Abstract
Direct cross-coupling between simple arenes and heterocyclic amines under mild conditions is undoubtedly important for C–N bonds construction. Selective C(sp2)-H amination is more valuable. Herein we show a selective C(sp2)-H amination of arenes (alkyl-substituted benzenes, biphenyl and anisole derivatives) accompanied by hydrogen evolution by using heterocyclic azoles as nitrogen sources. The reaction is selective for C(sp2)-H bonds, providing a mild route to N-arylazoles. The KIE (kinetic isotope effect) experiment reveals the cleavage of C–H bond is not involved in the rate-determining step. Kinetic studies indicate the first-order behaviour with respect to the arene component. It is interesting that this system works without the need for any sacrificial oxidant and is highly selective for C(sp2)-H activation, whereas C(sp3)-H bonds are unaffected. This study may have significant implications for the functionalization of methylarenes which are sensitive to oxidative conditions.
Functionalization of unactivated C–H bonds is an attractive strategy to introduce functionality without the need for pre-functionalized starting materials. Here the authors combine cobalt catalysis with photoredox catalysis, allowing arene-azole cross-coupling under oxidant-free conditions.
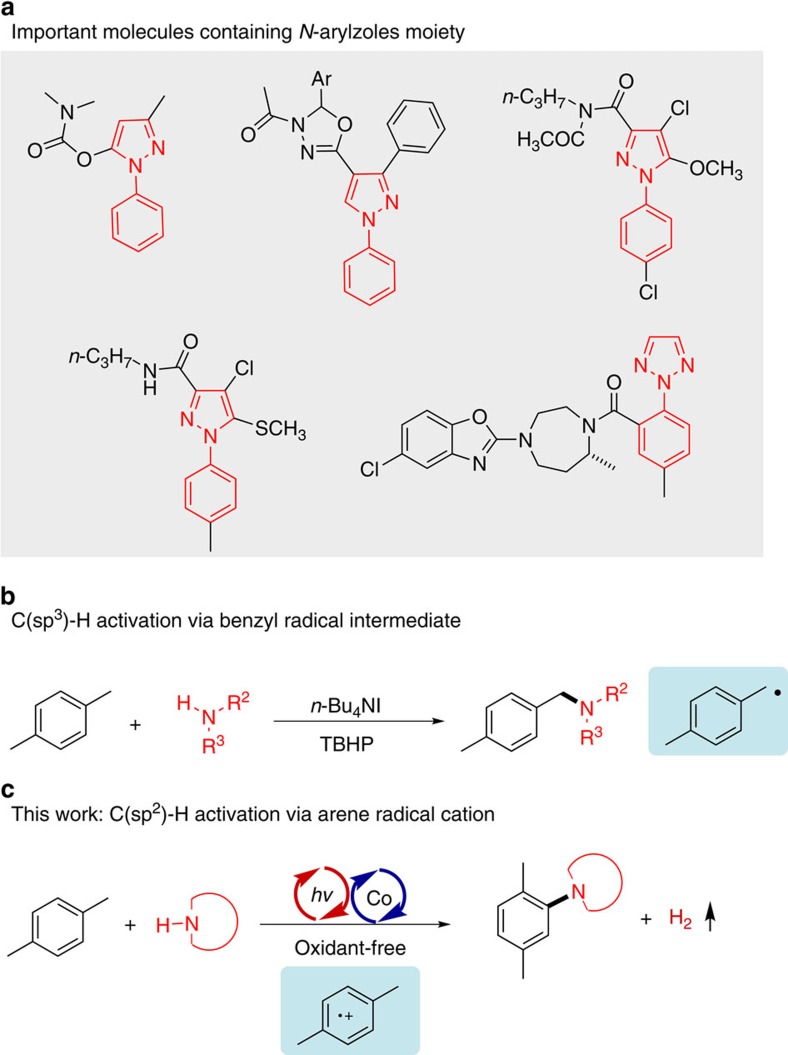
N-arylazoles, a particularly prevalent class of nitrogen-containing compounds, have long been privileged structures in agricultural chemical and biopharmaceuticals (Fig. 1a)1,2. Therefore, preparation of these valuable compounds in a sustainable way is always at the frontier of organic synthesis. The transition metal-catalysed (Pd, Cu and so on) cross-coupling of aryl halides (Ar–X) with azoles have served as a powerful tool for constructing C–N bonds3,4,5,6,7,8. Directly using simple arenes (Ar–H) to replace Ar–X is undoubtedly the most step- and atom-economical method, representing an ideal route for C–N bonds construction. To avoid the separate prefunctionalization and defunctionalization steps9,10,11, the new strategy that directly utilizes C–H/X–H (N, S, O and so on) of two nucleophiles is considered to be an ideal mode for bond formation. In recent years, the analogous technology for the oxidative C–H/N–H cross-coupling to form C–N bonds would be an important synthetic advance12,13,14. However, current strategies required high temperatures or stoichiometric quantities of oxidant, which caused the high consumption of material and energy15,16,17. Hence, more environmental and sustainable perspectives for the alternative catalytic system are therefore highly desirable for C–N construction.
