Abstract
We aimed to investigate pressure-dependent maternal uterine artery responses and vessel remodeling following gestational binge alcohol exposure. Two groups of pregnant rats were used: the alcohol group (28.5% wt/v, 6.0 g/kg, once-daily orogastric gavage in a binge paradigm between gestational day (GD) 5–19) and pair-fed controls (isocalorically matched). On GD20, excised, pressurized primary uterine arteries were studied following equilibration (60 mm Hg) using dual chamber arteriograph. The uterine artery diameter stabilized at 20 mm Hg, showed passive distension at 40 mm Hg, and redeveloped tone at 60 mm Hg. An alcohol effect (P=0.0025) was observed on the percent constriction of vessel diameter with greater pressure-dependent myogenic constriction. Similar alcohol effect was noted with lumen diameter response (P=0.0020). The percent change in media:lumen ratio was higher in the alcohol group (P<0.0001). Thus, gestational alcohol affects pressure-induced uterine artery reactivity, inward-hypotrophic remodeling, and adaptations critical for nutrient delivery to the fetus.
Keywords: FASD, pregnancy, uterine, alcohol
Introduction
Fetal Alcohol Spectrum Disorders (FASD) refers to the range of physical, mental, functional, and/or behavioral abnormalities following developmental alcohol exposure (Flak et al., 2014; Ramadoss, Lunde, Chen, West, & Cudd, 2007; Riley & McGee, 2005; Sokol, Delaney-Black, & Nordstrom, 2003). The United States Surgeon General and the American Academy of Pediatrics have issued recommendations to abstain from any alcohol use during pregnancy (General, 1981; U.S. Surgeon General & Vice Admiral Richard H. Carmona, 2005; Williams, Smith, & Committee On Substance, 2015). Despite these repeated advisories, the CDC recently estimated alcohol use in 10.1% of pregnant women aged 18–44 and binge drinking in 3.1% of pregnant women within the years 2011–2013 (Tan, Denny, Cheal, Sniezek, & Kanny, 2015). Among school-age children in the United States and some Western European countries the prevalence of FASD is estimated to be 2–5% (May et al., 2009).
In the past four decades, marked progress has been made to understand the effects of maternal alcohol consumption during pregnancy. Thus far, an extensive number of studies have focused on understanding alcohol’s teratogenic role in brain & neurodevelopment (Flak et al., 2014; Riley & McGee, 2005; Riley, McGee, & Sowell, 2004), while little is known about its effects on the mother, specifically the maternal uterine artery which delivers all the nutrients and oxygen to the developing fetus and its supporting tissues. We herein focus on the effects of binge alcohol exposure on remodeling of the maternal uterine artery. During normal pregnancy, the maternal uterine artery undergoes outward hypertrophic remodeling, characterized by an increased vessel cross sectional area and a decreased ratio of medial thickness to lumen diameter or media:lumen ratio. Systemic vascular adaptations to pregnancy include increases in vascular compliance and a decrease in mean arterial blood pressure (Barron, Mandala, & Osol, 2010; M. Cipolla & Osol, 1994; Osol & Mandala, 2009). These changes to the maternal vasculature are essential for a healthy pregnancy outcome‥
We and others have reported that alcohol consumption during pregnancy results in altered uterine endothelial adaptations (Ramadoss, Jobe, & Magness, 2011; Ramadoss & Magness, 2011, 2012a, 2012c), agonist-dependent vessel relaxation (Subramanian et al., 2014), spiral artery remodeling (Gundogan et al., 2008), and blood flow (Falconer, 1990). However, there exists no study on alcohol-related myogenic response, a critical gestational uterine vascular adaptation. In response to changes in the transmural pressure, the uterine artery will react by dilating or constricting to alter the resistance in order to maintain the perfusion rate, an intrinsic adaptation called myogenic reactivity (Barron et al., 2010; Bevan & Laher, 1991; Johansson, 1989; Telezhkin et al., 2008).The aim of this study was to investigate pressure-dependent maternal uterine artery responses and vessel remodeling parameters following chronic binge alcohol exposure during pregnancy.
