Abstract
In order to discover new antifungal agrochemicals that could have highly active and novel motifs, thirty-six new 2-acylaminocycloalkylsulfonamides (IV) were synthesized. Their structures were characterized and confirmed by 1H NMR, 13C NMR, IR, MS, elemental analysis and X-ray single crystal diffraction. In vitro and in vivo activities against various Botrytis cinerea strains were evaluated. Bioassay results revealed that most of the title compounds exhibited excellent in vitro fungicidal activity, in which compound IV-26 showed the highest activity against sensitive, low-resistant, moderate-resistant and high-resistant strains of B. cinerea compared with the positive fungicide procymidone. Meanwhile in vivo fungicidal activity of compound IV-31 was better than the commercial fungicides procymidone and chesulfamide in greenhouse trial. The structure activity relationship (SAR) was also discussed and the results were of importance to the structural optimization and development of more potent sulfonamides antifungal agents.
Botrytis cinerea (teleomorph: Botryotinia fuckeliana) is an airborne plant pathogen with a necrotrophic lifestyle attacking over 200 crop hosts worldwide. Many kinds of fungicides have been failed to control this plant disease due to its genetic plasticity1. Moreover, the continuous use of fungicides, such as carbendazim, diethofencarb, procymidone, and pyrimethanil etc, has led to the growing resistance of this plant pathogen to fungicides2. Thus, phytofungal disease control is urgently necessitated the discovery and development of new antifungal agents with highly active, low resistance and novel motifs for plant protection.
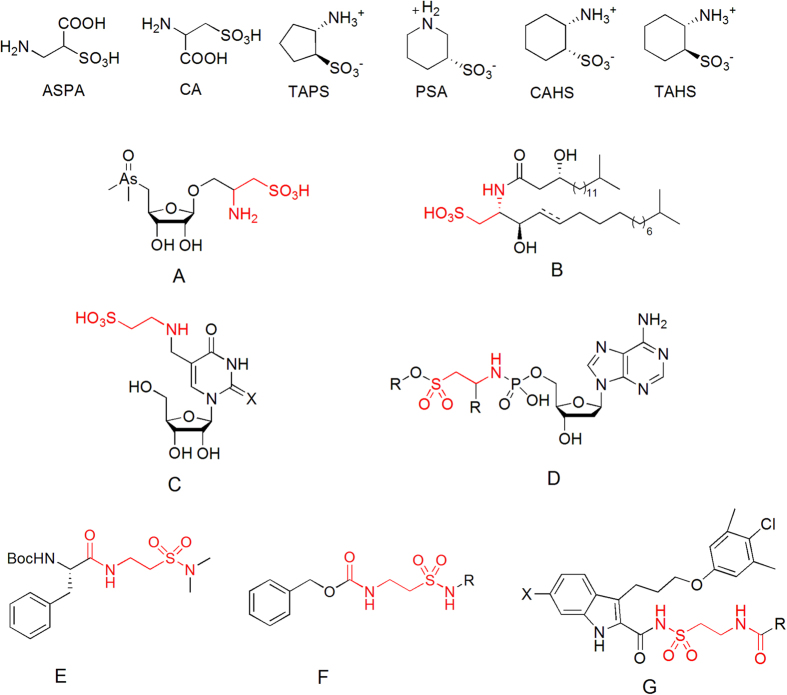
As very important sulfur-containing analogs of amino carboxylic acids, 2-aminoethanesulfonic acid was first isolated from ox bile in 19th century by Tiedemann and Gmelin, which name ‘taurine’ was attributed by Gmelin3,4. In addition, to be an essential amino acid of human body, taurine has also shown a variety of biological functions5,6,7,8,9,10,11,12,13. Its derivatives had been received much more attention around the world. For example, ASPA (3-amino-2-sulfopropanoic acid, Fig. 1) and CA (2-amino-3-sulfopropanoic acid, Fig. 1), the simple substituted taurines, showed some anti-inflammatory activities14. As representatives of cyclic taurine derivatives, TAPS ((1S,2S)-2-aminocyclopentane-1-sulfonic acid, Fig. 1) and PSA (piperidi-3-sulfonic acid, Fig. 1) gave different effects on ATP-dependent calcium ion uptake15, while CAHS ((1R,2S)-2-aminocyclohexane-1-sulfonic acid, Fig. 1) and TAHS ((1S,2S)-2-aminocyclohexane-1-sulfonic acid, Fig. 1) had the thermoregulation ability via interaction with the central serotonergic system16.
Figure 1. Taurine derivatives.
Besides, 2-aminoethanesulfonic acid had been found as key structural moieties in some natural products, such as dimethyl arsenic aminosulfonate (A, Fig. 1), which was isolated from Sargassum lacerifolium17. Flavocristamides (B, Fig. 1), isolated from a marine bacterium Flavobacterium sp., was able to inhibit the enzyme DNA polymerase α18. 5-Taurinomethyluridine (C, Fig. 1) was discovered in mammalian mitochondrial tRNAs19, which was considered to be responsible for precise codon recognition and the absence of these derivatives led to mitochondrial encephalomyopathic diseases. In addition, taurine deoxyadenosine monophosphates (Tau-dAMP, D, Fig. 1) were recently developed as potential substrates for the HIV-1 reverse transcriptase20.
Studies on the synthesis and biological activity of taurine analogues 2-acylaminoethylsulfonamides have been reported frequently. For instance, β-aminoethanesulfonyl azides E (Fig. 1)21 and taurine-containing peptidomimetics F (Fig. 1)22 were synthesized, and 2-indole-acylsulfonamides G (Fig. 1)23 was used as myeloid cell leukemia-1 inhibitors.
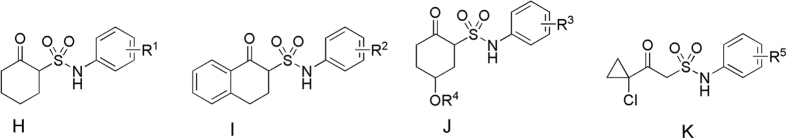
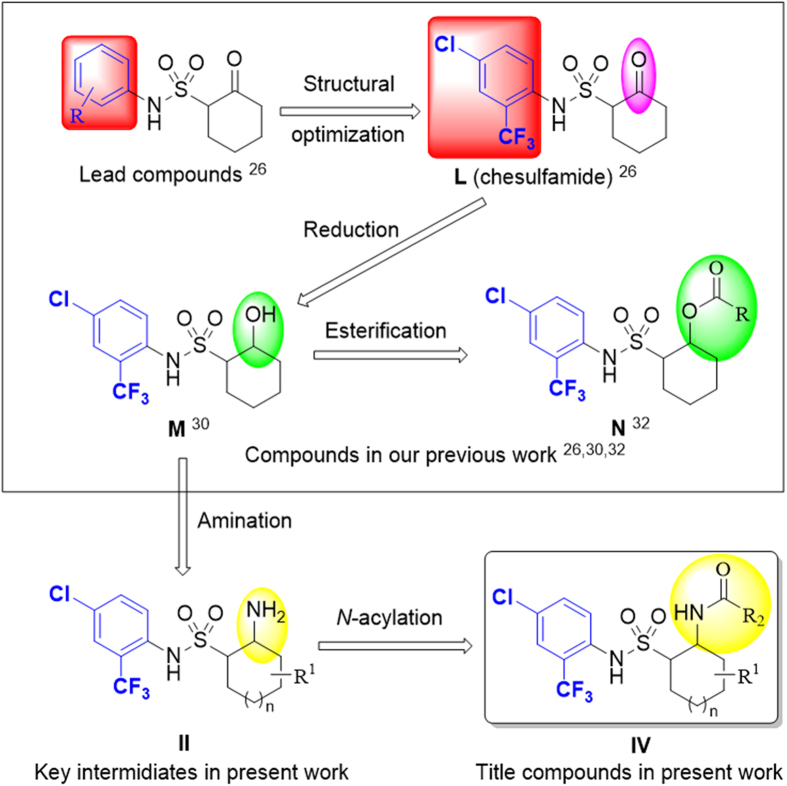
While 2-aminocycloalkylsulfonic acid and its derivatives were rarely reported so far24,25, and there are no reports on the synthesis of 2-acylaminocycloalkyl-sulfonamides. Although its application in field of medicine was primarily reported, in agricultural research was still poorly applied. Recently, our group reported a series of 2-oxycycloalkylsulfonamides (H-N, Figs 2 and 3), which possessed highly fungicidal activity26,27,28,29, of which compound L (chesulfamide, Fig. 3) could be great promise and a lead compound in fungicide research and development. Based on the lead structure of compound L, compounds M and N (Fig. 3) were designed and synthesized with much higher fungicidal activity30,31,32.
Figure 2. 2-Oxycycloalkylsulfonamides derivatives.
Figure 3. The designed strategy for the key intermediates II and title compounds IV.
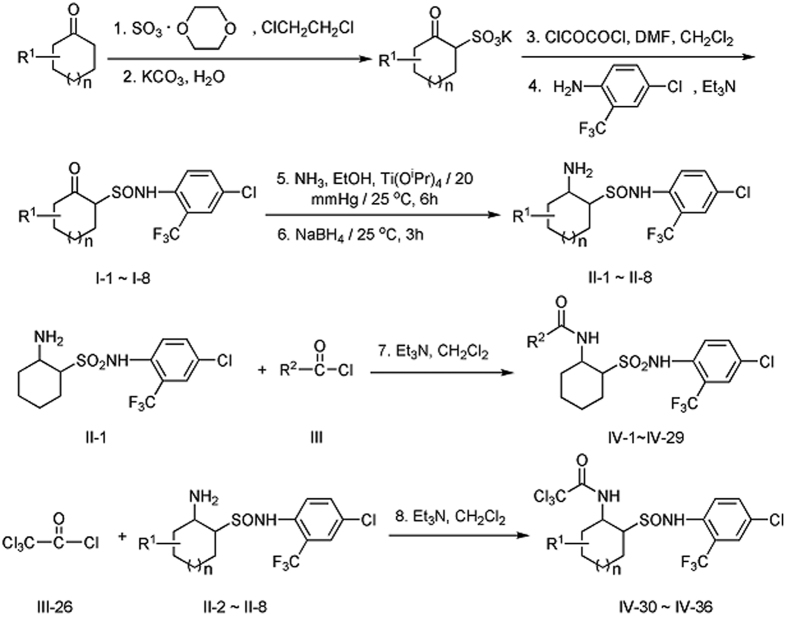
These findings encouraged us to further extend the structural modification of compound L with the aim to find more potent antifungal agents. In this paper, 2-acylaminocycloalkylsulfonamides (IV, Fig. 3) were constructed by reaction of reductive amination and acylation (Fig. 4). The single-crystal structure of the title compounds IV-3 and IV-31 were analyzed. The fungicidal activity of the title compounds against various B. cinerea strains was evaluated. According to their fungicidal activities, structure-activity relationship (SAR) was also discussed.
Figure 4. Synthetic route for the key intermediates II and the title compounds IV-1 to IV-36.
Results and Discussion
Synthesis and Structure Elucidation
The synthetic route of title compounds IV-1 to IV-36 was outlined in Fig. 4 using 2-oxycycloalkylsulfonamides as a starting material. Reductive amination method in ref. 30 was applied to the treatment of ketones with ammonia in ethanol and titanium (IV) isopropoxide, followed by in situ sodium borohydride reduction. In our experiments, however, the method is improved. For the synthesis of compounds II from compounds I, the ethanol solution of ammonia was replaced directly by continuous passing of ammonia gas. The reaction was completed in a short time by monitoring ammonia gas pressure upto 20 mmHg. It was easy to operate and, and the yield of compounds II were from 42% to 96%. In addition, the title compounds IV were easily obtained by the reaction of compounds II with acyl chloride. Yields of title compounds IV were generally high (over 90%).
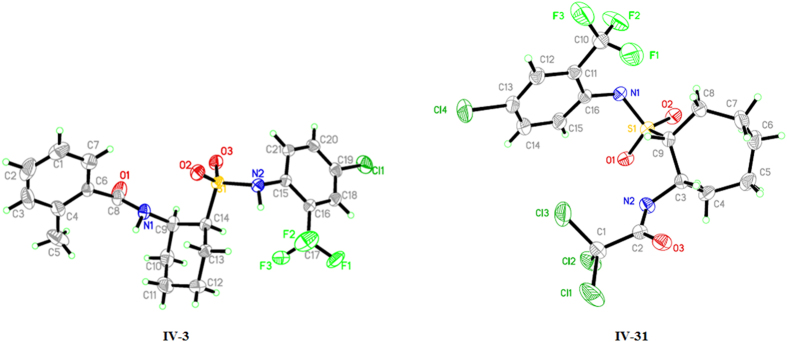
Crystal structures of compounds IV-3 and IV-31 were analyzed by X-ray single crystal diffraction. Their structures were shown in Fig. 5, and their crystal data were shown in Table S1 to Table S3. Compound IV-3 was typical chair conformation, in which the chiralities of the 9th and the 14th carbon atoms on cyclohexane were R and S respectively. In addition, the bulky sulfonamide group was on equatorial bond and the smaller amide group was on axial bond. The spatial configuration was presented as cis-1, 2-disubstituent. Two benzene rings were far apart, which avoided the steric hindrance effect. For compound IV-31, the chiralities of the 3th and the 9th carbon atoms on cycloheptane were S and R respectively. Similarly, that of the two groups was also on the same side of the ring plane and its space conformation was cis-1, 2-disubstituent. Specific optical rotation of compounds IV-3 and IV-31 were tested as −39.2° and −0.67° respectively. Compound IV-3 possessed specific optical rotation value of −39.2° due to the better stability of cyclohexane, while compound IV-31 was unstable in methanol solution, of which two conformations were mutually transformed to a raceme, resulting in the optical activity disappeared.