Figure 1. Reaction design.
(a) Important molecules containing N-arylzoles moiety. (b) Amination of benzylic C-H bond by Zhu and co-workers23. (c) The C(sp2)-H amination of methylarenes under oxidant-free conditions.
Methylarenes, a group of the most important raw chemical materials, have been widely utilized as acyl, alkyl, carboxylate and/or alcohol sources18, providing the selective formation of C–C and C-heteroatom bonds. Due to the fact that activation of methylarenes is always under oxidative conditions, the process is always coupled with the transfer of electrons and protons. For example, p-xylene can react with the oxidant to generate the radical cation primarily, which is prossibly transferred into benzyl radical19. The benzylic C(sp3)-H bonds functionalization has been proceeded via the potentially benzyl radical intermediate in numerous reports20,21,22. In 2013, Zhu and co-workers23 achieved the C(sp3)-H amination of methylarenes with azoles using n-Bu4NI as the catalyst and tert-butyl hydroperoxide as the oxidant (Fig. 1b). However, limited examples have been developed for the arylation of azoles using simple methylarenes.
Recently, photocatalysis mediated by visible light has been widely developed, serving as a mild and powerful tool for bond formation24,25,26,27,28,29,30. As pioneering work for arene C–H functionalization, the visible-light-mediated C(sp2)-H amination using azoles as nitrogen sources and O2 as terminal oxidant has been achieved recently31. Due to the fact that the methyl group of methylarene is sensitive to oxidative conditions, especially to dioxygen in photocatalytic system32, developing a new strategy for the selective C(sp2)-H functionalization of methylarenes is urgently required. It is always proposed that two-electron and two-proton were lost in traditional oxidative coupling. It demands extra stoichiometric oxidants and proton acceptors as the sacrificial reagents in the cross-coupling reactions, leading to a number of wasteful by-products or oxidative side reactions9,10,11. The oxidant-free condition combining photocatalyst with cobalt complex provides a new strategy for bond formation between two nucleophiles33,34,35. Inputting energy by photocatalysis and employing the cobalt catalyst to take two electrons, we show an external oxidant-free oxidative C(sp2)-H amination of arenes, especially the methylarenes, whereas the C(sp3)-H bonds of the methylarenes are unaffected (Fig. 1c). Selective C–N formation is achieved accompanying with H2 evolution in the same system. A variety of N-arylazoles can be successfully afforded under this oxidant-free condition. The arene radical cation species serves the key role for the selective C(sp2)-H amination.
Results
Investigation of reaction conditions
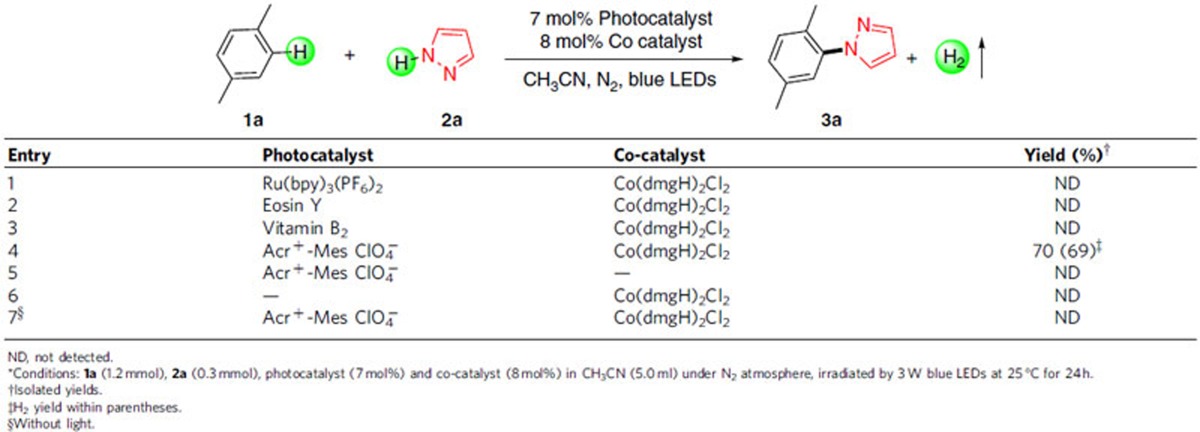
Initially, we optimized the conditions using the p-xylene (1a) and pyrazole (2a) as model substrates. On the basis of our previous studies in the field of oxidant-free system34, we firstly chose Ru(bpy)3(PF6)2 as photocatalyst and Co(dmgH)2Cl2 as the proton-reduction catalyst. Unfortunately, no desired product was achieved (Table 1, entry 1). Then, different commonly used photocatalysts were screened (Table 1, entries 2–4). To our delight, we successfully achieved our C–N coupling product 3a in 23% yield using acridinium moiety as the photocatalyst (Supplementary Table 1, entry 2), which has a strong oxidative ability36. After investigating the reaction conditions (Supplementary Table 1), we obtained a satisfactory yield of amination product with four equivalent p-xylene 1a and one equivalent pyrazole 2a as a suitable proportion of substrates (Table 1, entry 4). Almost the same yield of H2 was detected by gas chromatography-thermal conductivity detector (see Supplementary Methods for details). Additionally, the control experiments indicated that the cobalt catalyst, photocatalyst and visible light was indispensable for this transformation (Table 1, entries 5–7).
