sorted and transferred into Mst1flox/floxCd11c-Cre− (WT) and Mst1flox/floxCd11c-Cre+ (Mst1ΔDC) mice. After 24 h, recipient mice were injected s.c. with OVA323–339 or MOG35–55 in the presence of Complete Freund's adjuvant (CFA; Difco), LPS (Sigma). On day 8–9 after immunization, draining LN cells were harvested and stimulated with the cognate peptide for 2–3 days for cytokine mRNA and secretion analyses, or pulsed with PMA and ionomycin for 5 h for intracellular staining donor-derived T cells.
Cell cultures and flow cytometry
Spleens were digested with Collagenase D and DCs (CD11c+TCR−CD19−NK1.1−F4/80−Ly6G−) were sorted on a FACS caliber (Becton Dickinson, CA, USA). Lymphocytes were sorted to enrich for naive T cells. For DC-T-cell co-cultures, DCs and T cells (1:10) were mixed in the presence of 1 μg ml−1 OVA323–339 peptide and 100 ng ml−1 LPS. After 5 days of culture, live T cells were stimulated with PMA and ionomycin for intracellular cytokine staining, or with plate-bound α-CD3 to measure cytokine secretion and mRNA expression. T-cell proliferation was determined by pulsing the cells with 3H-thymidine for the final 12–16 h of culture. For drug treatments, cells were incubated with vehicle, SB205830 (5 μM; Calbiochem), U0126 (10 μM; Calbiochem) for 0.5–1 h before stimulation. For cytokine treatment, cultures were supplemented with 20 ng ml−1 IL-6 (R&D system). Flow cytometry was performed with antibodies from eBioscience or BD Biosciences. Anti-CD11c APC (clone N418; 1:400), anti-CD11c PE (clone N418; 1:400), anti-CD11c FITC (clone N418; 1:100), anti-CD4 APC-Cy7 (clone GK1.5; 1:100), anti-CD8α FITC (clone 53-6.7; 1:200), anti-CD11b FITC (clone M1/70; 1:200), anti-Ly6G PE (clone RB6-8C5; 1:500), anti-F4/80 PE (clone BM8; 1:400), anti-CD19 PE (clone 1D3; 1:400), anti-TCR FITC (clone H57-597; 1:200), anti-CD44 FITC (clone IM7; 1:200), anti-CD62L APC (clone MEL14; 1:400 ), anti-CD80 APC (clone 16-10A1; 1:400), anti-CD86 APC (clone GL1; 1:400), anti-CD40 APC (clone 1C10; 1:400), anti-CD54 FITC (clone YN1/1/7.4; 1:200 ), anti-MHCII APC (clone AF6-120.1; 1:300), anti-CD45 APC (clone 30-F11; 1:400), anti-NK1.1 PE (PK136; 1:400), anti-IL-17A APC (clone eBio17B7; 1:200), anti-IFNγ PE (clone XMG1.2; 1:200), anti-IL-4 PE (clone 11B11; 1:200), anti-Foxp3 PE (clone FJK-16s; 1:50). The anti-IL-6Rα PE (clone MP5-20F3; 1:100) was from Biolegend and the anti-IL-6Rβ APC (clone MAB4681; 1:100) from R&D systems. All antibodies were diluted in 2% bovine serum albumin. Flow cytometry data were acquired on a FACSCalibur (Becton Dickinson) or an Epics XL bench-top flow cytometer (Beckman Coulter, CA, USA) and the data analysed with FlowJo (Tree Star, San Carlos, CA, USA).
RNA analysis
RNA was extracted with an Rneasy kit (QIAGEN, Dusseldorf, Germany) and the cDNA synthesized using SuperScrip III reverse transcriptase (Invitrogen). An ABI 7900 real-time PCR system was used for quantitative PCR (qPCR), with the primer and probe sets obtained from Applied Biosystems (Carlsbad, CA, USA).The primers used in the present study are listed in Supplementary Table 1. Results were analysed using SDS 2.1 software (Applied Biosystems). The cycling threshold value of the endogenous control gene (Hprt1, encoding hypoxanthine guanine phosphoribosyl transferase) was subtracted from the cycling threshold. The expression of each target gene is presented as the fold change relative to that of control samples68.