Figure 5. Crystal structures of IV-3 and IV-31.
The structures of the synthesized compounds were confirmed by 1H NMR, 13C NMR, IR, LC-MS and elemental analysis. Due to the structural similarity, all the compounds showed similar spectroscopic characteristics. In 1H NMR spectra of compounds II and IV, the protons on the benzene ring appeared in low field in the range of δH 7.0 to 8.0 ppm, while cycloalkyl group gave signals in the range of δH 0 to 5.0 ppm appointed to the protons of CH3-, CH2- and CH-. In addition, active hydrogen atoms of -NH2 and SO2NH- in compounds II appeared around 8.3 ppm, and these two types of hydrogen signals were combined together to represent a broad singlet. The reason may be that active hydrogen of SO2NH- is transferred to -NH2, forming a structure of -NH3+. While that of O = C-NH and SO2NH- in compound IV appeared around 8.3 ppm and 9.3 ppm respectively.
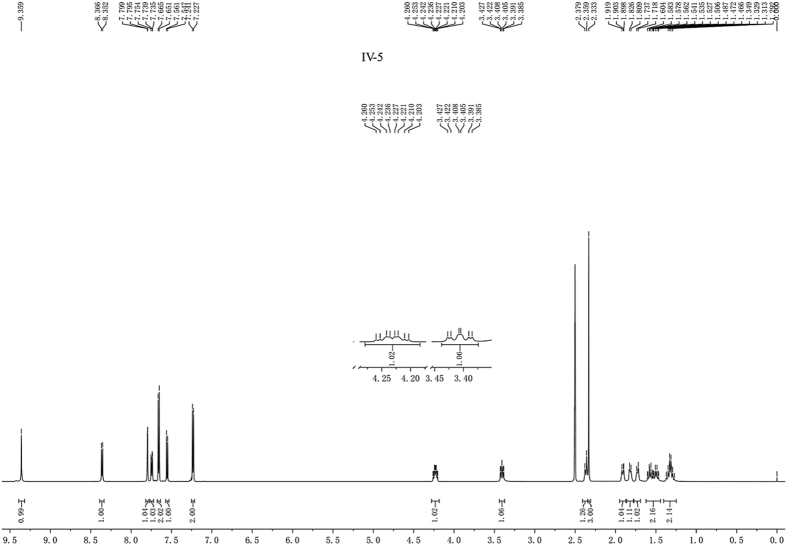
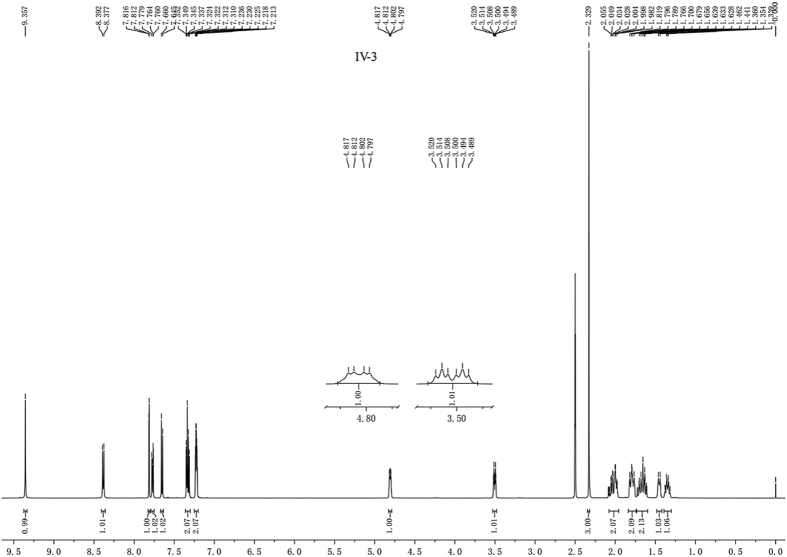
Coupling splitting of protons on CH-SO2, is very characteristic. Generally, the proton of CH-SO2 showed doublet of doublet of doublets (ddd), such as compounds IV-5, IV-7, IV-14, IV-21 and IV-23, and corresponding splitting of proton on CH-N was triplet of doublets (td) (Fig. 6). While in the spectra of some compounds, such as compound IV-3, the proton of CH-SO2 showed doublet of doublets (dd), instead of ddd, and corresponding splitting of proton on CH-N was doublet of triplets (dt) (Fig. 7). However, there were some compounds which coupling splitting of protons on CH-SO2 were special because the conformation was dynamic, such as compounds IV-13, IV-15, IV-16, IV-30 and IV-32 and so on, the protons of CH-SO2 and CH-N showed different signal peak type. This phenomenon was very interesting, but the reason was still unknown. We choose the dominant conformation to explain the normal splitting characteristic of CH-SO2.
Figure 6. 1H NMR spectrum of compound IV-5.
Figure 7. 1H NMR spectrum of compound IV-3.
Taking the 1H NMR spectra of compound IV-3 as an example (Fig. 7), according to the crystal structure, the conformation diagram (Fig. 8) of compound IV-3 was drawn to explain the reasons for this splitting characteristic. As shown in Fig. 8, the proton of C14 linked -SO2 was located in axial bond (C14-Ha) due to coupling splitting effects of equatorial bond (C9-He) on C9, equatorial bond (C13-He) and axial bond (C13-Ha) on C13. Normally the proton of C14 linked -SO2 showed ddd signal due to the magnetic non-equivalence of these three protons (C9-He, C13-He and C13-Ha), appeared as dd signal. Its spatial conformation was shown in Fig. 8(a), which can be determined from the single crystal structure in Fig. 5. It showed a strong coupling splitting effect due to C14-Ha, C9-He and C13-He lying on one side of cyclohexane plane and at a close distance, while it showed a weak coupling splitting effect due to C13-Ha and C14-Ha lying on both sides of cyclohexane plane and at a distant position, which led to its split signal invisible in the spectrum. Therefore, in 1H NMR spectra C14-Ha showed double doublets.
Figure 8. Conformation of compound IV-3 according to the crystal structure.
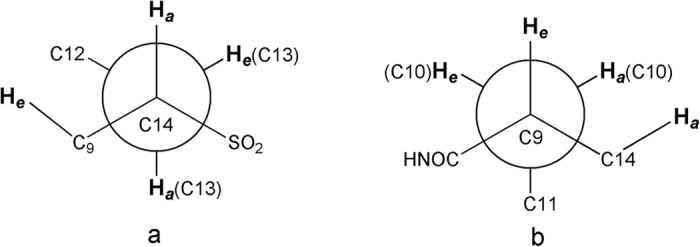
Correspondingly, the proton of C9-He linked -NH showed double triplets. The reason can be explained by its spatial conformation. As shown in Fig. 8(b), the difference of magnetic non-equivalence of two protons between C10-He and C10-Ha is small due to the protons adjacent C10-He and C10-Ha close to proton of C9-He. So proton of C9-He affected by protons of C10-He and C10-Ha, which signal showed coupling splitting of triplets, and protons of C9-He affected by protons of C14-He, which signal showed coupling splitting of doublets. Therefore, in 1H NMR spectra double triplets were assigned to the proton of C9-He.
In 13C NMR spectra (see supplementary information), compounds I, II and IV revealed signals of carbon in the range of δC 0 to 70 ppm assigned to methyl, methylene and methane on naphthene, and carbon signals of benzene ring and trifluoromethyl in the range of δC 115 to 140 ppm in low field. Compounds I and IV gave carbon signals around 202 ppm and 166 ppm respectively assigned to C = O.
In IR spectra of compounds I and IV, the absorption peak of carbonyl stretching vibration appeared around 1700 cm−1 and 1650 cm−1, respectively. While the absorption peak of imino group stretching vibration appeared around 3300 cm−1. In addition, the stretching vibration absorption (-NH2 and -SO2NH) of compounds II appeared around 3500 cm−1 and 3150 cm−1.
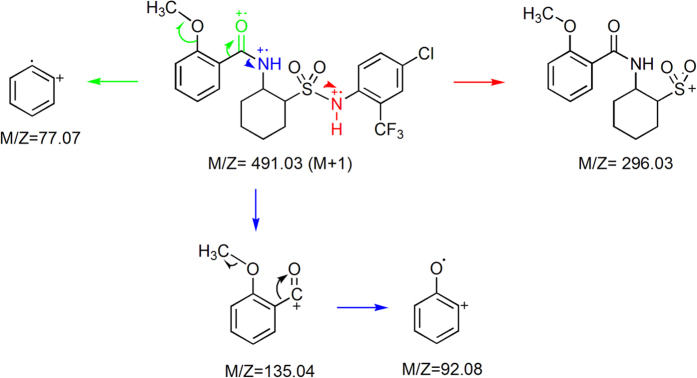
In LC-MS (ES+ mode) spectrum of IV-1 (Fig. 9), the quasi-molecular ion peak was 491 [M + H]+, which accorded with the nitrogen rule. Firstly, sulfonamide bond was broken into a characteristic ion peak at m/z 296, and then fragment ion peak of m/z 135 was obtained by amide bond fracture. Finally, fragment ion peaks of m/z 92 and m/z 77 were obtained via McLafferty rearrangement on the benzene ring and after losing a methylene, respectively. According to the above analysis, fragment missing was reasonable.
Figure 9. MS (ES+ mode) analysis of IV-1 with the fragmentation patterns.
Bioassay of Fungicidal Activities
B. cinerea strains showed multiple physiological characteristics because of the different living environment and fungicide application level. As a result, sensitivities of strains from different areas are also disparate to new compounds.
Fungicidal activity and structure-activity relationship of compounds IV-1~IV-29
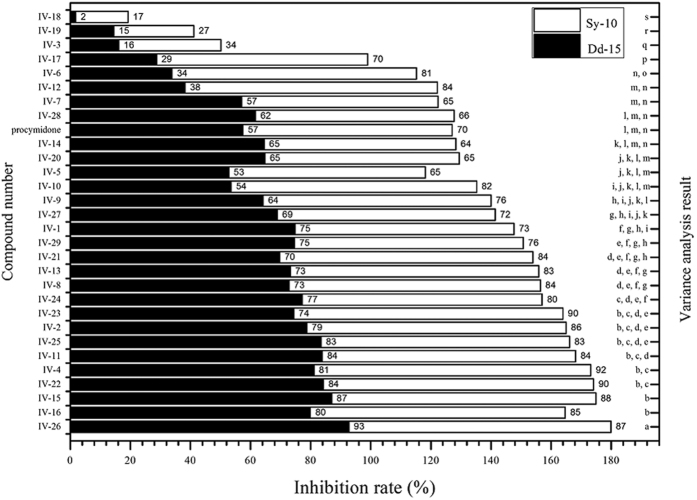
In order to screen out active compounds correctly and quickly, firstly the title compounds (IV-1~IV-29) were tested against two B cinerea strains (Dd-15 and Sy-10), which inhibition rates were shown in Fig. 10. Two-factor analysis of variance between strains and compounds was conducted by SPSS20.0. Analytical results showed that there were sensitivity differences in the twenty-nine new compounds against the two strains. For example, the activity of the title compounds against Dd-15 was generally high, and average inhibition rate was about 76.0%. While the activity was relatively low against Sy-10, which average inhibition rate was about 58.4%. According to the analysis of bioactivity against two strains, there were twenty-one compounds, which fungicidal activities were higher than that of the positive control procymidone.
Figure 10. Fungicidal activity of compounds IV-1~IV-29 against two B. cinerea strains (Sy-10 and Dd-15, 50 mg/L).
The preliminary structure-activity relationship can be summarized in four points. First, for substituent benzoyl chloride (IV-1~IV-17), fungicidal activity was mediocre on the benzene ring containing two substituents, and substituted phenyl groups at ortho- and para-position with methoxyl group and fluorine atom showed excellent activity. However, fungicidal activity was higher at meta-substituted methyl group and meta-substituted chlorine atom. When trifluoromethyl group was on the benzene ring such as compounds IV-15 and IV-16, fungicidal activity was the highest. Second, for alkylacyl chloride (IV-18~IV-23), fungicidal activity of compounds showed a rising trend with the increase of carbon number in the alkyl group, for example, that of which containing n-hexanoyl chloride (IV-22) or n-heptanoyl chloride (IV-23) was the highest. Third, for halogenated acetyl chloride (IV-24~IV-27), the bioactivity increased with the increase of chlorine atom number, and activity of chloro-substituted compounds was higher than that of the bromo-substituted ones. Finally, for 2-alkoxyl acetyl chloride (IV-28 and IV-29), the activity of 2-ethoxyl acetyl chloride was higher than that of the 2-methoxyl acetyl chloride. As a result, eleven highly active compounds were chosen as candidates in the second round screening.