Materials and Methods
Treatment groups and alcohol dosing paradigm
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) with approval by the Animal Care and Use Committee at the Texas A&M University. Rats were housed in a temperature-controlled room (23°C) with a 12:12-hour light–dark cycle. Timed pregnant Sprague–Dawley rats were purchased from Charles River (Wilmington, MA). Two groups were utilized including a nutritional pair-fed control and a binge alcohol group. The number of animals in the pair-fed control and alcohol groups were n=11 and n=12 respectively. Uterine arteries that were either mechanically damaged during tissue preparation for pressure myography or did not show any functional response by constricting at 60 mm Hg were not studied, and the number of these vessels was not different between treatment groups (Novak, Ramirez, Gandley, Sherwood, & Conrad, 2002; Veerareddy, Campbell, Williams, Baker, & Davidge, 2004). Maternal uterine arteries from a total of n=8 rats in each group exhibited pressure-dependent vascular response by constricting at 60 mm Hg and were all analyzed. Dams in the alcohol group received a binge-like- dose of 6 g/kg body-weight/day of 28.5% w/v, ethanol via oral gavage from GD 5–19 (Thomas, Idrus, Monk, & Dominguez, 2010; Thomas, Sather, & Whinery, 2008). The regimen of exposure utilized in this study is based on reported alcohol consumption patterns in pregnant women and FASD animal models (Caetano, Ramisetty-Mikler, Floyd, & McGrath, 2006; Church & Gerkin, 1988; Cudd, Chen, & West, 2002; May et al., 2013; Thomas et al., 2010; Thomas et al., 2008). Daily feed intake of the alcohol rat was measured. The pair-fed control dams were matched with alcohol-fed dams of similar weight, and food intake was measured daily and yoked (Thomas, Abou, & Dominguez, 2009). Pair-fed control animals were also gavaged daily with isocaloric maltose-dextrin to control for the calories derived from alcohol. There was no significant maternal weight difference between the groups on GD 20 (Pair-fed controls, 310 ± 8 g; Alcohol, 305 ± 7 g). Animals were sacrificed on GD 20, one day after the last alcohol exposure.
Tissue preparation
Following sacrifice, the whole uterus was transferred to a large 200 mm petri dish filled with solidified Sylgard containing cold HEPES – Bicarbonate Solution pH 7.4 (NaCl 130 mM; KCl 4 mM; MgSO4.7H2O 2.5 mM; NaHCO3 4.05 mM; CaCl2, 2.4 mM; HEPES 10 mM; KH2PO4 1.18 mM; Glucose 6 mM; EDTA 0.024 mM), where it was pinned to facilitate the cutting of the uterine artery. Primary uterine artery of approximately 3–5 mm was cut between bifurcations and washed in HEPES-Bicarbonate Solution, to remove excess fat and connective tissues. A dual-chamber arteriograph was used for our experiments, which permits vessels from a control and an alcohol-fed dam to be studied consecutively under identical experimental conditions
2.3 Pressure myography
A dual-chamber pressure myograph system (Living Systems Instruments, VT) was used for the experiments. The chamber consisted of an inflow and an outflow port to maintain the bath level such that the vessel remained submerged under the superfusate of warmed HEPES buffer. There were two glass cannulas on opposite sides of the chamber. The vessel was mounted on these cannulas positioned above a cover glass from where it was visualized utilizing an inverted microscope (Accu-Scope) mounted with a CCIR camera (IonOptix Corporations, MA). One of the cannula was connected to a pressure transducer, which converted fluid pressure into an electrical signal and sent this data to the pressure servo controller. Based on the output value, the pressure servo controller in-turn sent signal to the pressure pump to maintain the set pressure. The pressure was observed with the pressure monitor and was recorded with IonWizard software (IonOptix Corporations, MA). Simultaneously, the camera sent the image to the video dimension analyzer software (IonWizard, IonOptix Corporation, MA) which calculated various parameters such as vessel diameter, lumen diameter, media:lumen ratio, etc.
The chambers, cannulas, tubing, and stopcocks were primed with HEPES buffer prior to mounting the vessel, to ensure no air passed into the vessel. Each vessel was transferred to one of the two chambers of the dual-chamber pressure myograph system. Using a dissection microscope, the endothelium-intact vessel was then mounted on the glass cannulas that were connected to the pressure transducer and tied using two nylon ligatures. Excess blood was removed from inside the vessel by slowly injecting buffer through the stop valve using a syringe attached to a 45μm syringe filter. The opposite end was tied off using a nylon ligature, which was in-turn ligated to the opposite glass cannula. The chamber was placed on the inverted microscope to complete the setup described above (Figure 1). The pressure transducer tubing was filled with warm 37°C HEPES buffer, and were connected via stopcocks to the cannulas, creating a closed-pressure system.
Figure 1. Cannulated and pressurized uterine artery.
Representative picture of the maternal uterine artery (gestational day 20) mounted in a pressure arteriograph.
