Abstract
The Non-invasive Micro-test Technique (NMT) is used to measure dynamic changes of specific ions/molecules non-invasively, but information about hydrogen peroxide (H2O2) fluxes in different classes of roots by mycorrhiza is scarce in terms of NMT. Effects of Funneliformis mosseae on plant growth, H2O2, superoxide radical (O2·−), malondialdehyde (MDA) concentrations, and H2O2 fluxes in the taproot (TR) and lateral roots (LRs) of trifoliate orange seedlings under well-watered (WW) and drought stress (DS) conditions were studied. DS strongly inhibited mycorrhizal colonization in the TR and LRs, whereas mycorrhizal inoculation significantly promoted plant growth and biomass production. H2O2, O2·−, and MDA concentrations in leaves and roots were dramatically lower in mycorrhizal seedlings than in non-mycorrhizal seedlings under DS. Compared with non-mycorrhizal seedlings, mycorrhizal seedlings had relatively higher net root H2O2 effluxes in the TR and LRs especially under WW, as well as significantly higher total root H2O2 effluxes in the TR and LRs under WW and DS. Total root H2O2 effluxes were significantly positively correlated with root colonization but negatively with root H2O2 and MDA concentrations. It suggested that mycorrhizas induces more H2O2 effluxes of the TR and LRs, thus, alleviating oxidative damage of DS in the host plant.
Drought stress (DS) is usually regarded as one of the major abiotic factors limiting crop growth and yield, including citrus. DS generally triggers oxidative stress, due to the accumulation of reactive oxygen species (ROS) in plant cells. ROS, the byproduct of aerobic metabolism in vivo, mainly includes hydroxyl radical (OH·), superoxide radical (O2·−), and hydrogen peroxide (H2O2), and it can potentially damage a variety of biological molecules, such as proteins, lipids, and nucleic acids1,2. At the same time, plants also develop an elaborate antioxidant protection system to eliminate part of ROS in cells and tissues3,4.
H2O2 is considered to be not only a toxic molecule inducing oxidative damage, but also an intermediary molecule participating in a variety of physio-biochemical processes in plants. The intermediary function of H2O2 is closely related to its ability to travel in cellular membranes and migrate into different compartments2,5. Moreover, H2O2 is a reliable indicator for the oxidative burst, because it is more stable than O2·− 6. The formation of O2·− in mitochondria can be measured according to the production of H2O2 as determined by H2O2 effluxes7,8. H2O2 effluxes may be an important cellular mechanism against oxidative stress9,10. H2O2 is effused from the root surface into the rhizosphere in trifoliate orange11.
Arbuscular mycorrhizal fungi (AMF), a kind of beneficial soil microorganism, can create a symbiotic association with plant roots forming arbuscular mycorrhizas (AMs), which play a role in the regulation of plant growth12,13,14. AMF can mitigate the detrimental effect of DS through restraining overproduction of ROS15,16. Less accumulation of ROS in AMF-inoculated plants is due to the enhancement of antioxidant enzyme (e.g. superoxide dismutase and catalase) activities and/or non-enzymatic antioxidants (e.g. ascorbate and glutathione) concentrations17,18. In addition, AM structures (e.g. arbuscules and hyphae) are involved in H2O2 accumulation within the roots of the host plant19,20. Root H2O2 effluxes are one of the important ROS-scavenging mechanisms in plants subjected to DS, which were found in citrus plants under DS by using the Non-invasive Micro-test Technique (NMT)11. NMT can non-invasively preserve the integrity of the sample and provide dynamic information of specific ions/molecules (including K+, Na+, Ca2+, H+, Cl−, Mg2+, H2O2, IAA, and glucose) near material surfaces21,22. However, the response of H2O2 effluxes in different order roots and their contribution to root ROS removal are not fully known under DS conditions, as well as under mycorrhization.
In this context, the objectives of the present study were to investigate the effects of the AM fungus Funneliformis mosseae on plant growth, ROS (H2O2 and O2·−) levels, malondialdehyde (MDA) concentration, and H2O2 fluxes in different classes of roots of trifoliate orange [Poncirus trifoliata (L.) Raf.] seedlings under WW and DS conditions, and to highlight the distinct role of AMF-alleviated oxidative damage.
Results
Mycorrhizal colonization
Root mycorrhizas were found in roots of AMF-inoculated seedlings, but not in roots of non-AMF-inoculated seedlings (Fig. 1). Mycorrhizal seedlings had 54%, 82%, 50%, 74%, and 65% significantly higher AMF colonization in the taproot (TR), first-order lateral root (LR1), second-order lateral root (LR2), third-order lateral root (LR3), and average root (AR) under WW than under DS, respectively. Moreover, AMF mainly colonized LR2 and LR3 in whole root systems, regardless of WW and DS.
Figure 1. Mycorrhizal colonization in the taproot (TR), first-order lateral root (LR1), second-order lateral root (LR2), and third-order lateral root (LR3) of Funneliformis mosseae-colonized trifoliate orange (Poncirus trifoliata) seedlings under well-watered (WW) and drought stress (DS) conditions.
