Abstract
BACKGROUND
Current immunohistochemical and in situ hybridization (ISH) assays are generally inconclusive for clonality unless plasmacytic differentiation is present. This study examined a series of cytology specimens and explored the ability of a branched-chain RNA (bRNA) ISH assay for immunoglobulin κ constant (IGKC) and immunoglobulin λ constant (IGLC) to detect a clonal population of B lymphocytes.
METHODS
Pathology databases were used to identify fine-needle aspiration biopsies (n = 28) and exfoliative cytology samples (n = 20). Demographic, flow cytometry, and excision biopsy results were recorded. bRNA ISH was performed on the Leica Bond platform with the following probes: IGKC, IGLC, immunoglobulin λ-like polypeptide 5 (IGLL5), and a housekeeping gene (HKG).
RESULTS
The bRNA ISH assay was validated with 30 surgical biopsies. On bRNA ISH, a clonal B-cell population (light-chain ratio > 10:1) was detected in 22 of 28 cases with a final diagnosis of lymphoma. In 2 cases, a κ predominance was present, although the ratio was <10:1. Eleven of the 17 κ-clonal lymphomas also expressed IGLL5, the latter recognized by the presence of an intranuclear signal. Two B-cell lymphomas lacked IGKC and IGLC, whereas 2 cases were negative for the HKG. In 12 of the 20 cases with reactive lymphoid tissue, bRNA ISH identified a polyclonal lymphoid population. No light-chain messenger RNA was detected in 6 cases (typically those associated with very few B cells).
CONCLUSIONS
The automated bRNA ISH platform is a robust technique for detecting a clonal B-cell population in cytology material.
Keywords: B-cell lymphoma, immunoglobulin λ constant (IGLC), immunoglobulin κ constant (IGKC), immunoglobulin λ-like polypeptide 5 (IGLL5), in situ hybridization (ISH)
INTRODUCTION
Fine-needle aspiration cytology of lymph nodes is widely practiced, although the efficacy of this technique for the primary diagnosis of lymphoma remains controversial.1,2 Nevertheless, in conjunction with flow cytometry, fine-needle aspiration biopsy may confirm the diagnosis of lymphoma and, in select cases, accurately classify the neoplasm and obviate surgical biopsy.3–5 The identification of a clonal population of lymphocytes, a task generally performed with flow cytometry, is an essential and occasionally indispensable ancillary test.6 In a proportion of cases, material for flow cytometry is unavailable, or the aspirate lacks the necessary cellularity; this frustrates the cytopathologist as well as the oncologist. Furthermore, some lymphoma cells are fragile and do not survive flow cytometry, lack surface light-chain expression, or show minimal surface light-chain expression; this precludes a determination of clonality. Finally, at sites such as the pancreas and liver, the diagnosis is often unsuspected, and material is not available for flow cytometry analysis. Although the specificity and sensitivity of cytology are relatively high, false-negative results are seen most often when flow cytometry is unavailable.7 Other, albeit less commonly used means of detecting B-cell clonality include immunoglobulin heavy chain (IGH) gene rearrangements and split-signal IGH DNA fluorescence in situ hybridization (FISH), although the latter is effective only for tumors with rearrangement at the IGH locus.8
Theoretically, stains for the expression of immunoglobulin light chains κ (immunoglobulin κ constant [IGKC]) and λ (immunoglobulin λ constant [IGLC]) would be able to distinguish a polyclonal population of lymphocytes from a monoclonal one. Although conventional methods for detecting clonal B-cell populations such as immunohistochemistry and in situ hybridization (ISH) readily detect light chains in plasmacytomas as well as lymphomas with plasmacytic differentiation such as mucosa-associated lymphoid tissue (MALT) lymphomas, these techniques are generally unsuccessful with cytology preparations, primarily because of issues related to nonspecific staining as well as an inability to detect low-level protein and messenger RNA (mRNA) expression in the majority of B-cell lymphomas.
The availability of a branched-chain RNA (bRNA) ISH assay, a robust means of detecting mRNA in paraffin-embedded tissue, has the potential to transform current clinical practice.9,10 The increased sensitivity of this assay relies on signal amplification, and it has the potential to detect single-copy RNA transcripts.11,12 The assay detects light-chain mRNA in mature lymphocytes: conventional ISH techniques are seldom successful at detecting low-level mRNA expression in lymphocytes. Theoretically, the bRNA ISH platform could provide a robust means of assessing clonality.
However, the interpretation of this assay is associated with some unique challenges: κ light chain–positive B-cell lymphomas also express a surrogate λ light chain—immunoglobulin λ-like polypeptide 5 (IGLL5)—and as a result, κ light chain–producing lymphomas appear to be positive for both κ and λ light chains.12 This apparent coexpression of light chains could potentially erase the benefits associated with the high sensitivity of the bRNA ISH assay.
In this study, we examined fine-needle aspirates and exfoliative cytology samples and explored the ability of the bRNA ISH assay for IGKC and IGLC to detect B-cell clonality. We also evaluated an interpretive algorithm that facilitates the distinction of IGLC from IGLL5.
