Abstract
Increased awareness of organ donation has increased the availability of deceased donors, and it has boosted the opportunities for treating patients with multiple organ dysfunction. Simultaneously replacing two organs gives advantages of single surgery, lower immunosuppression dose and better survival than when one organ alone is transplanted. We present reports of management of three cases of combined liver and kidney transplantation (CLKT) from deceased donors. Based on management of these cases we discuss the importance of CLKT and anaesthetic concerns during such complex procedures.
Keywords: Anaesthesia for combined liver and kidney transplant, intraoperative continuous venovenous hemodiafiltraion, primary hyperoxalurea, simultaneous liver and kidney transplant
INTRODUCTION
End-stage renal disease (ESRD) can be associated with end-stage liver disease (ESLD) and vice versa. Simultaneously replacing both the organs gives advantages of single surgery, lower immunosuppression dose and better survival than when one organ alone is transplanted.[1]
Based on management of three cases of combined liver and kidney transplantation (CLKT), we discuss the importance of CLKT and anaesthetic concerns during such complex procedures.
CASE REPORTS
Case 1
A 17-year-old boy presenting with ESRD secondary to primary hyperoxaluria type 1 (PH 1) was on haemodialysis (HD) for the last 2 years. His ejection fraction (EF) was 39%, and serum oxalate was high. He was enlisted for cadaver programme, and daily high flux HD was initiated. After 1½ months, his serum oxalates reduced and EF improved to 60%. He received the organs from an 18-year-old deceased donor. Two years since transplant, he is doing well.
Case 2
A 5-year-old boy with PH 1 and ESRD requiring daily peritoneal dialysis (PD) was referred for CLKT. He also had uncontrolled hypertension, asthma and seizure disorder. A sequential liver and kidney transplant from his father was planned. Dialysis was converted from PD to HD through right internal jugular vein (IJV). The day before the planned living donor liver transplant (LDLT), we received donor organs from a 3-year-old brain dead child. Hence, he underwent simultaneous CLKT. The graft consisted of whole liver and en bloc kidneys with aorta and inferior venacava (IVC). Both organ functions were normal post-transplant but he succumbed to cytomegalovirus infection 2 months after transplant.
Case 3
A 54-year-old male patient with hepatitis C related ESLD and diabetic nephropathy was referred for CLKT. He was known case of diabetes mellitus (20 years) and hypertension (14 years). The patient had undergone deceased donor kidney transplantation 14 years back. Due to chronic rejection of the graft, he was on HD thrice weekly since 2 years. He had oesophageal varices and ascites requiring repeated large-volume paracentesis. He received the organs from a 52 year deceased donor. One year post–transplant, he is doing well.
Patients were shifted to designated operating room with all facilities for warming and infusing large volume of fluid quickly. Monitors were applied as per standard guidelines. Left radial artery was cannulated with 20-gauge (Insyte™) cannula under local anaesthesia (22-gauge in the child under general anaesthesia). After pre-oxygenation, induction was performed with injection midazolam 1 mg, injection fentanyl 2 μg/kg, injection propofol in titrated doses and injection atracurium 0.5 mg/kg. Trachea was intubated with appropriate size cuffed endotracheal tube. Right IJV, left femoral arterial and left femoral vein dialysis catheter of appropriate size were placed after induction. Anaesthesia was maintained with oxygen, air, isoflurane, injection atracurium infusion 0.3 mg/kg/h and injection fentanyl infusion 1 μg/kg/h.
In the first patient, pre-operative serum bicarbonate was 14 mmol/L and as time to optimise was less, continuous venovenous hemodiafiltration (CVVHDF) was started from the beginning of surgery. We discontinued it once graft kidney started functioning.
The second patient was well prepared for LDLT and third patient had undergone HD on the day of surgery. Hence, intraoperatively CVVHDF was kept as standby. Patients were monitored with hourly arterial blood gas (ABG), second hourly haemoglobin (Hb), platelet count, international normalised ratio (INR) and thromboelastography (TEG) when appropriate. Based on these reports, acid-base and electrolyte corrections were undertaken. Blood and blood products were administered with target Hb of ≥8 g%, and INR of 2–3.
In pre-anhepatic phase, because of the presence of renal failure, fluids were limited to potassium free crystalloids, blood and blood products. Crystalloids of choice were 0.9% or 0.45% saline and dextrose normal saline. Human albumin was avoided. Goal was to maintain central venous pressure (CVP) of 5–7 mmHg and stroke volume variation <10%. Hypotension was managed with phenylephrine and noradrenaline infusions. There was no significant blood loss in any of our patients.