Table 1. Optimization of the reaction conditions*.
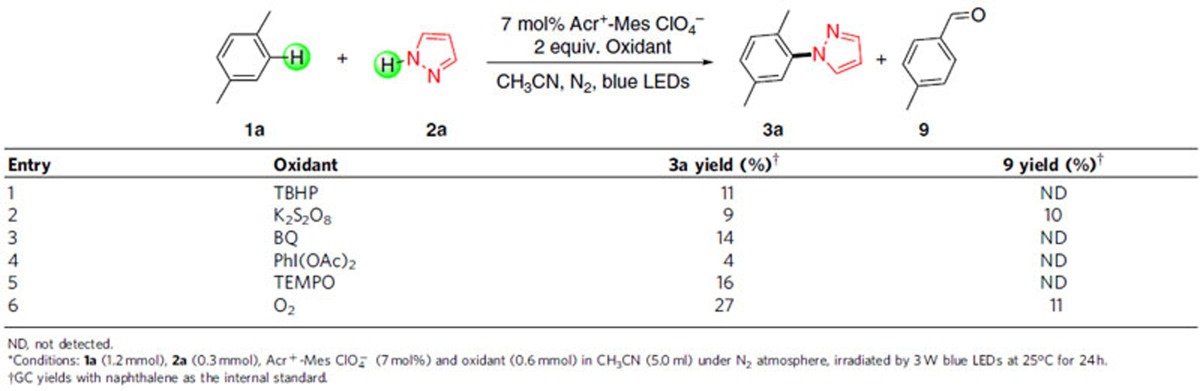
To highlight the advantage of our oxidant-free system, several oxidants were added into the system to replace the cobalt catalyst (Table 2). In general, the photocatalytic systems in the presence of oxidant show low reactivity for this C–N coupling reaction and afforded low yields of amination product 3a. tert-butyl hydroperoxide, K2S2O8, benzoquinone, PhI(OAc)2 and TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxyl) were not suitable for this amination process, showing very poor efficiency (Table 2, entries 1–5). Using O2 as oxidant, it did not make a satisfactory result, and a relatively small amount of aldehyde 9 was generated concomitantly, while the result was slightly improved (Table 2, entry 6). These results revealed that the oxidant-free system shows great superiority over photocatalyst/oxidant system for this selective C(sp2)-H amination.
Table 2. Comparison between photo/cobalt system and photo/oxidant system.*.