MATERIALS AND METHODS
This study was approved by the institutional review board at the Massachusetts General Hospital.
Study Cases
Clinical and pathology databases were used to identify fine-needle aspiration biopsies (n = 28) and exfoliative cytology samples (n = 20) evaluated at the Massachusetts General Hospital between January 2011 and December 2014. Inclusionary criterion included the presence of a prominent lymphoid population as well as a cell block preparation with at least 100 lymphocytes. Flow cytometry data were available in the majority of cases. Reactive lymphoid lesions, B-cell lymphomas, and 2 plasmacytic neoplasms were included in the study. The final diagnosis and subtyping were based on excisional biopsies in the majority of cases. The diagnosis of benign lymphoid proliferation was based on a combination of excisional biopsies, flow cytometry (when available), and clinical follow-up.
In an attempt to assess the validity of the probes, paraffin sections from 30 excisional biopsies were also evaluated. The cohort was composed of 21 B-cell lymphomas and 9 reactive lymph nodes. The excisional biopsies were independent of the fine-needle and exfoliative cytology samples. bRNA ISH was performed on the cytology samples as well as excisional biopsies.
Automated κ and λ Light-Chain RNA ISH Assay
All 48 cases were assayed with a commercially available automated RNA ISH platform for IGKC (catalog number ZVA1-15950), IGLC (catalog number ZVA1-15951), and IGLL5 ViewRNA eZ probes (catalog number DVA1-16581; Affymetrix, Santa Clara, Calif). The RNA integrity of the specimens was determined with a cocktail of glyceraldehyde 3-phosphate dehydrogenase, β-actin, and a peptidylprolyl isomerase B ViewRNA eZ probe (catalog number DVA1-16742), which served as a housekeeping gene (HKG). Automated ISH assays were performed with the ViewRNA eZ-L detection kit (catalog number 19500; Affymetrix) on the Bond RX automated immunohistochemistry and ISH staining system with BDZ 6.0 software (Leica Biosystems, Buffalo Grove, Ill). Sections from formalin-fixed, paraffin-embedded cell block preparations were processed automatically from deparaffinization through ISH staining to hematoxylin counterstaining. Briefly, 5-μm-thick sections were baked for 1 hour at 60°C and placed on the Bond RX for processing. The Bond RX user-selectable settings were as follows: ViewRNA 1 protocol, ViewRNA Dewax 1, ViewRNA HIER for 10 minutes and ER2 (90), ViewRNA Enzyme 2 for 7 minutes, and ViewRNA probe hybridization for 3 hours. With these settings, the RNA unmasking conditions for the cytology cell block sections consisted of a 10-minute incubation at 90°C in Bond Epitope Retrieval Solution 2 (Leica Biosystems) followed by a 7-minute incubation with proteinase K from the Bond enzyme pretreatment kit at a 1:1000 dilution (Leica Biosystems). IGKC and IGLC probes (Affymetrix) were diluted (1:10) in the ViewRNA probe diluent (Affymetrix). The pooled HKG probe was diluted (1:40) in the ViewRNA probe diluent (Affymetrix). Posthybridized slides were rinsed with water, air-dried for 30 minutes at room temperature, mounted with Histomount organic mounting media, and visualized with a standard bright-field microscope with 20× and 40× objectives. Positive ISH staining was defined as punctate red-colored dots present in the nucleus or cytoplasm of lymphocytes. Additional details on automation are provided at the Panomics Web site.13
A 2-color assay (n = 10) was performed for select cases in a manual format as described in a prior publication.9,10 The assay allowed the simultaneous detections of IGLC and IGKC on a single slide.10 The probes were Type 1 IGKC (catalog number VA1-11526) and Type 6 IGLC (catalog number VA6-13793; Affymetrix); the dilution was 1:20.
Flow Cytometry
Eight-color flow cytometry analyses were performed as previously described in a publication from this institution.14
Immunohistochemistry and Conventional ISH
The source of the antibodies and ISH probes and the antigen retrieval conditions are listed in Table 1. Immunohistochemistry for paired box 5 (PAX5) was performed for a subset of cases (n = 24), including all cases negative for κ and λ light chains. Immunohistochemistry and conventional ISH were performed for select cytology samples: immunohistochemistry for κ and λ light chains was performed in 16 cases, and conventional ISH for κ and λ light chains was performed in 19 cases. Immunohistochemistry for κ and λ light chains was performed for all excision specimens.
TABLE 1.