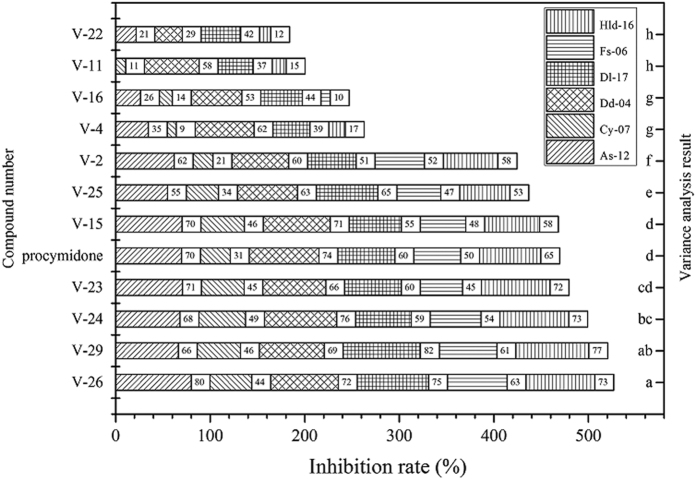
As shown in Fig. 11, eleven compounds were screened out to determine fungicidal activity against other six different B. cinerea strains. Two-factor analysis of variance results showed that there remained significant differences in sensitivities of the six B. cinerea strains to the title compounds. For example, the average inhibition rates of eleven compounds against As-12, Cy-07, Dd-04, Dl-17, Fs-06 and Hld-16 were 51.97%, 29.17%, 62.82%, 55.84%, 35.70% and 47.74% respectively. The activities of eleven compounds against the six B. cinerea strains could be divided into eight subsets (a–h), in which those of compounds IV-23, IV-24, IV-26 and IV-29 were higher than the positive control procymidone. These four compounds were selected to do the further study and their EC50 values were evaluated and shown in Table 1.
Figure 11. Fungicidal activity of title compounds IV against six B. cinerea strains (50 mg/L).
Table 1. EC50 values of title compounds IV against six B. cinerea strains.
| Compound Number | R2 | EC50 (mg/L) (95% confidence limits of EC50) |
|||||
|---|---|---|---|---|---|---|---|
| As-12 | Cy-07 | Dd-04 | Dl-17 | Fs-06 | Hld-16 | ||
| IV-23 ab | CH3(CH2)5― | 13.87 (9.25–20.81) | 18.13 (9.92–33.14) | 9.14 (6.34–13.18) | 15.19 (11.63–19.85) | 13.63 (10.12–18.36) | 19.40 (13.67–27.53) |
| IV-24 ab | ClCH2― | 16.14 (11.94–21.81) | 12.34 (8.44–18.05) | 8.89 (5.94–13.30) | 13.69 (10.06–18.62) | 25.81 (14.13–47.16) | 25.44 (19.80–32.68) |
| IV-26 a | Cl3C― | 4.79 (3.37–6.81) | 1.60 (0.65–3.91) | 1.97 (1.18–3.28) | 0.37 (0.07–2.08) | 7.56 (4.57–12.51) | 5.88 (3.36–10.30) |
| IV-29 ab | C2H5OCH2― | 13.27 (10.57–16.67) | 10.37 (6.78–15.88) | 5.29 (3.51–7.99) | 11.81 (8.50–16.39) | 8.94 (7.20–11.09) | 31.90 (19.81–51.36) |
| procymidone bc | — | 16.93 (10.28–27.88) | 21.36 (14.18–32.18) | 8.17 (5.80–11.49) | 2.49 (1.33–4.66) | 75.84 (36.17–159.00) | 12.91 (9.15–18.20) |
The letters a–c denoted the difference significance analysis results of the same compound against six different strains. Means followed by the same letter within the same column are not significantly different (p > 0.05, Fisher1s LSD multiple comparison test).
Fungicidal activities of all the four title compounds were higher than that of the positive fungicide procymidone. Overall, the fungicidal activities of compound IV-26 against six strains (As-12, Cy-07, Dd-04, Dl-17, Fs-06, and Hld-16), IV-29 against four strains (As-12, Cy-07, Dd-04, and Fs-06), IV-23 and IV-24 against three strains (As-12, Cy-07, and Fs-06) were higher than those of procymidone. Activities of the four title compounds against the six B. cinerea strains were different, for example, EC50 values of compound IV-26 against the six strains were 0.37~7.56 mg/L, while those of procymidone were 2.49~75.84 mg/L. Referring to resistant grading standards to procymidone33,34, Dd-04 and Dl-17 were low-resistant strains; As-12, Cy-07 and Hld-16 were moderate-resistant strains; Fs-06 was high-resistant strain. The results displayed that the in vitro activities of compound IV-26 against all the resistant strains were excellent.
After the above test, structure-activity relationship between acyl chloride and fungicidal activity was confirmed. It was to be sure that trichloroacetyl chloride had the greatest contribution to the fungicidal activity for the twenty-nine acyl chlorides. Therefore, trichloroacetyl chloride was marked as the active group in the later structural modification of cycloalkyl group (IV-30~IV-36). Moreover, compared to the fungicidal activity screened for one strain, it was more reliable for different strains from different areas to choose as the test targets.
Fungicidal activity and structure-activity relationship of compounds IV-26 and IV-30~IV-36
As shown in Table 2, after the structural modification of cycloalkyl group, compounds IV-30~IV-36 also had very high fungicidal activity against five other B. cinerea strains. Referring to resistant grading standards to procymidone33,34, As-11 was sensitive strain; Dl-11 was low-resistant strain; Cy-09 was moderate-resistant strain; Fs-11 and Hld-15 were high-resistant strains. The statistical results of SPSS showed that compounds IV-26, IV-30, IV-31, IV-32, IV-33 and IV-34 exhibited excellent activity. It was found that the size of cycloalkyl group was important factor to determine the fungicidal activity compared with that of IV-26. For example, the EC50 values of compounds IV-26, IV-30 and IV-31 were 0.15~3.64 mg/L, 0.66~11.68 mg/L and 0.82~9.49 mg/L, respectively. The activities of compound IV-26 containing 6-membered ring were better than those of compounds IV-30 and IV-31 respectively containing 5- and 7-membered ring. In addition, it was found that the types of substituent alkyl group on the cyclohexane had a significant effect on the fungicidal activity by comparing activities of compounds IV-32~IV-36. The activity decreased with the increase of alkyl carbon number. Moreover, the position of alkyl group also had effect on the activity. For example, compared with compounds IV-32, IV-33 and IV-34, their fungicidal activity was para-methyl > ortho-methyl > meta-methyl in the order.
Table 2. EC50 values of title compounds IV-26 and IV-30~IV-36 against five B. cinerea strains.
| Compound number | n | R1 | EC50 (mg/L) (95% confidence limits of EC50) |
||||
|---|---|---|---|---|---|---|---|
| As-11 | Cy-09 | Dl-11 | Fs-11 | Hld-15 | |||
| IV-26 a | 2 | H | 0.41 (0.08–2.01) | 1.13 (0.46–2.75) | 0.15 (0.02–1.26) | 3.64 (1.72–7.72) | 1.87 (1.06–3.31) |
| IV-30 a | 1 | H | 0.66 (0.17–2.48) | 2.28 (1.54–3.38) | 0.77 (0.32–1.87) | 11.68 (9.08–15.03) | 0.85 (0.39–1.86) |
| IV-31 a | 3 | H | 4.59 (2.87–7.33) | 1.36 (0.58–3.15) | 0.96 (0.47–1.99) | 9.49 (2.22–40.56) | 0.82 (0.20–3.26) |
| IV-32 a | 2 | 3-CH3 | 14.76 (2.92–74.55) | 10.71 (0.71–160.53) | 0.01 (0.00–25.75) | 7.93 (2.60–24.18) | 0.96 (0.13–7.13) |
| IV-33 a | 2 | 4-CH3 | 0.18 (0.01–2.95) | 2.23 (0.37–13.43) | 0.15 (0.02–1.32) | 15.56 (5.34–45.32) | 0.15 (0.01–2.67) |
| IV-34 a | 2 | 5-CH3 | 0.56 (0.06–4.85) | 6.19 (2.94–13.04) | 2.22 (1.37–3.59) | 16.75 (7.52–37.27) | 1.19 (0.47–2.98) |
| IV-35 ab | 2 | 5-C2H5 | 51.4 (2.52–1049.18) | 19.87 (1.42–277.31) | 0.22 (0.01–4.01) | 16.42 (5.30–50.84) | 31.99 (3.84–266.66) |
| IV-36 c | 2 | 5-C(CH3)3 | >100 | 44.12 (18.73–103.92) | 9.49 (6.30–14.28) | >100 | 30.76 (18.10–52.25) |
| procymidone bc | — | — | 0.22 (0.07–0.65) | 20.00 (14.52–27.55) | 4.40 (3.43–5.65) | >100 | >100 |
The letters a–d denoted the difference significance analysis results of the same compound against five different strains. Means followed by the same letter within the same column are not significantly different (p > 0.05, Fisher1s LSD multiple comparison test).
In vivo fungicidal activity against B. cinerea on leaves of cucumber (mycelium inoculation method)
Six compounds (IV-25, IV-26, IV-30, IV-31, IV-32, and IV-33) were tested for their in vivo fungicidal activity on leaves of cucumber, and the leading compound chesulfamide (L, Fig. 3) was used as the positive control. The bioassay results in Table 3 showed that the control efficiency of compound IV-31 was significantly higher than that of the positive control chesulfamide. Fungicidal activity of compounds IV-26, IV-30, IV-32 and IV-33 was equivalent to the chesulfamide.
Table 3. Control efficiency of compounds against B. cinerea on leaves of cucumber.
| Compd. | Inhibition rate (%) ± SEM |
|---|---|
| IV-25 | 24.54 ± 11.43 b |
| IV-26 | 37.58 ± 32.58 ab |
| IV-30 | 35.78 ± 16.95 ab |
| IV-31 | 64.30 ± 15.57 a |
| IV-32 | 39.37 ± 22.08 ab |
| IV-33 | 36.68 ± 34.42 ab |
| chesulfamide | 32.11 ± 23.32 ab |
The letters a–b denoted the results of difference significance analysis. Means followed by the same letter within the same column are not significantly different (p > 0.05, Fisher1s LSD multiple comparison test).
Compared with the previous work, the structure of the title compounds was modified and new. Meanwhile, their fungicidal activity had greater improvement than that of the lead compound. From the point of view of chemical synthesis, novel key intermediates 2-aminocycloalkylsulfonamides (II-1~II-8) were obtained, which had the vital significance for obtaining the title molecules with structural diversity. In addition, the effective improvements of synthesis method for compounds II were made, which greatly increased the yield and the reaction progress. On the other hand, structural characterization of title compounds IV was described in detail. In particular, the NMR spectra are very characteristic. The single crystal structure was obtained, which provided the basis for accurately structural analysis. According to the single crystal structure, the computer-aided design could be simulated, which is helpful to further molecular design and structural optimization. Preliminary mechnism study indicated that cyclohexyl alkyl sulfonamides might inhibit the growth of gray mould by affecting the synthesis of the internal substance35. The elucidation of the mode of action of these new compound is worth research, which will be studied in detail in the future.
Conclusion
In conclusion, we reported the synthesis of a new series of 2-acylaminocycloalkylsulfonamides and their in vitro and in vivo fungicidal activities against various B. cinerea strains were evaluated. Some title compounds showed notable activity, especially compound IV-31 was of great potential to be developed as new antifungal agents for plant protection. Moreover, single crystal structure of compound IV-31 was determined to assist the further molecular design and structural modification. In addition, the SAR results indicated that structure of acylchloride and naphthenic scaffold had significant effects on the activity. Thus, the present results were of great promise for the design and development of novel sulfonamides antifungal agents. Further research was necessary on the more extensive structural modification and the broad determination of the fungicidal spectra.
Materials and Methods
General
Nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 and DMSO-d6 unless indicated otherwise with a Bruker Avance III 600 MHz spectrometer (Bruker, Fallanden, Switzerland), using tetramethylsilane (TMS) as an internal standard. Infrared (IR) spectra were recorded on a Shimadzu IR Affinity-1 spectrophotometer (Shimadzu, Kyoto, Japan) with KBr disks. UPLC-MS/MS (Agilent, Palo Alto, CA. USA): ACQUITY UPLC BEH C18 chromatographic column (2.1 mm × 100 mm, 1.7 μm); column temperature: 40 °C; mobile phase: solvent A for acetonitrile, solvent B for 0.1% formic acid-water solution; gradient elution program: 10% A at the initial time of 0 min, and then 90% A~10% B in the range of 0 to 2.0 min, 50% A in the range of 2.0 to 4.0 min, 10% A~90% B in the range of 4.0 to 4.2 min, 10% A in the range of 4.2 to 5.2 min; velocity of flow: 0.2 mL/min; sampling volume: 3 μL. Ion source: ESI; acquisition methods: using multiple reaction monitoring and electrospray ionization in positive mode. Melting points were determined on an X-5 melting-point apparatus (Beijing Tech Instrument Co., Ltd., Beijing, China), and the thermometer was uncorrected. Optical rotation was measured on an automatic polarimeter (ATOGO AP-300; condition: λ = 589 nm, L = 100 mm, Temp. = 22.0 °C). The solvents and reagents were used as received or were dried prior to use, as needed. High resolution mass spectra for new compounds were recorded on a G2-XS QTof Mass Spectrometry Facility (Waters, Milford, MA, USA). Elemental analysis was carried out with a Flash EA 1112 elemantal analyzer (Thermo Finnigan, Bremen, Germany).
Botrytis cinerea strains
Thirteen different B. cinerea strains, Sy-10, Dd-04, Dd-15, Hld-15, Hld-16, Fs-06, Fs-11 Dl-11, Dl-17, Cy-07, Cy-09, As-11 and As-12, were isolated from damaged parts of tomato in a greenhouse in Shenyang, Dandong, Huludao, Fushun, Dalian, Chaoyang and Anshan respectively, Liaoning Province, China, in April 2014, and cultured on potato dextrose agar (PDA) at 28 °C and maintained at 4 °C with periodic subculturing.