Substrate scope
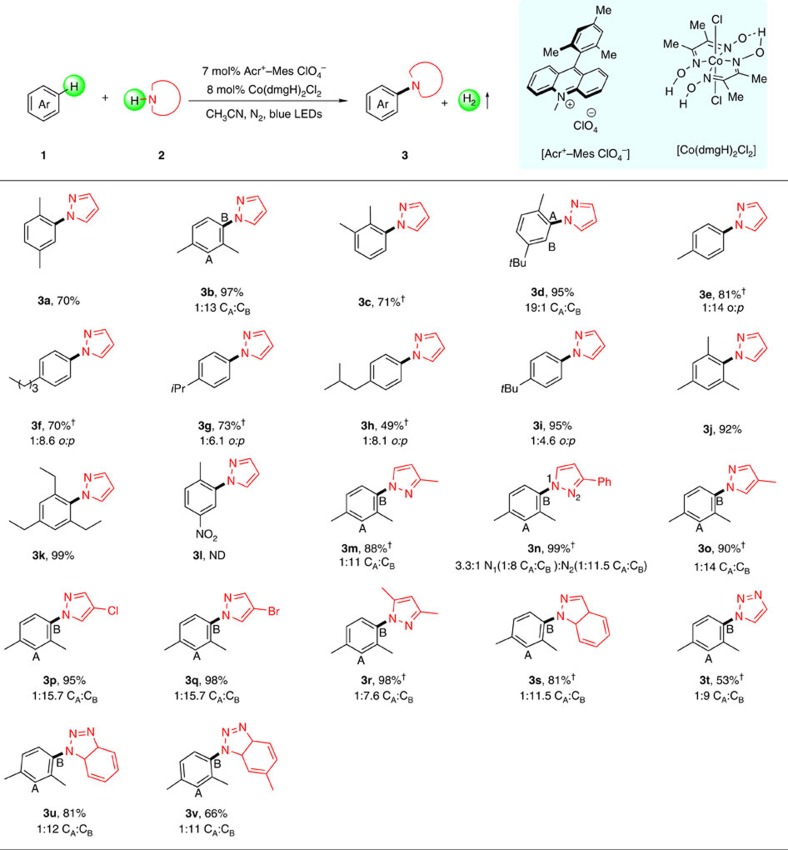
Having confirmed the high efficiency and unique advantages of this amination protocol without any sacrificial oxidant, we firstly examined the scope of methylarenes 1 with pyrazole 2a. To our delight, the current catalytic system was suitable for a wide range of methylarenes (Fig. 2). Other dialkyl-substituted benzenes like m-xylene, o-xylene and 1-(tert-butyl)-4-methylbenzene could be successfully converted into the corresponding amination products in good yields (3b–3d) and with high site-selectivity, especially for o-xylene. It was found that toluene was still effective in our catalytic system and can afford the C–N product in 81% yield (3e). Subsequently, a series of monoalkyl-substituted benzenes were examined under standard conditions, in which the functional groups such as methyl, n-Bu, i-Pr, i-Bu and t-Bu were surprisingly compatible with satisfactory efficiency (3f–3i). It is worth noting that trialkyl-substituted benzenes could also be suitable for this system, achieving the C(sp2)-H amination product in high efficiency (3j and 3k). In spite of trialkyl-substituted benzenes (mesitylene and 1,3,5-triethylbenzene) containing weak benzylic C–H bonds, which may be prone for benzylic C–H amination, notably only aromatic ring C(sp2)-H amination were resolutely observed, showing excellent chemoselectivity of this oxidant-free system. Furthermore, amination process can be conducted with methylarenes loadings as low as one equivalent relative to nitrogen nucleophiles, albeit with a slight decrease in chemical yield to 50% (Supplementary Fig. 55). However, there was no reaction for p-nitrotoluene due to the inhibition of nitro group, possibly indicating a radical process (3l). At the same time, a series of heterocyclic amines and its tolerance of functional groups were studied to expand the synthetic utility of this methodology. Delightfully, the pyrazoles with different substituent could be converted into the desired products (3m–3r) in excellent yields. Several functional groups on the pyrazole moiety (Cl and Br) were well tolerated (3p and 3q), which provides an opportunity for further transformation. It is very gratifying to see that 1H-indazole could also be transformed into the desired product in 81% yield (3s). Moreover, amination products were obtained in high efficiency for 1,2,3-1H-triazole and 1H-benzotriazole (3t–3v). Unfortunately, non-cyclic amines such as aniline, diphenylamine, dibenzylamine and N-acetylacetamide could not be used as suitable nitrogen sources under the same catalytic system.
Figure 2. Substrate scope of arenes 1 with azoles 2.
Reaction conditions: 1 (1.2 mmol), 2 (0.3 mmol), Acr+-Mes ClO4− (7 mol%) and Co(dmgH)2Cl2 (8 mol%) in CH3CN (5.0 ml) under N2 atmosphere, irradiated by 3 W blue LEDs at 25 °C for 24 h. The ratio of the isomer was determined by NMR. †Arenes 1 (2.0 ml) and CH3CN (3.0 ml) were used. Isolated yields are shown. ND, not detected.