IHC and Conventional ISH: Source of Antibody and Probes
| Source | Catalog Number | Dilution | Antigen/RNA Retrieval | |
|---|---|---|---|---|
| κ ISH (conventional) | Leica | 5748a | Ready to use | Enzyme 1 for 15 min |
| λ ISH (conventional) | Leica | 5770a | Ready to use | Enzyme 5 for 15 min |
| κ IHC | Leica | NCL-kap-581 | 1:100 | ER1 (citrate at pH 6) for 20 min |
| λ IHC | Leica | NCL-L-LAM570 | 1:7000 | ER1 for 10 min |
| PAX5 IHC | Leica | PA0552 | Ready to use | ER2 (EDTA at pH 9) for 20 min |
Abbreviations: EDTA, ethylenediaminetetraacetic acid; ER1, Epitope Retrieval Buffer 1; ER2, Epitope Retrieval Buffer 2; IHC, immunohistochemistry; ISH, in situ hybridization; PAX5, paired box 5.
RESULTS
Paraffin-Embedded Sections From Lymph Node Excisions
To validate the bRNA ISH technology, we evaluated paraffin-embedded sections from excised lymph nodes, including 21 lymphomas (11 diffuse large B-cell lymphomas, 6 follicular lymphomas, and 4 mantle cell lymphomas) and 9 reactive lymph nodes.
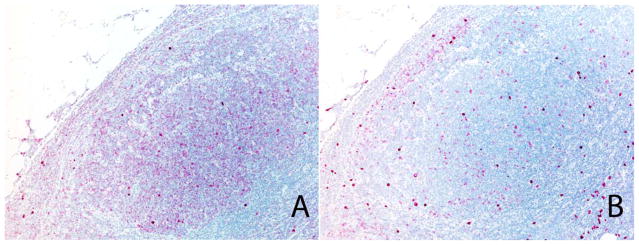
Robust staining was observed in plasma cells and lymphocytes, although the number of transcripts in mature lymphocytes was significantly lower than that in plasma cells. In contrast, clonality could not be determined from immunohistochemical stains for κ and λ light chains. As determined by bRNA ISH, 14 cases showed clonal IGKC-positive B-cell populations, whereas 7 cases were composed of IGLC-positive B cells. The malignant follicles were composed of clonal populations of B cells in all 6 follicular lymphomas, whereas the mantle zone and interfollicular tissue was composed of polyclonal populations of lymphocytes and plasma cells (Fig. 1). Notably, all 14 cases of κ-clonal lymphomas also showed staining for the IGLC probe; the latter was predominantly intranuclear. The IGLC nuclear signal in κ-clonal lymphocytes was a reflection of the IGLL5 transcripts and not IGLC mRNA; this finding was confirmed with a probe specific for IGLL5.
Figure 1.
Branched-chain in situ hybridization: excisional biopsy. (A) This follicular lymphoma shows strong immunoglobulin λ constant reactivity in the follicles. (B) The immunoglobulin κ constant stain shows positive cells in the mantle zone (in situ hybridization assay).
Six cases that were indeterminate on flow cytometry were identified to have light-chain restriction with bRNA ISH. In cases with measurable levels of surface or cytoplasmic light chains on flow cytometry, a perfect concordance between flow cytometry and bRNA ISH was achieved. In contrast, small lymphocytes in these samples were typically negative for light-chain expression with immunohistochemistry, but the plasma cells showed polytypic staining.
The 9 reactive lymph nodes showed a polyclonal population of IGKC-positive and IGLC-positive B cells. The germinal center and the mantle zone and interfollicular tissue were composed of polyclonal populations of lymphocytes and plasma cells. The T cell–rich zones were devoid of light-chain transcripts.
Cytology Samples
Aspirates from lymph nodes (n = 17) and pleural fluid (n = 14) were the 2 most common specimens evaluated in the study (Table 2). Fine-needle aspirates from other sites included the following: lung (n = 3), parotid gland (n = 3), pancreas (n = 2), subcutaneous soft tissue (n = 2), and stomach (n = 1). Other exfoliative samples included vitreous fluid (n = 3), ascitic fluid (n = 2), and pericardial fluid (n = 1).
TABLE 2.