Protein analysis
For detection of phosphorylated signalling proteins, purified cells activated with LPS (Sigma) were immediately fixed with Phosflow Perm buffer (BD Biosciences) and stained with PE or APC directly conjugated with an antibody to Erk phosphorylated at Thr202 and Tyr204 (clone 20A; BD Biosciences), p38MAPK phosphorylated at Thr180 and Thr182 (clone D3F9), JNK phosphorylated at Thr183 and Tyr185 (clone G9), STAT4 phosphorylated at Tyr701 and Ser727 (clone 58D6), STAT3 phosphorylated at Tyr705 (clone D3A7), AKT phosphorylated at Ser473 (clone D9E), AKT phosphorylated at Thr308 (clone D25E6), S6 Ribosomal Protein phosphorylated at Ser235/236 (clone D57.2.2E; all from Cell Signaling Technology and diluted at 1:100 in 2% bovine serum albumin), as described previously16. Immunoblot analysis was performed as described68 The primary antibodies against MST1 (catalogue number 3682), NF-kB p65 (clone D14E12), p38MAPK (catalogue number 9212), p-p38MAPK (Thr180/Thr183; clone 3D7), p-MK2 (Thr334; clone 27B7), p-MSK1 (Thr581; catalogue number 9595), p-CREB (Ser133; clone 87G3), p-C/EBPβ (Thr235; catalogue number 3084), p-c-Fos (Ser32; catalogue number 5348) and p-IκBα (Ser32; clone 14D4; all from Cell Signaling Technology and diluted at 1:1,000 in 2% bovine serum albumin) and along with an anti-β-actin (clone AC-15; Sigma-Aldrich; 1:2,000) antibody. Images have been cropped for presentation and full-size blot is shown in Supplementary Fig. 22.
Retroviral transduction
MST1 was cloned into a MSCV retroviral vector (Clontech Laboratories, Mountain View, CA, USA)17,69. Phoenic-Eco packaging cells (ABP-RVC-10001; Allele Biotechnology, San Diego, CA, USA) were transfected with Lipofectamine (Invitrogen) and recombinant retrovirus was collected 48 h after transfection. After sorted splenic DCs were stimulated for 24 h with LPS (Sigma), DC cells were transduced with retroviral supernatant by spin inoculation (650 g for 1 h). Cells were sorted based on the expression of GFP by flow cytometry and cytokine production and gene-expression analysis were performed.
Gene knockdown with RNAi
A gene-knockdown lentiviral construct was generated by subcloning gene-specific short RNA (siRNA) sequences into lentiviral siRNA expression plasmids (pMagic4.1)70. Lentiviruses were harvested from culture supernatant of 293T cells transfected with the siRNA vector. Sorted splenic DCs or OT-II CD4+ T cells were infected with recombinant lentivirus and GFP-expressing cells were isolated using fluorescence sorting 48 h later. Gene expression was confirmed using qPCR. The sorted DCs and T cells with either the control or siRNA vectors were used for functional assays.
Human DC and T-cell cultures
For assays of human DC-mediated T-cell activation and differentiation, normal human DCs (CC-2701; Lonza) were cultured and their populations expanded for 5 days with human granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (R&D system), followed by stimulation for 24 h with LPS (Sigma). DCs were washed extensively and cultured with human cord blood CD4+ T cells (2C-200; Lonza) at a ratio of 1:10. After 7 days of culture, live T cells were purified and then stimulated either with PMA and ionomycin for intracellular cytokine staining for 5 h or with plate-bound anti-CD3 for analysis of mRNA expression.
Statistical analysis
All data are presented as the mean±s.d. Student's unpaired t-test was applied for comparison of means to identify differences between groups. Comparison of the survival curves was performed using the log-rank (Mantel–Cox) test. A P-value (alpha-value) of <0.05 was considered to be statistically significant.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.
Additional information
How to cite this article: Li, C et al. Dendritic cell MST1 inhibits Th17 differentiation. Nat. Commun. 8, 14275 doi: 10.1038/ncomms14275 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures and Supplementary Table
Acknowledgments
Our research is supported by grants from the National Natural Science Foundation for General Programs of China (31671524, G.L.); National Basic Research Program of China (2013CB945300, W.T.); National Natural Science Foundation for General Programs of China (31171407 and 81273201, G.L.), Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality (12JC1400900, G.L.), and Innovation Program of Shanghai Municipal Education Commission (14ZZ009, G.L.).
Footnotes
The authors declare no competing financial interests.
Author contributions C.L., Y.B. and Y.L., designed and conducted the experiment with cells and mice, analysed data; H.Y., Q.Y., J.W. and Y.W. conducted the experiments with mice, analysed data; H.S., A.J., Y.H., L.H., J.Z. and S.L. assisted with analysis and interpretation of experiments and results; W.T. contributed to providing the Mst1flox/flox mice and revised the manuscript and G.L. developed the concept, designed and conducted the experiments with cells and mice, analysed data, wrote the manuscript and provided overall direction.
