Abstract
Background
In borderline personality disorder (BPD), attentional bias (AB) to emotional stimuli may be a core component in disorder pathogenesis and maintenance.
Sampling
11 emotional Stroop task (EST) studies with 244 BPD patients, 255 nonpatients (NPs) and 95 clinical controls and 4 visual dot-probe task (VDPT) studies with 151 BPD patients or subjects with BPD features and 62 NPs were included.
Methods
We conducted two separate meta-analyses for AB in BPD. One meta-analysis focused on the EST for generally negative and BPD-specific/personally relevant negative words. The other meta-analysis concentrated on the VDPT for negative and positive facial stimuli.
Results
There is evidence for an AB towards generally negative emotional words compared to NPs (standardized mean difference, SMD = 0.311) and to other psychiatric disorders (SMD = 0.374) in the EST studies. Regarding BPD-specific/personally relevant negative words, BPD patients reveal an even stronger AB than NPs (SMD = 0.454). The VDPT studies indicate a tendency towards an AB to positive facial stimuli but not negative stimuli in BPD patients compared to NPs.
Conclusions
The findings rather reflect an AB in BPD to generally negative and BPD-specific/personally relevant negative words rather than an AB in BPD towards facial stimuli, and/or a biased allocation of covert attentional resources to negative emotional stimuli in BPD and not a bias in focus of visual attention. Further research regarding the role of childhood traumatization and comorbid anxiety disorders may improve the understanding of these underlying processes.
Key Words: Borderline personality disorder, Emotion regulation, Attentional bias, Threat, Visual dot-probe task, Emotional Stroop task, Meta-analysis
Introduction
Borderline personality disorder (BPD) is characterized by instability in affect, self-image, and interpersonal relationships, and by impaired impulse control [1,2]. In the last two decades, research on emotion regulation has been increasingly linked to BPD [3,4], as maladaptive emotion-related cognitive processes have been shown to be related to the onset, development, and maintenance of various emotional disorders [5,6,7]. Studies in emotion-related cognitive processes show a BPD-specific memory bias towards generally negative and BPD-related material, and a BPD-specific cognitive bias towards distorted beliefs and schemata [4], whereas results regarding emotion responding are more mixed [3].
Another central emotion-related cognitive process is attention [8]. In this context, attentional bias (AB) reflects the tendency to preferentially focus or allocate attentional resources to certain types of emotional stimuli [7]. Moreover, AB has been studied in different mental disorders, particularly in anxiety disorders [9], but in other disorders also, such as depression [10] and eating disorders [11]. The results of a meta-analysis [9] reveal evidence of a threat-related AB both at the conscious (i.e. later) and nonconscious (i.e. early, automatic) stages of information processing in patients with anxiety disorders and in nonclinical highly anxious samples, but not in nonanxious subjects.
Regarding BPD, the review of Baer et al. [4] indicates mixed results concerning a BPD-specific AB. Their inconclusive results might be due to differences in experimental designs and paradigms that examine different underlying processes or different aspects of the same cognitive process. The emotional Stroop task (EST [12]) and visual dot-probe task (VDPT [13]) are the most prominent paradigms in AB assessment.
The EST assesses the ability to inhibit stimulus interference [14]. Emotional words in different colors are presented on a computer screen, and participants have to name the color of the word via button/keyboard (corresponding with the color) or verbally (a voice key detects response latency). Thus, two stimuli (i.e. emotional stimulus word vs. color of the word as the stimulus to be attended to) are presented simultaneously - spatially and temporally [15]. Stimuli can differ in their emotional valence and personal relevance to the corresponding psychopathology being examined. Interference is computed by subtracting reaction time (RT) to neutral words from RT to emotional words. Hence, in the EST the emotional stimulus words interfere with color naming in a different way compared to neutral stimuli by capturing attentional resources [16]. Three types of interference scores are distinguished: positive interference scores (attention allocation towards the emotional stimuli), negative interference scores (attention allocation away from the emotion stimuli) and interference scores around zero (no different attention allocation towards or away from emotional stimuli compared to neutral stimuli), indicating that such words capture covert attentional resources, causing deceleration in color-naming the words. Research shows that patients with emotional disorders react more slowly when naming the color when the words displayed are relevant to their psychopathology [14].
The VDPT assesses visual attention allocation. After the offset of a fixation cross, a pair of stimuli (primes), typically emotional and neutral faces or words, appears briefly on the screen. The primes disappear, and a dot (probe) appears either on the former location of the neutral (incongruent trial) or the emotional (congruent trial) prime. The task is to identify the location of the dot by pushing a button as fast as possible. In this context, the so-called congruency effect is defined by faster RT to congruent than to incongruent primes [17]. Positive bias scores are interpreted as attention towards the emotional prime, i.e. persistent vigilance, negative scores as attention away from the prime, i.e. initial vigilance followed by avoidance [18]. Moreover, shorter presentation times of the primes detect hypervigilance towards emotional stimuli; longer presentation times detect difficulties in disengagement, or avoidance [19,20]. Another variant of the dot-probe task uses an arrow pointing either up or down, whereby the arrows substitute the previously presented stimulus. Here, the task is to push one of two buttons to determine the arrow's direction as fast as possible. Still another dot-probe design compares RT on emotional primes with RT on exclusively neutral primes [17]. Vigilance, i.e. the facilitated detection of emotional (negative) information is reflected by faster RT to congruent trials compared with trials with two neutral stimuli. Difficulties in disengagement occur through slower RT to incongruent trials compared with trials with two neutral stimuli.
To sum up, the stimulus to be attended to (i.e. the dot-probe) in the VDPT is presented after the emotional stimulus. Consequently, all emotional and attended stimuli are presented in temporal separation, and half of the emotional and attended stimuli are presented in spatial separation [15]. Thus, the VDPT demands visual orientation and attention resources by scanning across different regions in the visual field [21].
To our knowledge, there has been no quantitative meta-analysis of AB studies in BPD yet. The present study aims to synthesize results from various studies on a quantitative basis, including a comparison between BPD and clinical controls (CCs). We can thus determine whether the AB found in BPD is specific to this disorder or due to psychopathology in general. Our meta-analysis includes experimental studies investigating AB in BPD with the EST [12,14] or the dot-probe task [13] as they are most often used to examine AB in BPD, and enough studies were available to conduct separate meta-analyses for each design.
Methods
Study Selection
We followed PRISMA guidelines for reporting meta-analyses [22]. We searched published sources using the databases PubMed, Medline and PsychInfo until January 2016. Reference lists of relevant articles, previous reviews and Google scholar were studied to identify further relevant sources. Search strategies were adapted to different databases. Key words were: attention*, bias*, threat, Stroop, dot-probe, and borderline*.
We included studies that investigated AB in BPD patients or individuals with BPD symptoms, CCs and/or nonpatients (NPs) (DSM-III-R, DSM-IV or DSM-5 criteria) and used either an EST or a VDPT. We set the limit to a minimum of 4 studies to conduct a meta-analysis. We included studies that enabled the computation of an effect size for at least a between-group comparison in interference scores, i.e. the reported biases measured the differences between BPD participants and NP control participants. Journal articles published until January 2016 were included.