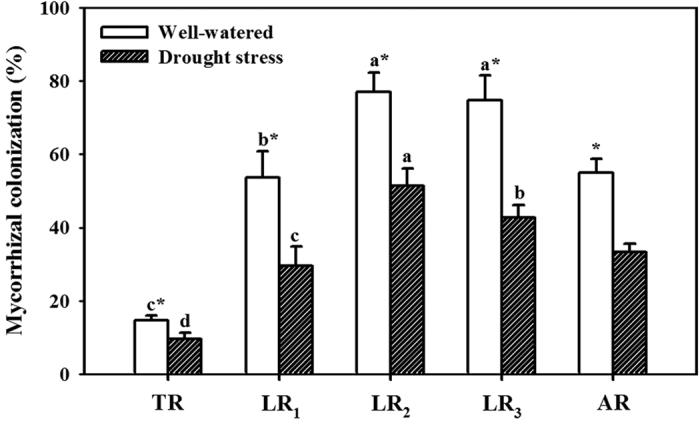
The average root mycorrhizal colonization (AR) was calculated as (TR+LR1+LR2+LR3)/4. Data (means ± SD, n = 4) followed by different letters (a, b, c, d) above the bars represent significant differences between the taproot and lateral roots for the same soil water statue, and an asterisk (*) above the bars represent significant differences between soil water treatments for the taproot or the same lateral roots at the 5% level.
Plant biomass and RWC
Mycorrhizal seedlings had significantly higher shoot, root, and total biomass than non-mycorrhizal seedlings: 190%, 62%, and 126% under WW and 466%, 148%, and 274% under DS, respectively (Table 1). Compared to non-AMF seedlings, AMF seedlings had 4% and 12% significantly higher RWC under WW and DS conditions, respectively (Table 1).
Table 1. Effects of Funneliformis mosseae (AMF) on plant biomass and leaf relative water content of trifoliate orange (Poncirus trifoliata) seedlings under well-watered (WW) and drought stress (DS) conditions.
| Treatments | Biomass (g FW/plant) |
Leaf relative water content (%) | ||
|---|---|---|---|---|
| Shoot | Root | Total | ||
| WW+AMF | 4.17 ± 0.31a | 2.33 ± 0.06a | 6.50 ± 0.29a | 89.40 ± 1.01a |
| WW−AMF | 1.44 ± 0.58c | 1.44 ± 0.27c | 2.88 ± 0.85c | 86.34 ± 0.87b |
| DS+AMF | 3.00 ± 0.23b | 1.98 ± 0.10b | 4.98 ± 0.28b | 86.60 ± 1.87b |
| DS−AMF | 0.53 ± 0.08d | 0.80 ± 0.20d | 1.33 ± 0.27d | 76.93 ± 2.95c |
Note: Data (means ± SD, n = 4) followed by different letters among treatments represent significant differences at the 5% level.
Number and surface area of lateral roots
Compared with non-AMF treatment, AMF inoculation significantly increased the number of LR1 and LR2 by 47% and 131% under WW condition and by 39% and 187% under DS condition, respectively (Fig. 2a). In addition, AMF treatment markedly stimulated LR3 formation under WW and DS conditions, but LR3 was not found in non-mycorrhizal seedlings. AMF inoculation notably increased the surface area of LR1 and LR2 by 85% and 182% under WW and by 65% and 280% under DS than non-AMF inoculation, while it did not significantly alter the surface area of the TR (Fig. 2b).
Figure 2.
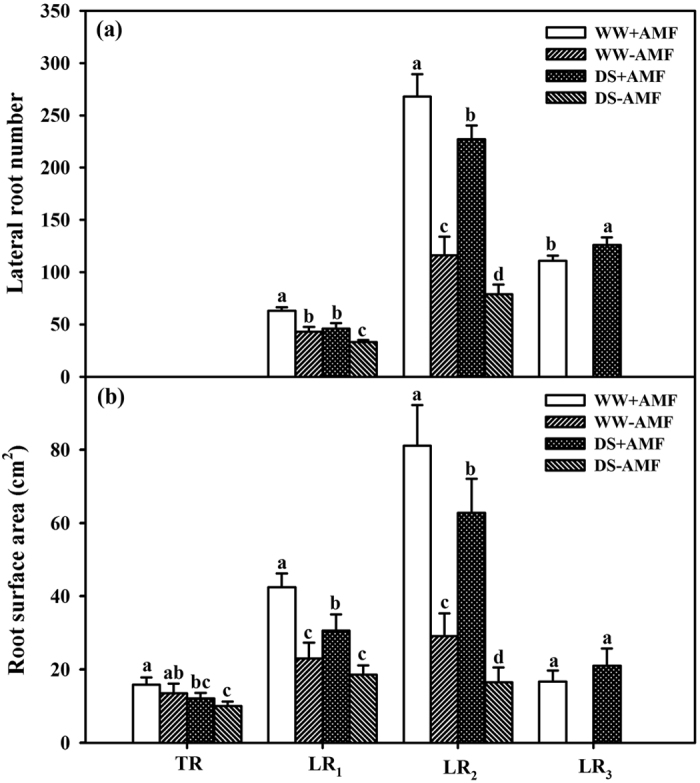
Effect of Funneliformis mosseae (AMF) on the number of lateral roots (a) and root surface area (b) of trifoliate orange (Poncirus trifoliata) seedlings under well-watered (WW) and drought stress (DS) conditions. Data (means ± SD, n = 4) followed by different letters (a,b,c,d) above the bars among treatments represent significant differences at the 5% level.
