Abstract
Body mass index (BMI) has been shown to be associated with host susceptibility to several infections. However, the link between BMI and the risk of tuberculosis (TB) infection has been sparsely studied in China and in worldwide. Based on the baseline survey of a population-based, prospective study in rural China, the association between BMI and TB infection among adults was estimated by means of cross-sectional analysis. TB infection status was tested using QuantiFERON-TB Gold In-Tube (QFT), a commercial of interferon-γ release assay (IGRA). Totally, 17796 eligible participants aged ≥18 years from 4 study sites, were included in the analysis. 21.76% (3873/17796) were observed to be QFT positive. Age and gender standardized prevalence ranged from 16.49% to 23.81% across the study sites. 42.19% study participants were obese/overweight with BMI ≥ 24.0 kg/m2. BMI ≥ 28.0 kg/m2 was observed to be independently associated with QFT positivity (adjusted odds ratio: 1.17, 95% confidence interval: 1.04–1.33). The strength of the association was found to be geographically diversity, which might be explained, at least partly, by the varied local TB epidemic status. Our results suggest that individuals with obesity might be one important target population for TB infection control in rural China.
Latent tuberculosis infection (LTBI) screening and preventive treatment among high risk populations has been a major component of tuberculosis (TB) control programs in low-burden countries, such as USA and Canada1. For the areas and countries in developing LTBI management guidelines, including China, identification of individuals susceptible to infection and those with highest likelihood of progression to active disease should be the first important issue for consideration. According to testing of TB infection, there are two available methods, Tuberculin Skin Test (TST) and Interferon-γ Gamma Release Assays (IGRAs). The TST measures type IV hypersensitivity in response to purified protein derivative (PPD), while IGRAs detect interferon–γ (IFN-γ) level after the in-vitro stimulation with specific Mycobacterium tuberculosis (MTB) antigens2. However, the longitudinal data provided compelling evidence that the TST results were influenced by several factors including Bacillus Calmette-Guerin (BCG) vaccination and old age3. According to high-risk populations for screening and prophylactic treatment of LTBI, only close contacts of patients with active pulmonary TB and HIV infections are recommended currently in China4.
Globally, risk factors of TB infection prevalence have been found to be varied across areas. Low body mass index (BMI) has been shown to be associated with host susceptibility to active TB development5,6. In addition, obesity (BMI ≥ 30 kg/m2) and overweight (25 kg/m2 ≤ BMI < 30 kg/m2) were observed to be significantly associated with decreased risk of developing active TB as compared with normal-weight (18.5 kg/m2 ≤ BMI < 25 kg/m2)7. Additionally, type 2 diabetes mellitus (T2DM) has been suggested to be a re-emerging risk factor for TB development8 and for TB infection as well9. However, the link between BMI and the risk for TB infection has not been widely studied in worldwide. Therefore, the present study aims to assess the association of BMI with TB infection in rural adults based on the baseline data of a population-based multi-center prospective study from China.
Results
Characteristics of the study participants
A total of 17796 eligible participants were included in this analysis. Basic characteristics of the study population were shown in Table 1. More than a half (55.03%) of them were females and three quarters (74.29%) were older than 40 years. Gender and age distributions differed significantly across study sites (p < 0.0001). As compared to the participants from the other three study sites, participants from Site C showed lower educational levels (89.21% with middle school levels or lower) and higher exposure to close contact with TB patients (12.83%). Nearly one third of the participants reported ever smoking (29.16%) and about one fifth reported alcohol drinking (22.25%). Nearly half of the participants (48.42%) presented a BCG scar. 5.03% participants had a self-reported history of T2DM or with a baseline fast blood glucose level ≥7.0 mmol/L. The median BMI of the participants was 23.19 kg/m2 (interquartile range [IQR]: 21.06–25.76 kg/m2) and 42.19% study participants were overweight or obese with BMI ≥ 24.0 kg/m2. The distributions of BMI across age groups and study sites by gender please refer to Supplementary Tables S1 and S2. Among the study population, 21.76% were QuantiFERON-TB Gold In-Tube (QFT; a commercial IGRA kit) positive. After standardization for age and gender, the QFT positivity prevalence was found to be varied from 16.49% for Site A to 23.81% for Site C (Fig. 1).
Table 1. Characteristics of the study population.