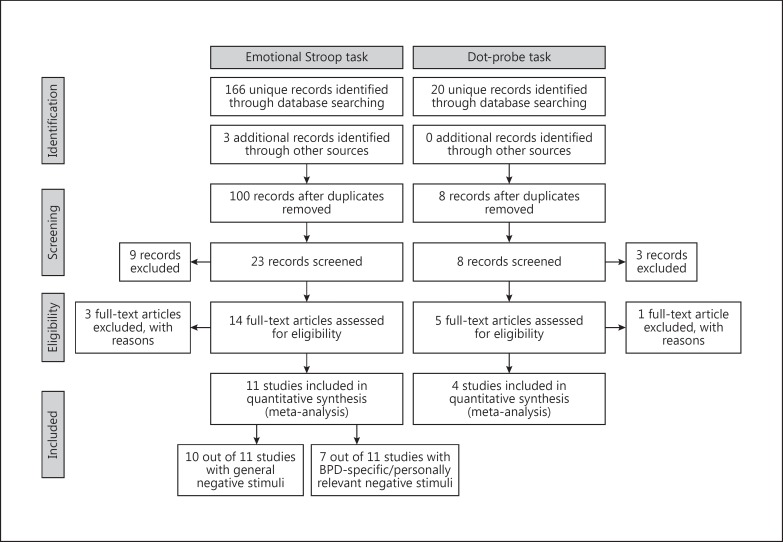
Corresponding authors were contacted if necessary. We received one unpublished study via personal communication [Button, postal delivery, September 21, 1999]. One non-English publication was translated. In total, we included 10 published and 1 unpublished EST involving 244 BPD patients (92% females), 255 NPs (89% females) and 95 CCs (94% females), and 4 VDPT studies involving 151 BPD patients or subjects with BPD features (83% females) and 62 NPs (84% females) (for flowchart, see fig. 1).
Fig. 1.
Flowchart diagram for meta-analyses: summary of the literature search and study selection.
Statistical Analysis
Meta-Analysis of EST Studies
Study characteristics are provided in table 1. We meta-analyzed standardized mean differences (SMDs) of interference scores (table 2), i.e. the difference between groups in mean interference (defined as the difference between RT of the target word compared to that of the neutral word) divided by the pooled standard deviation of interference scores. SMD is also known as Hedges' g and is an unbiased alternative to Cohen's d [23]. SMDs were analyzed instead of RT differences, as not all studies presented the descriptive statistics (means and SD of interference scores per group), and studies varied widely in design, therefore meaning that raw RT could not be directly compared. If descriptive statistics were lacking, we derived SMDs from test statistics of the pertinent group contrasts. In one case a correlation between the bias score and BPD features was reported instead of a group difference [24]. Here we converted Pearson's R to Hedges' g.
Table 1.
Cohort and study characteristics of all EST studies
| First author | Group | n | Females,% | Mean age ± SD, years | In/out | Medication,% | Comorbidity | Inferencescores | Presentation | Button |
|---|---|---|---|---|---|---|---|---|---|---|
| Arntz [30], 2000 | BPD | 16 | 100 | 29.8 | out | n.a. | 2.88AID, 3.31PD | neg.-neu.; neg. (BPD-spec.)-neu. | random | voice |
| CPD | 12 | 32.5 | 2.58AID, 1.83PD | |||||||
| NP | 15 | 35.0 | ||||||||
| Domes [57], 2006 | BPD | 28 | 100 | 24.93 ± 5.85 | in | - | -/- | neg.-neu. | random | button |
| NP | 30 | 23.9 ± 5.88 | ||||||||
| Portella [25], 2011 | BPD | 38 | 100 | 27.42 ± 5.8 | out | 81.6 | n.a. | neg.-neu.; neg. (BPD-spec.)-neu. | random | button |
| NP | 23 | 25.56 ± 2.3 | ||||||||
| Sieswerda [27], 2006 | BPD | 16 | 94 | 27 ± 6.8 | in/out | n.a. | 1.63AID, 1.56PD | neg.-neu.; neg. (BPD-spec.)-neu. | random | voice |
| CPD | 18 | 89 | 29 ± 9.2 | 1.67 1.06PD | ||||||
| AID | 16 | 75 | 30 ± 9.2 | 1−81AID | ||||||
| NP | 16 | 88 | 26 ± 5.4 | |||||||
| Sieswerda [58], 2007 | BPD | 24 | 88 | 29.6 ± 7.2 | out | n.a. | 71%MD, 96%AD, 42%CPD, 25%ASPD | neg.-neu.; neg. (BPD-spec.)-neu. | random | voice |
| NP | 23 | 91 | 34 ± 11 | |||||||
| Sprock [33], 2000 | BPD | 18 | 100 | 37.6 ± 5.3 | out | + | 72.2%DD, 27.8%dysthymia | neg.-neu. | blocks | voice |
| NP | 16 | 30.3 ± 5.9 | ||||||||
| DD | 17 | 32.7 ± 9.7 | ||||||||
| Völker [26], 2009 | BPD | 24 | 100 | 23.17 ± 4.74 | in | - | n.a. | neg.-neu. | random | button |
| DD | 22 | 20.41 ± 1.71 | ||||||||
| NP | 24 | 22.21 ± 4.69 | ||||||||
| Waller [29], 1996 | BPD | 10 | 100 | 26.6 ± 4.74 | in | n.a. | n.a. | neg.-neu. | blocks | voice |
| AID | 10 | 28.5 ± 10.8 | ||||||||
| NP | 20 | 25.1 ± 8.39 | ||||||||
| Wingenfeld [31], 2009 | BPD | 20 | 70 | 29.75 ± 13.2 | in | 60 | 25%PTSD, 25%MD, 15%ED, 5%SP, 5%SPD | neg.-neu.; neg. (pers.)-neu. | blocks | button |
| NP | 20 | 70 | 29.45 ± 12.4 | |||||||
| Wingenfeld [32], 2009 | BPD | 31 | 67.74 | 28.2 ± 11.1 | in | n.a. | 83.88%AID, 51.61%PTSD | neg.-neu.; neg. (pers.)-neu. | blocks | button |
| NP | 49 | 61.22 | 32.4 ± 11.8 | |||||||
| Winter [28], 2015 | BPD | 19 | 100 | 28.74 ± 8.07 | n.a. | - | 37%PTSD, 11%MD, 60%AD | neg.-neu. | blocks | button |
| NP | 19 | 28.05 ± 7.82 | ||||||||
F = Female; in/out = inpatients/outpatients; n.a. = not applicable; AID = axis I disorders; PD = personality disorder; neg. = negative; neu. = neutral; CPD = cluster C personality disorder; BPD-spec. = BPD-specific; MD = mood disorder; AD = anxiety disorder; ASPD = antisocial personality disorder; DD = depressive disorder; EgoThreat = Ego-threat words pooled; PTSD = posttraumatic stress disorder; ED = eating disorder; SP = social phobia; SPD = somatoform pain disorder; pers. = personal relevant words.
Table 2.