Experimental protocol and data analysis
Transmural vessel pressure was gradually increased and set to 60 mm Hg using the pressure servo controller. The vessels were allowed to equilibrate in the 37°C superfusate buffer for 60 min, or until the vessels started showing myogenic tone (Withers, Taggart, Baker, & Austin, 2009). The pressure was then lowered to 20 mm Hg where constriction was inhibited (Barron et al., 2010) and baseline measurements of vessel diameter, lumen diameter, and media:lumen ratio were recorded. The diameter was considered stable when the constrictions stabilized or there were no detectable changes in measured variables (3min – 5min). Subsequently, the pressure was increased by 20 mm Hg, starting 40 mm Hg until the pressure reached 120 mm Hg. After the measurements were complete, the data were transferred to a computer for analysis. The maximal contracted diameters at the stabilized state were determined for each pressure. The percent change from the baseline was analyzed by a two-way ANOVA with the treatment group as the between factor and the pressure as the within factor. Further pairwise comparisons were performed when appropriate using Fisher’s protected LSD. Level of significance was established a priori at P < 0.05.
Results
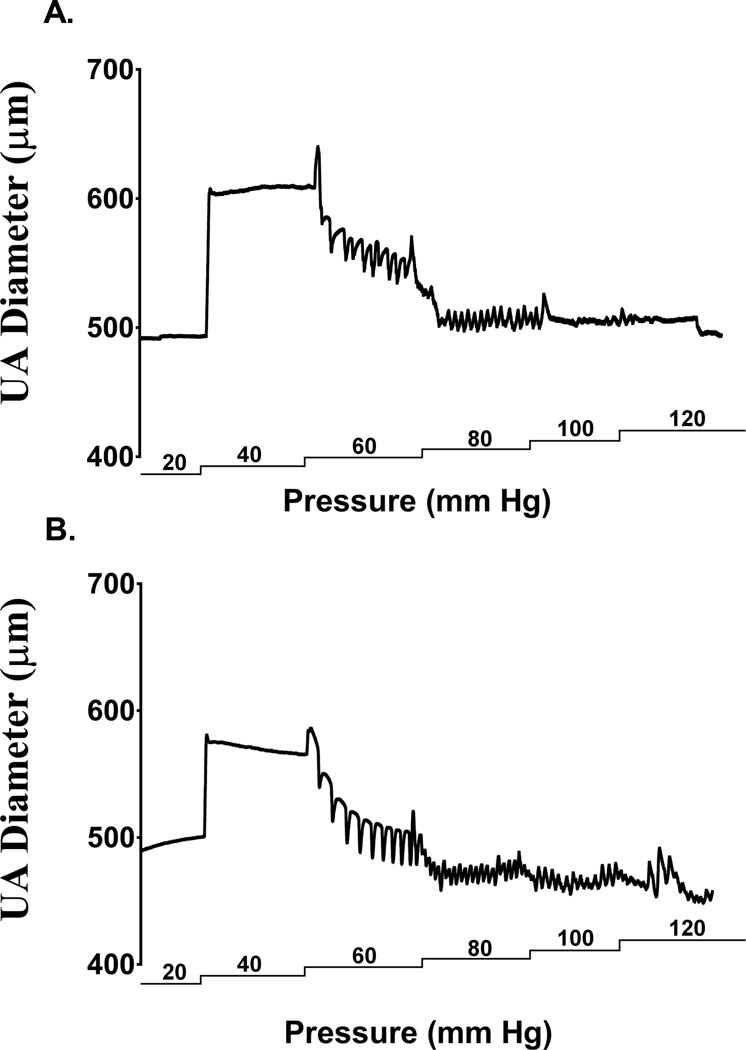
The dual-chamber arteriograph system described above is graphically represented (Figure 1). To illustrate the pressure-dependent response of a uterine artery, an experimental tracing from a control and an alcohol-fed pregnant dam is depicted in Figure 2. The maternal uterine artery diameter stabilized at 20 mm Hg, showed passive distension with increase in pressure to 40 mm Hg, and redeveloped tone at 60 mm Hg and was maintained until 120 mm Hg.
Figure 2. Uterine artery (UA) pressure arteriograph traces from (a) pair-fed control and (b) alcohol dams.
The maternal uterine artery diameter stabilized at 20 mm Hg, showed passive distension with increase in pressure to 40 mm Hg, and maintained tone between 60 mm Hg and 120 mm Hg.