| Total* (N = 17796) | Site A (n = 5068) | Site B (n = 4223) | Site C (n = 4395) | Site D (n = 4110) | p for χ2 test | |
|---|---|---|---|---|---|---|
| Gender | <0.0001 | |||||
| Male | 8003(44.97) | 2328(45.94) | 2089(49.47) | 1991(45.30) | 1595(38.81) | |
| Female | 9793(55.03) | 2740(54.06) | 2134(50.53) | 2404(54.70) | 2515(61.19) | |
| Age (years) | <0.0001 | |||||
| 18–29 | 2391(13.44) | 472(9.31) | 862(20.41) | 380(8.65) | 677(16.47) | |
| 30–39 | 2183(12.27) | 456(9.00) | 566(13.40) | 443(10.08) | 718(17.47) | |
| 40–49 | 4651(26.14) | 1260(24.86) | 1161(27.49) | 1084(24.66) | 1146(27.88) | |
| 50–59 | 3613(20.30) | 1174(23.16) | 746(17.67) | 1009(22.96) | 684(16.64) | |
| 60–69 | 3136(17.62) | 1069(21.09) | 539(12.76) | 944(21.48) | 584(14.21) | |
| ≥70 | 1822(10.24) | 637(12.57) | 349(8.26) | 535(12.17) | 301(7.32) | |
| Education level | <0.0001 | |||||
| Primary school or lower | 8900(50.01) | 2232(44.04) | 2001(47.38) | 2394(54.47) | 2273(55.30) | |
| Middle school | 6197(34.82) | 2043(40.31) | 1547(36.63) | 1527(34.74) | 1080(26.28) | |
| High school | 2052(11.53) | 608(12.00) | 489(11.58) | 388(8.83) | 567(13.80) | |
| College or higher | 647(3.64) | 185(3.65) | 186(4.40) | 86(1.96) | 190(4.62) | |
| Smoking status | <0.0001 | |||||
| Never smoked | 12605(70.84) | 3754(74.07) | 2878(68.18) | 2857(65.01) | 3116(75.82) | |
| Ever smoked | 5189(29.16) | 1314(25.93) | 1343(31.82) | 1538(34.99) | 994(24.18) | |
| Alcohol drinking | <0.0001 | |||||
| No | 13836(77.75) | 3873(76.42) | 2851(67.53) | 3589(81.66) | 3523(85.72) | |
| Yes | 3959(22.25) | 1195(23.58) | 1371(32.47) | 806(18.34) | 587(14.28) | |
| History of close contact with TB patients | <0.0001 | |||||
| No | 15826(95.72) | 4946(99.32) | 3976(96.76) | 3473(87.17) | 3431(99.13) | |
| Yes | 708(4.28) | 34(0.68) | 133(3.24) | 511(12.83) | 30(0.87) | |
| History of T2DM | <0.0001 | |||||
| No | 16396(94.97) | 4719(94.12) | 3966(95.77) | 3758(96.46) | 3953(93.83) | |
| Yes | 868(5.03) | 295(5.88) | 175(4.23) | 138(3.54) | 260(6.17) | |
| Number of BCG scars | <0.0001 | |||||
| 0 | 9178(51.58) | 3457(68.23) | 824(19.51) | 3960(96.35) | 937(21.32) | |
| 1 | 5182(29.12) | 1254(24.75) | 1490(35.28) | 143(3.48) | 2295(52.23) | |
| ≥2 | 3434(19.30) | 356(7.03) | 1909(45.20) | 7(0.17) | 1162(26.45) | |
| Cholesterol levela | <0.0001 | |||||
| Normal | 15065(84.67) | 4566(90.13) | 3299(78.12) | 3122(71.07) | 4078(99.22) | |
| Higher than normal | 2727(15.33) | 500(9.87) | 924(21.88) | 1271(28.93) | 32(0.78) | |
| Triglyceride levelb | <0.0001 | |||||
| Normal | 11431(64.25) | 3205(63.26) | 2376(56.26) | 2629(59.85) | 3221(78.37) | |
| Higher than normal | 6361(35.75) | 1861(36.74) | 1847(43.74) | 1764(40.15) | 889(21.63) | |
| LDL levelc | <0.0001 | |||||
| Normal | 14942(83.99) | 4934(97.41) | 3665(86.79) | 2708(61.64) | 3635(88.44) | |
| Higher than normal | 2849(16.01) | 131(2.59) | 558(13.21) | 1685(38.36) | 475(11.56) | |
| HDL leveld | <0.0001 | |||||
| Normal | 15702(88.25) | 4350(85.87) | 4193(99.29) | 3604(82.04) | 3555(86.50) | |
| Higher than normal | 2090(11.75) | 716(14.13) | 30(0.71) | 789(17.96) | 555(13.50) | |
| BMI (kg/m2) | ||||||
| Median (IQR)‡ | 23.29(21.06, 25.76) | 23.30(21.25, 25.41) | 24.31(21.92, 27.25) | 22.93(20.71, 25.54) | 22.68(20.58, 25.10) | <0.0001 |
| <18.5 | 927(5.21) | 220(4.34) | 127(3.01) | 288(6.55) | 292(7.10) | <0.0001 |
| 18.5–24.0 | 9362(52.61) | 2787(54.99) | 1851(43.83) | 2384(54.26) | 2340(56.93) | |
| 24.0–28.0 | 5496(30.89) | 1629(32.14) | 1402(33.20) | 1285(29.24) | 1180(28.71) | |
| ≥28.0 | 2010(11.30) | 432(8.52) | 843(19.96) | 437(9.95) | 298(7.25) | |
| QFT test | <0.0001 | |||||
| Negative | 13391(75.25) | 3962(78.18) | 3340(79.09) | 3040(69.17) | 3049(74.18) | |
| Positive | 3873(21.76) | 1052(20.76) | 801(18.97) | 1173(26.69) | 847(20.61) | |
| Indeterminate | 532(2.99) | 54(1.07) | 82(1.94) | 182(4.14) | 214(5.21) |
Abbreviations: BMI = body mass index. BCG = Bacillus Calmette-Guerin. HDL = high density lipoprotein. IQR = interquartile range. LDL = low density lipoprotein. QFT = QuantiFERON-TB Gold In-Tube. TB = tuberculosis. T2DM = type 2 diabetes mellitus.