Descriptive and statistical data of the meta-analysis of experimental studies using the EST
| First author | BPD group |
CC group |
SD pooled | SMD | 95% CI |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | lower | upper | ||||
| BPD versus NPs | |||||||||
| Arntz [30], 2000 | 16 | 40.46 ± 63.15 | 15 | 11.45 ± 38.79 | 54.22 | 0.535 | −0.182 | 1.252 | |
| Domes [57], 2006 | 28 | 12.37 ± 52.88 | 30 | 19.29 ± 44.84 | 49.43 | −0.140 | −0.655 | 0.376 | |
| Portella [25], 2011 | 38 | 14.31 ± 63.57 | 23 | 17.06 ± 91.18 | 76.06 | −0.036 | −0.554 | 0.482 | |
| Sieswerda [27], 2006 | 16 | 32.4 ± 40.7 | 16 | −1.35 ± 18.2 | 32.33 | 1.044 | 0.305 | 1.782 | |
| Sieswerda [58], 2007 | 24 | 27.26 ± 40.85 | 23 | 3.82 ± 23.51 | 34.06 | 0.688 | 0.099 | 1.277 | |
| Völker [26], 2009 | 24 | 2.94 ± 25.59 | 24 | −2.46 ± 21.81 | 23.775 | 0.223 | −0.344 | 0.791 | |
| Waller [29], 1996 | 10 | 13.3 ± 14.64 | 20 | 3.2 ± 7.81 | 10.79 | 0.936 | 0.141 | 1.731 | |
| Wingenfeld [31], 2009 | 20 | 17.6 ± 34.5 | 20 | 5.2 ± 34.5 | 35.23 | 0.352 | −0.272 | 0.977 | |
| Wingenfeld [32], 2009 | 31 | 14 ± 44 | 49 | 15 ± 39 | 41.67 | −0.024 | −0.474 | 0.426 | |
| Winter [28], 2015 | 19 | 11.7 ± 29.68 | 19 | 4.00 ± 39.36 | 35.65 | 0.216 | −0.421 | 0.854 | |
| BPD versus CCs | |||||||||
| Arntz [30], 2000 | 12 | 40.46 ± 63.15 | 12 | 29.44 ± 29.03 | 52.99 | 0.208 | −0.543 | 0.958 | |
| Sieswerda [27], 2006 | 16 | 32.4 ± 40.7 | 34 | 17.5 ± 23.73 | 30.53 | 0.488 | −0.114 | 1.089 | |
| Völker [26], 2009 | 24 | 2.94 ± 25.59 | 22 | − 2.25 ± 23.72 | 24.194 | 0.206 | −0.374 | 0.786 | |
| Waller[29], 1996 | 10 | 13.3 ± 14.64 | 10 | 4.467 ± 5.37 | 11.516 | 0.767 | −0.141 | 1.676 | |
| CCs versus NPs | |||||||||
| Arntz [30], 2000 | 12 | 29.44 ± 29.03 | 15 | 11.45 ± 38.79 | 35.90 | 0.501 | −0.270 | 1.272 | |
| Sieswerda [27], 2006 | 34 | 17.5 ± 23.73 | 16 | −1.350 ± 18.20 | 22.49 | 0.838 | 0.221 | 1.454 | |
| Völker [26], 2009 | 22 | −2.25 ± 23.72 | 24 | −2.46 ± 21.810 | 23.33 | 0.009 | −0.569 | 0.588 | |
| Waller [29], 1996 | 10 | 4.467 ± 5.37 | 20 | 3.2 ± 7.81 | 7.32 | 0.173 | −0.587 | 0.934 | |
In total, 11 studies were included in this meta-analysis. All studies presented the stimuli words supraliminally. Five studies included besides a NP control group, also a CC group. SMDs could not be estimated in 2 studies [25,26], but both first authors provided us with the necessary descriptive statistics [Portella, pers. commun., April 10, 2014; Völker, pers. commun., May 16, 2014]. For 1 study [27], we collapsed the two CC groups they used. In another study [28], one BPD subgroup underwent a dissociation induction before the EST. We did not include that BPD subgroup. We focused on interference scores for generally negative versus neutral and for BPD-specific negative/personally relevant versus neutral word comparisons. Ten of the 11 EST studies used generally negative words as stimuli (table 2a), and 7 studies used BPD-specific/personally relevant stimuli (table 2b). We merged the different categories of negative words from the Waller and Button [29] study into one category of generally negative stimuli. These stimulus words were being considered as generally negative words, since they had been used in a study on bulimia nervosa patients. The fourth author provided us with the descriptive data on the interference scores of generally negative-neutral words and the BPD-specific negative-neutral words, which had been merged in the original study [30]. The 2 studies by Wingenfeld et al. [31,32] utilized individual personally negative words with and without current relevance. In our meta-analysis, we focused exclusively on the individual personal negative words with current relevance. Sprock et al. [33] stated that they used anger words as BPD-specific stimuli and sad stimuli as depression-specific stimuli. Therefore, we included only the interference scores of anger-neutral words in the meta-analysis of EST studies for BPD-specific/personal relevant words.
Positive words were only used in 3 of the 11 EST studies [25,27,28]. Since we had set our limit to a minimum of 4 studies to conduct a meta-analysis, no meta-analysis of EST studies with positive words was made. However, exploration suggests no evidence for an AB towards positive words (SMD = -0.301, SE = 0.259, p = 0.245, 95% CI = -0.810 to 0.207).
OpenMetaAnalyst was used for computations [34]. Given the large variations between study designs, random effects models (DerSimonian-Laird estimate) were used. We assessed the sensitivity of the results for finding by a particular study by leave-one-out analyses. Meta-regression analyses were conducted for verbal versus button-pressing response type and random versus nonrandom (presented in one block or card) word category presentation (blocked presentations have been criticized as potentially triggering processes other than AB, e.g. worrying). Meta-regression analyses were done for measures that had been reported by at least 8 studies.
From one study [26] valence of the emotional words, response type and word category presentation (random vs. nonrandom) was not clear. The first author provided us with this information [Völker, pers. commun., May 16, 2014].
Meta-Analysis of VDPT Studies
Study characteristics and SMDs are provided in tables 3 and 4. In the meta-analysis of the VDPT studies, they were classified into 3 categories according to stimulus presentation time: subliminal (30 ms; 1 study) [35], medium (200-500 ms; 4 studies) [24,35,36,37] and lengthy (≥1,250 ms; 2 studies) [24,38], as early and later attentional processes often differ. Only the medium presentation category contained 4 studies that investigated AB for positive and negative faces, and thus enough studies to warrant a meta-analysis. Effect sizes were pooled when several classes of negative stimuli were studied [35]; one study already had collapsed data in this group [36]. SMDs of the AB index of the 4 studies were analyzed with OpenMetaAnalyst. Positive SMDs denote an AB towards emotional faces, negative SMDs an AB away from emotional faces (avoidance).
Table 3.