Clinical, Flow Cytometry and ISH Results
| Case No. | Age, y | Sex | Specimen Type | B-Cell Flow, % | Clonality on Flow | Final Diagnosis | Initial Diagnosis of Lymphoma | Clonal on bRNA ISH | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Female | Lung | 2 | Polyclonal | Reactive | NA | Polyclonal | |
| 2 | 84 | Male | Pleural fluid | 0 | No B cells | Reactive | NA | No B cells | |
| 3 | 47 | Female | Pleural fluid | 31 | Polyclonal | Reactive | NA | No B cells | |
| 4 | 58 | Male | Pleural fluid | 2 | Polyclonal | Reactive | NA | No B cells | |
| 5 | 45 | Male | Lymph node, groin | ND | ND | Reactive | NA | Polyclonal | |
| 6 | 61 | Male | Pleural fluid | 8 | Polyclonal | Reactive | NA | Polyclonal | |
| 7 | 63 | Male | Ascitic fluid | 22 | Polyclonal | Reactive | NA | Polyclonal | |
| 8 | 52 | Female | Pleural fluid | 7 | Polyclonal | Reactive | NA | Polyclonal | |
| 9 | 36 | Female | Pleural fluid | 2 | Polyclonal | Reactive | NA | Polyclonal | |
| 10 | 31 | Male | Lymph node | 20 | Polyclonal | Reactive | NA | Significant background | |
| 11 | 84 | Male | Pleural fluid | 0 | No B cells | Reactive | NA | No B cells | |
| 12 | 31 | Male | Lymph node, portal | ND | ND | Reactive | NA | Polyclonal | |
| 13 | 34 | Male | Vitreous fluid | ND | ND | Reactive | NA | No B cells | |
| 14 | 40 | Female | Lymph node, neck | 12 | Polyclonal | Reactive | NA | No B cells | |
| 15 | 75 | Female | Lung | 3 | Polyclonal | Reactive | NA | Polyclonal | |
| 16 | 63 | Male | Lymph node, neck | 4 | Polyclonal | Reactive | NA | Clonal κ | |
| 17 | 54 | Male | Lymph node, neck | ND | ND | Reactive | NA | Polyclonal | |
| 18 | 70 | Female | Lymph node, retroperitoneal | 25 | Polyclonal | Reactive | NA | Polyclonal | |
| 19 | 85 | Female | Pleural fluid | ND | ND | Reactive | NA | Polyclonal | |
| 20 | 82 | Female | Pleural fluid | 20 | Polyclonal | Reactive | NA | Polyclonal | |
| 21 | 85 | Female | Pleural fluid | 9 | κ | Low-grade B-cell lymphoma | NA | Clonal κ | |
| 22 | 39 | Male | Lymph node, neck | 46.00 | κ | Low-grade B-cell lymphoma | No | Clonal κ | |
| 23 | 48 | Female | Lung | 16 | κ | Low-grade B-cell lymphoma | No | Clonal κ | |
| 24 | 82 | Female | Soft tissue, paraspinal | 51 | κ | Low-grade B-cell lymphoma | No | Clonal κ | |
| 25 | 75 | Female | Lymph node, neck | 24 | λ | Follicular lymphoma grade 2/3 | Yes | Clonal λ | |
| 26 | 45 | Male | Lymph node, peripancreatic | 45 | Inconclusive | Follicular lymphoma grade 1–2/3 | Yes | Clonal κ | |
| 27 | 72 | Male | Pancreas | 52 | Inconclusive | Follicular lymphoma, grade 1–2 | No | No B cells | |
| 28 | 79 | Male | Lymph node, abdominal | 63 | κ | Follicular lymphoma | No | Clonal κ | |
| 29 | 76 | Male | Lymph node, abdominal | 46 | κ | Follicular lymphoma, grade 1 | No | κ predominance | |
| 30 | 92 | Female | Parotid | 41 | κ | Follicular lymphoma, grade 1–2 | Yes | Clonal κ | |
| 31 | 67 | Male | Lymph node, neck | 28 | λ | Follicular lymphoma | No | Clonal λ | |
| 32 | 62 | Male | Lymph node, neck | 71 | λ | SLL | Yes | Clonal λ | |
| 33 | 68 | Female | Parotid | 40 | κ | SLL | No | κ predominance | |
| 34 | 46 | Male | Parotid | Inconclusive | SLL | No | Clonal κ | ||
| 35 | 68 | Female | Pleural fluid | ND | ND | Plasma cell myeloma | No | Clonal κ | |
| 36 | 72 | Male | Stomach | ND | ND | MALT lymphoma | Yes | Clonal κ | |
| 37 | 65 | Female | Subcutaneous, chest wall | 16 | λ | Plasma cell myeloma | Yes | Clonal λ | |
| 38 | 40 | Female | Ascitic fluid | ND | ND | Burkitt | No | No B cells | |
| 39 | 79 | Male | Pleural fluid | 16 | λ | Mantle cell lymphoma | No | Clonal λ | |
| 40 | 65 | Male | Lymph node | 83 | κ | Burkitt | No | Clonal κ | |
| 41 | 85 | Male | Pleural fluid | 28 | Inconclusive | DLBCL | No | Clonal κ | |
| 42 | 67 | Male | Vitreous fluid | ND | ND | DLBCL | Yes | Clonal κ | |
| 43 | 61 | Female | Lymph node, neck | 26 | κ | DLBCL | Yes | Clonal κ | |
| 44 | 86 | Female | Vitreous fluid | ND | ND | DLBCL | Yes | Clonal κ | |
| 45 | 73 | Female | Pancreas | ND | ND | DLBCL | Yes | Assay failed | |
| 46 | 69 | Male | Pericardial | 48 | κ | DLBCL | No | Clonal κ | |
| 47 | 54 | Male | Pleural fluid | 2 | Inconclusive | DLBCL | No | Clonal κ | |
| 48 | 66 | Male | Lymph node, retroperitoneal | 30 | κ | DLBCL | Yes | Assay failed | |
Abbreviations: bRNA, branched-chain RNA; DLBCL, diffuse large B-cell lymphoma; ISH, in situ hybridization; MALT, mucosa-associated lymphoid tissue; NA, not available; ND, not done; SLL, small cell lymphoma. Bold font represents cases that do not have a prior history of lymphoma and lack tissue for flow cytometry.