a,b,c,dRanges of normal value for blood tests in different study sites: Cholesterol: Site A = 2.59–5.17 mmol/L, Site B = 3.1–5.17 mmol/L, Site C = 0–5.17 mmol/L, Site D = 0–5.17 mmol/L. Triglyceride: Site A = 0–1.7 mmol/L, Site B = 0.56–1.7 mmol/L, Site C = 0–1.71 mmol/L, Site D = 0–2.3 mmol/L. HDL: Site A = >0.9 mmol/L, Site B = 0.9–2.28 mmol/L, Site C = 1.16–1.55 mmol/L, Site D = 1.05–1.74 mmol/L. LDL: Site A = 0–4.11 mmol/L, Site B = 0-3.36 mmol/L, Site C = 0–3.12 mmol/L, Site D = <3.1 mmol/L.
*Sum might not always be in total because of missing data. Frequency of missing data did not differ significantly between sites. ‡Kruskal-Wallis test.
Figure 1. Age and gender-standardized prevalence of QFT positivity by study site.
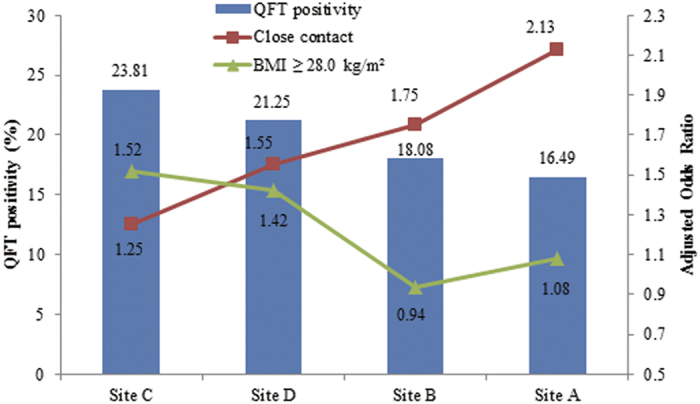
The associations of QFT positivity with close contact with active TB patients and BMI ≥ 28.0 kg/m2 were shown by study site as well. The strength of the association for close contact was strongest at the site with the lowest prevalence of infection. Inversely, the association of BMI ≥ 28.0 kg/m2 was strongest for the site with the highest prevalence of infection.
Factors independently associated with QFT positivity
For the association analysis (Table 2), QFT positivity has been observed to be associated with study site, age, gender, education level, smoking, close contact with TB patients, low density lipoprotein (LDL) level and BMI. History of T2DM was not observed to be related with QFT positivity. In addition, as shown in Table 3, BMI ≥ 28.0 kg/m2 (obesity) was observed to be independently associated with QFT positivity (with an adjusted odds ratio [OR]: 1.17, 95% confidence interval [CI]: 1.04–1.33). Male sex, increasing age, ever smoked, close contact with TB patients, and LDL level were also independently related with QFT positivity.
Table 2. Univariate and multivariate analysis for QFT positivity.
| n/N (%) | p for χ2 test | Adjusted OR* (95% CI) | |
|---|---|---|---|
| Study sites | <0.0001 | ||
| Site A | 1052/5014(20.98) | Reference | |
| Site B | 801/4141(19.34) | 1.03(0.90–1.17) | |
| Site C | 1173/4213(27.84) | 1.34(1.19–1.51) | |
| Site D | 847/3896(21.74) | 1.37(1.21–1.56) | |
| Gender | <0.0001 | ||
| Female | 1793/9415(19.04) | Reference | |
| Male | 2080/7849(26.50) | 1.29(1.15–1.44) | |
| Age (years) | <0.0001 | ||
| 18–29 | 194/2344(8.28) | Reference | |
| 30–39 | 313/2116(14.79) | 1.81(1.47–2.22) | |
| 40–49 | 876/4513(19.41) | 2.46(2.04–2.96) | |
| 50–59 | 934/3479(26.85) | 3.81(3.16–4.58) | |
| 60–69 | 966/3047(31.70) | 4.97(4.10–6.04) | |
| ≥70 | 590/1765(33.43) | 5.67(4.61–6.98) | |
| Education level | <0.0001 | ||
| Primary school or lower | 2175/8573(25.37) | Reference | |
| Middle school | 1264/6044(20.91) | 1.10(1.00–1.21) | |
| High school | 367/2014(18.22) | 1.06(0.92–1.22) | |
| College or higher | 67/633(10.58) | 0.95(0.71–1.26) | |
| Smoking status | <0.0001 | ||
| Never smoked | 2405/12176(19.75) | Reference | |
| Ever smoked | 1468/5086(28.86) | 1.28(1.15–1.43) | |
| Alcohol drinking | <0.0001 | ||
| No | 2877/13373(21.51) | Reference | |
| Yes | 996/3890(25.60) | 0.99(0.89–1.10) | |
| History of close contact with TB patients | <0.0001 | ||
| No | 3480/15826(21.99) | Reference | |
| Yes | 221/708(31.21) | 1.38(1.16–1.64) | |
| History of T2DM | |||
| No | 3627/16396(22.12) | Reference | |
| Yes | 246/868(28.34) | 1.04(0.88–1.22) | |
| Number of BCG scars | |||
| 0 | 2021/8891(22.73) | Reference | |
| 1 | 1049/5030(20.85) | 0.98(0.88–1.10) | |