Cohort and study characteristics of all dot-probe studies
| First author | Group | n | Females, % | Mean age ± SD, years | In/out | Medication, % | Comorbidity | Presentation time, ms | Emotional face | Examined AB |
|---|---|---|---|---|---|---|---|---|---|---|
| Berenson [24]1, 2009 | BPD | 43 | 79.31 | 22.75 ± 5.57 | NP | -/- | -/- | 500 | threat | RTincongruent to RTcongruent |
| NP | 44 | pleasant | ||||||||
| Brüne [37], 2013 | BPD | 13 | 61.54 | 26.8 ± 7.22 | in | + | n.a. | 200 + 5002 | angry | RTincongruent to RTcongruent |
| NP | 13 | 76.92 | 25.7 ± 6.79 | happy | ||||||
| Von Ceumern-Lindenstjerna [36], 2010 | BPD | 30 | 100 | 16.13 ± 1.48 | in/out | 6.6 | 100% with ≥1comorbid AID | 500 | negative | RTincongruent to RTcongruent |
| AID | 29 | 100 | 15.31 ± 1.11 | 10.34 | 100% with ≥1comorbid AID | positive | ||||
| NP | 30 | 100 | 15.73 ± 1.46 | |||||||
| Jovev [35], 2012 | BPD | 21 | 85.71 | 18.90 ± 3.1 | out | +3 | 100% with ≥1comorbid AID | 500 | fear-angry | RTbias = RTincongruent to RTcongruent |
| pooled | RTvigilance = RTneutral to RTcongruent | |||||||||
| NP | 20 | 60 | 20.4 ± 2.72 | happy | RTadherence = RTincongruent to RTneutral | |||||
F = Female; in/out = inpatients/outpatients; PT = psychotherapy; n.a. = not applicable; AID = axis I disorders.
Nonclinical adolescents with high and low features of BPD.
Data were pooled.
Antidepressives (n = 52), mood stabilizers (n = 10), and antipsychotics (n = 5).
Table 4.
Descriptive and statistical data of the meta-analysis of experimental studies using the VDPT: BPD versus NPs
| First author | BPD, n | NPs, n | SMDs | 95% CI |
|
|---|---|---|---|---|---|
| lower | upper | ||||
| Negative versus neutral faces | |||||
| Berenson [24]1, 2009 | 43 | 44 | −0.140 | −0.560 | 0.280 |
| Brüne [37], 2013 | 13 | 13 | −1.115 | −1.884 | −0.346 |
| Von Ceumern-Lindenstjerna [36], 2010 | 30 | 30 | 0.539 | −0.073 | 1.151 |
| Jovev [35], 2012 | 21 | 30 | 0.058 | −0.448 | 0.564 |
| Positive versus neutral faces | |||||
| Berenson [24]1, 2009 | 43 | 44 | 0.181 | −0.239 | 0.601 |
| Brüne [37], 2013 | 13 | 13 | 0.247 | −0.522 | 1.016 |
| Von Ceumern-Lindenstjerna [36], 2010 | 30 | 30 | 0.448 | 0.090 | 0.806 |
| Jovev [35], 2012 | 21 | 20 | −0.054 | −0.482 | 0.374 |
Nonclinical adolescents with high and low features of BPD.
Results
Meta-Analysis of EST Studies
BPD versus NPs
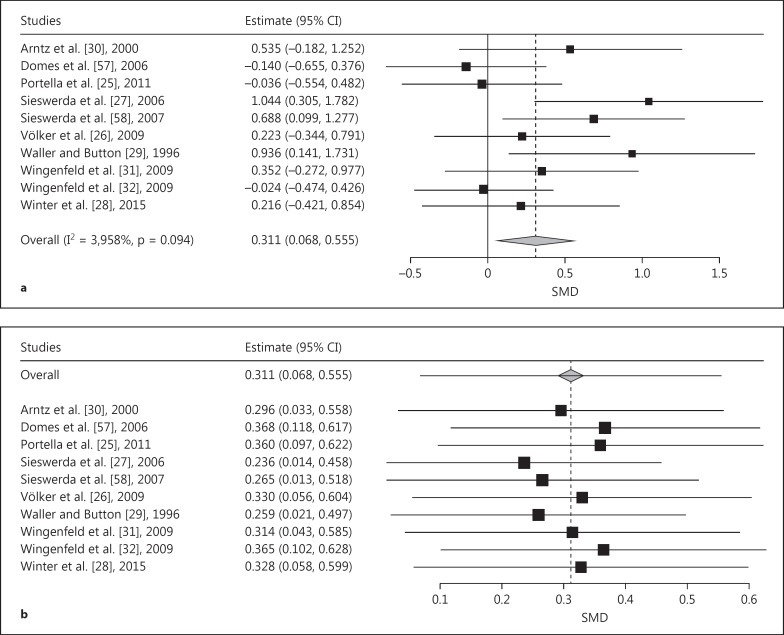
The pooled effect size for generally negative words was in the small-to-medium range (SMD = 0.311, SE = 0.124, p = 0.001) and significantly favors the hypothesis that BPD is characterized by an AB towards generally negative stimuli (fig. 2a). The leave-one-out analysis did not cause the pooled EST to become nonsignificant and indicated robust results (fig. 2b). The meta-regression resulted in a nonsignificant effect of random versus blocked stimulus category presentation (p = 0.880); however, we observed a significant effect of response type (β = -0.713, SE = 0.21, p = 0.001). The 6 studies using button press responses failed to yield a significant pooled effect (pooled SMD = 0.07, 95% CI = -0.16 to 0.29), whereas the 4 studies using voice detection exhibited a significant pooled effect (SMD = 0.78, 95% CI = 0.43-1.13).
Fig. 2.
a Forest plot of a meta-analysis of EST studies comparing patients with BPD with NPs. Positive effects denote stronger AB in BPD for generally negative emotional words. SMDs of interference scores were meta-analyzed. b Leave-one-out meta-analysis plot of the EST studies comparing patients with BPD with NPs and using generally negative emotional words as stimuli. The pooled SMDs prove to be quite stable when individual studies are omitted.
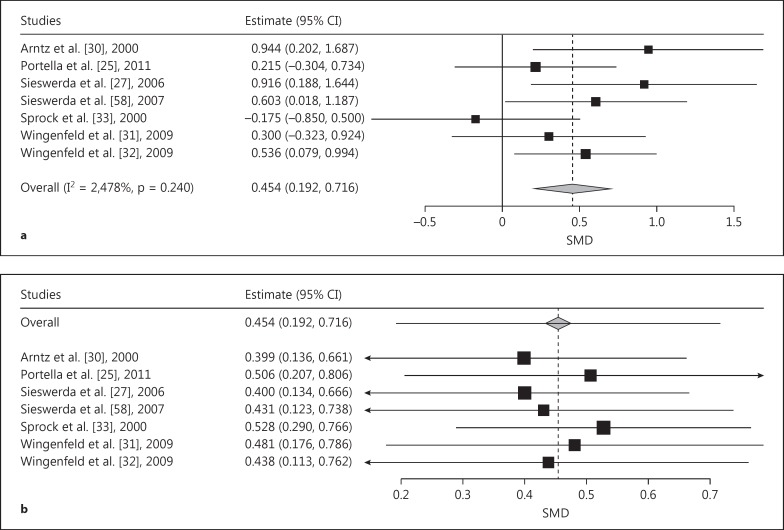
The pooled effect size for BPD-specific negative words was medium (SMD = 0.454, SE = 0.134, p < 0.001) and is significantly in line with the hypothesis that BPD is defined by an AB towards BPD-specific stimuli (fig. 3a). Again, the leave-one-out analysis did not cause the pooled ES to become nonsignificant and indicated robust results (fig. 3b). The meta-regression resulted in a nonsignificant effect of random versus blocked stimulus category presentation (β = -0.271, SE = 0.229, p = 0.236) and of response type (β = 0.017, SE = 0.230, p = 0.454).