Synthesis
The synthetic routes of the key intermediates II and title compounds IV were outlined in Fig. 4.
Synthesis of N-(2-trifluoromethyl-4-chlorophenyl)-2-oxocyclohexylsulfonamides I-1~I-8
Compounds I were synthesized according to the method given in the ref. 26. The synthetic route of compounds I-1 to I-8 was outlined in Fig. 4. I-1 (n = 1, R1 = H), I-2 (n = 0, R1 = H), I-3 (n = 2, R1 = H) were already known30 and I-4~I-8 are new compounds. Their physical data and spectra data were shown as follows:
N-(2-trifluoromethyl-4-chlorophenyl)-3-methyl-2-oxocyclohexylsulfonamide (I-4)
(n = 1, R1 = 3-Me) Colorless crystal; yield, 71%; mp 108–109 °C; 1H NMR (CDCl3) δ: 1.11 (d, J = 6.4 Hz, 3H, CH3), 1.47–2.64 (m, 7H, C4H7), 3.97 (dd, J = 13.4, 5.3 Hz, 1H, CH-SO2), 7.37 (s, 1H, SO2-NH), 7.51–7.71 (m, 3H, Ph-H); 13C NMR (DMSO-d6) δ: 14.41, 23.59, 30.20, 35.94, 45.28, 70.74, 118.99, 121.97, 123.79, 126.94, 131.63, 132.20, 133.43, 204.54; IR (ν, cm−1): 3344, 1708; MS (z/e): 369(M+), 195, 175, 111, 83, 55; Anal. Calcd for C14H15ClF3NO3S: C, 45.47; H, 4.09; N, 3.79; found: C, 45.31; H, 3.94; N, 3.92.
N-(2-trifluoromethyl-4-chlorophenyl)-4-methyl-2-oxocyclohexylsulfonamide (I-5)
(n = 1, R1 = 4-Me) Colorless crystal; yield, 91%; mp 97–99 °C; 1H NMR (CDCl3) δ: 1.34–2.62 (m, 10H, C5H10), 3.90 (dd, J = 13.0, 5.7 Hz, 1H, CH-SO2), 7.35 (s, 1H, SO2-NH), 7.51–7.71 (m, 3H, Ph-H); 13C NMR (DMSO-d6) δ: 21.84, 26.24, 28.86, 34.55, 48.07, 69.24, 118.64, 118.99, 125.61, 126.98, 132.23, 133.28, 145.66, 202.41; IR (ν, cm−1): 3365, 1708; MS (z/e): 369(M+), 148, 131, 126, 120, 91; Anal. Calcd for C14H15ClF3NO3S: C, 45.47; H, 4.09; N, 3.79; found: C, 45.63; H, 3.98; N, 3.57.
N-(2-trifluoromethyl-4-chlorophenyl)-5-methyl-2-oxocyclohexylsulfonamide(I-6)
(n = 1, R1 = 5-Me) Colorless crystal; yield, 94%; mp 104–105 °C; 1H NMR (CDCl3) δ: 1.07–2.63 (m, 10H, C5H10), 3.99 (dd, J = 13.3, 5.4 Hz, 1H, CH-SO2), 7.37 (s, 1H, SO2-NH), 7.51–7.69 (m, 3H, Ph-H); 13C NMR (DMSO-d6) δ: 21.16, 26.44, 30.32, 34.52, 36.63, 69.63, 119.00, 121.97, 123.78, 126.91, 131.69, 132.19, 133.44, 203.09; IR (ν, cm−1): 3367, 1710; MS (z/e): 369(M+), 352, 306, 195, 175, 55; Anal. Calcd for C14H15ClF3NO3S: C, 45.47; H, 4.09; N, 3.79; found: C, 45.66; H, 4.31; N, 3.59.
N-(2-trifluoromethyl-4-chlorophenyl)-5-ethyl-2-oxocyclohexylsulfonamide (I-7)
(n = 1, R1 = 5-Et) Colorless crystal; yield, 99%; mp 90~93 °C; 1H NMR (CDCl3) δ: 0.96–2.66 (m, 12H, C6H12), 3.98 (dd, J = 12.5, 5.4 Hz, 1H, CH-SO2), 7.38 (s, 1H, SO2-NH), 7.52–7.70 (m, 3H, Ph-H); 13C NMR (DMSO-d6) δ: 11.80, 28.21, 32.23, 34.41, 36.63, 41.27, 69.70, 121.97, 123.79, 126.91, 131.70, 132.21, 133.46, 133.82, 203.20; IR (ν, cm−1): 3375, 1714; MS (z/e): 383(M+), 366, 320, 195, 175, 55; MS (z/e): 383(M+), 366, 320, 195, 175, 55; Anal. Calcd for C15H17ClF3NO3S: C, 46.94; H, 4.46; N, 3.65; found: C, 47.11; H, 4.37; N, 3.78.
N-(2-trifluoromethyl-4-chlorophenyl)-5-tertiarybutyl-2-oxocyclohexylsulfonamide (I-8)
(n = 1, R1 = 5-t-Bu) Colorless crystal; yield, 93%; mp 86–89 °C; 1H NMR (CDCl3) δ: 0.93–2.70 (m, 16H, C8H16), 3.95 (dd, J = 13.3, 5.3 Hz, 1H, CH-SO2), 7.38 (s, 1H, SO2-NH), 7.52–7.70 (m, 3H, Ph-H); 13C NMR (DMSO-d6) δ: 8.97, 27.63, 30.23, 32.64, 41.31, 44.82, 69.96, 119.01, 123.78, 126.91, 131.76, 132.28, 133.49, 133.99, 203.08; IR (ν, cm−1): 3329, 1714; MS (z/e): 280, 194, 175, 154, 69, 57; Anal. Calcd for C17H21ClF3NO3S; C, 49.57; H, 5.14; N, 3.40; found; C, 49.68; H, 4.95; N, 3.61.
Synthesis of the key intermediates N-(2-trifluoromethyl-4-chlorophenyl)-2-aminocycloalkylsulfonamides II-1~II-8
The synthetic route of compounds II-1 to II-8 was outlined in Fig. 4, according to the method given in the ref. 36, under a nitrogen atmosphere, compounds I (30 mmol) and titanium (IV) isopropoxide (17 mL, 60 mmol) in dry ethyl alcohol (150 mL) were stirred, while the ammonia gas passed through the reaction mixture and maintained the pressure of ammonia upto 20 mmHg at room temperature for 6 h, which was monitored by TLC analysis. Then sodium borohydride (1.7 g, 45 mmol) was added slowly to the resulting mixture at room temperature and stirred for 3 h. The reaction was quenched by addition of ammonium hydroxide solution (2 M, 120 mL). The resulting inorganic precipitate was filtered off, and washed with ethyl acetate (150 mL). The filtrate was concentrated under reduced pressure to remove ethyl acetate, and then extracted with ethyl acetate (200 mL). The combined organic extracts were washed with brine (300 mL), dried over anhydrous Na2SO4, evaporated under reduced pressure, and recrystallized from methanol to afford pure key intermediates II. Their physical and spectra data were shown as follows.
N-(2-trifluoromethyl-4-chlorophenyl)-2-aminocyclohexylsulfonamide (II-1)
(n = 1, R1 = H) Colorless crystal, yield, 73%; mp 252–254 °C; 1H NMR (DMSO-d6) δ: 1.32–2.00 (m, 8H, 4CH2), 2.89 (dt, J = 12.4, 3.1 Hz, 1H, CH-N), 3.79 (d, J = 2.1 Hz, 1H, CH-SO2), 7.27–7.42 (m, 3H, Ph-H), 8.21 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 24.07, 24.17, 25.09, 30.36, 50.21, 60.99, 118.65, 122.25, 123.87, 125.68, 125.84, 132.23, 147.54; IR (ν, cm−1): 3516, 3078; MS (z/e): 357[M + H]+, 175, 162, 98, 81; Anal. Calcd for C17H21ClF3NO3S: C, 43.76; H, 4.52; N, 7.85. found: C, 43.88; H, 4.69; N, 7.61.
N-(2-trifluoromethyl-4-chlorophenyl)-2-aminocyclopentylsulfonamide (II-2)
(n = 0, R1 = H) White powder; yield, 95%; mp 183–186 °C; 1H NMR (DMSO-d6) δ: 1.52–2.05 (m, 6H, 3CH2), 3.40–3.44(m, 1H, CH-N), 3.64 (dd, J = 11.6, 6.6 Hz, 1H, CH-SO2), 7.27–7.47 (m, 3H, Ph-H), 8.14 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 21.72, 26.00, 30.71, 51.90, 61.45, 118.71, 121.93, 123.88, 125.68, 125.86, 132.24, 147.70; IR (ν, cm−1): 3614, 3198; MS (z/e): 342(M+), 196, 176, 148, 84, 67; Anal. Calcd for C12H14ClF3N2O2S: C, 42.05; H, 4.12; N, 8.17; found: C, 41.92; H, 3.98; N, 8.35.
N-(2-trifluoromethyl-4-chlorophenyl)-2-aminocycloheptyl sulfonamide (II-3)
(n = 2, R1 = H) White powder; yield, 91%; mp 230–232 °C; 1H NMR (DMSO-d6) δ: 1.41–2.28 (m, 10H, 5CH2), 2.92 (dd, J = 10.0, 2.2 Hz, 1H, CH-N), 3.99 (td, J = 5.5, 2.4 Hz, 1H, CH-SO2), 7.27–7.39 (m, 3H, Ph-H), 8.27 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 21.81, 22.09, 25.95, 27.41, 32.06, 49.81, 61.71, 118.64, 122.28, 123.92, 125.73, 125.80, 132.23, 147.29; IR (ν, cm−1): 3523, 3095; MS (z/e): 370, 194, 174; Anal. Calcd for C14H18ClF3N2O2S; C, 45.35; H, 4.89; N, 7.55; found; C, 45.21; H, 5.02; N, 7.63.
N-(2-trifluoromethyl-4-chlorophenyl)-3-methyl-2-aminocyclohexylsulfonamide (II-4)
(n = 1, R1 = 3-Me) White powder; yield, 79%; mp 213–216 °C; 1H NMR (DMSO-d6) δ: 0.85–2.11 (m, 10H, C5H10), 2.83 (td, J = 11.5, 3.5 Hz, 1H, CH-N), 3.18 (td, J = 11.4, 4.4 Hz, 1H, CH-SO2), 7.27–7.42 (m, 3H, Ph-H), 8.34 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 17.05, 19.17, 21.96, 24.89, 31.34, 52.13, 56.10, 118.64, 122.26, 123.87, 125.68, 125.79, 132.20, 147.29; IR (ν, cm−1): 3599, 3140; MS (z/e): 370(M+), 176, 112, 95, 67; Anal. Calcd for C14H18ClF3N2O2S: C, 45.35; H, 4.89; N, 7.55; found: C, 45.18; H, 4.62; N, 7.69.
N-(2-trifluoromethyl-4-chlorophenyl)-4-methyl-2-aminocyclohexylsulfonamide (II-5)
(n = 1, R1 = 4-Me) White powder; yield, 86%; mp 230–233 °C; 1H NMR (DMSO-d6) δ: 0.74–2.08 (m, 10H, C5H10), 3.11 (s, 1H, CH-N), 3.29 (s, 1H, CH-SO2), 7.22–7.51 (m, 3H, Ph-H), 8.25 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 22.27, 25.40, 28.00, 31.26, 33.51, 50.43, 55.57, 118.60, 122.07, 123.86, 125.66, 125.83, 132.15, 147.74; IR (ν, cm−1): 3523, 3072; MS (z/e): 370(M+), 176, 112, 95, 67, 55; Anal. Calcd for C14H18ClF3N2O2S: C, 45.35; H, 4.89; N, 7.55; found: C, 45.56; H, 4.69; N, 7.41.
N-(2-trifluoromethyl-4-chlorophenyl)-5-methyl-2-aminocyclohexylsulfonamide (II-6)
(n = 1, R1 = 5-Me) White powder; yield, 85%; mp 250–252 °C; 1H NMR (DMSO-d6) δ: 0.90–2.12 (m, 10H, C5H10), 2.83 (td, J = 11.5, 3.5 Hz, 1H, CH-N), 3.18 (td, J = 11.4, 4.4 Hz, 1H, CH-SO2), 7.28–7.42 (m, 3H, Ph-H),8.35 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 22.14, 25.68, 30.73, 32.35, 50.00, 55.70, 60.67, 118.52, 118.67, 122.14, 122.28, 125.85, 132.23, 147.44; IR (ν, cm−1): 3523, 3170; MS (z/e): 370(M+), 278, 250, 197; Anal. Calcd for C14H18ClF3N2O2S: C, 45.35; H, 4.89; N, 7.55; found: C, 45.57; H, 4.98; N, 7.38.
N-(2-trifluoromethyl-4-chlorophenyl)-5-ethyl-2-aminocyclohexylsulfonamide (II-7)
(n = 1, R1 = 5-Et) White powder; yield, 54%; mp 227–230 °C; 1H NMR (DMSO-d6) δ: 0.81–2.04 (m, 12H, C6H12), 3.05–3.06 (m, 1H, CH-N), 3.55–3.56 (m, 1H, CH-SO2), 7.26–7.46 (m, 3H, Ph-H), 8.19 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 12.02, 24.62, 27.03, 29.29, 32.53, 37.50, 46.60, 55.72, 118.55, 122.07, 122.27, 125.84, 132.15, 132.23, 147.67; IR (ν, cm−1): 3523, 3277; MS (z/e): 384(M+), 194, 174, 95, 67, 56; Anal. Calcd for C15H20ClF3N2O2S: C, 46.81; H, 5.24; N, 7.28; found: C, 47.02; H, 5.06; N, 7.51.