During anhepatic phase, if pH was <7.1, it was treated with CVVHDF in case of first patient and 1 ml/kg boluses of sodium bicarbonate in the next two patients. Fluid boluses and inotropes were administered to prepare the patient for IVC trial and final cross-clamping. The second patient required IVC clamping twice, once for liver implantation and second time for en bloc kidney implantation. All patients tolerated the cross clamp well. Tranexamic acid 10 mg/kg over 30 min followed by 1 mg/kg/h infusion was started. Blood products, electrolytes (calcium) and dextrose were administered based on ABG, TEG and laboratory results. After reperfusion, drop in blood pressure was managed with phenylephrine and inotropes.
After hepatic arterial anastomosis, urology team began kidney transplantation. CVP was gradually raised to 9–10 mmHg carefully avoiding fluid overload. Mannitol 0.5 g/kg and frusemide 0.5 mg/kg were administered. Urine output was adequate after the reperfusion in all the patients. Tranexamic acid infusion was stopped 2 h post-reperfusion. Hypothermia gradually normalised and injection insulin was used to control blood sugars. Patients were shifted to the intensive care unit and were extubated after 6–8 h of elective ventilation. Postoperatively both organ functions were optimal and there was no need of dialysis.
Immunosuppression was initiated with injection basiliximab (20 mg for first and third patient, 10 mg for the second patient) and injection methylprednisolone10 mg/kg. Follow-up is shown in Figures 1–4.
Figure 1.
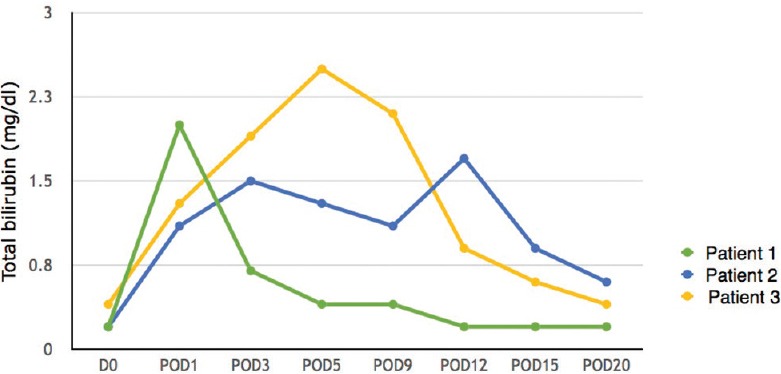
Total bilirubin levels (POD – Postoperative day)
Figure 4.
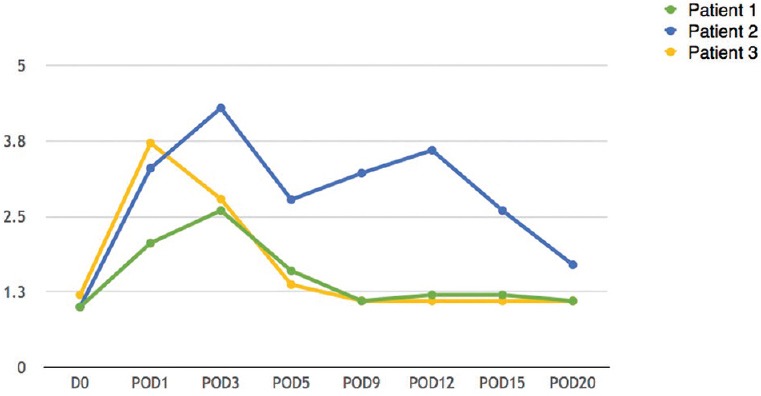
International normalised ratio levels (POD – Postoperative day)
Figure 2.