Concentrations of O2 ·−, H2O2 and MDA
AMF seedlings exhibited significantly lower O2·− concentrations than non-AMF seedlings regardless of water treatments: 30% and 36% lower in leaves and 18% and 28% lower in roots under WW and DS conditions, respectively (Fig. 3a). Similarly, compared with non-AMF seedlings, AMF seedlings had 14% and 22% significantly lower leaf H2O2 concentrations and 27% and 28% lower root H2O2 concentrations under WW and DS conditions, respectively (Fig. 3b). AMF inoculation notably decreased MDA concentrations by 13% and 15% in leaves and 17% and 20% in roots under WW and DS conditions, respectively (Fig. 3c).
Figure 3.
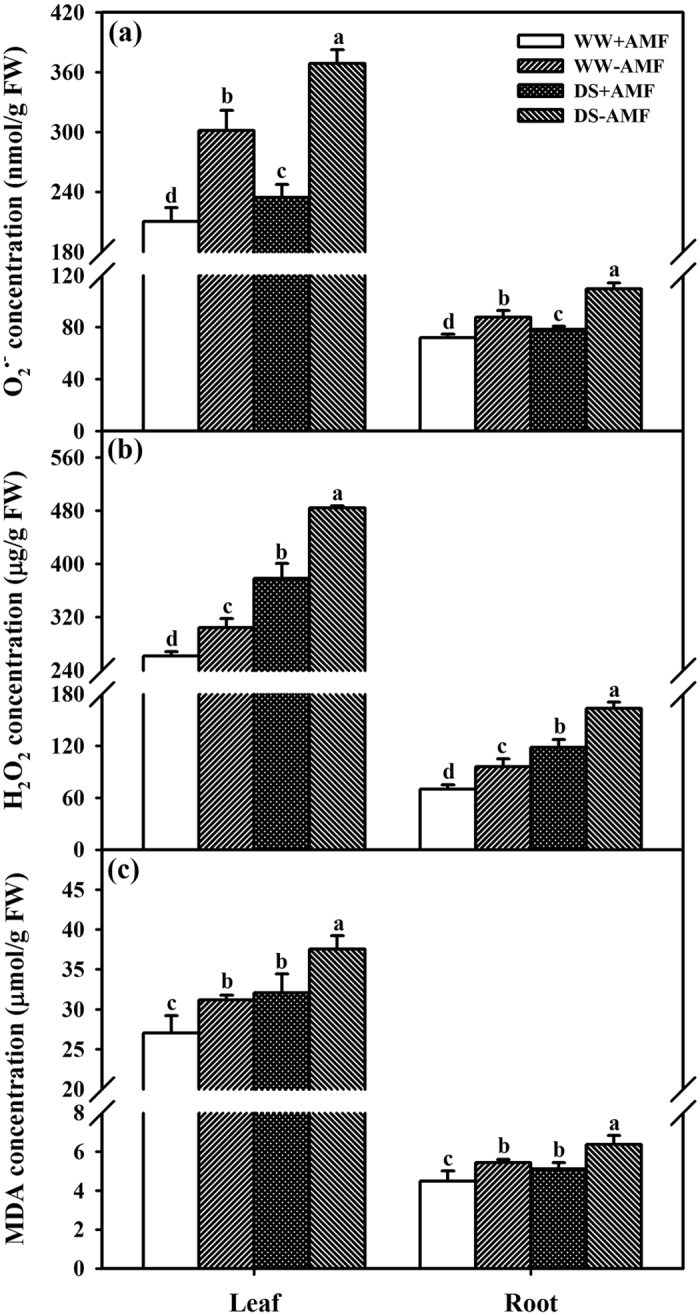
Effects of Funneliformis mosseae (AMF) on superoxide radical (O2·−) (a), hydrogen peroxide (H2O2) (b), and malondialdehyde (MDA) (c) concentrations of trifoliate orange (Poncirus trifoliata) seedlings under well-watered (WW) and drought stress (DS) conditions. Data (means ± SD, n = 4) followed by different letters (a,b,c,d) above the bars among treatments represent significant differences at the 5% level.
Root H2O2 effluxes
Net root H2O2 effluxes (real-time effluxes of H2O2 in the 0.5 cm length from the tip in each order root) were the highest in the TR among different classes of roots, regardless of AMF or soil water status (Fig. 4a). Compared to non-AMF seedlings, AMF seedlings showed significantly higher net root H2O2 effluxes in the TR, LR1, and LR2 by 21%, 59%, and 24% under WW condition, respectively. Under DS, AMF inoculation significantly increased net root H2O2 effluxes by 91% in the TR, but they were not notably altered in LR1 and LR2, compared with non-AMF inoculation. In addition, net root H2O2 effluxes in LR3 were decreased by 40% in mycorrhizal seedlings under DS conditions than under WW conditions. Average net root H2O2 effluxes were not significantly different between mycorrhizal and non-mycorrhizal seedlings under WW and DS conditions.
Figure 4.
