Abstract
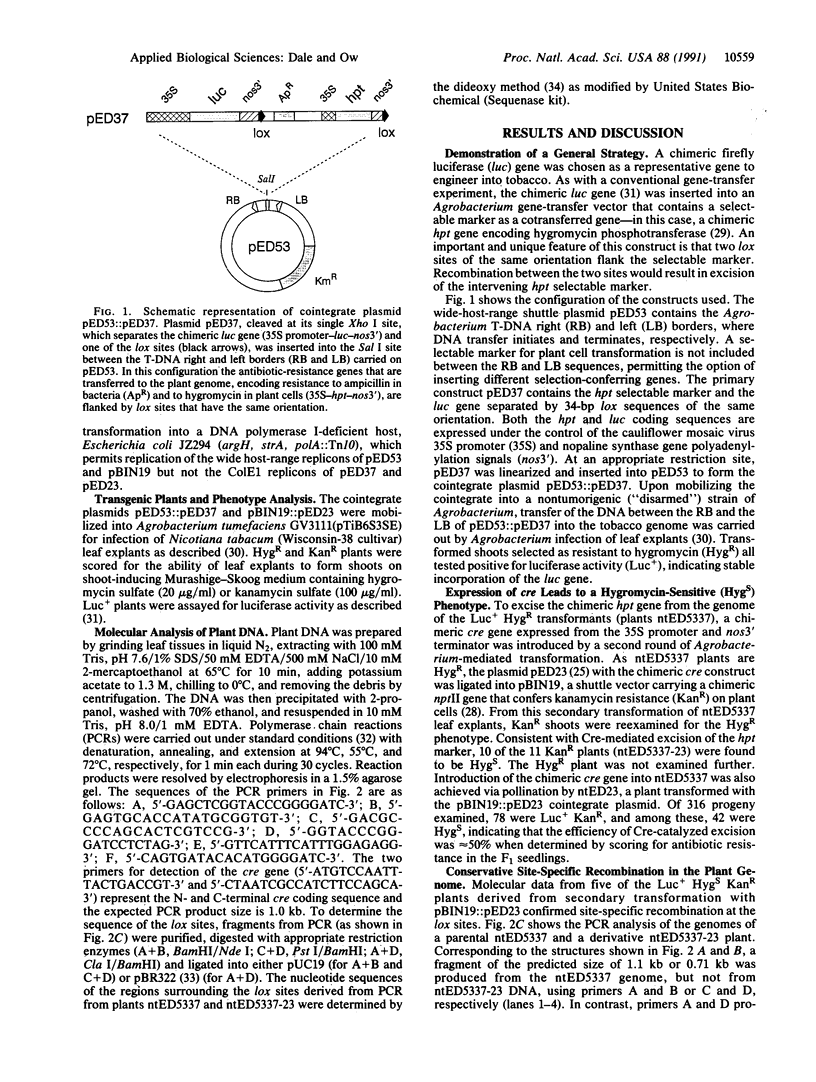
A general method of gene transfer that does not leave behind a selectable marker in the host genome is described. A luciferase gene was introduced into the tobacco genome by using the hygromycin phosphotransferase gene (hpt) as a linked selectable marker. Flanked by recombination sites from the bacteriophage P1 Cre/lox recombination system, the hpt gene was subsequently excised from the plant genome by the Cre recombinase. The Cre-catalyzed excision event in the plant genome was precise and conservative--i.e., without loss or alteration of nucleotides in the recombinant site. After removal of the Cre-encoding locus by genetic segregation, plants were obtained that had incorporated only the desired transgene. Gene transfer without the incorporation of antibiotic-resistance markers in the host genome should ease public concerns over the field release of transgenic organisms expressing such traits. Moreover, it would obviate the need for different selectable markers in subsequent rounds of gene transfer into the same host.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Angenent G. C., Van den Ouweland J. M., Bol J. F. Susceptibility to virus infection of transgenic tobacco plants expressing structural and nonstructural genes of tobacco rattle virus. Virology. 1990 Mar;175(1):191–198. doi: 10.1016/0042-6822(90)90199-2. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Dale E. C., Ow D. W. Intra- and intermolecular site-specific recombination in plant cells mediated by bacteriophage P1 recombinase. Gene. 1990 Jul 2;91(1):79–85. doi: 10.1016/0378-1119(90)90165-n. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Fraley R. T. Genetically engineering plants for crop improvement. Science. 1989 Jun 16;244(4910):1293–1299. doi: 10.1126/science.244.4910.1293. [DOI] [PubMed] [Google Scholar]
- Golemboski D. B., Lomonossoff G. P., Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6311–6315. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. M., Hauptli H., Crossway A., Knauf V. C. Gene transfer in crop improvement. Science. 1987 Apr 3;236(4797):48–54. doi: 10.1126/science.236.4797.48. [DOI] [PubMed] [Google Scholar]