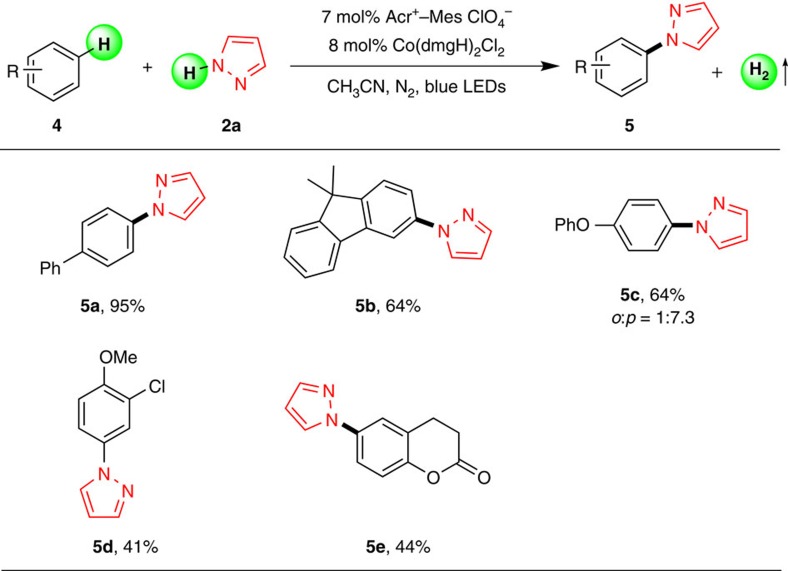
To further expand the potential usage of our oxidant-free amination methodology, a variety of other aromatic rings were also investigated and the results are summarized in Fig. 3. The good yields and high selectivity for biphenyl and 9,9-dimethyl-9H-fluorene were effortlessly obtained (5a and 5b). Importantly, the electron-rich arenes such as diphenyl oxide and anisole, which have been regarded as commonly used arenes in C(sp2)-H amination, were also suitable for this system and achieved the desired products in moderate yields (5c and 5d). In particular, dihydrocoumarin was smoothly tolerated in this amination protocol (5e), which has exhibited interesting biological activities37.
Figure 3. Substrate scope of pyrazole 2a with other aromatic arenes 4.
Reaction conditions: 4 (1.2 mmol), 2a (0.3 mmol), Acr+-Mes ClO4− (7 mol%) and Co(dmgH)2Cl2 (8 mol%) in CH3CN (5.0 ml) under N2 atmosphere, irradiated by 3 W blue LEDs at 25 °C for 24 h. The ratio of the isomer was determined by nuclear magnetic resonance. Isolated yields are shown.
Discussion
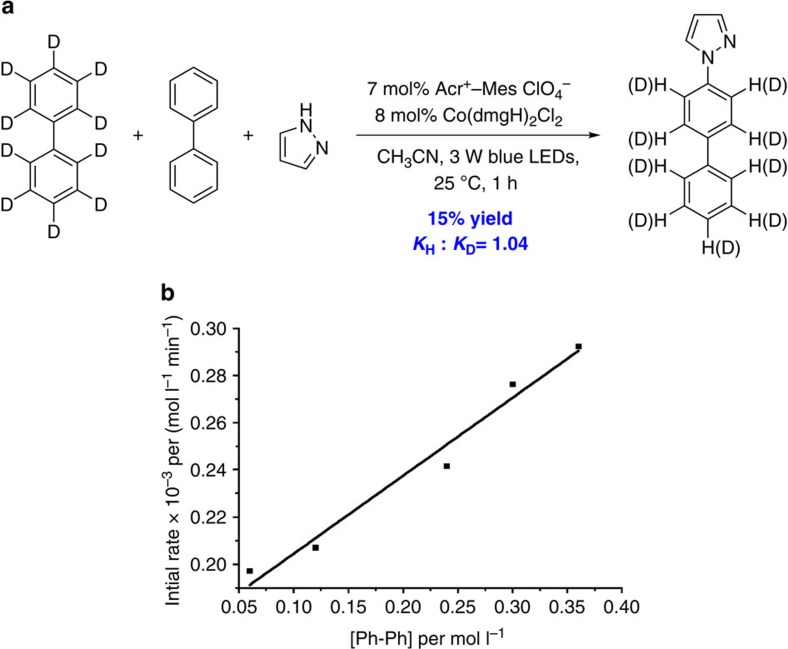
An intermolecular kinetic isotope effect experiment was carried out to further study this oxidant-free C–H/N–H cross-coupling reaction. As shown in Fig. 4a, the KIE (kinetic isotope effect) of 1.04 was observed from an intermolecular competition (see Supplementary Fig. 56 for details). It revealed that the C–H bond cleavage of arene might not be involved in the rate-determining step. Next, the further detailed kinetic behaviour of biphenyl was also tested (see Supplementary Methods). As depicted in Fig. 4b, the initial reaction rate was affected by changing the concentration of the biphenyl, demonstrating first-order dependencies on biphenyl.
Figure 4. Investigation for mechanistic insights.
(a) The intermolecular kinetic of isotopic effect experiment. (b) Kinetic plots of the reactions with different concentrations of biphenyl 4a.