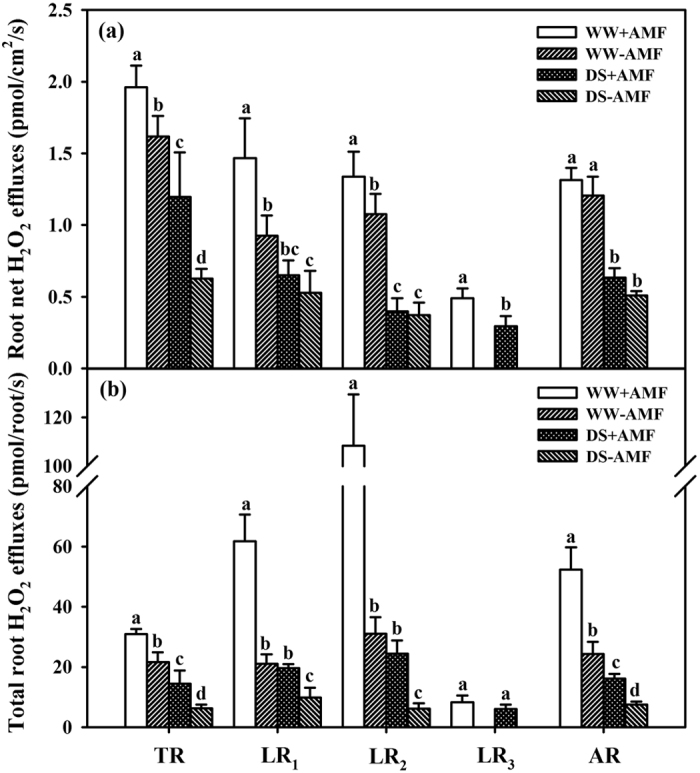
Effects of Funneliformis mosseae (AMF) on net root hydrogen peroxide (H2O2) effluxes (a) and total root H2O2 effluxes (b) in the taproot (TR), first-order lateral root (LR1), second-order lateral root (LR2), and third-order lateral root (LR3) of trifoliate orange (Poncirus trifoliata) seedlings under well-watered (WW) and drought stress (DS) conditions. Average root H2O2 effluxes (AR) were calculated as (TR+LR1+LR2+LR3)/4. Total root H2O2 fluxes of the taproot and lateral roots were calculated as (root surface area × root net H2O2 effluxes in the corresponding root classes). Data (means ± SD, n = 4) followed by different letters (a,b,c,d) above the bars represent significant differences between soil water treatments for the taproot or the same lateral roots at the 5% level.
Mycorrhizal seedlings showed 43%, 194%, and 249% higher total root H2O2 effluxes (real-time effluxes of H2O2 in whole root systems) in the TR, LR1, and LR2 under WW and 130%, 99%, and 300% higher under DS condition, respectively, as compared with non-mycorrhizal seedlings (Fig. 4b). The AMF treatment significantly increased average root H2O2 effluxes by 113% under WW conditions and by 118% under DS conditions, compared to the non-AMF treatment.
Correlation studies
Total root H2O2 effluxes were significantly positively correlated with root mycorrhizal colonization (Fig. 5a), but negatively correlated with root H2O2 concentrations (Fig. 5b) and root MDA concentrations (Fig. 5c).
Figure 5.
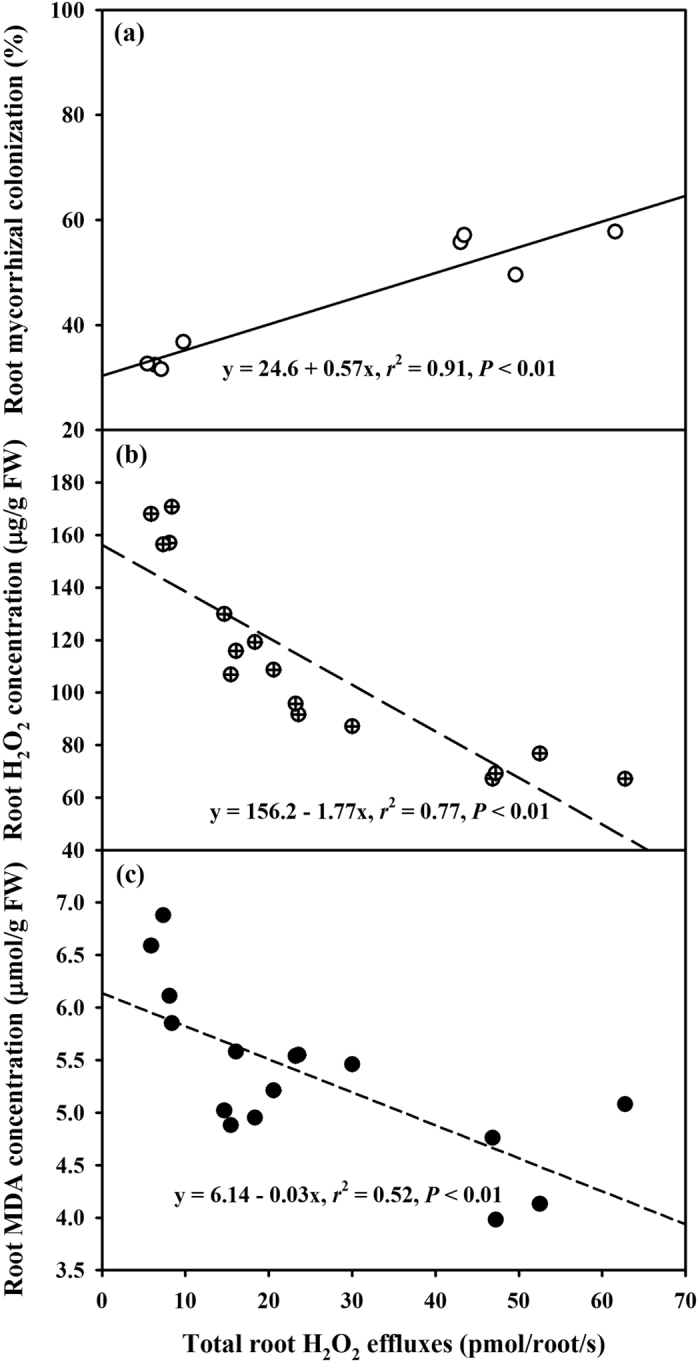
Linear regression between total root hydrogen peroxide (H2O2) effluxes and root mycorrhizal colonization (a, n = 8), root H2O2 concentration (b, n = 16), or root MDA concentration (c, n = 16) of trifoliate orange (Poncirus trifoliata) seedlings colonized by Funneliformis mosseae under well-watered (WW) and drought stress (DS) conditions.