| ≥2 | 803/3341(24.03) | 1.05(0.93–1.20) | |
| Cholesterol levela | <0.0001 | ||
| Normal | 3168/14632(21.65) | Reference | |
| Higher than normal | 704/2628(26.79) | 1.04(0.92–1.19) | |
| Triglyceride levelb | <0.0001 | ||
| Normal | 2380/11072(21.50) | Reference | |
| Higher than normal | 1492/6188(24.11) | 1.04(0.96–1.13) | |
| LDL levelc | <0.0001 | ||
| Normal | 3117/14538(21.44) | Reference | |
| Higher than normal | 754/2721(27.71) | 1.12(1.03–1.16) | |
| HDL leveld | 0.9913 | ||
| Normal | 3420/15246(22.43) | ||
| Higher than normal | 452/2014(22.44) | ||
| BMI (kg/m2) | 0.0028 | ||
| <18.5 | 168/908(18.50) | 0.80(0.66–1.01) | |
| 18.5–24.0 | 1987/9046(21.97) | Reference | |
| 24.0–28.0 | 1254/5350(23.44) | 1.07(0.98–1.17) | |
| ≥28.0 | 464/1960(23.67) | 1.15(1.01–1.31) | |
Abbreviations: BMI = body mass index. BCG = Bacillus Calmette-Guerin. CI = confidence interval. HDL = high density lipoprotein. LDL = low density lipoprotein. OR = odds ratio. QFT = QuantiFERON-TB Gold In-Tube. TB = tuberculosis. T2DM = type 2 diabetes mellitus.
*Controlling for variables with p < 0.05 in the univariate analysis.
a,b,c,dRanges of normal value for blood tests in different study sites: Cholesterol: Site A = 2.59–5.17 mmol/L, Site B = 3.1–5.17 mmol/L, Site C = 0–5.17 mmol/L, Site D = 0–5.17 mmol/L. Triglyceride: Site A = 0–1.7 mmol/L, Site B = 0.56–1.7 mmol/L, Site C = 0–1.71 mmol/L, Site D = 0–2.3 mmol/L. HDL: Site A = >0.9 mmol/L, Site B = 0.9–2.28 mmol/L, Site C = 1.16–1.55 mmol/L, Site D = 1.05–1.74 mmol/L. LDL: Site A = 0–4.11 mmol/L, Site B = 0–3.36 mmol/L, Site C = 0–3.12 mmol/L, Site D = <3.1 mmol/L.
Table 3. Potential independent factors associated with QFT positivity of the study population.
| Variables | n/N(%) | p for χ2 test | Adjusted OR* (95% CI) |
|---|---|---|---|
| Study site | <0.0001 | ||
| Site A | 1052/5014(20.98) | Reference | |
| Site B | 801/4141(19.34) | 1.05(0.94–1.17) | |
| Site C | 1173/4213(27.84) | 1.39(1.26–1.54) | |
| Site D | 847/3896(21.74) | 1.31(1.17–1.46) | |
| Gender | <0.0001 | ||
| Female | 1793/9415(19.04) | Reference | |
| Male | 2080/7849(26.50) | 1.30(1.17–1.44) | |
| Age (years) | <0.0001 | ||
| 18–29 | 194/2344(8.28) | Reference | |
| 30–39 | 313/2116(14.79) | 1.84(1.51–2.25) | |
| 40–49 | 876/4513(19.41) | 2.50(2.10–2.97) | |
| 50–59 | 934/3479(26.85) | 3.85(3.24–4.59) | |
| 60–69 | 966/3047(31.70) | 4.93(4.14–5.86) | |
| ≥70 | 590/1765(33.43) | 5.60(4.65–6.74) | |
| Smoking status | <0.0001 | ||
| Never smoked | 2405/12176(19.75) | Reference | |
| Ever smoked | 1468/5086(28.86) | 1.28(1.15–1.43) | |
| History of close contact with TB patients | <0.0001 | ||
| No | 3480/15826(21.99) | Reference | |
| Yes | 221/708(31.21) | 1.38(1.16–1.65) | |
| LDL levela | <0.0001 | ||
| Normal | 3117/14538(21.44) | Reference | |
| Higher than normal | 754/2721(27.71) | 1.14(1.02–1.27) | |
| BMI (kg/m2) | 0.0028 | ||
| <18.5 | 168/908(18.50) | 0.89(0.76–1.03) | |
| 18.5–24.0 | 1987/9046(21.97) | Reference | |
| 24.0–28.0 | 1254/5350(23.44) | 1.09(0.99–1.18) | |
| ≥28.0 | 464/1960(23.67) | 1.17(1.04–1.33) |
Abbreviations: BMI = body mass index. CI = confidence interval. LDL = low density lipoprotein. OR = odds ratio. QFT = QuantiFERON-TB Gold In-Tube. TB = tuberculosis.
*Controlling for study site, age, gender, smoking status, LDL level, history of close contact with TB, and BMI when appropriated.
aRange of normal value in different study sites: Site A = 0–4.11 mmol/L, Site B = 0–3.36 mmol/L, Site C = 0–3.12 mmol/L, Site D = <3.1 mmol/L.