Cases With a Final Diagnosis of Reactive Lymphoid Tissue (n = 20)
The mean age of this cohort was 57 years (range, 31–85 years) with 11 males and 9 females. The cytology smears and the corresponding cell block preparations showed mature lymphocytes. Plasma cells were observed in half of the cases (n = 9). The lymph node aspirates (n = 7) showed a predominantly small lymphocytic population: lymphoid tangles and tingible body macrophages were identified in 3 of the 7 lymph nodes. No atypical lymphoid cells were noted. On follow-up (median, 18 months), these cases did not progress to lymphoma.
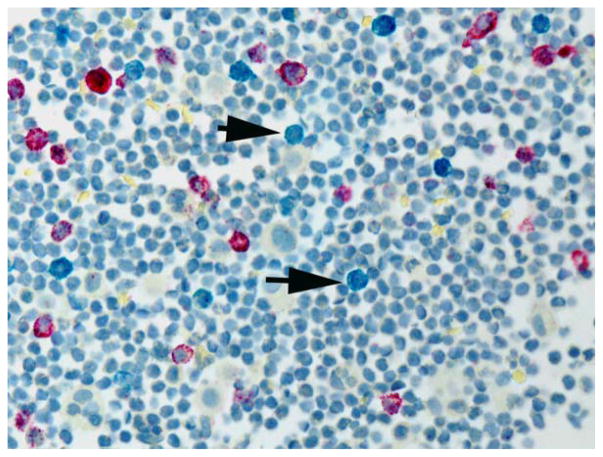
Flow cytometry was performed for 15 of the 20 cases: 13 cases with detectable B cells and plasma cells were interpreted as polyclonal, and 2 cases showed insufficient B cells. In comparison, bRNA ISH identified 12 cases with polyclonal lymphocytes/plasma cells: the κ/λ ratio ranged from 2:1 to 4:1 (Fig. 2). No staining for κ or λ was observed in 6 cases; however, a few scattered B cells were identified on the Pax5 stain, and this suggested that not all mature lymphocytes showed detectable levels of light-chain mRNA. One case showed significant background staining, likely a result of poor RNA preservation, as determined by an evaluation of the HKG.
Figure 2.
Two-color in situ hybridization assay performed on a pleural fluid with reactive lymphocytes and plasma cells. Both immunoglobulin κ constant–positive (red chromogen) and immunoglobulin λ constant–positive (blue chromogen) lymphocytes and plasma cells are present. The blue chromogen (arrow) is significantly less prominent than the red chromogen (in situ hybridization assay).
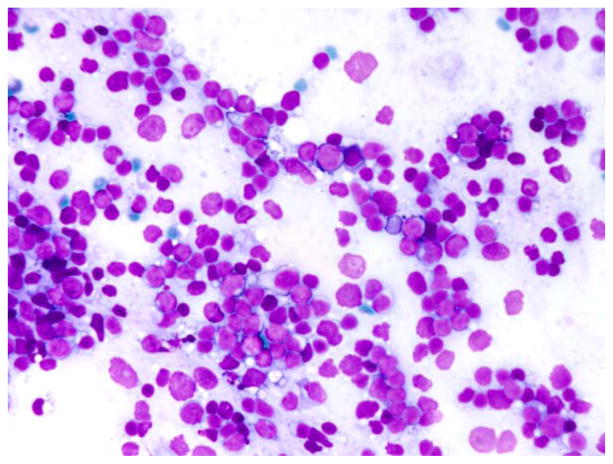
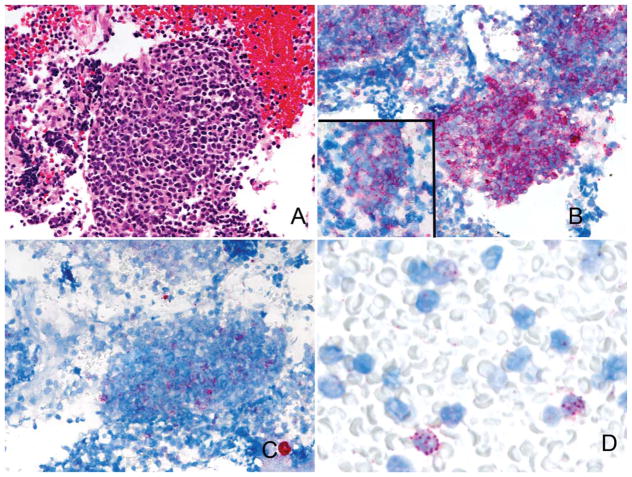
Case 16, originally interpreted as a reactive lymph node, showed a clonal κ population on bRNA ISH. A polymorphic population of lymphocytes was noted on the Giemsa-stained preparation, although the aspirate was devoid of tingible body macrophages (Fig. 3). Flow cytometry samples showed 4% B cells; however, the percentage of B cells as determined by the Pax5 stain was significantly higher (>80%). The patient was lost to follow-up, and thus the significance of this clonal B-cell population is uncertain.