Discussion
AMF colonization was considerably higher in LRs than in the TR and higher under WW than under DS. This is in agreement with the findings of Ding et al.23, who reported that F. mosseae colonization in LRs of soybean were higher than those in the TR at 56 days after sowing. The decrease of root mycorrhizal colonization under DS compared to under WW ascribed to the reduction of spore germination, root exudates, and root carbohydrate supply24. Possibly, greater LR formation in AMF plants, especially LR2, could provide more chances to be colonized by mycorrhizal hyphae, resulting in higher mycorrhizal colonization in LRs than in the TR25. On the other hand, mycorrhizal plants may tend to have more fine roots and less coarse roots26,27, which can be successfully colonized.
AMF inoculation with F. mosseae alleviated the negative effect of DS on LRs development, indicating the significantly higher number and surface area of LR1 and LR2 in AMF plants than in non-AMF plants. This is consistent with previous reports28,29. In general, taproots have a large diameter and play a role in fixing plants and guiding the direction of root growth, while LRs carry out absorption30. The LR formation, especially higher order LRs (e.g., 3rd order LR formation in this study), was stimulated by AMF, which helped the host plant to absorb more water and nutrients, thus potentially enhancing drought tolerance in AMF seedlings. Maillet et al.31 also reported that root branching of the legume Medicago truncatula was stimulated by Rhizoglomus intraradices. Greater root branching in AMF seedling may be due to the fact that AMF can produce indole-3-acetic acid and polyamines and also further stimulate these phytohormone synthesis in roots32,33.
Our study showed an efflux behavior of root H2O2 from roots into the rhizosphere. Compared with WW, DS resulted in lower net root H2O2 effluxes and total root H2O2 effluxes, regardless of the TR and LRs. Earlier studies confirmed that H2O2 effluxes are closely related with cell membrane permeability34. As a result, DS strongly destroyed cell membranes and resulted in less effluxes of H2O2, thereby, inducing the oxidative burst, compared with WW. Root H2O2 effluxes are a way of physiological metabolism of intracellular H2O2 overproduction and the response of plant self protection to DS11. Bienert et al.2 first provided molecular genetic evidence that indicated diffusivity of H2O2 through specific members of the aquaporin family. The decrease in root H2O2 effluxes by DS would induce more H2O2 accumulation in roots, thereby resulting in a stronger oxidative burst in plants exposed to DS (e.g., a higher MDA was found under DS than under WW). Generally, net root H2O2 effluxes gradually decreased with an increase of root order, from the TR to LRs. H2O2 can be transported long distances across cell membranes from its generation site35. Possibly, the taproot as the first arising root in the whole root system has greater capacity to transfer H2O2 from the extensive original sites (the TR, as well LRs) to the epidermis of the TR, further effluxing into the rhizosphere, as compared with LRs. On the other hand, mycorrhizal structures, including intercellular hyphae and arbuscules, could accumulate H2O219,20, thereby decreasing H2O2 diffusion in cell membranes. Since root mycorrhizal colonization increased with the increase of root order, from the TP to LRs, a comparatively greater mycorrhizal status in LRs would accumulate more H2O2 in mycorrhizas, resulting in a decrease of root H2O2 effluxes, compared with the TR.