Fig. 3.
a Forest plot of a meta-analysis of EST studies comparing patients with BPD with NPs. Positive effects denote stronger AB in BPD for BPD-specific and personally relevant negative emotional words. SMDs of interference scores were meta-analyzed. b Leave-one-out meta-analysis plot of the EST studies comparing patients with BPD with NPs and using BPD-specific and personally relevant negative emotional words as stimuli. The pooled SMDs prove to be quite stable when individual studies are omitted.
BPD versus CCs
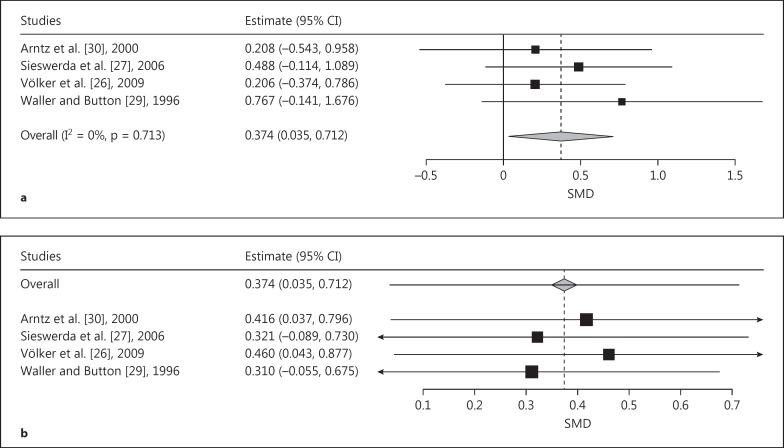
Four studies using generally negative stimuli words reported BPD versus CC comparisons. The pooled effect size was in the small-to-medium domain (SMD = 0.374, SE = 0.173, p = 0.031; fig. 4a) and significantly favored a stronger AB in BPD than in CCs. The pooled SMD remained in the same domain with the leave-one-out analysis (-0.089 to 0.877), although 95% CIs covered zero when we omitted Sieswerda et al. [27] or Waller and Button [29] (fig. 4b). Given the low number of studies, a meta-regression was not conducted to explore further results. However, we found that omitting the study of Völker et al. [26] (which did not use a voice-based response) led to a greater effect (SMD = 0.460, SE = 0.213, p = 0.031). Due to the low number of studies that used BPD-specific or personally relevant negative stimuli and reported BPD versus CC comparisons, we did not conduct meta-analysis in this case.
Fig. 4.
a Forest plot of a meta-analysis of EST studies comparing patients with BPD with CCs. Positive effects denote stronger AB in BPD for generally negative emotional words. SMDs of interference scores were meta-analyzed. b Leave-one-out meta-analysis plot of the EST studies comparing patients with BPD with CCs and using generally negative words as stimuli. The effect remains in the range from -0.09 to 0.88, though leaving out the study of Sieswerda et al. [27] or of Waller and Button [29] leads to the 95% CI covering zero.
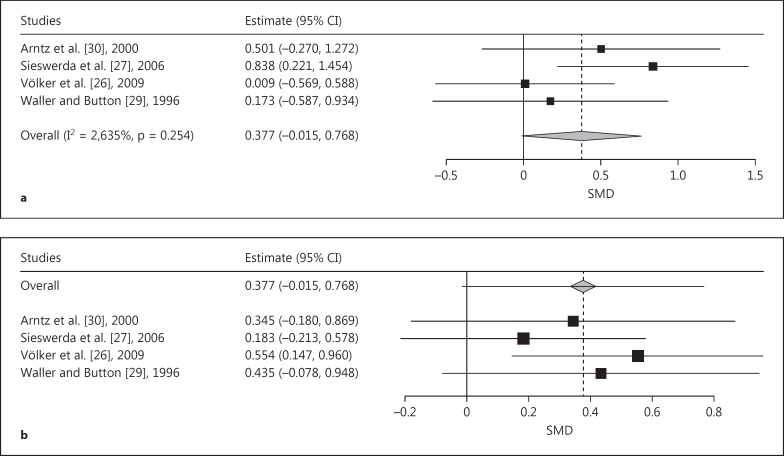
CCs versus NPs
The comparison of CCs with NPs revealed a trend towards a significant pooled effect size of a stronger AB in CCs than in NPs (SMD = 0.377, SE = 0.200, p = 0.059; fig. 5a). The leave-one-out analysis indicated wide variance in the pooled SMD from 0.183 (leaving out Sieswerda et al. [27]; fig. 5b) to 0.544 (leaving out Völker et al. [26]). Only omission of the study by Völker et al. [26] led to a significant effect (SMD = 0.554, SE = 208, p = 0.008).
Fig. 5.
a Forest plot of a meta-analysis of EST studies comparing CCs with NPs. Positive effects denote a trend towards a stronger AB for generally negative emotional words in CCs. SMDs of interference scores were meta-analyzed. b Leave-one-out meta-analysis plot of the EST studies comparing CCs with NPs and using generally negative words as stimuli. The leave-one-out analysis indicated a wide variance in the pooled SMD from 0.183 (leaving out the study of Völker et al. [26]) to 0.544 (leaving out the study of Sieswerda et al. [27]).
Meta-Analysis of VDPT Studies
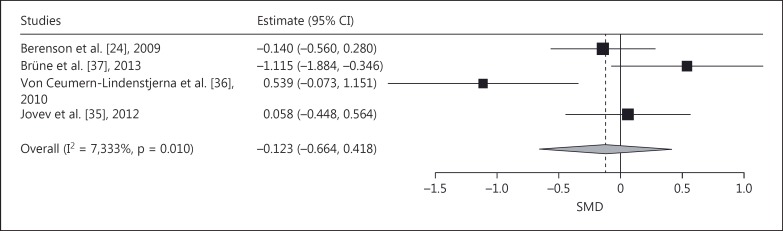
As to the negative versus neutral faces contrast, the meta-analysis revealed no evidence for a significant pooled effect (pooled SMD = -0.123, SE = 0.276, p = 0.66; fig. 6). Leaving individual studies out, the analysis yielded evidence for a significant effect.
Fig. 6.
Forest plot of a meta-analysis of the dot-probe studies with negative versus neutral faces and with medium presentation time (200-500 ms) comparing patients with BPD with NPs. SMDs denote AB toward, negative SMDs AB away from negative faces (avoidance).