References
- Zhu J., Yamane H. & Paul W. E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel K. M., Lee D. U. & Rao A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 4, 616–623 (2003). [DOI] [PubMed] [Google Scholar]
- Zhou L., Chong M. M. & Littman D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009). [DOI] [PubMed] [Google Scholar]
- Murphy K. M. & Reiner S. L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4(+) T cells. Immunity 44, 1337–1349 (2016). [DOI] [PubMed] [Google Scholar]
- Torchinsky M. B., Garaude J., Martin A. P. & Blander J. M. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458, 78–82 (2009). [DOI] [PubMed] [Google Scholar]
- Chung Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E. et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38, 336–348 (2013). [DOI] [PubMed] [Google Scholar]
- Jones G. W. et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 184, 2130–2139 (2010). [DOI] [PubMed] [Google Scholar]
- Dileepan T. et al. Robust antigen specific th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 7, e1002252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M. et al. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J. Immunol. 186, 6746–6752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I. et al. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J. Exp. Med. 205, 2281–2294 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 23, 1–21 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yang K., Burns S., Shrestha S. & Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 11, 1047–1056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. et al. Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1. Proc. Natl Acad. Sci. USA 112, E957–E965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Wang Y., Shi L. Z., Kanneganti T. D. & Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity 35, 45–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. et al. Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat. Immunol. 13, 152–161 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S. et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z. et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 6, 8357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. D. et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S. et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J. Clin. Invest. 111, 1463–1474 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar B. et al. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J. 26, 4523–4534 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc. Natl Acad. Sci. USA 105, 20321–20326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl Acad. Sci. USA 107, 1431–1436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani A. et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat. Med. 20, 385–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F. et al. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J. Biol. Chem. 286, 6940–6945 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K. et al. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J. 28, 1319–1331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K., Imamura M. & Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 7, 919–928 (2006). [DOI] [PubMed] [Google Scholar]
- Dong Y. et al. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J. Immunol. 183, 3865–3872 (2009). [DOI] [PubMed] [Google Scholar]
- Ueda Y. et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat. Commun. 3, 1098 (2012). [DOI] [PubMed] [Google Scholar]
- Mou F. et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J. Exp. Med. 209, 741–759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J. Immunol. 192, 1525–1535 (2014). [DOI] [PubMed] [Google Scholar]
- Praskova M., Xia F. & Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J. et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 16, 1142–1152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V. et al. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J. Exp. Med. 208, 2465–2476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. L., Schreiner B., McMahon E. J. & Miller S. D. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007). [DOI] [PubMed] [Google Scholar]
- Karulin A. Y., Quast S., Hesse M. D. & Lehmann P. V. Neuroantigen-specific CD4 cells expressing interferon-gamma (IFN-gamma), interleukin (IL)-2 and IL-3 in a mutually exclusive manner prevail in experimental allergic encephalomyelitis (EAE). Cells 1, 576–596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson M., Lundgren B. A., Ahlgren K. M., Kampe O. & Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur. J. Immunol. 42, 2555–2563 (2012). [DOI] [PubMed] [Google Scholar]
- Huang G., Shi L. Z. & Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine 48, 161–169 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammit A. J. et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 15, 1212–1214 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 16, 1423–1430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. D., Draves K. E., Gotoh Y., Krebs E. G. & Clark E. A. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J. Biol. Chem. 276, 14909–14915 (2001). [DOI] [PubMed] [Google Scholar]
- Creasy C. L., Ambrose D. M. & Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271, 21049–21053 (1996). [DOI] [PubMed] [Google Scholar]
- Schinkmann K. & Blenis J. Cloning and characterization of a human STE20-like protein kinase with unusual cofactor requirements. J. Biol. Chem. 272, 28695–28703 (1997). [DOI] [PubMed] [Google Scholar]
- Creasy C. L. & Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J. Biol. Chem. 270, 21695–21700 (1995). [DOI] [PubMed] [Google Scholar]
- Rawat S. J. & Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem. Sci. 40, 149–156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J. et al. Protein kinases of the Hippo pathway: regulation and substrates. Semin. Cell Dev. Biol. 23, 770–784 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E., Rushworth L., Baccarini M. & Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306, 2267–2270 (2004). [DOI] [PubMed] [Google Scholar]
- Del Re D. P. et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol. Cell 54, 639–650 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y. et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 19, 1478–1488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K. F., Zhang X. & Thomas D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013). [DOI] [PubMed] [Google Scholar]
- Yu F. X. & Guan K. L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. & Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q. & Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13, 188–192 (2008). [DOI] [PubMed] [Google Scholar]
- Abdollahpour H. et al. The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme N. T. et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M. & Kuchroo V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009). [DOI] [PubMed] [Google Scholar]
- Iwasaki A. & Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638 (2007). [DOI] [PubMed] [Google Scholar]
- Jirmanova L., Giardino Torchia M. L., Sarma N. D., Mittelstadt P. R. & Ashwell J. D. Lack of the T cell-specific alternative p38 activation pathway reduces autoimmunity and inflammation. Blood 118, 3280–3289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubade R. et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 118, 3290–3300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commodaro A. G. et al. p38α MAP kinase controls IL-17 synthesis in Vogt-Koyanagi-Harada syndrome and experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 51, 3567–3574 (2010). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Kinase AKT1 negatively controls neutrophil recruitment and function in mice. J. Immunol. 191, 2680–2690 (2013). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid-derived suppressor cells. J. Immunol. 192, 3068–3079 (2014). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood 121, 519–529 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Supplementary Table
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.