AMF colonization by F. mosseae significantly increased total root H2O2 effluxes under WW and DS and net root H2O2 effluxes in the TR, LR1 and LR2 under WW and in the TR under DS. Ding et al.23 reported that H+ effluxes in the TR of soybean seedlings were higher than that in LRs, and in extraradical hyphae of the TR than in extraradical hyphae of LRs. Li et al.36 recently cloned two functional aquaporin genes from G. intraradices, which can express in extraradical mycelium under DS conditions. Since H2O2 diffusion can be through aquaporins2, higher root H2O2 effluxes in AMF seedlings than in non-AMF seedlings might be involved in the H2O2 release of aquaporins by extraradical hyphae. Total root H2O2 effluxes were significantly positively correlated with root AMF colonization, strengthening the functioning of AMs on H2O2 effluxes. Further studies are needed to demonstrate whether AM extraradical hyphae release H2O2, and if H2O2 in root cells is transported from arbuscules to extraradical hyphae and then completely effluxed through special aquaporins. In whole root systems, LR2 had the highest total root H2O2 effluxes, which is due to the fact that LR2 had a distinctly higher root mycorrhizal colonization, root surface area and number. In the present study, the significantly negative correlation of total root H2O2 effluxes with root H2O2 or MDA concentrations indicated that root H2O2 effluxes result in less accumulation of root H2O2, thus mitigating damage of membrane lipid peroxidation.
In conclusion, inoculation with F. mosseae induced considerably higher root H2O2 effluxes in LRs and the TP, which might result in a lower oxidative damage in roots under DS.
Methods
Experimental design
The experiment was a completely randomized block design with two factors: (1) two mycorrhizal treatments, inoculation with Funneliformis mosseae (+AMF) and without F. mosseae (−AMF), and (2) two soil water levels, well-watered (WW, 75% of maximum water holding capacity of soil) and drought stress (DS, 55% of maximum water holding capacity of soil). Each of the four treatments had four replicates, with a total of 16 pots.
Mycorrhizal inoculum
The AM fungus strain, Funneliformis mosseae (Nicol. & Gerd.) Schüßler & Walker, was isolated from the rhizosphere of Incarvillea younghusbandii in Dangxiong (90°45′E and 29°31′N, 4,300 m above the sea level), Tibet, and further propagated using white clover (Trifolium repens) as a host plant for 16 weeks under potted conditions. For the AMF treatment (+AMF), 100 g inoculum (15 spores/g), including spores, infected root segments of white clover plants, and growth substrates was mixed with the 1.2 kg soil at transplanting. The non-AMF-inoculated treatment (-AMF) received the same amount of sterilized inoculums together with 2 mL inoculums filtrate (25 μm filter) to maintain a similar microbial community except for F. mosseae.
Plant material
Seeds of trifoliate orange were surface-sterilized with 70% alcohol for 10 min, rinsed five times with distilled water, and placed in autoclaved (0.11 Mpa, 121 °C, 2 h) sands at 26 °C for germination. Subsequently, four-leaf-old seedlings of the same size were transferred into a 1.1 L plastic pot containing 1.2 kg autoclaved (0.11 Mpa, 121 °C, 2 h) soil from a citrus orchard of the Yangtze University campus. The soil collected here had a pH of 6.0, 12.1 mg/kg KMnO4-N, 15.7 mg/kg Bray-P, 22.3 mg/kg neutral NH4OAc-K, and 38% maximum water holding capacity. All the seedlings were placed in a glasshouse (photosynthetic photon flux density was 982 μmol m−2 s−1, day/night temperature 27/20 °C, and relative humidity 80%) from March 24 to August 19, 2014.
Soil water treatment
According to Zou et al.11, WW treatment was selected as 75% of maximum water holding capacity, and DS was considered as 55% of maximum water holding capacity of soil. Before DS was begun, soil water of these pots was kept in WW. After 99 days, half of the seedlings were kept in DS status for 50 days and the other seedlings were still kept in WW status for 50 days. The soil water levels in the pots were determined daily through weighing, and the amount of water lost was supplied to each pot to reach the designed soil water levels. During the experiment, a 50 mL of Hoagland solution with half of P strength was biweekly added into each pot for normal growth of seedlings.
Variable determinations
After 50 days of DS treatment, all the plants were harvested, and plant height, stem diameter and leaf number per plant were recorded. Subsequently, the plants were divided into shoots and roots, whose fresh weights were measured. According to Hackket37, root systems were divided into the TP and LRs. The number of LR1, LR2, and LR3 was carefully counted. Ten 1 cm long root segments per pot of the TR, LR1, LR2, and LR3 were stained with 0.05% (w/v) trypan blue for 5 min38 and observed by the LEICA DME bio-microscope for AM structures. Root AMF colonization was calculated as the percentage of colonized root lengths by AMF against observed total root length.
Different classes of roots per plant were scanned with an Epson Perfection V700 Photo (Seiko Epson Corp, Japan), and then the scanned root images were analyzed to obtain the root surface area using a WinRHIZO professional 2007b (Regent Instruments Incorporated, Canada).
The fourth fully expanded top leaf was used to determine leaf RWC by the method outlined by Bajji et al.39. The leaf RWC was calculated by the following formula: RWC (%) = 100 × (FW-DW)/(SW-DW), where FW refers to fresh weight, DW to dry weight (oven-dried, at 75 °C for 48 h), and SW to saturated weight after leaf rehydration for 24 h.