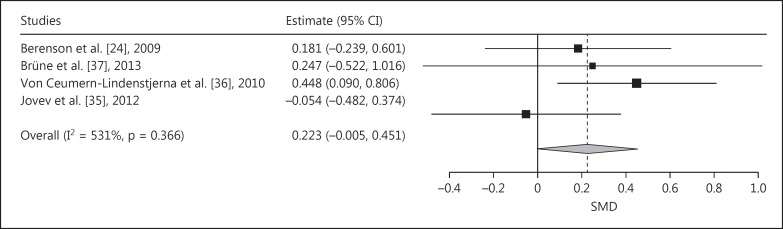
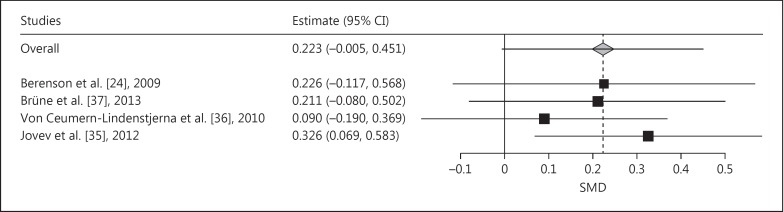
Meta-analysis of the SMDs of the difference between BPD and NPs of the AB index towards positive faces (vs. neutral faces) demonstrated a positive pooled SMD (indicating AB towards positive faces), which almost reached significance (pooled SMD = 0.223, SE = 0.116, p = 0.055; fig. 7). Leaving the study of Jovev et al. [35] out of the analysis would lead to a significant AB towards positive faces (pooled SMD = 0.326, p < 0.05; fig. 8).
Fig. 7.
Forest plot of a meta-analysis of the dot-probe studies with positive versus neutral faces and with medium presentation time (200-500 ms) comparing patients with BPD with NPs. Positive SMDs denote AB toward positive faces, negative SMDs AB away from positive faces.
Fig. 8.
Leave-one-out meta-analysis plot of the dot-probe studies with positive versus neutral faces and with medium presentation time (200-500 ms) comparing patients with BPD with NPs. Positive SMDs denote AB toward positive faces, negative SMDs AB away from positive faces.
Discussion
The present study is the first meta-analysis investigating AB in BPD. We conducted two separate meta-analyses focusing on AB in BPD assessed with either the EST or with the VDPT.
Our meta-analysis of the EST studies revealed evidence for the existence of an AB to generally negative stimuli in BPD compared to NPs and CCs. Compared to NPs, AB in BPD became stronger when we excluded studies using button press rather than oral response. Thus, the increased AB for generally negative stimuli seems to be specific to BPD. In addition, we observed clear and relatively stronger evidence of an AB towards BPD-specific/personally relevant negative stimuli in BPD patients compared to NPs. These results therefore suggest the existence of a threat-related AB in BPD patients which becomes stronger when the negative stimulus words are BPD schema congruent. The AB towards BPD-schema-congruent stimulus words in BPD is comparable to that seen in anxiety disorders [9]. Furthermore, we noted a trend towards evidence of an AB to generally negative words in CCs compared to NPs.
Cisler and Koster [39] claimed there is evidence for the AB effect's equal magnitude in all anxiety disorders and that threat-related AB in anxiety disorders can be observed in several different paradigms. Indeed, an AB for threat is commonly observed across anxiety disorders [9,14,39]. Anxiety disorders were represented in CCs of the EST studies, either as the primary diagnosis or (probably) a secondary diagnosis (e.g. many cluster C personality disorder patients have comorbid anxiety disorders). However, the CC groups' heterogeneity might explain the smaller effect size in CCs in the present study, and comparisons between BPD and anxiety disorder groups might be worth pursuing.
Interestingly, verbal responses yielded much stronger AB effects to generally negative stimuli than button pressing. This is in line with findings indicating that the response type influences the magnitude of the Stroop effect, or, to be more precise, using verbal responses exhibited higher interference scores than using button responses [40,41]. This was also found regarding the EST in a clinical sample of people who stutter [42]. The EST effect might thus arise mainly with verbal responses, probably because reading and speaking are intrinsically related (reading automatically leading to the preparation of speaking), which is not the case for reading and pressing a button. Perhaps the Stroop effect's underlying cognitive process is masked by the variance in reaction times arising from manual responses (i.e. like button pressing) due to the additionally required motoric involvement, unlike the EST effect with voice detection [43]. The EST involving the verbal response type might therefore yield a more pure and reliable estimate of interference by word content. However, verbal responses yielded no stronger AB effects towards BPD-specific/personally relevant negative stimuli. This might be due to the lower number of studies using button response and BPD-specific/personally relevant negative stimuli.
Not all EST studies included in the present meta-analysis reported details on design and statistical outcomes. Word contents were often not reported, as were attempts to control for word length and word use in the general population. Lacking information on these details makes it impossible to assess to what degree the AB in BPD is dependent on the relevance of the words' content for BPD.
Our meta-analysis of the VDPT studies revealed inconclusive findings. There was no evidence for an AB in BPD for negative emotional faces in conjunction with medium presentation times (200-500 ms), but there was initial evidence for an AB for positive emotional faces in BPD compared to NPs - but not in the study of Jovev et al. [35]. These results contradict Linehan's [44] postulation of hypersensitivity to any emotional stimuli in BPD patients. We cannot take a position as to whether the AB in BPD is borderline specific, since only 1 study included a patient control group [36]. These inconclusive results could be due to different patient cohorts (e.g. NPs [24] vs. adolescents not meeting the full threshold of BPD criteria [35,36] vs. adults fulfilling all the BPD criteria [37]).
Moreover, findings from the dot-probe studies are limited as our meta-analysis was only feasible for stimuli presented at a medium range of 200-500 ms. For stimuli presented in a shorter or longer time range, a meta-analysis was impossible due to the paucity of studies. Further research is needed to investigate the association between different attentional stages, the valence of facial stimuli, and their effects on the AB in BPD via the VDPT. In addition, some studies reported very poor reliability estimates of the dot-probe task [45,46], which might explain the inconsistent findings in the literature. To improve the facial dot-probe task's sensitivity to detect subtle group differences in visual attention, a greater effort is needed to increase its reliability [47].
Another possible reason why our meta-analysis supports AB in BPD as assessed with the EST, but not the VDPT, has to do with the fact that the two paradigms assess different underlying mechanisms or different aspects of the same cognitive process. The EST assesses the degree to which processing resources are captured by a word's emotional content. Since the emotional and attended stimuli in the EST are presented spatially and temporally simultaneously, it is not visual but rather covert attention that is involved. In contrast, the dot-probe task assesses the focus of visual attention by requiring that the field of view be scanned for the attended stimuli that do not coincide with the emotional stimuli. Furthermore, the EST usually uses verbal stimuli rather than pictures. Those words covered very different domains in the studies we included, while the pictures in the dot-probe tasks were always emotional human faces. On the other hand, words might represent more accurately the typical stimuli for which BPD patients might be vigilant for (like abuse, abandonment, rejection) than emotional faces. Moreover, BPD patients might find neutral faces ambiguous [48] and difficult to tolerate; they might be as attention-getting as emotional faces (e.g. in creating anxiety [49]), making the control stimulus category ineffective in the studies so far. One conceivable approach to test whether BPD reveals an AB to any face could be to compare faces and nonfacial stimuli [50].
Thus, the evidence for EST effects and the absence of dot-probe effects in BPD might indicate that it is not the focus of visual attention that is biased in BPD, but rather the processing of emotional material. However, whether such differences are due to the different processes or stimuli involved cannot be judged from our analyses. Further research is needed to clarify this issue.