N-(2-trifluoromethyl-4-chlorophenyl)-5-tertiarybutyl-2-aminocyclohexylsulfonamide (II-8)
(n = 1, R1 = 5-t-Bu) White powder; yield, 42%; mp 230–233 °C; 1H NMR (DMSO-d6) δ: 0.77–2.45 (m, 16H, C8H16), 2.87 (d, J = 12.2 Hz, 1H, CH-N), 3.76 (s, 1H, CH-SO2), 7.27–7.41 (m, 3H, Ph-H), 8.20 (s, 3H, NH2 + NH); 13C NMR (DMSO-d6) δ: 19.56, 22.51, 27.66, 28.86, 32.74, 46.15, 46.23, 60.46, 118.64, 122.32, 123.90, 125.70, 125.82, 132.18, 147.31; IR (ν, cm−1): 3502, 3109; MS (z/e): 412(M+), 397, 355, 194, 154; Anal. Calcd for C17H24ClF3N2O2S: C, 49.45; H, 5.86; N, 6.78; found: C, 49.25; H, 6.04; N, 6.66.
Synthesis of acyl chlorides III
Substituent benzoyl chlorides (III-1~III-17), acetyl chlorides (III-18~III-23), halogenated acetyl chlorides (III-24~III-27), alkoxylacetyl chlorides (III-28~III-29) were synthesized according to the given method in the ref. 32.
Synthesis of title compounds 2-acylaminocycloalkylsulfonamides IV-1~IV-29 and IV-30~IV-36
Under nitrogen, acyl chlorides III (3 mmol) were dropwise added to the solution of II (3 mmol) and triethylamine (Et3N, 3.9 mmol) in dry dichloromethane (40 mL). (Fig. 4) The solution was stirred at room temperature for 2 h. The mixture was filtered and washed with 3 M HCl (30 mL), saturated NaHCO3 (30 mL), and brine (40 mL). After dried by anhydrous Na2SO4 and concentrated in vacuo, the crude product was recrystallized with the acetone/petroleum ether to afford pure IV. Their physical data and spectra data were shown as follows.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-methoxybenzoylamino) cyclohexylsulfonamide (IV-1)
(R2 = 2-CH3OC6H4) White solid; yield, 95%; mp 129–130 °C; 1H NMR (DMSO-d6) δ: 1.48–2.14 (m, 8H, 4CH2), 3.55 (dt, J = 11.9, 3.3 Hz, 1H, CH-N), 3.94 (s, 3H, OCH3), 4.64 (dd, J = 6.9, 3.4 Hz, 1H, CH-SO2), 7.05–7.89 (m, 7H, Ph-H), 8.60 (d, J = 7.3 Hz, 1H, CO-NH), 9.57 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 20.07, 22.53, 24.01, 29.88, 45.54, 56.60, 62.79, 112.70, 121.11, 121.96, 122.02, 126.85, 127.07, 131.24, 131.29, 131.49, 133.21, 133.64, 133.84, 157.79, 164.40; IR (ν, cm−1): 3370, 3121, 1649; MS (z/e): 491[M + H]+, 296, 135, 92, 77; Anal. Calcd for C21H22ClF3N2O4S: C,51.38; H, 4.52; N, 5.71; found: C, 51.62; H, 4.69; N, 5.52.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(4-methoxybenzoylamino) cyclohexylsulfonamide (IV-2)
(R2 = 4-CH3OC6H4) White solid; yield, 86%; mp 139–141 °C; 1H NMR (DMSO-d6) δ: 1.37–2.17 (m, 8H, 4CH2), 3.51 (dt, J = 11.8, 3.4 Hz, 1H, CH-N), 3.81 (s, 3H, OCH3), 4.72 (dd, J = 7.9, 3.6 Hz, 1H, CH-SO2), 6.97–7.79 (m, 7H, Ph-H), 7.91 (d, J = 8.5 Hz, 1H, CO-NH), 9.43 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.71, 21.72, 24.15, 30.65, 45.26, 55.74, 63.08, 113.62, 113.62, 122.05, 123.87, 126.55, 127.07, 127.41, 129.98, 131.01, 131.26, 133.62, 134.04, 161.95, 166.86; IR (ν, cm−1): 3390, 3080, 1631; HRMS-ESI, m/z calcd for C21H23ClF3N2O4S, [M + H]+491.1019; found, 491.1022.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-methylbenzoylamino) cyclohexylsulfonamide (IV-3)
(R2 = 2-CH3C6H4) White crystal; yield, 96%; mp 192–194 °C; 1H NMR (DMSO-d6) δ: 1.36–1.96 (m, 8H, 4CH2), 2.33 (s, 3H, CH3), 3.50 (dt, J = 11.9, 3.6 Hz, 1H, CH-N), 4.81 (dd, J = 8.9, 3.1 Hz, 1H, CH-SO2), 7.20–7.81 (m, 7H, Ph-H), 8.38 (d, J = 9.2 Hz, 1H, CO-NH), 9.36 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.40, 19.55, 21.36, 24.39, 31.18, 44.43, 63.36, 122.08, 123.89, 125.57, 126.97, 128.00, 129.56, 130.46, 130.68, 131.03, 133.62, 134.15, 135.86, 137.37, 169.89; IR (ν, cm−1): 3388, 3074, 1645; MS (z/e): 475[M + H]+, 280, 216, 119, 91; Anal. Calcd for C21H22ClF3N2O3S: C,53.11; H, 4.67; N, 5.90; found: C, 53.35; H, 4.50; N, 6.09.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3-methylbenzoylamino) cyclohexylsulfonamide (IV-4)
(R2 = 3-CH3C6H4) White crystal; yield, 43%; mp 176–177 °C; 1H NMR (DMSO-d6) δ: 1.32–2.12 (m, 8H, 4CH2), 2.36 (s, 3H, CH3), 3.54–3.49 (m, 1H, CH-N), 4.74 (d, J = 4.3 Hz, 1H, CH-SO2), 7.34–7.80 (m, 7H, Ph-H), 8.04 (d, J = 8.6 Hz, 1H, CO-NH), 9.42 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.71, 21.32, 21.71, 24.15, 30.61, 45.27, 63.04, 122.05, 123.87, 125.31, 127.02, 128.30, 128.60, 130.95, 131.23, 132.01, 133.62, 134.03, 135.29, 137.60, 167.60; IR (ν, cm−1): 3385, 3046, 1629; MS (z/e): 475[M + H]+, 280, 216, 119, 91; Anal. Calcd for C21H22ClF3N2O3S: C, 53.11; H, 4.67; N, 5.90; found: C, 52.94; H, 4.57; N, 5.71.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(4-methylbenzoylamino) cyclohexylsulfonamide (IV-5)
(R2 = 4-CH3C6H4) White solid; yield, 98%; mp 217–219 °C; 1H NMR (DMSO-d6) δ: 1.32–2.37 (m, 8H, 4CH2), 2.33 (s, 3H, CH3), 3.41 (td, J = 11.1, 2.9 Hz, 1H, CH-N), 4.23 (ddd, J = 19.2, 10.6, 4.0 Hz, 1H, CH-SO2), 7.23–7.80 (m, 7H, 7H, Ph-H), 8.36 (d, J = 8.5 Hz, 1H, CO-NH), 9.36 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 21.32, 24.36, 24.45, 27.08, 32.95, 48.53, 65.11, 122.03, 123.84, 126.78, 126.97, 127.69, 128.91, 128.98, 131.28, 131.33, 132.37, 133.58, 134.25, 141.24, 165.99; IR (ν, cm−1): 3346, 3045, 1630; MS (z/e): 475[M + H]+, 280, 216, 119, 91; Anal. Calcd for C21H22ClF3N2O3S: C, 53.11; H, 4.67; N, 5.90; found: C, 53.31; H, 4.88; N, 6.12.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,4-dimethylbenzoylamino) cyclohexylsulfonamide (IV-6)
(R2 = 2,4-(CH3)2C6H3) White solid; yield, 95%; mp 179–181 °C; 1H NMR (DMSO-d6) δ: 1.35–2.01 (m, 8H, 4CH2), 2.30 (d, J = 5.4 Hz, 6H, CH3 + CH3), 3.50 (dt, J = 11.6, 3.6 Hz, 1H, CH-N), 4.78 (dd, J = 8.8, 3.1 Hz, 1H, CH-SO2), 7.03–7.81 (m, 6H, Ph-H), 8.27 (d, J = 9.1 Hz, 1H, CO-NH), 9.37 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.41, 19.59, 21.16, 21.36, 24.37, 31.13, 44.46, 63.39, 122.08, 123.89, 126.02, 126.96, 128.20, 130.59, 130.97, 131.14, 133.62, 134.18, 134.40, 136.01, 139.11, 170.00; IR (ν, cm−1): 3392, 3053, 1645; MS (z/e): 489[M + H]+, 294, 133, 105, 79; Anal. Calcd for C22H24ClF3N2O3S: C, 54.04; H, 4.95; N, 5.73; found: C, 53.89; H, 5.08; N, 5.54.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3,5-dimethylbenzoylamino) cyclohexylsulfonamide (IV-7)
(R2 = 3,5-(CH3)2C6H3) White solid; yield, 93%; mp 193–195 °C; 1H NMR (DMSO-d6) δ: 1.33–2.31 (m, 8H, 4CH2), 2.28 (s, 6H, CH3 + CH3), 3.41 (td, J = 11.0, 2.8 Hz, 1H, CH-N), 4.23 (ddd, J = 19.2, 10.5, 4.0 Hz, 1H, CH-SO2), 7.12–7.79 (m, 6H, 6H, Ph-H), 8.35 (d, J = 8.5 Hz, 1H, CO-NH), 9.33 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 21.21, 24.34, 24.45, 27.04, 32.87, 48.47, 63.01, 65.13, 122.04, 123.85, 125.37, 125.82, 126.99, 131.07, 131.16, 132.65, 133.58, 134.28, 135.17, 137.46, 137.52, 166.38; IR (ν, cm−1): 3324, 2977, 1626; MS (z/e): 489[M + H]+, 294, 230, 133, 105; Anal. Calcd for C22H24ClF3N2O3S: C, 54.04; H, 4.95; N, 5.73; found: C, 54.27; H, 4.77; N, 5.93.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-fluorobenzoylamino) cyclohexylsulfonamide (IV-8)
(R2 = 2-FC6H4) White solid; yield, 96%; mp 167–169 °C; 1H NMR (DMSO-d6) δ: 1.38–2.11 (m, 8H, 4CH2), 3.54 (m, 1H, CH-N), 4.76 (s, 1H, CH-SO2), 7.26–7.83 (m, 7H, Ph-H), 8.29 (dd, J = 7.2, 65.3 Hz, 1H, CO-NH), 9.41 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.65, 21.76, 24.12, 30.55, 45.14, 62.95, 116.41, 122.04, 123.86, 124.63, 127.02, 130.71, 130.98, 131.28, 132.74, 133.63, 133.97, 158.90, 160.55, 164.18; IR (ν, cm−1): 3308, 3077, 1635; MS (z/e): 479[M + H]+, 284, 220, 123, 95; Anal. Calcd for C20H19ClF4N2O3S: C, 50.16; H, 4.00; N, 5.85; found: C, 50.38; H, 4.14; N, 5.77.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3-fluorobenzoylamino) cyclohexylsulfonamide (IV-9)
(R2 = 3-FC6H4) White solid; yield, 94%; mp 192–194 °C; 1H NMR (DMSO-d6) δ: 1.31–2.20 (m, 8H, 4CH2), 3.52 (d, J = 11.8 Hz, 1H, CH-N), 4.76 (s, 1 H, CH-SO2), 7.38–7.80 (m, 7H, Ph-H), 8.21 (d, J = 8.5 Hz, 1H, CO-NH), 9.41 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.65, 21.65, 24.13, 30.61, 45.33, 62.96, 115.08, 118.36, 122.04, 123.85, 124.39, 127.09, 130.54, 130.60, 131.19, 131.36, 133.62, 133.96, 137.66, 166.04; IR (ν, cm−1): 3378, 3050, 1630; MS (z/e): 479[M + H]+, 284, 220, 123, 95; Anal. Calcd for C20H19ClF4N2O3S: C, 50.16; H, 4.00; N, 5.85; found: C, 50.31; H, 3.83; N, 6.02.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-chlorobenzoylamino) cyclohexylsulfonamide (IV-10)