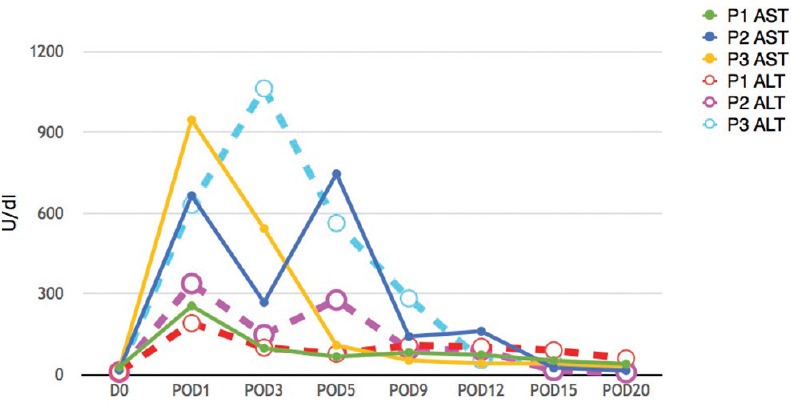
Aspartate aminotransferase and alanine aminotransferase levels (POD – Postoperative day)
Figure 3.
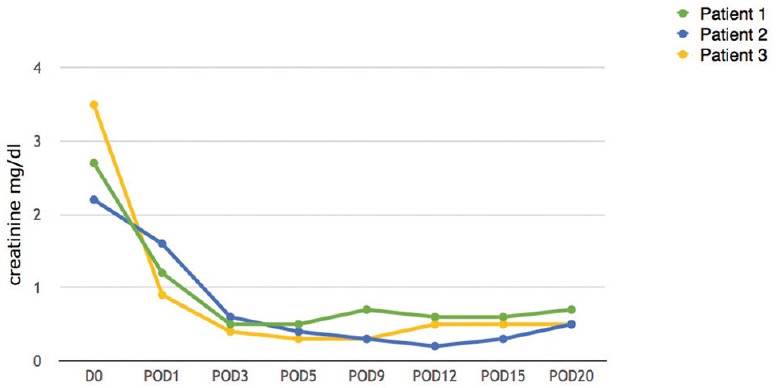
Creatinine levels (POD – Postoperative day)
DISCUSSION
CLKT is the procedure of choice for patients with both liver and kidney failure.[1] Renal failure in patients with ESLD is associated with high morbidity and mortality even after liver transplantation.[2,3] Use of calcineurin inhibitors, sepsis and haemodynamic insults might be the cause.[3] Patients who need dialysis therapy post-liver transplant have higher mortality compared to the others and it is mostly due to vascular access related sepsis.[4] Similarly, patients with ESRD and ESLD might suffer from perioperative liver decompensation if kidney alone is transplanted. The decision to transplant both organs is difficult when kidney dysfunction is temporary as seen with hepatorenal syndrome (HRS) or acute kidney injury in presence of ESLD. The long waiting time will deteriorate the kidney function in HRS cases and good renal recovery is seen only if transplant is done in early stages.[4] There are published criteria to guide in decision-making.[2,5,6] Two of our patients had ESRD secondary to metabolic disease (PH 1) and third patient had dual organ failure. These two are the most common indications for CLKT.[7] In metabolic diseases where the defect lies in liver and the organ affected is kidney, it is logical to transplant both simultaneously. Few case reports[8,9] support sequential transplantation, as it reduces nephrotoxic metabolite before kidney transplant and there is no need to use renal sparing immunosuppressants. However, when living donor is unavailable, CLKT becomes natural choice and it gives advantage of single donor, single surgery and common immunosuppression regime.[1] There is a proposition of protective effect of liver over kidney graft as well.[4,7] This is secondary to the secretion of soluble human leukocyte antigens by the liver and phagocytosis of reactive antibodies by Kupffer cells. In terms of survival, excellent 5 years survival was seen with CLKT than with sequential transplant (64% vs. 53.3%).[10,11]
Anaesthetic management of CLKT is challenging due to multi system involvement[12] and the complex surgical procedure. The presence of ESLD can be associated with hepatopulmonary syndrome, portopulmonary hypertension, hepatic hydrothorax, HRS, ascites, varices, coagulopathy, cardiac involvement, encephalopathy, hyponatraemia and hypocalcaemia. ESRD can cause hyperkalaemia, platelet dysfunction, pulmonary oedema, pericardial effusion and coronary disorders. Combined disease can lead to significant acidosis, anaemia and reduced drug metabolism. Systemic oxalosis seen in PH 1 can manifest as cardiac conduction defects, myocardial depression and pathological fractures. Fluid overload is a major concern during CLKT and hence restricted fluid replacement (CVP 5–7 mmHg) is recommended in pre-anhepatic phase.[13,14] CVVHDF is indicated in unoptimised recipient, prolonged anhepatic phase, severe acidosis, hyperkalaemia and fluid overload.[14,15] Problems due to CVVHDF are circuit or filter clotting, hypotension, air embolism, disconnection, haemorrhage and post-operative vascular thrombosis. If the anhepatic phase is shorter and there are no signs of fluid overload, acidosis can be managed with boluses of sodium bicarbonate as we did in two of our patients. Tranexamic acid is used to prevent post-reperfusion fibrinolysis.[12] The infusion should be started in anhepatic phase and discontinued within 2 h of liver reperfusion to avoid any possibility of hypercoagulation and hepatic artery thrombosis.[12]
CONCLUSION
CLKT is the best option for patients who need transplantation of both organs, especially when living donor is unavailable. With multiple advantages it gives better results than when done sequentially. Strict inclusion criteria should be followed as there is long waiting list for either organ.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We sincerely thank the Department of Anaesthesiology and Hepatobiliary surgery, Kerala Institute of Medical Sciences for their support and technical help.