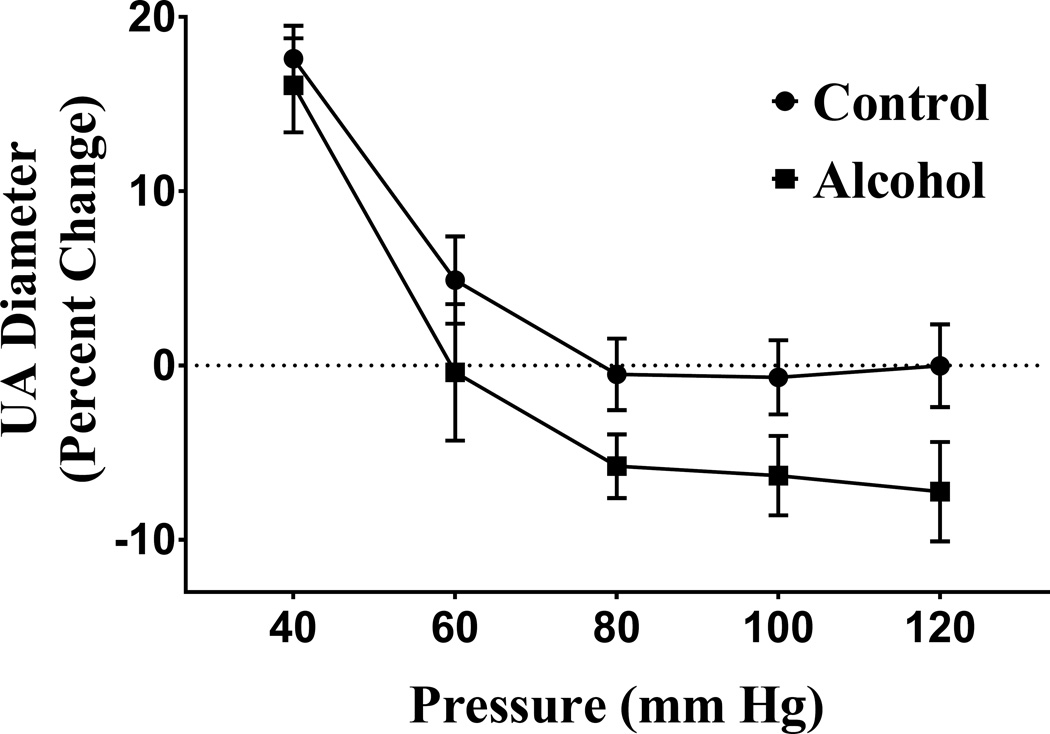
In both control and alcohol treatment groups, maternal uterine artery exhibited pressure-dependent myogenic constriction as pressure was increased from 40 mm Hg (Figure 3). The percent change in the uterine artery vessel diameter from baseline showed a significant main effect of pressure in both the control and alcohol groups (pressure effect, P < 0.000l). There was also a main effect of alcohol on the percent constriction of the uterine artery vessel diameter (alcohol effect, P = 0.0025); the alcohol group exhibited significantly greater pressure-dependent constriction compared to the controls.
Figure 3. Effect of alcohol on percent change of uterine artery (UA) diameter with pressure.
Percent change (from baseline) in uterine artery vessel diameter was significantly greater in the alcohol group compared to the control group and the alcohol group exhibited greater pressure-dependent constriction (pressure effect, P < 0.0001; alcohol effect, P = 0.0025).
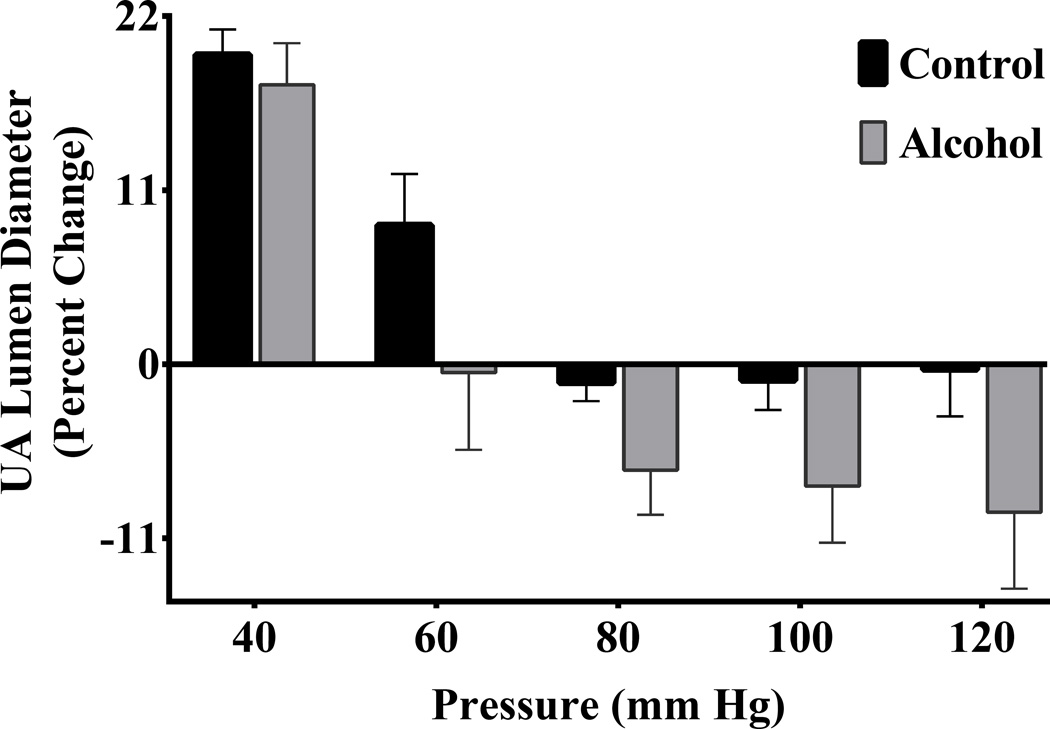