(R2 = 2-ClC6H4) White solid; yield, 97%; mp 199–201 °C; 1H NMR (DMSO-d6) δ: 1.33–2.20 (m, 8H, 4CH2), 3.52 (m, 1H, CH-N), 4.78 (dd, J = 3.0, 8.9 Hz, 1H, CH-SO2), 7.46–7.81 (m, 7H, Ph-H), 8.58 (d, J = 9.2 Hz, 1H, CO-NH), 9.37 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.43, 21.41, 24.31, 30.94, 44.55, 63.14, 122.06, 123.88, 127.02, 127.20, 129.59, 129.74, 130.53, 130.92, 130.99, 131.18, 133.64, 134.07, 137.17, 166.65; IR (ν, cm−1): 3386, 3083, 1655; MS (z/e): 495[M + H]+, 300, 236, 139, 111; Anal. Calcd for C20H19Cl2F3N2O3S: C, 48.49; H, 3.87; N, 5.66; found: C, 48.62; H, 4.01; N, 5.39.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3-chlorobenzoylamino) cyclohexylsulfonamide (IV-11)
(R2 = 3-ClC6H4) White solid; yield, 95%; mp 184–186 °C; 1H NMR (DMSO-d6) δ: 1.33–2.07 (m, 8H, 4CH2), 3.51–3.53 (m, 1H, CH-N), 4.76 (s, 1H, CH-SO2), 7.46–7.81 (m, 7H, Ph-H), 8.58 (d, J = 8.6 Hz, 1H, CO-NH), 9.37 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.66, 21.65, 24.13, 30.62, 45.32, 62.96, 122.04, 123.86, 126.98, 127.08, 128.02, 130.41, 131.12, 131.25, 131.33, 133.15, 133.62, 133.97, 137.34, 166.04; IR (ν, cm−1): 3353, 3069, 1638; MS (z/e): 495[M + H]+, 300, 236, 139, 111; Anal. Calcd for C20H19Cl2F3N2O3S: C, 48.49; H, 3.87; N, 5.66; found: C, 48.70; H, 3.66; N, 5.87.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(4-chlorobenzoylamino) cyclohexylsulfonamide (IV-12)
(R2 = 4-ClC6H4) White solid; yield, 67%; mp 188–190 °C; 1H NMR (DMSO-d6) δ: 1.29–2.17 (m, 8H, 4CH2), 3.51 (dt, J = 11.9, 3.5 Hz, 1H, CH-N), 4.74 (dd, J = 8.1, 3.6 Hz, 1H, CH-SO2), 7.50–7.86 (m, 7H, Ph-H), 8.19 (d, J = 8.7 Hz, 1H, CO-NH), 9.40 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.67, 21.66, 24.13, 30.62, 45.32, 62.99, 122.04, 123.86, 126.61, 126.81, 127.04, 128.45, 130.12, 131.09, 131.31, 133.62, 133.99, 134.06, 136.25, 166.41; IR (ν, cm−1): 3386, 3081, 1634; MS (z/e): 495[M + H]+, 300, 236, 139, 111; Anal. Calcd for C20H19Cl2F3N2O3S: C, 48.49; H, 3.87; N, 5.66; found: C, 48.33; H, 4.10; N, 5.81.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,6-dichlorobenzoylamino) cyclohexylsulfonamide (IV-13)
(R2 = 2,6-Cl2C6H3) White solid; yield, 90%; mp 216–218 °C; 1H NMR (DMSO-d6) δ: 1.34–2.19 (m, 8H, 4CH2), 3.35–3.38 (m, 1H, CH-N), 4.45–4.49 (m, 1H, CH-SO2), 7.41–7.81 (m, 6H, Ph-H), 8.86 (d, J = 7.8 Hz, 1H, CO-NH), 9.46 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 21.81, 22.31, 24.27, 29.38, 46.63, 62.44, 121.98, 123.79, 127.17, 127.73, 128.18, 128.40, 131.33, 131.69, 131.80, 131.85, 133.63, 134.03, 136.63, 163.20; IR (ν, cm−1): 3309, 3110, 1645; HRMS-ESI, m/z calcd for C20H19Cl3F3N2O3S [M + H]+529.0134; found, 529.0128.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3,5-dichlorobenzoylamino) cyclohexylsulfonamide (IV-14)
(R2 = 3,5-Cl2C6H3) White solid; yield, 98%; mp 236–238 °C; 1H NMR (DMSO-d6) δ: 1.33–2.38 (m, 8H, 4CH2), 3.39 (td, J = 11.2, 3.0 Hz, 1H, CH-N), 4.21 (ddd, J = 19.3, 10.7, 4.0 Hz, 1H, CH-SO2), 7.56–7.80 (m, 6H, Ph-H), 8.70 (d, J = 8.5 Hz, 1H, CO-NH), 9.39 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 24.30, 24.38, 27.03, 32.83, 48.76, 64.92, 120.19, 122.01, 123.83, 126.45, 126.95, 127.03, 127.15, 130.84, 131.50, 133.60, 134.13, 134.51, 138.39, 163.14; IR (ν, cm−1): 3268, 3086, 1644; MS (z/e): 529[M + H]+, 334, 270, 173, 145; Anal. Calcd for C20H18Cl3F3N2O3S: C, 45.34; H, 3.42; N, 5.29; found: C, 45.27; H, 3.67; N, 5.04.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-trifluoromethylbenzoylamino) cyclohexylsulfonamide (IV-15)
(R2 = 2-CF3C6H4) Colorless crystal; yield, 90%; mp 88–89 °C; 1H NMR (DMSO-d6) δ: 1.34–2.03 (m, 8H, 4CH2), 3.49–3.51 (m, 1H, CH-N), 4.80 (s, 1H, CH-SO2), 7.56–7.81 (m, 7H, Ph-H), 8.67 (d, J = 5.5 Hz, 1H, CO-NH), 9.33 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.25, 21.37, 24.33, 30.78, 44.42, 63.10, 122.06, 123.29, 123.87, 125.11, 126.31, 127.06, 129.18, 129.86, 131.21, 131.33, 132.52, 133.63, 134.03, 136.78, 167.22; IR (ν, cm−1): 3338, 3195, 1657; MS (z/e): 529[M + H]+, 334, 270, 173, 145; Anal. Calcd for C21H19ClF6N2O3S: C, 47.69; H, 3.62; N, 5.30; found: C, 47.89; H, 3.52; N, 5.21.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(3-trifluoromethylbenzoylamino) cyclohexylsulfonamide (IV-16)
(R2 = 3-CF3C6H4) White solid; yield, 89%; mp 178–179 °C; 1H NMR (DMSO-d6) δ: 1.42–2.19 (m, 8H, 4CH2), 3.52 (d, J = 11.3 Hz, 1H, CH-N), 4.77 (s, 1H, CH-SO2), 7.60–8.11 (m, 7H, Ph-H), 8.41 (d, J = 8.7 Hz, 1H, CO-NH), 9.41 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.75, 21.74, 24.06, 30.57, 45.42, 62.88, 122.04, 123.51, 123.85, 124.86, 125.31, 127.06, 128.03, 129.05, 129.26, 129.69, 131.13, 132.32, 133.63, 136.29, 166.09; IR (ν, cm−1): 3381, 3092, 1636; MS (z/e): 529[M + H]+, 334, 270, 173, 145; Anal. Calcd for C21H19ClF6N2O3S: C, 47.69; H, 3.62; N, 5.30; found: C, 47.55; H, 3.76; N, 5.13.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-methoxy-5-chlorobenzoylamino) cyclohexylsulfonamide (IV-17)
(R2 = 2-CH3O-5-ClC6H3) White solid; yield, 97%; mp 140–141 °C; 1H NMR (DMSO-d6) δ: 1.44–2.15 (m, 8H, 4CH2), 3.53–3.56 (m, 1H, CH-N), 3.93 (s, 3H, OCH3), 4.63 (dd, J = 7.0, 3.4 Hz, 1H, CH-SO2), 7.22–7.80 (m, 6H, Ph-H), 8.58 (d, J = 7.4 Hz, 1H, CO-NH), 9.54 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 20.01, 22.44, 23.96, 29.84, 45.62, 57.02, 62.71, 114.86, 122.01, 123.83, 124.09, 124.98, 127.08, 130.23, 131.29, 131.53, 132.40, 133.64, 133.79, 156.49, 163.29; IR (ν, cm−1): 3380, 3125, 1653; MS (z/e): 525[M + H]+, 266, 169, 126, 111; Anal. Calcd for C21H21Cl2F3N2O4S: C, 48.01; H, 4.03; N, 5.33; found: C, 48.26; H, 3.90; N, 5.62.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(acetylamino) cyclohexylsulfonamide (IV-18)
(R2 = Me) Colorless crystal; yield, 99%; mp 145–146 °C; 1H NMR (DMSO-d6) δ: 1.34–2.03 (m, 8H, 4CH2), 1.87 (s, 3H, CH3), 3.39 (dt, J = 12.1, 3.2 Hz, 1H, CH-N), 4.55 (dd, J = 8.8, 2.9 Hz, 1H, CH-SO2), 7.60–7.78 (m, 3H, Ph-H), 7.95 (d, J = 9.2 Hz, 1H, CO-NH), 9.26 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.49, 21.40, 23.08, 24.24, 30.87, 44.07, 63.19, 122.06, 123.87, 126.91, 129.99, 130.72, 133.59, 134.22, 170.44; IR (ν, cm−1): 3390, 3037, 1657; MS (z/e): 398[M]+, 194, 159, 140; Anal. Calcd for C15H18ClF3N2O3S: C, 45.17; H, 4.55; N, 7.02; found: C, 44.95; H, 4.21; N, 7.26.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(propionylamino) cyclohexylsulfonamide (IV-19)
(R2 = Et) Colorless crystal; yield, 98%; mp 165–167 °C; 1H NMR (DMSO-d6) δ: 0.98 (t, J = 7.6 Hz, 3H, CH3), 1.29–2.00 (m, 8H, 4CH2), 2.15 (q, J = 7.6 Hz, 2H, CH2), 3.39 (dt, J = 12.0, 3.3 Hz, 1H, CH-N), 4.56 (dd, J = 8.8, 3.1 Hz, 1H, CH-SO2), 7.59–7.79 (m, 3H, Ph-H), 7.84 (d, J = 9.2 Hz, 1H, CO-NH), 9.25 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 10.19, 19.52, 21.45, 24.24, 28.76, 30.87, 43.94, 63.27, 122.05, 123.87, 126.94, 130.24, 130.80, 133.56, 134.19, 174.04; IR (ν, cm−1): 3375, 3095, 1643; MS (z/e): 412[M]+, 218, 154, 69, 57; Anal. Calcd for C16H20ClF3N2O3S: C, 46.55; H, 4.88; N, 6.79; found: C, 46.27; H, 5.01; N, 6.58.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(n-butyrylamino) cyclohexylsulfonamide (IV-20)
(R2 = n-propyl) Colorless crystal; yield, 93%; mp 119–121 °C; 1H NMR (DMSO-d6) δ: 0.86 (t, J = 4.8 Hz, 3H, CH3), 1.32–2.14 (m, 12H, 6CH2), 3.35–3.40 (m, 1H, CH-N), 4.56 (d, J = 4.0 Hz, 1H, CH-SO2), 7.59–7.80 (m, 3H, Ph-H), 7.87 (d, J = 6.4 Hz, 1H, CO-NH), 9.29 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 13.98, 19.10, 19.49, 21.46, 24.26, 30.98, 37.56, 43.96, 63.26, 122.06, 123.87, 126.98, 130.35, 130.85, 133.59, 134.19, 173.11; IR (ν, cm−1): 3392, 3101, 1658; HRMS-ESI, m/z calcd for C17H23ClF3N2O3S [M + H]+427.1070; found, 427.1076.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(n-valerylamino) cyclohexylsulfonamide (IV-21)
(R2 = n-butyl) White crystal; yield, 92%; mp 175–177 °C; 1H NMR (DMSO-d6) δ: 0.84 (t, J = 6.9 Hz, 3H, CH3), 1.23–2.24 (m, 14H, 7CH2), 3.20 (td, J = 10.1, 3.4 Hz, 1H, CH-N), 4.06 (ddd, J = 18.7, 9.3, 4.0 Hz, 1H, CH-SO2), 7.57–7.80 (m, 3H, Ph-H), 7.93 (d, J = 8.5 Hz, 1H, CO-NH), 9.31 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 14.26, 22.34, 23.81, 25.31, 26.31, 28.64, 31.41, 35.89, 47.42, 65.02, 122.02, 123.83, 126.92, 130.97, 131.09, 133.55, 134.25, 172.43; IR (ν, cm−1): 3360, 1647; MS (z/e): 440[M]+, 246, 195, 57; Anal. Calcd for C18H24ClF3N2O3S: C, 49.03; H, 5.49; N, 6.35; found: C, 49.25; H, 5.30; N, 6.28.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(n-hexanoylamino) cyclohexylsulfonamide (IV-22)