MDA, a by-product of oxidative damage to lipids in tissues, was measured following the method described by Sudhakar et al.40. Fresh samples (0.1 g) of leaves and roots were homogenized with 5 mL 0.1% (w/v) trichloroacetic acid and centrifuged at 3,000 × g for 10 min. The mixture including 0.5 mL supernatant and 1.5 mL 0.67% thiobarbituric acid was incubated at 100 °C for 0.5 h and centrifuged at 3,000 × g for 10 min. The absorbance at 450, 532, and 600 nm was recorded, and the MDA concentration (μmol/L) was calculated by the formula: 6.45 (A532 − A600) − 0.56A450.
The H2O2 concentration in leaves and roots was determined according to Velikova et al.41. Fresh samples (0.1 g) were homogenized with 5 mL 0.1% (w/v) trichloroacetic acid and centrifuged at 12,000 × g for 15 min. A 4 mL mixture was comprised of 1 mL 10 mM potassium phosphate buffers (pH 7.0), 2 mL 1 M KI, and 1 mL supernatant. The absorbance at 390 nm was recorded.
The levels of O2·− in tissues were measured using the protocol described by Wang and Luo42. Fresh leaf or root samples (0.15 g) were homogenized in 5 mL 0.1 M phosphate buffer (pH 7.8) and centrifuged at 4,000 × g for 10 min at 4 °C. The 0.5 mL supernatant was mixed with 0.5 mL 50 mM phosphate buffers (pH 7.8) and 0.1 mL 10 mM hydroxylamine chloride for reaction for 1 h at 25 °C. Subsequently, 1 mL 17 mM sulfanilamide and 1 mL 7 mM α-naphthylamine were added to the mixture at 25 °C for 20 min, and the absorbance was recorded at 530 nm.
Net root H2O2 fluxes in a 0.5 cm length from the tip of the taproot and different order lateral roots were measured using the Scanning Ion-Selective Electrode Technique (SIET) system (BIO-001A, Younger USA Sci. & Tech. Corp., Amherst, MA, USA) in the Xuyue Beijing NMT Service Center (Xuyue Beijing Sci. and Tech. Co., Ltd., Beijing, China). An H2O2-sensitive microsensor was polarized at +600 mV against an Ag/AgCl reference electrode before use6. Root systems in vivo were placed in a Petri dish and immersed in an assay solution, comprised of 0.1 mM CaCl2, 0.1 mM KCl, and 0.3 mM MES (pH 6.0). The H2O2 microsensor was placed 30 μm from the root surface. Net root H2O2 fluxes were calculated by Fick’s law of diffusion: J (pmol/cm2/s) = −Do × (dc/dx), where Do indicates the diffusion constant, dc the concentration gradient, and dx the distance (30 μm)43. Total root H2O2 fluxes were counted according to J × surface area of the lateral root.
Statistical analysis
Data (means ± SD, n = 4) were statistically analyzed by the variance (ANOVA) with SAS 8.1 software (SAS Institute Inc., Cary, NC, USA), and the significant differences between the treatments were tested by Duncan’s Multiple Range Tests at P < 0.05.
Additional Information
How to cite this article: Huang, Y.-M. et al. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 7, 42335; doi: 10.1038/srep42335 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was supported by the Plan in Scientific and Technological Innovation Team of Outstanding Young, Hubei Provincial Department of Education (T201604), the National Natural Science Foundation of China (31101513), and the Open Fund of the Institute of Root Biology, Yangtze University (R201401). Thanks to Dr. Edward C. Mignot, Shandong University, for linguistic advice.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.M.H., Y.N.Z., and Q.S.W. conceived the experiment, Y.M.H. conducted the experiment, and Y.M.H., Y.N.Z. and Q.S.W. analyzed the results. All authors reviewed the manuscript.
References
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141, 312–322 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 (2007). [DOI] [PubMed] [Google Scholar]
- Mittler R. et al. ROS signaling: the new wave? Trends Plant Sci. 16, 300–309 (2011). [DOI] [PubMed] [Google Scholar]
- Ahmad P. et al. [Oxidative damage and antioxidants in plants] Oxidative damage to plants Ahmad P. (ed.) 345–367 (Elsevier, 2014).