- Hemenway C., Fang R. X., Kaniewski W. K., Chua N. H., Tumer N. E. Analysis of the mechanism of protection in transgenic plants expressing the potato virus X coat protein or its antisense RNA. EMBO J. 1988 May;7(5):1273–1280. doi: 10.1002/j.1460-2075.1988.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Ziese M., Sternberg N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3398–3402. doi: 10.1073/pnas.79.11.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster K. R., Burgett S. G., Rao R. N., Ingolia T. D. Analysis of a bacterial hygromycin B resistance gene by transcriptional and translational fusions and by DNA sequencing. Nucleic Acids Res. 1983 Oct 11;11(19):6895–6911. doi: 10.1093/nar/11.19.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C., Kaniewski W., Haley L., Rozman R., Newell C., Sanders P., Tumer N. E. Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Y in transgenic Russet Burbank. Biotechnology (N Y) 1990 Feb;8(2):127–134. doi: 10.1038/nbt0290-127. [DOI] [PubMed] [Google Scholar]
- Loesch-Fries L. S., Merlo D., Zinnen T., Burhop L., Hill K., Krahn K., Jarvis N., Nelson S., Halk E. Expression of alfalfa mosaic virus RNA 4 in transgenic plants confers virus resistance. EMBO J. 1987 Jul;6(7):1845–1851. doi: 10.1002/j.1460-2075.1987.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J., Caimi P., Sauer B., Russell S. Site-directed recombination in the genome of transgenic tobacco. Mol Gen Genet. 1990 Sep;223(3):369–378. doi: 10.1007/BF00264442. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Perlak F. J., Deaton R. W., Armstrong T. A., Fuchs R. L., Sims S. R., Greenplate J. T., Fischhoff D. A. Insect resistant cotton plants. Biotechnology (N Y) 1990 Oct;8(10):939–943. doi: 10.1038/nbt1090-939. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Cre-stimulated recombination at loxP-containing DNA sequences placed into the mammalian genome. Nucleic Acids Res. 1989 Jan 11;17(1):147–161. doi: 10.1093/nar/17.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., O'connell K. M., Nelson R. S., Sanders P. R., Beachy R. N., Fraley R. T., Shah D. M. Expression of alfalfa mosaic virus coat protein gene confers cross-protection in transgenic tobacco and tomato plants. EMBO J. 1987 May;6(5):1181–1188. doi: 10.1002/j.1460-2075.1987.tb02352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall D. J. Gene therapy in perspective. Nature. 1991 Jan 24;349(6307):275–276. doi: 10.1038/349275a0. [DOI] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Dun C. M., Bol J. F. Transgenic tobacco plants accumulating tobacco rattle virus coat protein resist infection with tobacco rattle virus and pea early browning virus. Virology. 1988 Dec;167(2):649–652. doi: 10.1016/s0042-6822(88)90131-6. [DOI] [PubMed] [Google Scholar]