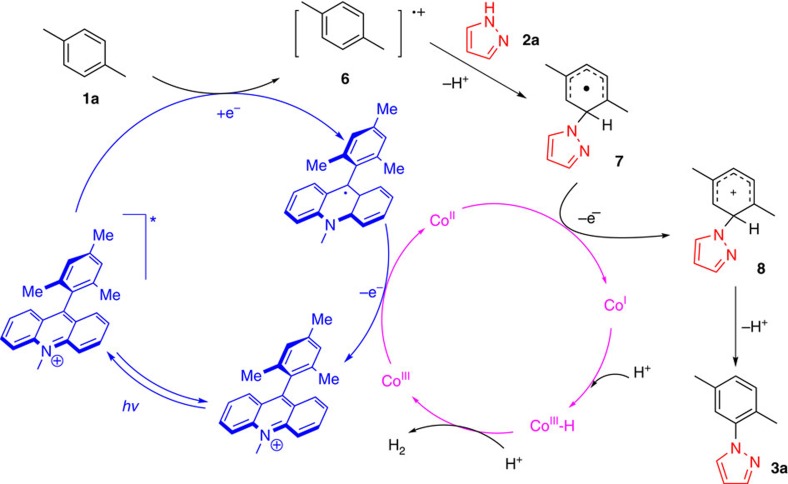
Based on the above kinetic data and previous reports, a plausible reaction mechanism was illustrated in Fig. 5. The photocatalyst and cobalt synergistic catalysis is hypothesized to be responsible for the electron transfer and proton release. Initially, photocatalyst acridinium is excited to *Mes-Acr-Me+ (E1/2 red=+2.06 V) by blue light-emitting diodes38, which is capable of oxidizing methylarenes 1 or biphenyl 4a (E1/2 red=+1.96 V) to corresponding radical cation 6. On the other hand, Co (III) catalyst could oxidize the Mes-Acr-Me radical (E1/2 red=−0.49 V)36 to finish the cycle of the photocatalyst and generate the Co (II) species at the same time. It is considered that the generated aromatic ring radical cation is attacked by nucleophile pyrazole 2a (E1/2 red=+2.27 V)31, which is difficult to be oxidized by the excited state acridinium, to intermediate 7. Then, the radical adduct 7 is likely to undergo a single electron transfer with Co (II) catalyst to 8, which quickly loses one proton and generates the amination product. At the same time, Co (I) could capture the present proton in the reaction system, yielding Co (III)-H. As a result, H2 can be released and the Co (III) is regenerated, as well through the protonation of Co (III)-H39,40.
Figure 5. Proposed mechanism for oxidant-free C–H amination.
The plausible mechanism involves oxidation of arene to generate arene radical cation, nucleophilic attack of azoles and visible–light photoredox cobalt-catalysed formation of amination product and H2.
In conclusion, we have developed an oxidant-free and selective C(sp2)-H bond amination of arenes utilizing a photocatalyst and cobalt oxime complex. A series of alkyl-substituted benzenes, biphenyl and anisole derivatives under current catalytic system could be well tolerated, providing a new way to N-arylazoles synthesis. The KIE experiment and kinetic study provide important mechanistic insights. Our further efforts in this study using another nucleophile for the C(sp2)-H functionalization are currently underway.
Methods
General procedure (3a)
A solution of p-xylene 1a (1.2 mmol), 1H-pyrazole 2a (0.3 mmol), photocatalyst (7 mol%) and CoIII(dmgH)2Cl2 (8 mol%) in degassed CH3CN (5.0 ml) were stirred under nitrogen atmosphere and irradiated by 3 W blue light-emitting diodes at 25 °C for 24 h. After completion of the reaction, H2 was detected by gas chromatography-thermal conductivity detector. The aqueous solution was extracted with ethyl acetate (3 × 10 ml) and the combined extracts were dried with anhydrous Na2SO4. The solvent was removed under reduced pressure by rotary evaporation, and then the pure product was obtained by flash column chromatography on silica gel (eluent: petroleum ether/ethyl acetate=25:1), directly to give the desired product 3a in 70% yield as a colourless liquid. For 1H NMR and 13C NMR spectra of compounds 3a–3k, 3m–3v and 5a–5e see Supplementary Fig. 1–54. Full experimental details can be found in the Supplementary Methods.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information Files.
Additional information
How to cite this article: Niu, L. et al. Photo-induced oxidant-free oxidative C–H/N–H cross-coupling between arenes and azoles. Nat. Commun. 8, 14226 doi: 10.1038/ncomms14226 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary table, supplementary methods and supplementary references.