Figure 3.
Case 16. This lymph node aspirate shows a polymorphic population of lymphocytes (May-Grünwald-Giemsa stain).
Conventional ISH and immunohistochemistry for κ and λ, performed for 5 and 6 cases, respectively, failed to detect a sufficient signal within the lymphoid population to allow the determination of clonality.
Cases With a Final Diagnosis of a B-Cell Lymphoma (n = 28)
The mean age of this group was 67 years (range, 39–92 years) with 16 males and 12 females. Based on analyses of excision biopsies and immunohistochemical and flow cytometry data, the diagnoses were as follows: diffuse large B-cell lymphoma (n = 8), follicular lymphoma (n = 7), small lymphocytic lymphoma (n = 3), plasmacytoma (n = 2), Burkitt lymphoma (n = 2), mucosa-associated lymphoid tissue lymphoma (n = 1), and mantle cell lymphoma (n = 1). Four cases lacked both excisional biopsies and diagnostic features on flow cytometry and were classified as low-grade B-cell lymphomas.
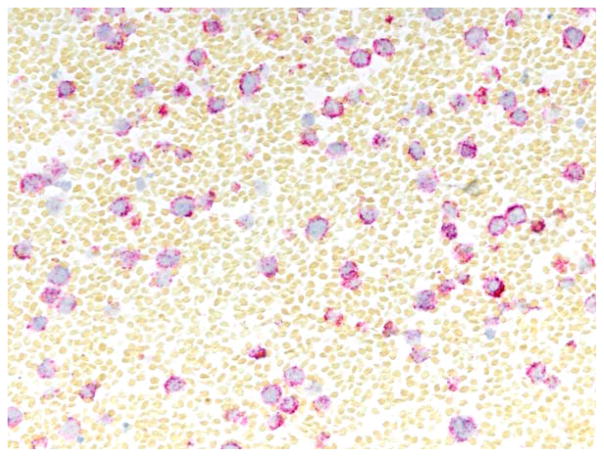
In 22 cases, bRNA ISH showed a predominance of a single immunoglobulin light chain (ratio > 10:1; Fig. 4). Two cases showed κ predominance; however, the ratio was <10:1 but >4:1. Among the 17 κ-clonal lymphomas, cytoplasmic reactivity for IGKC in 11 cases was also accompanied by nuclear reactivity for IGLC (Figs. 5 and 6). This pattern was interpreted as a clonal κ-positive population with coexpression of IGLL5: nuclear IGLL5 reactivity was confirmed on ISH for IGLL5. The 5 IGLC clonal lymphomas lacked IGLL5 reactivity (Fig. 7). bRNA ISH was negative in 2 cases: PAX5 staining was positive, whereas the HKG confirmed the presence of preserved RNA. This suggested that these cells either lacked light-chain transcripts or were below the detectable limits. Two additional cases were negative for the HKG, and this suggested a lack of RNA preservation.
Figure 4.
Two-color in situ hybridization performed on a diffuse large B-cell lymphoma positive for immunoglobulin κ constant (red chromogen) and supporting the diagnosis of κ-clonal proliferation. Immunoglobulin λ constant (blue chromogen) is not seen (2-color in situ hybridization assay).
Figure 5.
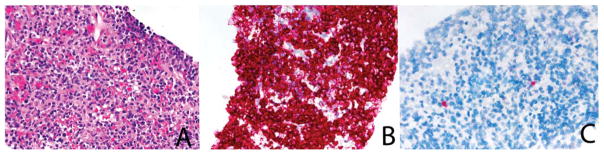
(A) Plasma cell myeloma (H & E) with (B) intense reactivity for immunoglobulin κ constant. (C) The immunoglobulin λ constant stain shows a single positive plasma cell (in situ hybridization assay).
Figure 6.
(A) Peripancreatic lymph node from a patient with a grade 1 follicular lymphoma (H & E). (B) The immunoglobulin κ constant stain is intensely positive, and a follicular pattern is appreciable. The negatively stained cells represent T cells (results not shown). (C) Occasional cells are positive on the immunoglobulin λ constant stain. (D) However, as shown at a high-power magnification, these cells show predominantly nuclear reactivity, a pattern typical of immunoglobulin λ-like polypeptide 5 reactivity (in situ hybridization assay).
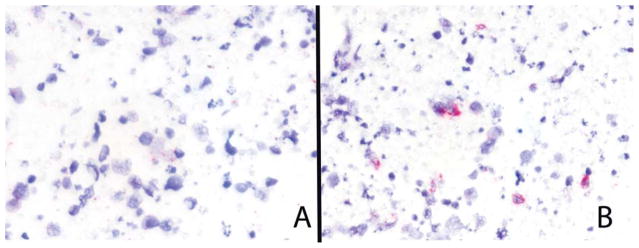
Figure 7.