The uterine artery lumen diameter displayed a significant main effect of pressure in both control and alcohol groups (pressure effect, P < 0.0001; Figure 4). Similar to the uterine artery vessel diameter, percent change in uterine artery lumen diameter was significantly greater in the alcohol group; the alcohol group exhibited greater pressure-dependent constriction of lumen diameter compared to the control group (alcohol effect, P = 0.0020).
Figure 4. Effect of alcohol on percent change of uterine artery (UA) lumen diameter with pressure.
Percent change (from baseline) in uterine artery lumen diameter was significantly greater in the alcohol group; alcohol group exhibited greater pressure-dependent constriction of lumen diameter compared to the control group (pressure effect, P < 0.0001; alcohol effect, P = 0.0020).
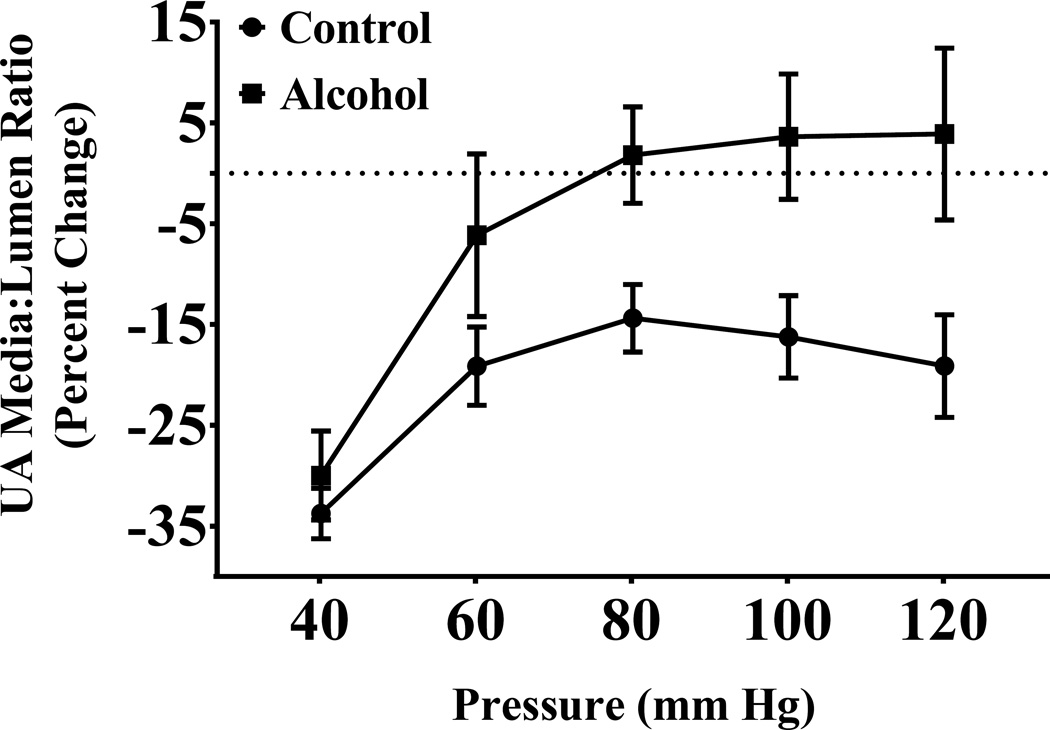
The percent change in the media:lumen ratio, an indicator of vessel remodeling, was significantly higher in the alcohol group compared to the controls (alcohol effect, P < 0.0001; Figure 5). Both the control and the alcohol groups displayed a significant main effect of pressure (pressure effect, P < 0.0001).