(R2 = n-pentyl) White crystal; yield, 87%; mp 119–121 °C; 1H NMR (DMSO-d6) δ: 0.86 (t, J = 7.3 Hz, 3H, CH3), 1.21–2.19 (m, 16H, 8CH2), 3.40 (d, J = 11.9 Hz, 1H, CH-N), 4.57 (d, J = 5.4 Hz, 1H, CH-SO2), 7.60–7.86 (m, 3H, Ph-H), 7.79 (s, 1H, CO-NH), 9.26 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 14.28, 22.34, 23.91, 25.31, 26.31, 28.64, 31.41, 32.30, 35.88, 47.41, 64.99, 109.90, 122.02, 123.84, 126.99, 131.01, 133.57, 134.24, 172.41; IR (ν, cm−1): 3376, 3029, 1646; HRMS-ESI, m/z calcd for C19H27ClF3N2O3S [M + H]+455.1383; found, 455.1389.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(n-heptanoylamino) cyclohexylsulfonamide (IV-23)
(R2 = n-hexyl) White crystal; yield, 95%; mp 105–106 °C; 1H NMR (DMSO-d6) δ: 0.84 (t, J = 7.4 Hz, 3H, CH3), 1.20–2.24 (m, 18H, 9CH2), 3.20 (td, J = 10.1, 3.4 Hz, 1H, CH-N), 4.06 (ddd, J = 18.7, 9.4, 4.0 Hz, 1H, CH-SO2), 7.57–7.80 (m, 3H, Ph-H), 7.93 (d, J = 8.5 Hz, 1H, CO-NH), 9.32 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 14.11, 22.14, 23.82, 23.93, 26.34, 27.51, 32.33, 35.61, 40.44, 47.42, 65.03, 122.03, 123.84, 126.98, 130.99, 131.09, 133.57, 134.25, 172.41; IR (ν, cm−1): 3325, 3110, 1643; MS (z/e): 468[M]+, 274, 195, 113; Anal. Calcd for C20H28ClF3N2O3S: C, 51.22; H, 6.02; N, 5.97; found: C, 51.42; H, 5.90; N, 6.15.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-chloroacetylamino) cyclohexylsulfonamide (IV-24)
(R2 = ClCH2) Gray solid; yield, 94%; mp 121–124 °C; 1H NMR (DMSO-d6) δ: 1.28–2.03 (m, 8H, 4CH2), 3.42 (dt, J = 12.1, 3.4 Hz, 1H, CH-N), 4.10 (dd, J = 53.0, 12.9 Hz, 2H, CH2), 4.52 (dd, J = 8.3, 3.3 Hz, 1H, CH-SO2), 7.59–7.80 (m, 3H, Ph-H), 8.19 (d, J = 8.8 Hz, 1H, CO-NH), 9.30 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.63, 21.70, 24.05, 30.49, 43.00, 44.89, 62.73, 122.02, 123.84, 127.04, 131.06, 131.33, 133.64, 133.92, 166.10; IR (ν, cm−1): 3383, 3180, 1678; HRMS-ESI, m/z calcd for C15H18Cl2F3N2O3S [M + H]+433.0367; found, 433.0371.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,2-dichloroacetylamino) cyclohexylsulfonamide (IV-25)
(R2 = Cl2CH) Colorless crystal; yield, 82%; mp 179–180 °C; 1H NMR (DMSO-d6) δ: 1.44–1.95 (m, 8H, 4 CH2), 3.44 (dt, J = 3.3, 12.2 Hz, 1H, CH-N), 4.49 (dd, J = 8.2, 3.5 Hz, 1H, CH-SO2),6.54 (s, 1H, CH-Cl2), 7.50–7.81 (m, 3H, Ph-H), 8.47 (d, J = 8.6 Hz, 1H, CO-NH), 9.41 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 14.47, 19.61, 23.98, 30.25, 45.35, 62.37, 66.82, 122.01, 123.83, 127.12, 131.58, 133.65, 133.80, 162.80, 163.24; IR (ν, cm−1): 3367, 3273, 1672; MS (z/e): 468(M+), 274, 210, 130, 81, 64; Anal. Calcd for C15H16ClF3N2O3S: C, 38.52; H, 3.45; N, 5.99; found: C, 38.66; H, 3.59; N, 5.73.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,2,2-chloroacetylamino) cyclohexylsulfonamide (IV-26)
(R2 = Cl3C) Colorless crystal; yield, 88%; mp 151–154 °C; 1H NMR (DMSO-d6) δ: 1.28–2.37 (m, 8H, 4CH2), 3.51–3.58 (m, 1H, CH-N), 3.95–4.04 (m, 1H, CH-SO2), 7.55–7.81 (m, 3H, Ph-H), 8.85 (d, J = 8.4 Hz, 1H, CO-NH), 9.46 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 24.27, 27.11, 28.68, 31.88, 50.33, 63.74, 93.26, 121.99, 123.81, 127.05, 131.72, 132.05, 133.64, 134.09, 160.27; IR (ν, cm−1): 3421, 3311, 1708; MS (z/e): 306, 242, 161; Anal. Calcd for C15H15Cl4F3N2O3S: C, 35.88; H, 3.01; N, 5.58; found: C, 36.01; H, 3.22; N, 5.47.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-bromoacetylamino) cyclohexylsulfonamide (IV-27)
(R2 = BrCH2) Colorless crystal; yield, 90%; mp 131–132 °C; 1H NMR (DMSO-d6) δ: 1.33–2.08 (m, 8H, 4CH2), 3.41 (m, 1H, CH-N), 3.88–4.16 (m, 2H, CH2-Br), 4.52 (s, 1H, CH-SO2), 7.59–7.81 (m, 3H, Ph-H), 8.33 (dd, J = 9.0, 61.8 Hz, 1H, CO-NH), 9.32 (d, J = 18.6 Hz, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.63, 21.70, 24.06, 30.50, 43.00, 44.89, 62.74, 122.02, 123.84, 127.06, 131.05, 131.32, 133.62, 133.92, 166.11; IR (ν, cm−1): 3383, 3090, 1678; MS (z/e): 477[M + H]+, 80; Anal. Calcd for C15H17BrClF3N2O3S: C, 37.71; H, 3.59; N, 5.86; found: C, 37.94; H, 3.47; N, 5.62.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-methoxyacetylamino) cyclohexylsulfonamide (IV-28)
(R2 = CH3OCH2) White solid; yield, 79%; mp 125–127 °C; 1H NMR (DMSO-d6) δ: 1.38–2.10 (m, 8 H, 4CH2), 3.31 (s, 3H, OCH3), 3.47 (dt, J = 11.5, 3.3 Hz, 1H, CH-N), 3.79–3.86 (m, 2H, OCH2), 4.51 (dd, J = 7.4, 3.5 Hz, 1H, CH-SO2), 7.55 (d, J = 8.0 Hz, 1H, CO-NH), 7.60–7.81 (m, 3H, Ph-H), 9.42 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 19.80, 21.98, 23.87, 30.17, 44.51, 58.98, 62.62, 71.52, 122.02, 123.83, 127.10, 127.15, 131.05, 131.36, 133.66, 169.47; IR (ν, cm−1): 3394, 3099, 1647; MS (z/e): 428[M]+, 234, 195, 170; Anal. Calcd for C16H20ClF3N2O4S: C, 44.81; H, 4.70; N, 6.53; found: C, 45.03; H, 4.52; N, 6.77.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2-ethoxyacetylamino) cyclohexylsulfonamide (IV-29)
(R2 = C2H5OCH2) Colorless crystal; yield, 32%; mp 125–127 °C; 1H NMR (DMSO-d6) δ: 1.14 (t, J = 7.0 Hz, 3H, CH3) 1.30–2.07 (m, 8H, 4CH2), 3.46–3.51 (m, 3H, OCH2-CO, CH-N), 3.86 (q, J = 15.3 Hz, 2H, OCH2), 4.47 (dd, J = 7.3, 3.6 Hz, 1H, CH-SO2), 7.53 (d, J = 7.8 Hz, 1H, CO-NH), 7.60–7.81 (m, 3H, Ph-H), 9.48 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 15.32, 19.88, 22.11, 23.82, 29.99, 44.66, 62.51, 66.62, 69.67, 109.90, 115.73, 122.01, 123.82, 127.07, 131.18, 133.66, 169.71; IR (ν, cm−1): 3412, 3070, 1681; HRMS-ESI, m/z calcd for C17H23ClF3N2O4S [M + H]+443.1019; found, 443.1024.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,2,2-trichloroacetylamino) cyclopentylsulfonamide (IV-30)
(n = 0, R1 = H) Colorless crystal; yield, 98%; mp 86–88 °C; 1H NMR (DMSO-d6) δ: 1.63–2.28 (m, 6H, 3CH2), 3.94 (q, J = 7.5 Hz, 1H, CH-N), 4.40 (p, J = 7.1 Hz, 1H, CH-SO2), 7.59–7.82 (m, 3H, Ph-H), 8.65 (d, J = 6.9 Hz, 1H, CO-NH), 9.72 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 21.44, 26.54, 30.73, 53.99, 63.48, 92.81, 121.93, 123.75, 127.25, 131.84, 132.01, 133.56, 133.73, 161.25; IR (ν, cm−1): 3363, 3190, 1726; MS (z/e): 488(M)+, 294, 230, 164, 67; Anal. Calcd for C14H13Cl4F3N2O3S: C, 34.45; H, 2.68; N, 5.74; found: C, 34.56; H, 2.87; N, 5.49.
N-(2-trifluoromethyl-4-chlorophenyl)-2-(2,2,2-trichloroacetylamino) cycloheptylsulfonamide (IV-31)
(n = 2, R1 = H) Colorless crystal; yield, 97%; mp 114–115 °C; 1H NMR (DMSO-d6) δ: 1.17–2.17 (m, 10H, 5CH2), 3.57–3.59 (m, 1H, CH-N), 4.57 (s, 1H, CH-SO2), 7.60–7.78 (m, 3H, Ph-H), 8.34 (d, J = 6.5 Hz, 1H, CO-NH), 9.58 (s, 1H, SO2-NH); 13H NMR (DMSO-d6) δ: 8.96, 22.60, 23.79, 25.95, 27.86, 31.06, 46.04, 50.26, 65.05, 93.06, 122.09, 123.90, 125.72, 127.12, 130.96, 133.59, 160.72; IR (ν, cm−1): 3381, 3242, 1710; MS (z/e): 530(M)+, 320, 256, 162, 95, 67; Anal. Calcd for C16H17Cl4F3N2O3S: C, 37.23; H, 3.32; N, 5.43; found: C, 37.55; H, 3.21; N, 5.62.
N-(2-trifluoromethyl-4-chlorophenyl)-3-methyl-2-(2,2,2-trichloroacetylamino) cyclohexylsulfonamide (IV-32)
(n = 1, R1 = 3-Me) Colorless crystal; yield, 96%; mp 137~139 °C; 1H NMR (DMSO-d6) δ: 0.93–2.35 (m, 10H, 5CH2), 3.75 (dd, J = 9.4, 4.6 Hz, 1H, CH-N), 3.97 (td, J = 8.6, 4.2 Hz, 1H, CH-SO2), 7.54–7.81 (m, 3H, Ph-H), 8.55 (d, J = 8.2 Hz, 1H, CO-NH), 9.66 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 18.24, 19.65, 24.93, 31.96, 50.48, 55.50, 60.87, 92.94, 121.97, 123.79, 127.19, 130.90, 131.59, 133.66, 133.73, 161.15; IR (ν, cm−1): 3398, 3250, 1707; MS (z/e): 516(M)+, 322, 258, 164, 95, 67; Anal. Calcd for C16H17Cl4F3N2O3S: C, 37.23; H, 3.32; N, 5.43; found: C, 37.42; H, 3.12; N, 5.60.
N-(2-trifluoromethyl-4-chlorophenyl)-4-methyl-2-(2,2,2-trichloroacetylamino) cyclohexylsulfonamide (IV-33)
(n = 1, R1 = 4-Me) Colorless crystal; yield, 93%; mp 123–124 °C; 1H NMR (DMSO-d6) δ: 0.93 (d, J = 6.0 Hz, 3H, CH3), 1.40–2.40 (m, 7H, C4H7), 3.82 (s, 1H, CH-N), 4.10–4.20 (m, 1H, CH-SO2), 7.53–7.82 (m, 3H, Ph-H), 8.81 (d, J = 7.2 Hz, 1H, CO-NH), 9.85 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 22.18, 25.42, 28.17, 31.15, 34.89, 51.80, 59.87, 92.66, 121.92, 123.74, 127.29, 131.20, 131.89, 133.53, 133.80, 160.81; IR (ν, cm−1): 3360, 3226, 1693; MS (z/e): 516(M)+, 259, 224, 202, 112, 81; Anal. Calcd for C16H17Cl4F3N2O3S: C, 37.23; H, 3.32; N, 5.43; found: C,36.99; H, 3.21; N, 5.60.
N-(2-trifluoromethyl-4-chlorophenyl)-5-methyl-2-(2,2,2-trichloroacetylamino) cyclohexylsulfonamide (IV-34)
(n = 1, R1 = 5-Me) White crystal; yield, 98%; mp 144–145 °C; 1H NMR (DMSO-d6) δ: 0.95–2.33 (m, 10H, 5CH2), 3.64 (td, J = 11.6, 2.9 Hz, 1H, CH-N), 3.90–4.03 (m, 1H, CH-SO2), 7.55–7.81 (m, 3H, Ph-H), 8.83 (d, J = 8.4 Hz, 1H, CO-NH), 9.45 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 22.11, 31.03, 31.71, 32.72, 35.13, 50.22, 63.45, 93.26, 122.00, 123.81, 127.07, 131.67, 132.05, 133.61, 134.11, 160.32; IR (ν, cm−1): 3431, 3336, 1687; MS (z/e): 516(M)+, 322, 258, 95, 67, 55; Anal. Calcd for C16H17Cl4F3N2O3S: C, 37.23; H, 3.32; N, 5.43; found: C, 37.08; H, 3.53; N, 5.27.