Acknowledgments
This work was supported by the 973 Program (2011CB808600, 2012CB725302, 2013CB834804), the National Natural Science Foundation of China (21390400, 21272180, 21302148, 2109343 and 21402217) and the Research Fund for the Doctoral Program of Higher Education of China (20120141130002) and the Ministry of Science and Technology of China (2012YQ120060) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.
Footnotes
The authors declare no competing financial interests.
Author contributions A.L. conceived the project. L.N., H.Y., S.W., T.L. and J.L. performed the experiments, analysed the data and discussed the results. L.N., H.Y. and A.L. wrote the paper. L.N. and H.Y. wrote the Supplementary Information and contributed other related materials. L.N. and H.Y. contributed equally. We thank Zahra, Dong Liu, Atul K. Singh and Xu Zhang for kind suggestions.
References
- Cox C. D. et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J. Med. Chem. 53, 5320–5332 (2010). [DOI] [PubMed] [Google Scholar]
- Vicentini C. B., Romagnoli C., Andreotti E. & Mares D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J. Agric. Food. Chem. 55, 10331–10338 (2007). [DOI] [PubMed] [Google Scholar]
- Cristau H.-J., Cellier P. P., Spindler J.-F. & Taillefer M. Highly efficient and mild copper-catalyzed N- and C-arylations with aryl bromides and iodides. Chem. Eur. J. 10, 5607–5622 (2004). [DOI] [PubMed] [Google Scholar]
- Correa A. & Bolm C. Iron-catalyzed N-arylation of nitrogen nucleophiles. Angew. Chem. Int. Ed. 46, 8862–8865 (2007). [DOI] [PubMed] [Google Scholar]
- Paul F., Patt J. & Hartwig J. F. Palladium-catalyzed formation of carbon-nitrogen bonds. Reaction intermediates and catalyst improvements in the hetero cross-coupling of aryl halides and tin amides. J. Am. Chem. Soc. 116, 5969–5970 (1994). [Google Scholar]
- Taillefer M., Xia N. & Ouali A. Efficient iron/copper co-catalyzed arylation of nitrogen nucleophiles. Angew. Chem. Int. Ed. 46, 934–936 (2007). [DOI] [PubMed] [Google Scholar]
- Wolfe J. P., Wagaw S., Marcoux J.-F. & Buchwald S. L. Rational development of practical catalysts for aromatic carbon–nitrogen bond formation. Acc. Chem. Res. 31, 805–818 (1998). [Google Scholar]
- Monnier F. & Taillefer M. Catalytic C–C, C–N, and C–O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 48, 6954–6971 (2009). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015). [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang H., Shi W. & Lei A. Bond formations between two nucleophiles: transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 111, 1780–1824 (2011). [DOI] [PubMed] [Google Scholar]
- Yeung C. S. & Dong V. M. Catalytic dehydrogenative cross-coupling: forming carbon−carbon bonds by oxidizing two carbon–hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011). [DOI] [PubMed] [Google Scholar]
- Louillat M.-L. & Patureau F. W. Oxidative C–H amination reactions. Chem. Soc. Rev. 43, 901–910 (2014). [DOI] [PubMed] [Google Scholar]
- Jiao J., Murakami K. & Itami K. Catalytic methods for aromatic C–H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 6, 610–633 (2015). [Google Scholar]
- Kim H. & Chang S. Transition-metal-mediated direct C–H amination of hydrocarbons with amine reactants: the most desirable but challenging C–N bond-formation approach. ACS Catal. 6, 2341–2351 (2016). [Google Scholar]
- Kantak A. A., Potavathri S., Barham R. A., Romano K. M. & DeBoef B. Metal-free intermolecular oxidative C–N bond formation via tandem C–H and N–H bond functionalization. J. Am. Chem. Soc. 133, 19960–19965 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Kim J., Cho S. H. & Chang S. Intermolecular oxidative C–N bond formation under metal-free conditions: control of chemoselectivity between aryl sp2 and benzylic sp3 C–H bond imidation. J. Am. Chem. Soc. 133, 16382–16385 (2011). [DOI] [PubMed] [Google Scholar]
- Shrestha R., Mukherjee P., Tan Y., Litman Z. C. & Hartwig J. F. Sterically controlled, palladium-catalyzed intermolecular amination of arenes. J. Am. Chem. Soc. 135, 8480–8483 (2013). [DOI] [PubMed] [Google Scholar]
- Vanjari R. & Singh K. N. Utilization of methylarenes as versatile building blocks in organic synthesis. Chem. Soc. Rev. 44, 8062–8096 (2015). [DOI] [PubMed] [Google Scholar]