- Petrov V. D. & Van Breusegem F. Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants. pls014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Jung S. K., Messerli M. A., Smith P. J. S. & Shirihai O. S. Real-time detection of reactive oxygen intermediates from single microglial cells. Biol. Bull. 201, 261–262 (2001). [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A. & Flohe L. Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett. 33, 84–87 (1973). [DOI] [PubMed] [Google Scholar]
- Treberg J. R., Quinlan C. L. & Brand M. D. Hydrogen peroxide efflux from mitochondria underestimates matrix superoxide production—a correction using glutathione depletion. FEBS J. 277, 2766–2778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Lopes A., Antunes F., Cyrne L. & Marinho H. S. Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Lett. 587, 152–156 (2004). [DOI] [PubMed] [Google Scholar]
- Branco M. R., Marinho H. S., Cyrne L. & Antunes F. Decrease of H2O2 plasma membrane permeability during adaptation to H2O2 in Saccharomyces cerevisiae. J. Biol. Chem. 279, 6501–6506 (2004). [DOI] [PubMed] [Google Scholar]
- Zou Y. N., Huang Y. M., Wu Q. S. & He X. H. Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+ influxes in trifoliate orange roots under drought stress. Mycorrhiza 25, 143–152 (2015). [DOI] [PubMed] [Google Scholar]
- Smith S. E. & Smith F. A. Roles of arbuscular mycorrhizas in plant nutrient and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250 (2011). [DOI] [PubMed] [Google Scholar]
- Wagg C., Jansa J., Schmid B. & van der Heijden M. G. A. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 14, 1001–1009 (2011). [DOI] [PubMed] [Google Scholar]
- Philippot L., Raaijmakers J. M., Lemanceau P. & van der Putten W. H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799 (2013). [DOI] [PubMed] [Google Scholar]
- Wu Q. S., Xia R. X. & Zou Y. N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 163, 1101–1110 (2006). [DOI] [PubMed] [Google Scholar]
- Huang Y. M. et al. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 5, 682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. C., Song F. B. & Liu S. Q. Arbuscular mycorrhiza impacts on drought stress of maize plants by lipid peroxidation, proline content and activity of antioxidant system. J. Food Agric. Environ. 9, 583–587 (2011). [Google Scholar]
- Bompadre M. J. et al. Arbuscular mycorrhizal fungi alleviate oxidative stress in pomegranate plants growing under different irrigation conditions. Botany 92, 187–193 (2014). [Google Scholar]
- Salzer P., Corbiere H. & Boller T. Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208, 319–325 (1999). [Google Scholar]
- Fester T. & Hause T. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15, 373–379 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Wang W. J., Pan L. J., Xu Y. & Zhang Z. M. Measuring Ca2+ influxes of TRPC1-dependent Ca2+ channels in HL-7702 cells with Non-invasive Micro-test Technique. World J. Gastroentero. 15, 4150–4155 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Q., Yu N., Ye S. N., Yang S. M. & Zhai S. Q. Measurement of Ca2+ flow in cochlear cells using Non-invasive Micro-test Technique. J. Otol. 5, 90–96 (2010). [Google Scholar]
- Ding X. D. et al. Synergistic interactions between Glomus mosseae and Bradyrhizobium japonicum in enhancing proton release from nodules and hyphae. Mycorrhiza 22, 51–58 (2012). [DOI] [PubMed] [Google Scholar]
- Fagbola O., Osonubi O., Mulongoy K. & Odunfa S. A. Effects of drought stress and arbuscular mycorrhiza on the growth of Gliricidia sepium (Jacq). Walp, and Leucaena leucocephala (Lam.) de Wit. in simulated eroded soil conditions. Mycorrhiza 11, 215–223 (2001). [Google Scholar]
- Oláh B., Brière C., Bécard G., Dénarié J. & Gough C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 44, 195–207 (2005). [DOI] [PubMed] [Google Scholar]
- Wu Q. S., Zou Y. N., He X. H. & Luo P. Arbuscular mycorrhizal fungi can alter some root characters and physiological status in trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Plant Growth Regul. 65, 273–278 (2011). [Google Scholar]
- Wang P., Wu S. H., Wen M. X., Wang Y. & Wu Q. S. Effects of combined inoculation with Rhizophagus intratradices and Paenibacillus mucilaginosus on plant growth, root morphology and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Sci. Hortic. 205, 97–105 (2016). [Google Scholar]
- Berta G., Fusconi A., Trotta A. & Scannerini S. Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol. 114, 207–215 (1990). [Google Scholar]
- Schellenbaum L. et al. Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann. Bot. 68, 135–141 (1991). [Google Scholar]
- Guo D. L. et al. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 180, 673–683 (2008). [DOI] [PubMed] [Google Scholar]
- Maillet F. et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469, 58–63 (2011). [DOI] [PubMed] [Google Scholar]
- Hodge A., Berta G., Doussan C., Merchan F. & Crespi M. Plant root growth, architecture and function. Plant Soil 321, 153–187 (2009). [Google Scholar]
- Wu Q. S. et al. Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 68, 27–35 (2012). [Google Scholar]
- Li J. G. et al. The fluxes of H2O2 and O2 can be used to evaluate seed germination and vigor of Caragana korshinskii. Planta 239, 1363–1373 (2014). [DOI] [PubMed] [Google Scholar]
- Ren G. W. et al. Effects of aphids Myzus persicae on the changes of Ca2+ and H2O2 flux and enzyme activities in tobacco. J. Plant Interact. 9, 883–888 (2014). [Google Scholar]
- Li T. et al. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 197, 617–630 (2013). [DOI] [PubMed] [Google Scholar]
- Hackket C. A study of the root system of barley. І. Effects of nutrition on two varieties. New Phytol. 67, 287–300 (1968). [Google Scholar]
- Phillips J. M. & Hayman D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161 (1970). [Google Scholar]
- Bajji M., Lutts S. & Kinet J. M. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 160, 669–681 (2001). [DOI] [PubMed] [Google Scholar]
- Sudhakar C., Lakshmi A. & Giridarakumar S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 161, 613–619 (2001). [Google Scholar]
- Velikova V., Yordanov I. & Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151, 59–66 (2000). [Google Scholar]
- Wang A. G. & Luo G. H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commu. 26, 55–57 (1990). [Google Scholar]
- Sun J. et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 33, 943–958 (2010). [DOI] [PubMed] [Google Scholar]