N-(2-trifluoromethyl-4-chlorophenyl)-5-ethyl-2-(2,2,2-trichloroacetylamino) cyclohexylsulfonamide (IV-35)
(n = 1, R1 = 5-Et) White crystal; yield, 93%; mp 122–125 °C; 1H NMR (DMSO-d6) δ: 0.84(t, J = 7.4 Hz, 3H, CH3), 1.15–2.35 (m, 9H, C5H9), 3.83 (d, J = 4.4 Hz, 1H, CH-N), 4.17 (dd, J = 10.4, 6.7 Hz, 1H, CH-SO2), 7.55–7.84 (m, 3H, Ph-H), 8.67 (d, J = 6.6 Hz, 1H, CO-NH), 9.81 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 11.75, 26.04, 27.69, 28.54, 30.29, 32.20, 51.19, 60.10, 92.73, 121.94, 123.76, 125.58, 127.26, 131.13, 131.84, 133.76, 160.91; IR (ν, cm−1): 3394, 3261, 1705; MS (z/e): 530(M)+, 320, 256, 162, 95, 67; Anal. Calcd for C17H19Cl4F3N2O3S: C, 38.51; H, 3.61; N, 5.28; found: C, 38.69; H, 3.50; N, 5.04.
N-(2-trifluoromethyl-4-chlorophenyl)-5-tertiarybutyl-2-(2,2,2-trichloroacetylamino) cyclohexylsulfonamide (IV-36)
(n = 1, R1 = 5-t-Bu) Colorless crystal; yield, 90%; mp 124–126 °C; 1H NMR (DMSO-d6) δ: 0.87–2.21 (m, 16H, C8H16), 3.57 (dt, J = 12.9, 3.3 Hz, 1H, CH-N), 4.38 (dd, J = 5.9, 3.1 Hz, 1H, CH-SO2), 7.61–7.83 (m, 3H, Ph-H), 7.97 (d, J = 6.0 Hz, 1H, CO-NH), 9.85 (s, 1H, SO2-NH); 13C NMR (DMSO-d6) δ: 20.55, 22.95, 27.55, 32.69, 46.21, 47.02, 62.47, 92.92, 121.95, 123.77, 127.12, 132.13, 132.36, 133.42, 133.72, 160.95; IR (ν, cm−1): 3400, 3284, 1708; MS (z/e): 530(M)+, 109, 67; Anal. Calcd for C19H23Cl4F3N2O3S: C, 40.88; H, 4.15; N, 5.02; found: C, 41.02; H, 3.98; N, 5.21.
Bioassays
In vitro fungicidal activity
In vitro effects of compounds against B. cinerea were evaluated by mycelium growth rate method30,31,32. The tested compounds were dissolved in DMSO (dimethyl sulfoxide) and mixed with sterile molten potato dextrose agar (PDA) to a final concentration of 50 mg/L. EC50 values were estimated using logit analysis. The concentration gradients were 50, 12.5, 3.13, 0.78 mg/L on PDA and a commercial fungicide procymidone was used as the positive control. EXCEL 2010 was used to analyze bioassay data. The variance analysis was carried out by using SPSS 20.0 software for the inhibition rate, EC50 and control efficiency.
The relative inhibition rate of the synthetic compounds compared to blank control was calculated via the following equation (1):
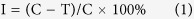 |
In which, I stands for the rate of inhibition (%), C is the diameter of mycelia in the blank control test (in mm), and T is the diameter of mycelia in the presence of tested compounds (in mm).
In vivo antifungal activity
In vivo effects were checked on leaves of cucumber (Cucumis sarivus L.) by mycelium inoculation method with pot cultural test in greenhouse37,38,39,40. The cucumber seedlings at 2–3 leaf stages were used to assay the fungicidal activity against B. cinerea. The compounds were confected to 2.5% EC (emulsifiable concentrate) formulation. The formulation was diluted to 500 mg/L with water and sprayed on the surface of the cucumber leaves. After air drying, the surface of the leaves was inoculated with 6 mm plugs of B. cinerea, which was maintained on potato dextrose agar (PDA). This procedure was repeated three times, and nine replicates were performed per treatment. The chesulfamide (L, Fig. 3) was used as the positive control.
The fungicidal activity was assessed when the untreated cucumber plant (blank control) fully developed symptoms. The area of inoculated leaves covered by disease symptoms was evaluated and compared to that of untreated ones to determine the average disease index. The relative control efficacy of compounds compared to the blank assay was calculated via the following equation (2):
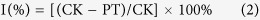 |
where I is relative control efficacy, CK is the average disease index during the blank assay and PT is the average disease index after treatment during testing.
Additional Information
How to cite this article: Liu, C.-H. et al. Synthesis, Fungicidal Activity, and Structure Activity Relationship of β-Acylaminocycloalkylsulfonamides against Botrytis cinerea. Sci. Rep. 7, 42096; doi: 10.1038/srep42096 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31101466 and 31570122), the National Key Project for Basic Research (973 Project, 2015CB150600), the Natural Science Foundation of Liaoning Province (2015020766), the Pearl River S & T Nova Program of Guangzhou (201506010029), the Opening Foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University (2015GDGP0101).
Footnotes
The authors declare no competing financial interests.
Author Contributions X.H.L. and Z.N.C. conceived and designed the experiments; X.H.L., C.H.L. and X.Y.C. performed the experiments; X.H.L., C.H.L., X.Y.C. and P.W.Q. analyzed the data; Z.Q.Q., X.Y.L. and M.S.J. contributed reagents, materials, and analysis tools; Z.N.C., X.H.L. and P.V.B. wrote the paper.
References
- Williamson B., Tudzynski B., Tudzynski P. & van Kan J. A. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8, 561–580 (2007). [DOI] [PubMed] [Google Scholar]
- Liu S. M., Che Z. P. & Chen G. Q. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Prot 84, 56–61 (2016). [Google Scholar]
- Tiedemann F. & Gmelin L. Einige neue bestandtheile der galle des ochsen. Annalen der Physik 85, 326–337 (1827). [Google Scholar]
- Demarcay H. Ueber die natur der Galle. Annalen der Pharmacie 27, 270–291 (1838). [Google Scholar]
- Takahashi K. et al. Taurine renders the cell resistant to ischemia-induced injury in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol. 41, 726–733 (2003). [DOI] [PubMed] [Google Scholar]
- Militante J. D. & Lombardini J. D. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids 23, 381–393 (2002). [DOI] [PubMed] [Google Scholar]
- Yokogoshi H. & Oda H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. Amino Acids 23, 433–439 (2002). [DOI] [PubMed] [Google Scholar]
- Matsushima Y. et al. Effects of taurine on serum cholesterol levels and development of atherosclerosis in spontaneously hyperlipidaemic mice. Clin Exp Pharmacol Physiol 30, 295–299 (2003). [DOI] [PubMed] [Google Scholar]
- Hilgier W., Anderzhanova E., Oja S. S., Saransaari P. & Albrecht J. Taurine reduces ammonia-and N-methyl-D-aspartate-induced accumulation of cyclic GMP and hydroxyl radicals in microdialysates of the rat striatum. Eur J Pharmacol 468, 21–25 (2003). [DOI] [PubMed] [Google Scholar]
- Kirchner A., Breustedt J., Rosche B., Heinemann U. F. & Schmieden V. Effects of taurine and glycine on epileptiform activity induced by removal of Mg2+ in combined rat entorhinal cortex–hippocampal slices. Epilepsia 44, 1145–1152 (2003). [DOI] [PubMed] [Google Scholar]
- Davison A. N. & Kaczmarek L. K. Taurine—a possible neurotransmitter? Nature 234, 107–108 (1971). [DOI] [PubMed] [Google Scholar]
- Saad S. Y. & Al-Rikabi A. C. Protection effects of taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy 48, 42–48 (2002). [DOI] [PubMed] [Google Scholar]
- Hwang D. F. & Wang L. C. Effect of taurine on toxicity of cadmium in rats. Toxicology 167, 173–180 (2001). [DOI] [PubMed] [Google Scholar]
- Pokhrel P. K. & Lau-Cam C. A. In vitro and in vivo effects of taurine and structurally related sulfur-containing compounds against phenylhydrazine-induced oxidative damage to erythrocytes. In Taurine 4 9780306468384, Della Corte L., Huxtable R. J., Sgaragli G. & Tipton K. F., Kluwer Academic/Plenum Publisher: New York, 483, 503–522 (2002). [DOI] [PubMed] [Google Scholar]
- Liebowitz S. M., Lombardini J. B. & Allen C. I. Sulfone analogues of taurine as modifiers of calcium uptake and protein phosphorylation in rat retina. Biochem Pharmacol 38, 399–406 (1989). [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S. & Camerino D. C. Effect of taurine depletion on excitation-contraction coupling and C1− conductance of rat skeletal muscle. Eur J Pharmacol 296, 215–222 (1996). [DOI] [PubMed] [Google Scholar]
- Francesconi K. A., Edmonds J. S., Stick R. V., Skelton B. W. & White A. H. Arsenic-containing ribosides from the brown alga Sargassum lacerifolium: X-ray molecular structure of 2-amino-3-[5′-deoxy-5′-(dimethylarsinoyl) ribosyloxy] propane-1-sulphonic acid. J Chem Soc Perkin Trans 1 11, 2707–2716 (1991). [Google Scholar]
- Kohayashi J. I. et al. Flavocristamides A and B, new DNA polymerase α inhibitors from a marine bacterium Flavobacterium sp. Tetrahedron 51, 10487–10490 (1995). [Google Scholar]
- Ogata T. et al. Chemical synthesis and properties of 5-taurinomethyluridine and 5-taurinomethyl-2-thiouridine. J Org Chem 74, 2585–2588 (2009). [DOI] [PubMed] [Google Scholar]
- Yang S., Froeyen M., Lescrinier E., Marlière P. & Herdewijn P. 3-Phosphono-L-alanine as pyrophosphate mimic for DNA synthesis using HIV-1 reverse transcriptase. Org Biomol Chem 9, 111–119 (2011). [DOI] [PubMed] [Google Scholar]
- Brouwer A. J., Merkx R., Dabrowska K., Rijkers D. T. & Liskamp R. M. Synthesis and applications of β-aminoethanesulfonyl azides. Synthesis, 455–460 (2006). [Google Scholar]
- Vertesaljai P. et al. Synthesis of taurine-containing peptides, sulfonopeptides, and N-and O-conjugates. J Org Chem 79, 2688–2693 (2014). [DOI] [PubMed] [Google Scholar]
- Pelz N. F. et al. Discovery of 2-indole-acylsulfonamide myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods. J Med Chem 59, 2054–2066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka A., Teranishi K., Matsushita Y. & Tamura N. Optically active antifungal azoles III. Chem Pharm Bull 42, 85–94 (1994). [DOI] [PubMed] [Google Scholar]
- Machetti F. et al. Synthesis of taurine analogues from alkenes. In Taurine 4, 9780306468384, Della Corte L., Huxtable R. J., Sgaragli G. & Tipton K. F., Kluwer Academic/Plenum Publisher: New York, 483, 399–401 (2002). [Google Scholar]
- Li X. H. et al. Synthesis and biological activities of 2-oxocycloalkylsulfonamides. Bioorg Med Chem 16, 4538–4544 (2008). [DOI] [PubMed] [Google Scholar]
- Liang X. M. et al. Preparation method and fungicide application of 1-oxotetralyl-2-sulfonamides. CN101503381B, September 5, 2012.
- Liang X. M., Zhang J. J., Kong H. C. & Wang D. Q. Preparation method and application of 5-alkoxy-2-oxo cyclohexyl sulfonamide compounds. CN104151209A, November 19, 2014.
- Liang X. M., Zhang J. J., Hu S. & Wang D. Q. Preparation method and application of cycloalkyl sulfonamide compounds. CN104211621A, December 17, 2014.
- Li X. H., et al. Synthesis, fungicidal activity, and structure-activity relationship of 2-oxo- and 2-hydroxycycloalkylsulfonamides. J Agric Food Chem 58, 11384–11389 (2010). [DOI] [PubMed] [Google Scholar]
- Li X. H. et al. Synthesis of 2-amino-6-oxocyclohexenylsulfonamides and their activity against Botrytis cinerea. Pest Manag Sci 67, 986–992 (2011). [DOI] [PubMed] [Google Scholar]
- Li X. H. et al. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int J Mol Sci 14, 22544–22557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. Y. et al. Multiple resistance of Botrytis cinerea from vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China. Plant Disease 94, 551–556 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang C. Y., Huang J. P. & Wang R. H. Resistance detection of Botrytis cinerea isolate to procymidone. J Anhui Agric Sci 32, 310–311 (2004). [Google Scholar]
- Qi Z. Q., Sun Q. B., Li X. H., Gu Z. M., Li X. W. & Ji M. S. Inhibitory effect of N-(2, 4, 5)-trichlorophenyl)-oxocyclohexylsulfonamide against Botrytis cinerea. Chin J Pestic Sci 5, 523–528 (2014). [Google Scholar]
- Miriyala B., Bhattacharyya S. & Williamson J. S. Chemoselective reductive alkylation of ammonia with carbonyl compounds: synthesis of primary and symmetrical secondary amines. Tetrahedron 60, 1463–1471 (2004). [Google Scholar]
- Li X. H., Pan Q., Cui Z. N., Ji M. S. & Qi Z. Q. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo-and 2-hydroxycycloalkylsulfonamides. Lett Drug Des Discov 10, 353–359 (2013). [Google Scholar]
- Cui Z. N. et al. Synthesis and fungicidal activity of novel 2,5-disubstituted-1,3,4- thiadiazole derivatives containing 5-phenyl-2-furan. Sci Rep 6, 20204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. N., Ito J., Dohi H., Amemiya Y. & Nishida Y. Molecular design and synthesis of novel salicyl glycoconjugates as elicitors against plant diseases, PLOS One 9, e108338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. N. et al. Synthesis and fungicidal activity of novel 2, 5-disubstituted-1, 3, 4-oxadiazole derivatives. J Agric Food Chem 60, 11649−11656 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.