Figure 5. Effect of alcohol on percent change of uterine artery (UA) media:lumen ratio.
Percent change in uterine artery media:lumen ratio was significantly higher in the alcohol group compared to the control group (pressure effect, P < 0.0001; alcohol effect, P < 0.0001).
Discussion
The purpose of this study was to investigate the functional response of the maternal uterine artery to changes in intra-luminal pressure following chronic binge alcohol exposure during pregnancy. Three salient findings can be gleaned from the current study: First, gestational alcohol exposure results in greater pressure-dependent uterine artery constriction. Second, uterine artery vessel/lumen diameters and the medial:lumen ratio together indicate gestational alcohol-induced inward hypotrophic remodeling. Third, our overall findings suggest that in vivo binge-alcohol exposure leads to altered gestational uterine arterial adaptations.
In this study, both the excised uterine arties of control and alcohol-fed rats exhibited decreases in the uterine artery diameter as the intraluminal pressure was increased from 40 mm Hg. However, compared to the controls, gestational alcohol exposure resulted in greater pressure-dependent uterine artery constriction, and the effect was most pronounced at physiologic pressures of 80 mm Hg – 120 mm Hg. Interestingly, we observed that the uterine arteries of the control group maintained their diameter throughout this range of pressures, whereas the alcohol group exhibited lower vessel diameter at the same pressures. We can deduce using Poiseuille's equation that resistance is inversely proportional to the radius to the fourth power, and thus changes in diameter observed in our study can lead to large significant decreases in uterine blood flow. These data are consistent with previous reports demonstrating ~40% decrease in uterine blood flow (UBF) following chronic binge alcohol exposure in sheep (Sawant, Ramadoss, Hankins, Wu, & Washburn, 2014). Chronic alcohol-induced UBF decrease of about ~40% would lead to a reduction in the uterine artery radius to normalize the shear, as noted in our study. Previous studies demonstrate that uterine artery myogenic constriction is decreased in pregnancy compared to non-pregnant mice and sheep (Veerareddy, Cooke, Baker, & Davidge, 2002; Xiao, Buchholz, & Zhang, 2006), whereas others have shown increased myogenic reactivity in myoendometrial arteries of rabbits and radial uterine arteries of rats (M. J. Cipolla, Binder, & Osol, 1997; Osol & Cipolla, 1993). Although the current manuscript is the first to report alcohol-induced myogenic constriction, other rat model studies investigating developmental insults have demonstrated that maternal undernutrition during pregnancy resulted in significantly increased myogenic tone in radial uterine arteries on GD 20 when compared with controls (Veerareddy et al., 2004). It is well established that myogenic adaptations are important during pregnancy to regulate blood flow to the feto-placental compartment. Collectively, our data suggest that alcohol-induced uterine artery adaptations may contribute to altered hemodynamics in the utero-placental circulation which are critical for delivery of gas and nutrients (Veerareddy et al., 2002; Washburn, Sawant, Lunde, Wu, & Cudd, 2013).
We herein demonstrate that the uterine arteries of the alcohol-administered rats undergo inward hypotrophic remodeling, as measured by a decrease in lumen diameter and an increased media:lumen ratio (M. J. Mulvany, 1999). This is in contrast to normal pregnancy uteroplacental vascular adaptations, which is associated with outward-hypertrophic remodeling, i.e. an increased lumen diameter and a decreased media:lumen ratio, resulting in decreased resistance and increased blood flow (R. Magness, 1998; Osol & Mandala, 2009; Rosenfeld, 1977). An increase in media:lumen ratio corresponds to increased resistance to blood flow (Folkow, Hallback, Lundgren, & Weiss, 1970; M. Mulvany, Hansen, & Aalkjaer, 1978). Future studies on elastin, collagen, and smooth muscle remodeling are warranted to further characterize alcohol-induced uterine artery programing.