The present study has some limitations. Conclusions have been drawn from studies applying very different methods and experimental designs. We only partly reached our goal to specifically focus on the comparison between BPD and CCs because of the scarcity of studies that include CCs - a problem that continues to frustrate our field and that hinders us from properly assessing the degree to which AB is specific to BPD. Other disorders (e.g. anxiety or depression) are known to be characterized by AB. Although CCs of the studies we meta-analyzed included patients with these disorders, it is not possible at the moment to clarify whether the AB in BPD assessed via the EST is comparable to or stronger than the AB in other CCs, or whether stimulus specificity plays a role. Moreover, we need to disentangle the influence of BPD and traumatic experiences/posttraumatic stress disorder on bias attention processes, since traumatic experiences are very common in BPD subjects; nearly all of them report some kind of abuse [51], and up to 70% report having suffered childhood sexual abuse [52]. Thirty to 60% of BPD patients reveal comorbid posttraumatic stress disorder [53,54].
Finally, the severity of BPD and of comorbid disorders, as well as medications, should be taken into account. The majority of the studies we analyzed had patient cohorts consisting of mainly or entirely females. Research is needed to draw conclusions from results on male patients also. Concerning the divergent results of the meta-analysis of the VDPT and EST studies, note that only 4 studies could be included in the meta-analysis of the VDPT studies whereas the meta-analysis of the EST studies included 11.
Moreover, both the EST and VDPT studies reported on cohorts with either inpatients or outpatients or in- and outpatients. Some studies did not inform whether their samples consisted of in- or outpatients [28], or if inpatients had just begun treatment or had already recovered in response to (inpatient) treatment [37]. All in all, the potential influence of the in- versus the outpatient setting cannot be excluded.
It should be noted that paradigms other than EST and VDPT have been used to examine attentional preferences and biases in BPD, such as the ‘attentional blink paradigm’ [55] and visual search tasks [56]. We could not include those studies in this meta-analysis because of the low number of published studies.
Conclusion
Our results from this meta-analysis of the EST studies display evidence of an AB for generally negative words and even stronger AB effects for BPD-specific/personally relevant words in BPD patients, an effect that seems to be specific for BPD and not for its psychopathology in general. The results of our meta-analysis of the VDPT studies do not confirm an AB for visual attentional focus for negative and/or threatening faces. Interestingly, the present results from the VDPT meta-analysis indicate a tendency towards an AB to positive facial stimuli in BPD. In sum, we can assume that not visual attentional, but rather covert attentional resources are drawn by threat signals in BPD patients. Alternatively, words better represent the specific threats that are relevant for BPD than do emotional faces. Further research into the impact of childhood traumatization and/or comorbid diagnoses like anxiety disorders and posttraumatic stress disorder will help to shed light onto (threat-related) AB in BPD.
b.
With BPD-specific or personally relevant negative stimuli
| First author | BPD group |
CC group |
SD pooled | SMD | 95% CI |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | lower | upper | ||||
| BPD versus NPs | |||||||||
| Arntz [30], 2000 | 16 | 28.45 ± 29.98 | 15 | 0.37 ± 27.84 | 29.75 | 0.944 | 0.202 | 1.687 | |
| Portella [25], 2011 | 38 | 13.04 ± 78.29 | 23 | −4.494 ± 84.33 | 81.56 | 0.215 | −0.304 | 0.734 | |
| Sieswerda [27], 2006 | 16 | 8.48 ± 50.7 | 16 | −28.3 ± 22.2 | 40.16 | 0.916 | 0.188 | 1.644 | |
| Sieswerda [58], 2007 | 24 | 19.09 ± 37.24 | 23 | 0.1 ± 23.58 | 31.83 | 0.603 | 0.018 | 1.187 | |
| Sprock [33], 2000 | 18 | −0.33 ± 6.03 | 16 | 0.75 ± 6.03 | 6.17 | −0.175 | −0.850 | 0.500 | |
| Wingenfeld [31], 2009 | 20 | 41.1 ± 31.5 | 20 | 29.6 ± 42.7 | 38.33 | 0.300 | −0.323 | 0.924 | |
| Wingenfeld [32], 2009 | 31 | 67 ± 78 | 49 | 36 ± 39 | 57.84 | 0.536 | 0.079 | 0.994 | |
Acknowledgment
This research was conducted in the program ‘Open Research Area in the Social Sciences’, funded by the Netherlands' Organization for Scientific Research (NWO) to A.A. (464-10-080) and the German Research Foundation to G.A.J. and G.D. (JA1785/3-1).
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 2.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: a review of the research literature. Clin Psychol Rev. 2008;28:75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Baer R, Peters JR, Eisenlohr-Moul T, Geiger PJ, Sauer SE. Emotion-related cognitive processes in borderline personality disorder: a review of the empirical literature. Clin Psychol Rev. 2012;32:359–369. doi: 10.1016/j.cpr.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Alloy LB, Riskind JH. Cognitive Vulnerability to Emotional Disorders. Hillsdale: Erlbaum; 2006. [Google Scholar]
- 6.Harvey A, Watkins E, Mansell W, Shafran R. Cognitive Behavioural Processes across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford: University of Oxford; 2004. [Google Scholar]
- 7.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 8.Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2:271–299. [Google Scholar]
- 9.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Epp A, Dobson KS, Dozois DJ, Frewen PA. A systematic meta-analysis of the Stroop task in depression. Clin Psychol Rev. 2012;32:316–328. doi: 10.1016/j.cpr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychol Med. 2010;40:1887–1897. doi: 10.1017/S0033291710000036. [DOI] [PubMed] [Google Scholar]
- 12.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 13.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Brosschot JF, De Ruiter C, Kindt M. Processing bias in anxious subjects and repressors, measured by emotional Stroop interference and attentional allocation. Pers Individ Dif. 1999;26:777–793. [Google Scholar]
- 16.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cogn Emot. 2000;14:789–808. [Google Scholar]
- 19.Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in sub-clinical anxiety? J Exp Psychol. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- 20.Fox E, Russo R, Bowels R, Dutton K. Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cogn Emot. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogg K, Bradley BP, Dixon C, Fisher S, Twelftree H, McWilliams A. Trait anxiety, defensiveness and selective processing of threat: An investigation using two measures of attentional bias. Pers Individ Dif. 2000;28:1063–1077. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Academia and clinic annals of internal medicine preferred reporting items for systematic reviews and meta-analyses. Annu Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, Version 5. Chichester: Wiley & Sons; 2008. [Google Scholar]