Mantle cell lymphoma. The tumor cells are relatively poorly preserved. (A) Immunoglobulin κ constant is negative. (B) Immunoglobulin λ constant shows occasional cells with strong reactivity confined to the cytoplasm of the tumor cells (in situ hybridization assay).
Flow cytometry detected a clonal lymphoid population in 17 of 21 cases. Two diffuse large B-cell lymphomas and 2 follicular lymphomas were negative for a clonal population of lymphocytes: bRNA ISH recognized light-chain restriction in 3 of the 4 cases.
Conventional ISH and immunohistochemistry for κ and λ were performed for 11 and 13 cases, respectively. Both techniques detected a clonal population in cases 35 and 37: plasma cell myeloma. In the other cases (all B-cell neoplasms), these conventional techniques failed to detect a clonal B-cell population.
DISCUSSION
Identifying a clonal B-cell population is a critical and often indispensable step in the diagnosis of B-cell lymphoma; this distinguishes a lymphoproliferative disease from reactive lymphoid proliferation.2,3,5,15,16 One exception to this rule is diffuse large B-cell lymphoma, which in most cases does not require clonality studies to establish a diagnosis. Most non-Hodgkin lymphomas express low levels of IGKC and IGLC mRNA transcripts, and typically κ and λ light chains cannot be detected by immunohistochemistry or conventional ISH. The absence of tissue architecture on fine-needle aspiration and exfoliative cytology samples enhances this dependence on ancillary tests. For cytology specimens, flow cytometry is the most commonly used assay to assess clonality: the assay is sensitive and, in certain scenarios, obviates open biopsy, particularly at sites at which excisional biopsy may prove difficult. Commonly, fresh cells, a prerequisite for flow cytometry, may not be available, particularly when suspicion for a lymphoma is relatively low. It is notable that neither immunohistochemistry nor any other currently available automated ISH assay represents a robust means for detecting B-cell clonality in cases other than those with plasmacytic differentiation.
In the study, we have validated an automated bRNA ISH assay for κ and λ light chains on resected lymph nodes. We have also shown that this novel ISH assay distinguishes a monoclonal B-cell population from a polyclonal B-cell population in cytology preparations. The evaluation of a reactive lymphoid population was often further simplified by the relative paucity of B lymphocytes: several reactive lymphoid infiltrates showed only rare B lymphocytes, as indicated by rare PAX5-positive lymphocytes. As with conventional ISH assays, plasma cells were readily recognized as polyclonal. The majority of B-cell lymphomas were classified as monoclonal, and they included a diverse collection of B-cell lymphomas: diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, and Burkitt lymphoma. The bright cytoplasmic staining of mRNA transcripts allowed easy interpretation. However, a fairly wide range of light-chain expression was noted, with a minority of cases showing only a few transcripts. The increased distance between individual lymphocytes allowed a clear distinction of nuclear and cytoplasmic reactivity; cytology samples proved easier to interpret than resected lymph nodes.
Six cases (highlighted in bold in Table 2) that did not have a prior history of lymphoma and lacked tissue for flow cytometry provide a glimpse into the ability of the assay to redefine the current diagnostic algorithm. An aspirate from the vitreous fluid of a 67-year-old male (case 42) was interpreted as a reactive lymphoid infiltrate. The bRNA ISH stain revealed a κ-positive B-cell lymphocyte population. Flow cytometry analysis of a subsequent vitrectomy specimen confirmed the diagnosis of a B-cell lymphoma. The diagnosis of vitreoretinal lymphoma remains a diagnostic challenge, and the application of this sensitive platform to detect clonality is likely to facilitate the distinction of inflammatory diseases from B-cell lymphomas.17
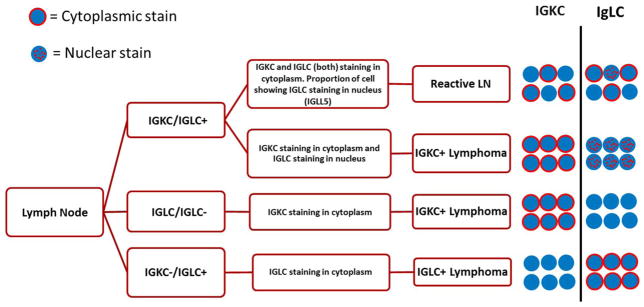
One predictable interpretation difficulty in using this highly sensitive bRNA ISH assay is the presence of a λ-like pseudogene (IGLL5) in κ-producing lymphomas and within reactive germinal centers. The apparent coexpression of κ and λ in κ-positive lymphoma cells represents IGLL5 and not λ light-chain mRNA transcripts. Interestingly, IGLL5 is detected only in κ-producing lymphocytes and is undetectable in λ-positive lymphocytes. The IGLL5 gene is located in the immunoglobulin λ locus (22q), but unlike the λ locus, it does not undergo somatic rearrangement. It should be noted that significant homology between IGLC and IGLL5 precludes the development of a λ-specific probe. However, it is feasible to develop a probe specific for IGLL5 because the first exon is unrelated to the λ locus. This restriction of IGLL5 to κ-producing lymphocytes was confirmed on a probe specific for IGLL5. Notably and in contrast to IGLC, IGLL5 is localized predominantly to the nucleus. Moreover, the IGLL5 intranuclear dots are significantly larger than IGLC; that is, larger intranuclear dots with the IGLC probe suggest IGLL5 cross-reactivity and not λ reactivity (see the algorithm in Fig. 8).