Our overall findings suggest that in vivo binge-alcohol exposure leads to altered gestational uterine arterial adaptations. During a healthy pregnancy, gestation-induced 30–50 fold increase in uterine blood flow is critical to meet the developmental requirements of the growing fetus (Rosenfeld, 1977). In women, uterine vascular resistance and resistance index decrease from 1.93 ± 0.22 mm Hg·ml−1·min and 0.89 in the non-pregnant state to 0.14 ± 0.01 mm Hg·ml−1·min and 0.52 by 34 weeks of gestation, respectively (Browne et al., 2011; Zamudio et al., 1995). Furthermore, animal models employing microspheres have validated that cardiac output to the uterus increases from 0.5% in non-pregnant state to 7.7% and 15.7% by the 2nd and 3rd trimester-equivalents of pregnancy, respectively (R. R. Magness, 1998; Rosenfeld, 1977). The aforementioned uterine vascular adaptations are essential for guaranteeing the nutrient requirements of the fetus be met throughout pregnancy and thus for normal fetal growth and development (R. R. Magness, 1998; Myers, Sparks, Makowski, Meschia, & Battaglia, 1982; Stock, 1994). Our current findings of alcohol-induced altered uterine artery adaptations are supported by earlier work on the impact of alcohol on uterine vasculature, specifically uterine artery endothelial mRNA (Ramadoss & Magness, 2012b) and protein profile (Ramadoss & Magness, 2012a), endothelial derived vasodilatory pathways (Magness, Sullivan, Li, Phernetton, & Bird, 2001), endothelial cell proliferation (Jobe et al., 2010), and spiral artery remodeling (Gundogan et al., 2008).
From the current study and others, it is evident that chronic binge alcohol exposure disrupts maternal uterine artery adaptations during pregnancy. The underlying mechanisms of alcohol-mediated pressure-dependent vascular response of the maternal uterine artery remain to be determined. Alcohol consumption during pregnancy is linked with intrauterine growth restriction and low birthweight (Kuehn et al., 2012), hallmark features of Fetal Alcohol Syndrome, and we hypothesize that maladaptive changes to uterine circulation may play a causal role in these abnormalities. Thus, we aim to continue to explore the consequences of alcohol exposure on the maternal vascular compartment so that we may better understand how these changes impact the fetus.
Highlights.
Gestational alcohol exposure increases pressure-dependent uterine artery constriction
Gestational alcohol induces inward hypotrophic remodeling of the uterine artery
Binge-alcohol exposure during pregnancy leads to altered uterine arterial adaptations
Acknowledgments
Grants: NIH AA19446, AA23520, AA23035 (JR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- Barron C, Mandala M, Osol G. Effects of pregnancy, hypertension and nitric oxide inhibition on rat uterine artery myogenic reactivity. J Vasc Res. 2010;47(6):463–471. doi: 10.1159/000313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan JA, Laher I. Pressure and flow-dependent vascular tone. FASEB J. 1991;5(9):2267–2273. doi: 10.1096/fasebj.5.9.1860618. [DOI] [PubMed] [Google Scholar]
- Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, et al. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1221–R1229. doi: 10.1152/ajpregu.91046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30(6):1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82(2):147–154. [PubMed] [Google Scholar]
- Cipolla M, Osol G. Hypertrophic and Hyperplastic Effects of Pregnancy on the Rat Uterine Arterial-Wall. American Journal of Obstetrics and Gynecology. 1994;171(3):805–811. doi: 10.1016/0002-9378(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Binder ND, Osol G. Myoendometrial versus placental uterine arteries: structural, mechanical, and functional differences in late-pregnant rabbits. Am J Obstet Gynecol. 1997;177(1):215–221. doi: 10.1016/s0002-9378(97)70464-2. [DOI] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, West JR. Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcohol Clin Exp Res. 2002;26(1):53–58. [PubMed] [Google Scholar]
- Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol. 1990;25(4):413–416. [PubMed] [Google Scholar]
- Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res. 2014;38(1):214–226. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- Folkow B, Hallback M, Lundgren Y, Weiss L. Background of Increased Flow Resistance and Vascular Reactivity in Spontaneously Hypertensive Rats. Acta Physiologica Scandinavica. 1970;79(2) A42-&. [PubMed] [Google Scholar]
- General S. Surgeon General's Advisory on Alcohol and Pregnancy. FDA Drug Bulletin. 1981;11(2):9–10. [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, et al. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29(2):148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55(4):1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl. 1989;7(4):S5–S8. discussion S9. [PubMed] [Google Scholar]
- Kuehn D, Aros S, Cassorla F, Avaria M, Unanue N, Henriquez C, et al. A prospective cohort study of the prevalence of growth, facial, and central nervous system abnormalities in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2012;36(10):1811–1819. doi: 10.1111/j.1530-0277.2012.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness R. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. The Endocrinology of Pregnancy. 1998:507–539. [Google Scholar]