- Ohkubo K. et al. Simultaneous production of p-tolualdehyde and hydrogen peroxide in photocatalytic oxygenation of p-xylene and reduction of oxygen with 9-mesityl-10-methylacridinium ion derivatives. Chem. Commun. 46, 601–603 (2010). [DOI] [PubMed] [Google Scholar]
- Hou T., Lu P. & Li P. Visible-light-mediated benzylic sp3 C–H bond functionalization to C–Br or C–N bond. Tetrahedron Lett. 57, 2273–2776 (2016). [Google Scholar]
- Shi J.-L. et al. Fe-promoted chlorobenzylation of terminal alkynes through benzylic C(sp3)-H bond functionalization. Org. Lett. 18, 1238–1241 (2016). [DOI] [PubMed] [Google Scholar]
- Yi H., Bian C., Hu X., Niu L. & Lei A. Visible light mediated efficient oxidative benzylic sp3 C–H to ketone derivatives obtained under mild conditions using O2. Chem. Commun. 51, 14046–14049 (2015). [DOI] [PubMed] [Google Scholar]
- Xue Q., Xie J., Li H., Cheng Y. & Zhu C. Metal-free, highly efficient organocatalytic amination of benzylic C–H bonds. Chem. Commun. 49, 3700–3702 (2013). [DOI] [PubMed] [Google Scholar]
- Hari D. P. & Konig B. The photocatalyzed meerwein arylation: classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed. 52, 4734–47439 (2013). [DOI] [PubMed] [Google Scholar]
- Narayanam J. M. & Stephenson C. R. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011). [DOI] [PubMed] [Google Scholar]
- Prier C. K., Rankic D. A. & MacMillan D. W. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli D., Protti S. & Fagnoni M. Carbon-carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 116, 9850–9913 (2016). [DOI] [PubMed] [Google Scholar]
- Xuan J. & Xiao W. J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 51, 6828–6838 (2012). [DOI] [PubMed] [Google Scholar]
- Yoon T. P., Ischay M. A. & Du J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010). [DOI] [PubMed] [Google Scholar]
- Hopkinson M. N., Sahoo B., Li J.-L. & Glorius F. Dual catalysis sees the light: combining photoredox with organo-, acid, and transition-metal catalysis. Chem. Eur. J. 20, 3874–3886 (2014). [DOI] [PubMed] [Google Scholar]
- Romero N. A., Margrey K. A., Tay N. E. & Nicewicz D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015). [DOI] [PubMed] [Google Scholar]
- Yamada Y., Maeda K., Ohkubo K., Karlin K. D. & Fukuzumi S. Improvement of durability of an organic photocatalyst in p-xylene oxygenation by addition of a Cu(II) complex. Phys. Chem. Chem. Phys. 14, 9654–9659 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q. Y. et al. A cascade cross-coupling hydrogen evolution reaction by visible light catalysis. J. Am. Chem. Soc. 135, 19052–19055 (2013). [DOI] [PubMed] [Google Scholar]
- Hang G. et al. External oxidant-free oxidative cross-coupling: a photoredox cobalt-catalyzed aromatic C–H thiolation for constructing C–S bonds. J. Am. Chem. Soc. 137, 9273–9280 (2015). [DOI] [PubMed] [Google Scholar]
- Zheng Y. W. et al. Photocatalytic hydrogen-evolution cross-couplings: benzene C–H amination and hydroxylation. J. Am. Chem. Soc. 138, 10080–10083 (2016). [DOI] [PubMed] [Google Scholar]
- Fukuzumi S. et al. Electron-transfer state of 9-Mesityl-10-methylacridinium ion with a much longer lifetime and higher energy than that of the natural photosynthetic reaction center. J. Am. Chem. Soc. 126, 1600–1601 (2004). [DOI] [PubMed] [Google Scholar]
- Posakony J., Hirao M., Stevens S., Simon J. A. & Bedalov A. Inhibitors of sir2: evaluation of splitomicin analogues. J. Med. Chem. 47, 2635–2644 (2004). [DOI] [PubMed] [Google Scholar]
- Romero N. A. & Nicewicz D. A. Mechanistic insight into the photoredox catalysis of anti-markovnikov alkene hydrofunctionalization reactions. J. Am. Chem. Soc. 136, 17024–17035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero V., Chavarot-Kerlidou M. & Fontecave M. Splitting water with cobalt. Angew. Chem. Int. Ed. 50, 7238–7266 (2011). [DOI] [PubMed] [Google Scholar]
- Dempsey J. L., Brunschwig B. S., Winkler J. R. & Gray H. B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 42, 1995–2004 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary table, supplementary methods and supplementary references.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information Files.