Association between QFT positivity and BMI (kg/m2) categories by sites
The association between BMI and QFT positivity was found to be geographically diverse as shown in Table 4. The strength of the association between QFT positivity and BMI ≥ 28.0 kg/m2 were statistically significant for Site C (adjusted OR: 1.52, 95% CI: 1.20–1.94) and Site D (adjusted OR: 1.42, 95% CI: 1.05–1.91).
Table 4. Association between QFT positivity and BMI (kg/m2) categories by sites.
| BMI categories | QFT positivity |
Adjusted OR† (95% CI) | |
|---|---|---|---|
| n/N | % | ||
| Site A | |||
| <18.5 kg/m2 | 41/217 | 18.89 | 0.98(0.68–1.41) |
| 18.5–24.0 kg/m2 | 553/2755 | 20.07 | Reference |
| 24.0–28.0 kg/m2 | 363/1609 | 22.56 | 1.10(0.95–1.29) |
| ≥28.0 kg/m2 | 95/433 | 21.94 | 1.04(0.81–1.34) |
| Site B | |||
| <18.5 kg/m2 | 20/126 | 15.87 | 0.75(0.45–1.26) |
| 18.5–24.0 kg/m2 | 350/1804 | 19.40 | Reference |
| 24.0–28.0 kg/m2 | 280/1384 | 20.23 | 1.00(0.83–1.20) |
| ≥28.0 kg/m2 | 151/827 | 18.26 | 0.93(0.74–1.16) |
| Site C | |||
| <18.5 kg/m2 | 58/279 | 20.79 | 0.67(0.48–0.92) |
| 18.5–24.0 kg/m2 | 616/2281 | 27.01 | Reference |
| 24.0–28.0 kg/m2 | 360/1236 | 29.13 | 1.20(1.01–1.41) |
| ≥28.0 kg/m2 | 139/417 | 33.33 | 1.52(1.20–1.94) |
| Site D | |||
| <18.5 kg/m2 | 49/286 | 17.13 | 0.87(0.59–1.29) |
| 18.5–24.0 kg/m2 | 468/2206 | 21.21 | Reference |
| 24.0–28.0 kg/m2 | 251/1121 | 22.39 | 1.00(0.82–1.20) |
| ≥28.0 kg/m2 | 79/283 | 27.92 | 1.42(1.05–1.91) |
Abbreviations: BMI = body mass index. CI = confidence interval. OR = odds ratio. QFT = QuantiFERON-TB Gold In-Tube. TB = tuberculosis.
†Controlling for age, gender, smoking status, LDL level, and history of close contact with TB patients.
Stratified analysis of the covariates on the association of BMI (kg/m2) with QFT positivity
In Table 5, potential impact of other independent factors on the relation of BMI with QFT positivity was evaluated by means of stratified analysis. Participants who were females (adjusted OR: 1.26, 95% CI: 1.08–1.48) or older than 60 years (adjusted OR: 1.31, 95% CI: 1.07–1.60) were found to be under much higher risk of QFT positivity if they were BMI ≥ 28.0 kg/m2.
Table 5. The association of BMI with QFT positivity stratified by various independent factors.
| Variables | BMI | n/N(%) | Adjusted OR* (95% CI) |
|---|---|---|---|
| Gender | |||
| Female | <28.0 kg/m2 | 1540/8643(17.82) | Reference |
| ≥28.0 kg/m2 | 253/1149(22.02) | 1.26(1.08, 1.48) | |
| Male | <28.0 kg/m2 | 1869/7142(26.17) | Reference |
| ≥28.0 kg/m2 | 211/861(24.51) | 0.97(0.82, 1.15) | |
| Age (years) | |||
| <60 | <28.0 kg/m2 | 2023/11308(17.89) | Reference |
| ≥28.0 kg/m2 | 294/1529(19.23) | 1.03(0.90, 1.19) | |
| ≥60 | <28.0 kg/m2 | 1386/4477(30.96) | Reference |
| ≥28.0 kg/m2 | 170/481(35.34) | 1.31(1.07, 1.60) | |
| Smoking status | |||
| Never smoked | <28.0 kg/m2 | 2088/11140(18.74) | Reference |
| ≥28.0 kg/m2 | 317/1464(21.65) | 1.18(1.03, 1.35) | |
| Ever smoked | <28.0 kg/m2 | 1321/4643(28.45) | Reference |
| ≥28.0 kg/m2 | 147/546(26.92) | 0.99(0.80, 1.22) | |
| History of close contact with TB patients | |||
| No | <28.0 kg/m2 | 3058/14414(21.22) | Reference |
| ≥28.0 kg/m2 | 422/1878(22.47) | 1.11(0.99, 1.25) | |
| Yes | <28.0 kg/m2 | 197/666(29.58) | Reference |
| ≥28.0 kg/m2 | 24/75(32.00) | 1.17(0.696, 1.98) | |
| LDL levela | |||
| Normal | <28.0 kg/m2 | 2778/13396(20.74) | Reference |
| ≥28.0 kg/m2 | 339/1545(21.94) | 1.11(0.97, 1.27) | |
| Higher than normal | <28.0 kg/m2 | 631/2387(26.43) | Reference |
| ≥28.0 kg/m2 | 123/462(26.62) | 1.12(0.88, 1.41) | |
Abbreviations: BMI = body mass index. CI = confidence interval. LDL = low density lipoprotein. OR = odds ratio. QFT = QuantiFERON-TB Gold In-Tube. TB = tuberculosis.