- Magness RR. The Endocrinology of Pregnancy. New York: Humana Press; 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy; pp. 507–539. [Google Scholar]
- Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x) Am J Physiol Heart Circ Physiol. 2001;280(4):H1692–H1698. doi: 10.1152/ajpheart.2001.280.4.H1692. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37(5):818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mulvany M, Hansen O, Aalkjaer C. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circulation Research. 1978;43(6):854–864. doi: 10.1161/01.res.43.6.854. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ. Vascular remodelling of resistance vessels: can we define this? Cardiovascular Research. 1999;41(1):9–13. doi: 10.1016/s0008-6363(98)00289-2. [DOI] [PubMed] [Google Scholar]
- Myers SA, Sparks JW, Makowski EL, Meschia G, Battaglia FC. Relationship between placental blood flow and placental and fetal size in guinea pig. Am J Physiol. 1982;243(3):H404–H409. doi: 10.1152/ajpheart.1982.243.3.H404. [DOI] [PubMed] [Google Scholar]
- Novak J, Ramirez RJJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2002;283(2):R349–R355. doi: 10.1152/ajpregu.00635.2001. [DOI] [PubMed] [Google Scholar]
- Osol G, Cipolla M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am J Obstet Gynecol. 1993;168(2):697–705. doi: 10.1016/0002-9378(93)90519-o. [DOI] [PubMed] [Google Scholar]
- Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Jobe SO, Magness RR. Alcohol and maternal uterine vascular adaptations during pregnancy-part I: effects of chronic in vitro binge-like alcohol on uterine endothelial nitric oxide system and function. Alcohol Clin Exp Res. 2011;35(9):1686–1693. doi: 10.1111/j.1530-0277.2011.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol Clin Exp Res. 2007;31(10):1738–1745. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. 2-D DIGE uterine endothelial proteomic profile for maternal chronic binge-like alcohol exposure. J Proteomics. 2011;74(12):2986–2994. doi: 10.1016/j.jprot.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Alcohol-induced alterations in maternal uterine endothelial proteome: a quantitative iTRAQ mass spectrometric approach. Reprod Toxicol. 2012a;34(4):538–544. doi: 10.1016/j.reprotox.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Multiplexed digital quantification of binge-like alcohol-mediated alterations in maternal uterine angiogenic mRNA transcriptome. Physiol Genomics. 2012b;44(11):622–628. doi: 10.1152/physiolgenomics.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012c;303(4):H414–H421. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127C(1):35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232(3):H231–H235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- Sawant OB, Ramadoss J, Hankins GD, Wu G, Washburn SE. Effects of L-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol. Amino Acids. 2014;46(8):1981–1996. doi: 10.1007/s00726-014-1751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290(22):2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Stock MKMJ. Maternal physiology during gestation. In: E a N Knobil JD, editor. The physiology of reproduction. New York: Raven; 1994. pp. 947–983. [Google Scholar]
- Subramanian K, Naik VD, Sathishkumar K, Yallampalli C, Saade GR, Hankins GD, et al. Chronic binge alcohol exposure during pregnancy impairs rat maternal uterine vascular function. Alcohol Clin Exp Res. 2014;38(7):1832–1838. doi: 10.1111/acer.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol Use and Binge Drinking Among Women of Childbearing Age - United States, 2011–2013. Mmwr-Morbidity and Mortality Weekly Report. 2015;64(37):1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(1):H272–H284. doi: 10.1152/ajpheart.00216.2007. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122(6):1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Surgeon General, & Vice Admiral Richard H. Carmona, M., MPH, FACS. A 2005 Message to Women from the U.S. Surgeon General: Advisory on Alcohol Use in Pregnancy. 2005 [Google Scholar]
- Veerareddy S, Campbell ME, Williams SJ, Baker PN, Davidge ST. Myogenic reactivity is enhanced in rat radial uterine arteries in a model of maternal undernutrition. American Journal of Obstetrics and Gynecology. 2004;191(1):334–339. doi: 10.1016/j.ajog.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283(6):H2226–H2233. doi: 10.1152/ajpheart.00593.2002. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Sawant OB, Lunde ER, Wu G, Cudd TA. Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids. 2013;45(3):543–554. doi: 10.1007/s00726-012-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JF, Smith VC Committee On Substance, A. Fetal Alcohol Spectrum Disorders. Pediatrics. 2015;136(5):e1395–e1406. doi: 10.1542/peds.2015-3113. [DOI] [PubMed] [Google Scholar]
- Withers SB, Taggart MJ, Baker P, Austin C. Responses of isolated pressurised rat uterine arteries to changes in pressure: effects of pre-constriction, endothelium and pregnancy. Placenta. 2009;30(6):529–535. doi: 10.1016/j.placenta.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290(6):H2337–H2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol (1985) 1995;79(1):7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]