REFERENCES
- 1.Chava SP, Singh B, Stangou A, Battula N, Bowles M, O’Grady J, et al. Simultaneous combined liver and kidney transplantation: A single center experience. Clin Transplant. 2010;24:E62–8. doi: 10.1111/j.1399-0012.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 2.Phuong-Thu TP. Clinical decision-making dilemma: Liver alone or simultaneous liver-kidney transplantation? J Transplant Technol Res. 2012;2:1–2. [Google Scholar]
- 3.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94:411–6. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 4.Davis CL, Gonwa TA, Wilkinson AH. Identification of patients best suited for combined liver-kidney transplantation: Part II. Liver Transpl. 2002;8:193–211. doi: 10.1053/jlts.2002.32504. [DOI] [PubMed] [Google Scholar]
- 5.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, et al. Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant. 2012;12:2901–8. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg C. Combined liver and kidney transplantation: Who and when? Pediatr Transplant. 2015;19:810–2. doi: 10.1111/petr.12632. [DOI] [PubMed] [Google Scholar]
- 7.Fagundes C, Guevara M. In: Combined liver and kidney transplantation. Understanding the Complexities of Kidney Transplantation. 1st ed. Ortiz J, André J, editors. Barcelona: InTech Open Access Publishers; 2011. pp. 349–58. [Google Scholar]
- 8.Nakamura M, Fuchinoue S, Nakajima I, Kitajima K, Tojimbara T, Takasaki K, et al. Three cases of sequential liver-kidney transplantation from living-related donors. Nephrol Dial Transplant. 2001;16:166–8. doi: 10.1093/ndt/16.1.166. [DOI] [PubMed] [Google Scholar]
- 9.Mor E, Nesher E, Ben-Ari Z, Weissman I, Shaharabani E, Eizner S, et al. Sequential liver and kidney transplantation from a single living donor in two young adults with primary hyperoxaluria type 1. Liver Transpl. 2013;19:646–8. doi: 10.1002/lt.23642. [DOI] [PubMed] [Google Scholar]
- 10.Demirci G, Becker T, Nyibata M, Lueck R, Bektas H, Lehner F, et al. Results of combined and sequential liver-kidney transplantation. Liver Transpl. 2003;9:1067–78. doi: 10.1053/jlts.2003.50210. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz R, Jennings LW, Kim P, Tomiyama K, Chinnakotla S, Fischbach BV, et al. Indications for combined liver and kidney transplantation: Propositions after a 23-year experience. Clin Transplant. 2010;24:807–11. doi: 10.1111/j.1399-0012.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- 12.Randolph HS, Christopher LW. In: Anaesthesia for abdominal organ transplantation. Miller's Anesthesia. 8th ed. Miller RD, editor. Canada: Elsevier Saunders Publishers; 2010. pp. 2262–91. [Google Scholar]
- 13.Kim JH, Lim BR, Jung JY. Intraoperative fluid management in combined liver kidney transplantation. Korean J Crit Care Med. 2013;28:309–10. [Google Scholar]
- 14.Townsend DR, Bagshaw SM, Jacka MJ, Bigam D, Cave D, Gibney RT. Intraoperative renal support during liver transplantation. Liver Transpl. 2009;15:73–8. doi: 10.1002/lt.21650. [DOI] [PubMed] [Google Scholar]
- 15.Aneja S, Upwar S. Anaesthesia for living donor combined liver kidney transplantation. Apollo Medicine. 2011;8:142–4. [Google Scholar]