- 24.Berenson KR, Gyurak A, Ayduk O, Downey G, Garner MJ, Mogg K, et al. Rejection sensitivity and disruption of attention by social threat cues. J Res Pers. 2009;43:1064–1072. doi: 10.1016/j.jrp.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portella MJ, Soler J, Tejero A, Barrachina J, Tiana T, Pascual JC, et al. Slow processing in borderline personality disorder: The Emotional Stroop Paradigm. Actas Esp Psiquiatr. 2011;39:356–362. [PubMed] [Google Scholar]
- 26.Völker KA, Spitzer C, Limberg A, Grabe H-J, Freyberger HJ, Barnow S. Executive dysfunctions in female patients with borderline personality disorder with regard to impulsiveness and depression. Psychother Psychosom Med Psychol. 2009;59:264–272. doi: 10.1055/s-2008-1067437. [DOI] [PubMed] [Google Scholar]
- 27.Sieswerda S, Arntz A, Mertens I, Vertommen S. Hypervigilance in patients with borderline personality disorder: specificity, automaticity, and predictors. Behav Res Ther. 2006;45:1011–1024. doi: 10.1016/j.brat.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Winter D, Krause-Utz A, Lis S, Chiu C, Lanius RA, Schriner F, et al. Dissociation in borderline personality disorder: disturbed cognitive and emotional inhibition and its neural correlates. Psychiatry Res Neuroimaging. 2015;233:339–351. doi: 10.1016/j.pscychresns.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Waller G, Button J. Processing of threat cues in borderline personality disorder. 1996 Unpublished. [Google Scholar]
- 30.Arntz A, Appels C, Sieswerda S. Hypervigilance in borderline disorder: a test with the emotional Stroop paradigm. J Pers Disord. 2000;14:366–373. doi: 10.1521/pedi.2000.14.4.366. [DOI] [PubMed] [Google Scholar]
- 31.Wingenfeld K, Rullkoetter N, Mensebach C, Beblo T, Mertens M, Kreisel S, et al. Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology. 2009;34:571–586. doi: 10.1016/j.psyneuen.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Wingenfeld K, Mensebach C, Rullkoetter N, Schlosser N, Schaffrath C, Woermann FG, et al. Attentional bias to personally relevant words in borderline personality disorder is strongly related to comorbid posttraumatic stress disorder. J Pers Disord. 2009;23:141–155. doi: 10.1521/pedi.2009.23.2.141. [DOI] [PubMed] [Google Scholar]
- 33.Sprock J, Rader TJ, Kendall JP, Yoder CY. Neuropsychological functioning in patients with borderline personality disorder. J Clin Psychol. 2000;56:1587–1600. doi: 10.1002/1097-4679(200012)56:12<1587::AID-9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:1–12. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovev M, Green M, Chanen A, Cotton S, Coltheart M, Jackson H. Attentional processes and responding to affective faces in youth with borderline personality features. Psychiatry Res. 2012;199:44–50. doi: 10.1016/j.psychres.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Von Ceumern-Lindenstjerna I-A, Brunner R, Parzer P, Mundt C, Fiedler P, Resch F. Initial orienting to emotional faces in female adolescents with borderline personality disorder. Psychopathology. 2010;43:79–87. doi: 10.1159/000274176. [DOI] [PubMed] [Google Scholar]
- 37.Brüne M, Ebert A, Kolb M. Oxytocin influences avoidant reactions to social threat in adults with borderline personality disorder. Hum Psychopharmacol Clin Exp. 2013;28:552–561. doi: 10.1002/hup.2343. [DOI] [PubMed] [Google Scholar]
- 38.Von Ceumern-Lindenstjerna I-A, Brunner R, Parzer P, Mundt C, Fiedler P, Resch F. Attentional bias in later stages of emotional information processing in female adolescents with borderline personality disorder. Psychopathology. 2010;43:25–32. doi: 10.1159/000255960. [DOI] [PubMed] [Google Scholar]
- 39.Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin Psychol Rev. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClain L. Effects of response type and set size on Stroop color-word performance. Percept Mot Skills. 1983;56:735–743. doi: 10.2466/pms.1983.56.3.735. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler DD. Locus of interference on the Stroop test. Percept Mot Skills. 1977;45:263–266. doi: 10.2466/pms.1977.45.1.263. [DOI] [PubMed] [Google Scholar]
- 42.Hennessey NW, Dourado E, Beilby JM. Anxiety and speaking in people who stutter: an investigation using the emotional Stroop task. J Fluency Disord. 2014;40:44–57. doi: 10.1016/j.jfludis.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda Y, Hirata S, Okuzumi H, Kokubun M. Features of Stroop and reverse-Stroop interference: analysis by response modality and evaluation. Percept Mot Skills. 2010;110:654–660. doi: 10.2466/PMS.110.2.654-660. [DOI] [PubMed] [Google Scholar]
- 44.Linehan MM. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York: Guilford; 1993. [Google Scholar]
- 45.Schmukle SC. Unreliability of the dot probe task. Eur J Pers. 2005;19:595–605. [Google Scholar]
- 46.Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychol Sci Q. 2009;51:339–350. [Google Scholar]
- 47.Waechter S, Nelson AL, Wright C, Hyatt A, Oakman J. Measuring attentional bias to threat: reliability of dot probe and eye movement indices. Cogn Ther Res. 2013;38:313–333. [Google Scholar]
- 48.Daros AR, Zakzanis KK, Ruocco AC. Facial emotion recognition in borderline personality disorder. Psychol Med. 2013;43:1953–1963. doi: 10.1017/S0033291712002607. [DOI] [PubMed] [Google Scholar]
- 49.Donegan NH, Sanislow C, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: Implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 50.Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Battle CL, Shea MT, Johnson DM, Yen S, Zlotnick C, Zanarini MC, et al. Childhood maltreatment associated with adult personality disorders: findings from the collaborative longitudinal personality disorders study. J Pers Disord. 2004;18:193–211. doi: 10.1521/pedi.18.2.193.32777. [DOI] [PubMed] [Google Scholar]
- 52.Lobbestael J, Arntz A, Bernstein DP. Disentangling the relationship between different types of childhood maltreatment and personality disorders. J Pers Disord. 2010;24:285–295. doi: 10.1521/pedi.2010.24.3.285. [DOI] [PubMed] [Google Scholar]
- 53.Pagura J, Stein MB, Bolton JM, Cox BJ, Grant B, Sareen J. Comorbidity of borderline personality disorder and posttraumatic stress disorder in the US population. J Psychiatr Res. 2010;44:1190–1198. doi: 10.1016/j.jpsychires.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733–1739. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- 55.Schulze L, Domes G, Köppen D, Herpertz SC. Enhanced detection of emotional facial expressions in borderline personality disorder. Psychopathology. 2013;46:217–224. doi: 10.1159/000341730. [DOI] [PubMed] [Google Scholar]
- 56.Hagenhoff M, Franzen N, Gerstner L, Koppe G, Sammer G, Netter P, et al. Reduced sensitivity to emotional facial expressions in borderline personality disorder: effects of emotional valence and intensity. J Pers Disord. 2013;27:19–35. doi: 10.1521/pedi.2013.27.1.19. [DOI] [PubMed] [Google Scholar]
- 57.Domes G, Winter B, Schnell K, Vohs K, Fast K, Herpertz SC. The influence of emotions on inhibitory functioning in borderline personality disorder. Psychol Med. 2006;36:1163–1172. doi: 10.1017/S0033291706007756. [DOI] [PubMed] [Google Scholar]
- 58.Sieswerda S, Arntz A, Kindt M. Successful psychotherapy reduces hypervigilance in borderline personality disorder. Behav Cogn Psychother. 2007;35:387–402. [Google Scholar]