*Controlling for study site, age, gender, smoking status, LDL level, and history of close contact with TB.
aRange of normal value in different study sites: Site A = 0–4.11 mmol/L, Site B = 0–3.36 mmol/L, Site C = 0–3.12 mmol/L, Site D = <3.1 mmol/L.
Discussion
In this population-based, multicenter study conducted in rural China, BMI ≥ 28.0 kg/m2 was found to be independently associated with host susceptibility to TB infection. The strength of such an association was stronger in the study sites with higher prevalence of TB infection. The increased risk of TB infection among individuals with obesity was observed to be modified by other potential independent factors such as gender, age and smoking. High risk populations for TB infection, such as elderly with obesity found in this study, should be paid more attention as potential target populations for TB infection monitoring and preventive intervention in China.
The association between BMI and TB infection has not been comprehensively understood, despite years of research on its links with active TB disease10,11. Our study provided an opportunity, with large sample size and different study sites with various TB epidemics, to explore the relation between BMI and TB infection in the general rural population in China. In addition, IGRA has been approved by the American Centers for Disease Control and Prevention (CDC) as an alternative screening strategy to TST for LTBI testing currently12. Based on our previous results, the TST results were found to be influenced by several factors including old age and the status of BCG vaccination13. Therefore, the present study used IGRAs rather than TST to define the status of TB infection. Participants aged 18 years or lower were excluded in the present study to minimize the potential bias caused by the dynamic change in BMI for growing children.
Contrary to the link between lower BMI and increased risk of active TB14, our results showed that BMI ≥ 28.0 kg/m2 was independently associated with TB infection. It has been suggested that excess adiposity negatively impacts immune function and host defense in obese individuals15,16,17. Additionally, the accumulation of adipose tissue might attenuate host pulmonary defense through metabolic disturbances18,19. Therefore, the potential mechanism underlying the relation of obesity and TB infection might be that adipose tissue takes effect through a variety of immune mediators5. Animal results proved that leptin-deficient (ob/ob) mice were highly susceptible to pulmonary TB infection20. The prevalence of TB infection prevalence increased gradually from 18.50% for underweight subjects to 23.67% for obese subjects in the present study would be consistent with the above speculation. If BMI is causally linked to the risk of latent TB in the way that our data presented here suggest, then promoting weight loss in overweight or obese populations and shifting the overall BMI distribution to lower values, would further reduce latent TB incidence, especially for people who may become TB infected. However, due to the limitation of cross-sectional study design, it should be critical to confirm the directionality of association between BMI and TB infection. In addition, we could not exclude another possibility that individuals with low BMI were under higher risk of developing active disease and then those with normal and high BMI stayed latent infection. Therefore, prospective studies were needed to clarify the underlying mechanisms for the observed relation between obesity and increased risk of TB infection. To put this in context, our data might strengthen the rationale to assess and implement strategies aimed at stopping increasing overweight and obesity trends in China and mitigating their public health effects.
In the present study, the strength of the association between BMI and TB infection was found to be geographically diverse. As shown in Fig. 1, the prevalence of TB infection in Site A was much lower than the other three sites, TB infection showed stronger association with close contact with active TB patients but relatively modest relation to BMI. It suggests that the effect of BMI on TB infection might be neglected among regions with lower TB epidemics. At the same time, certain regional disparities in TB prevalence suggest that screening and identification of LTBI in contacts of TB patients might be seen as a good clinical practice in low-endemic settings and tuberculosis-control activities should be regularly evaluated for effectiveness against changing epidemiological information. In addition, such geographical diversity might be explained by different distributions of the other covariates associated with TB infection such as age, gender and smoking.
Given that obesity is one main cause of T2DM, it has given strength about the pertinence of this study. Data showed that 201 (23.16%) of 868 participants with a history of T2DM had the status of BMI ≥ 28.0 kg/m2 (Supplementary Table S3). However, logistic regression analysis revealed that the relation between T2DM and TB infection was non-significant in our study population. It might further suggest that high BMI was independently correlated with TB infection. Although T2DM is a cause of comorbidity for people with active TB21,22, its role in TB infection need further explored.
Due to the fact that our data were cross-sectional design based, our study has several limitations. First, the causal nature between BMI and QFT positivity is uncertain, even the biologic mechanism is more plausible to support attenuated immune function and increased susceptibility to infections for individuals with obesity. Second, some covariant factors such as history of close contact with TB patients were collected by a face-to-face interviewed questionnaire. Potential recall bias caused by inaccurate response could therefore not be excluded. Silicosis and HIV infection have been suggested to be potential risk factors for TB infection9, however, no participant in our study was self-reported or officially registered patients with silicosis and/or HIV infection. Low prevalence of silicosis and HIV infection in the general rural population in China23,24,25 limited our analysis to explore their potential impact on TB infection. Third, a standard 0.35 IU/mL cutoff was used for QFT in this study which might not be the most appropriate cutoff for this population, because it has not been extensively validated in China. Finally, suspected cases with active TB based on the diagnosis of chest X-ray were excluded from the prevalence analysis of TB infection in the present study. Considering lacking pathogenic evidence, such as sputum smear, sputum culture or gene X-pert test, potential bias caused by misclassification of disease status could not be completely excluded. Despite these limitations, our results provide valuable insight into the relation of BMI with infectious diseases.
Conclusions
In summary, a positive association between obesity and TB infection was observed in our study population. It suggests that BMI management should be attached attention for the public health including infectious diseases control in China. In a community level, high-risk subgroups including the elderly with obesity found in this study should be prioritized for TB infection control in China and areas with a similar epidemiological profile.
Methods
Ethics consideration
Ethics approval was obtained from the ethics committee of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (Beijing, China) (No: IPB-2013–5). All experiments and study procedures were performed in accordance with relevant guidelines and regulations, and including any relevant details. All participants gave written informed consent.
Study design and participants
A population-based, prospective cohort study (LATENTTB-NSTM) addressing TB infection and active disease development was conducted at four study sites (Site A: eastern China, plains; Site B: central China, plains; Site C: western China, hills; or Site D: western China, basin.) in rural China during 2013–2015. Registered rural residents (5 years older) at the four study sites were the target population of the study. The baseline survey was conducted during July to September 2013, which has been reported in detail previously13.
The present study was restricted to the adults participated in the baseline survey. The inclusion criteria were: aged 18 years or older (referenced as June 1, 2013); registered resident or with continuous residence at the study site for ≥6 months over the past year; able to complete the investigations and tests during the study duration; and provision of voluntary written informed consent. The exclusion criteria were: current active TB, self-reported history of TB, and pregnancy.
Data collection
For each study participant, socio-demographic information was collected by a standardized questionnaire administered by trained interviewers. Data collected included age, gender, educational level, history of close contact with a TB patient, smoking status (never smoked or ever smoked), alcohol use status (never used or ever used) and history of T2DM (self-reported and/or fast blood glucose higher than 7.0 mmol/L at baseline examination)26. Ever smoked was defined as those who had smoked more than 5 cigarettes/month. Ever alcohol use was defined as those who had consumed alcohol for the last year. Digital chest radiography (CXR) was performed on all study participants over 15 years of age. Individuals with suspected TB (defined by radiographic abnormalities consistent with active TB) were not included in the LTBI analysis. Height, weight, pulse and the presence of a BCG scar were examined as well.
Venous blood was collected for QFT and blood biochemical examinations, including fast blood glucose, cholesterol, triglyceride, LDL, and high density lipoprotein (HDL) levels. The cutoff value of the blood test index is suggested according to the instruction manual. As a product of IGRA, QFT detected IFN-γ in vitro responses to peptide antigens that were associated with MTB infection27. Briefly, each QFT consisted of three tubes: (1) a TB antigen tube containing the specific MTB antigens, (2) a mitogen control tube containing a non-specific T-cell-stimulating antigen and serving as a positive control, and (3) a Nil control tube containing no antigens and serving as a negative control. QFT was performed as recommended by the manufacturer using a cutoff value of ≥0.35 IU/ml. The positivity of QFT was defined as TB antigen minus Nil ≥ 0.35 IU/ml and ≥ 25% of Nil value, together with Nil ≤ 8.0 IU/ml according to the manufacturer. The positive results might imply the presence of TB infection.
Statistical analysis
Questionnaire data, physical examination data (height, weight, pulse, and presence of BCG scar) and laboratory results (QFT and blood biochemical examination) were double entered into a spreadsheet and checked by web-based project-specific data collection and management software. After cleaning, the data were then converted and analyzed using Statistical Analysis System (SAS 9.2; SAS Institute Inc., NC, USA).
BMI was calculated as weight over height squared (kg/m2), and was presented as median and IQR when was recognized as continuous variable. BMI was further categorized as underweight (<18.5 kg/m2), normal weight (18.5 to 24.0 kg/m2), overweight (24.0 to 28.0 kg/m2), or obese (≥28.0 kg/m2)28. It has demonstrated that obesity is in correlation with blood glucose, cholesterol, blood glucose, triglycerides and LDL/HDL29,30. Therefore, these blood biochemical parameters were included in the analysis. The frequency of categorical variables in the study participants was compared between the study sites using Pearson’s chi-square test. To identify potential variables related with QFT positivity, univariate analysis were performed using Pearson’s chi-square test. All variables with p-values < 0.05 in univariate analysis were entered into the multiple logistic regression analyses, and the associations were then assessed by means of OR and 95% CI. Stepwise backward multiple logistic regression analysis was then used to identify the variables independently associated with QFT positivity. The significance level for the variables stayed in the model was 0.05. To explore the potential modifying effect of the covariates on the association of BMI with QFT positivity, stratified analysis were conducted (BMI was dichotomized at cutoff of 28.0 kg/m2).
Additional Information
How to cite this article: Zhang, H. et al. Association of Body Mass Index with the Tuberculosis Infection: a Population-based Study among 17796 Adults in Rural China. Sci. Rep. 7, 41933; doi: 10.1038/srep41933 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Prof. Shuigao Jin from Chinese Center for Disease Prevention and Control, and Prof. Yude Chen from Peking University for their help on study design. We thank all the consortium members for the LATENTTB-NSTM study team across the study sites for their contribution to the site work at baseline survey and follow-up examinations. This work was supported by the National Science and Technology Major Project of China (grant number: 2013ZX10003004-002, 2014ZX10003001-001), and the Program for Changjiang Scholars and Innovative Research Team in University of China (grant number: IRT13007).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.G. designed the study. H.R.Z., X.W.L., M.F.L., H.J.L. and H.N.X. were in charge of data management. H.R.Z., H.N.X., and L.G. did data analysis and wrote the report. Q.J. commented on the report and improved English writing. W.L., L.Q.B., X.H.W., and J.M.L. organised invtigations at the study sites. All authors contributed to review and revision and have seen and approved the final version of manuscript.
References
- Pai M. & Rodrigues C. Management of latent tuberculosis infection: an evidence-based approach. Lung India. 32, 205–207, doi: 10.4103/0970-2113.156210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella M., Ekaterini Z., Olga P. & Petros E. Screening optimization of latent tuberculosis infection in rheumatoid arthritis patients. Arthritis. 2015, 569620, doi: 10.1155/2015/569620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L. et al. Annual risk of tuberculosis infection in rural China: a population-based prospective study. Eur Respir J. 48, 168–178, doi: 10.1183/13993003.00235-2016 (2016). [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China. Administrative Measures of the Prevention and Treatment for Tuberculosis 2013.
- Falagas M. E. & Kompoti M. Obesity and infection. Lancet Infect Dis. 6, 438–446, doi: 10.1016/S1473-3099(06)70523-0 (2006). [DOI] [PubMed] [Google Scholar]
- Semunigus T., Tessema B., Eshetie S. & Moges F. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in Dessie and Debre Birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob. 15, 50, doi: 10.1186/s12941-016-0165-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. C. et al. Lower risk of tuberculosis in obesity. Arch Intern Med. 167, 1297–1304 (2007). [DOI] [PubMed] [Google Scholar]
- Abdelbary B. E., Garcia-Viveros M., Ramirez-Oropesa H., Rahbar M. H. & Restrepo B. I. Tuberculosis-diabetes epidemiology in the border and non-border regions of Tamaulipas, Mexico. Tuberculosis. S1472–9792, 30409–7. doi: 10.1016/j.tube.2016.09.024 (2016). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection 2015. [PubMed]
- Hanrahan C. F. et al. Body mass index and risk of tuberculosis and death. AIDS. 24, 1501–1508, doi: 10.1097/QAD.0b013e32833a2a4a (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro I. et al. Low BMI and falling BMI predict HIV-associated tuberculosis: a prospective study in Tanzania. Int J Tuberc Lung Dis. 14, 1447–1453 (2010). [PMC free article] [PubMed] [Google Scholar]
- Mazurek G. H. et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection- United States, 2010. MMWR Recomm Rep. 59, 1–25 (2010). [PubMed] [Google Scholar]
- Gao L. et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 15, 310–319, doi: 10.1016/S1473-3099(14)71085-0 (2015). [DOI] [PubMed] [Google Scholar]
- Lonnroth K., Williams B. G., Cegielski P. & Dye C. A. Consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 39, 149–155, doi: 10.1093/ije/dyp308 (2010). [DOI] [PubMed] [Google Scholar]
- Milner J. J. & Beck M. A. The impact of obesity on the immune response to infection. Proc Nutr Soc. 71, 298–306, doi: 10.1017/S0029665112000158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago R., Gomez R., Lago F., Gomez-Reino J. & Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 252, 139–145, doi: 10.1016/j.cellimm.2007.09.004 (2008). [DOI] [PubMed] [Google Scholar]
- Meckenstock R. & Therby A. Modifications of immunity in obesity: The impact on the risk of infection. Rev Med Interne. 36, 760–768, doi: 10.1016/j.revmed.2015.07.013 (2015). [DOI] [PubMed] [Google Scholar]
- Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 43, 157–168 (2009). [PubMed] [Google Scholar]
- Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Ther. 26, 412–419, doi: 10.1016/j.pupt.2012.04.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland C. W. et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol. 17, 1399–1408 (2005). [DOI] [PubMed] [Google Scholar]
- Jeon C. Y. & Murray M. B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 5, e152, doi: 10.1371/journal.pmed.0050152 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B. Diabetes and tuberculosis: an unhealthy partnership. Lancet Infect Dis. 7, 444, doi: 10.1016/s1473-3099(07)70144-5 (2007). [DOI] [PubMed] [Google Scholar]
- State Administration of Work Safety of China. National occupational diseases report for 2014 2015.
- State council AIDS working committee office and UN theme group on AIDS. A Joint Assessment of HIV/AIDS Prevention, Treatment and Care in China (2007) 2007.
- Zhang L. et al. HIV prevalence in China: integration of surveillance data and a systematic review. Lancet Infect Dis. 13, 955–963, doi: 10.1016/S1473-3099(13)70245-7 (2013). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Dellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Dellitus 1999.
- Andersen P. et al. Specific immune-based diagnosis of tuberculosis. Lancet. 356, 1099–1104 (2000). [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China. Criteria of Weight for Adults (WS/T428-2013) 2013.
- Lichtash C. T. et al. Body adiposity index versus body mass index and other anthropometric traits as correlates of cardiometabolic risk factors. PLoS One. 8, e65954, doi: 10.1371/journal.pone.0065954 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmer A. et al. Body adiposity index and other indexes of body composition in the SAPHIR study: association with cardiovascular risk factors. Obesity (Silver Spring). 21, 775–781, doi: 10.1002/oby.20289 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