Figure 8.
Algorithm for interpreting IGLC and IGKC in situ hybridization stains. Abbreviations: IGKC, immunoglobulin κ constant; IGLC, immunoglobulin λ constant; IGLL5, immunoglobulin λ-like polypeptide 5; LN, lymph node.
The bRNA ISH assay provides a robust means of detecting a clonal population of B lymphocytes in cytology samples; however, some additional caveats apply, particularly for those with limited numbers of B lymphocytes. These samples may show an artifactually skewed κ/λ ratio. As a result, clonality as defined by RNA ISH requires the overwhelming dominance (>10:1) of a clone.
This study identified 1 significant outlier: case 16. Although the bRNA ISH identified a clonal population of lymphocytes, flow cytometry failed to detect a clonal population; however, the sample appeared to be composed predominantly of blood. Although the patient was lost to follow-up, the possibility that this patient harbored a lymphoproliferative disease could not be excluded. Clonal B-cell populations in nonlymphomatous processes have been rarely reported in reactive lymph nodes and have been mainly observed in patients with viral illnesses or auto-immune or immunodeficiency syndromes.18,19 However, because both polymerase chain reaction–based studies and flow cytometry are more sensitive, we predict that the incidence of these benign clonal populations on bRNA ISH is likely to be lower. Nevertheless, this case underscores the need for a consolidated and thorough review of all available morphological, immunohistochemical, ISH, flow cytometry, and molecular data before a diagnosis of a B-cell lymphoma is confirmed.
In conclusion, the automated bRNA ISH platform represents a robust means of distinguishing a monoclonal B-cell/plasma cell population from a polyclonal one in both fine-needle aspiration biopsies and exfoliative cytology samples. The automated version of the assay fits easily into the workflow of most modern clinical laboratories. Although this analysis was performed on paraffin-embedded material, the assay could also be adapted for use with alcohol or air-dried smears. One significant advantage of cell block–based testing is that subsequent sections can be used for immunohistochemistry, FISH-based assays, and molecular and next-generation sequencing.20 Although IGLL5 represents a confounding factor, nuclear localization generally allows for the recognition of this pseudogene. The 2-color assay would address, in part, this shortcoming: cells with dual reactivity (ie, red dots [IGKC] in the cytoplasm and blue dots [IGLC] in the nucleus) would identify IGLL5-bearing, κ-positive lymphocytes. However, although the single-color assay is currently automated, an automated 2-color assay is yet to be validated. Although flow cytometry is more sensitive than bRNA ISH, this assay is likely to be immensely helpful in several clinical scenarios: 1) material for flow cytometry is unavailable; 2) the sample for flow cytometry lacks adequate cellularity or is composed predominantly of blood; and 3) a way of validating flow cytometry data, particularly samples with equivocal results, is needed. The availability of a robust assay for detecting clonality in cell block preparations would bring cytology closer to the goal of averting open biopsy at inaccessible sites. This assay adds to the growing list of ancillary techniques, which include flow cytometry, polymerase chain reaction for B- and T-cell gene rearrangements, FISH, and next-generation sequencing, and brings the field closer to the often stated goal of sparing patients from excisional biopsy in select cases.
Footnotes
FUNDING SUPPORT
No specific funding was disclosed.
CONFLICT OF INTEREST DISCLOSURES
Manoj Gandhi has a pending patent application entitled “Diagnosis of Multiple Myeloma and Lymphoma.” Miguel Rivera reports a sponsored research agreement with Affymetrix; in addition, Rivera has a patent pending for κ and λ in situ hybridization. David Ting reports a sponsored research agreement with Affymetrix; in addition, Ting has a patent pending for κ and λ in situ hybridization. Vikram Deshpande reports research support from Affymetrix during the conduct of the study; in addition, Deshpande has a patent pending for κ and λ in situ hybridization.
References
- 1.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–3052. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 2.Frederiksen JK, Sharma M, Casulo C, Burack WR. Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch Pathol Lab Med. 2015;139:245–251. doi: 10.5858/arpa.2013-0674-RA. [DOI] [PubMed] [Google Scholar]
- 3.Caraway NP. Evolving role of FNA biopsy in diagnosing lymphoma: past, present, and future. Cancer Cytopathol. 2015;123:389–393. doi: 10.1002/cncy.21551. [DOI] [PubMed] [Google Scholar]
- 4.Young NA. Grading follicular lymphoma on fine-needle aspiration specimens—a practical approach. Cancer. 2006;108:1–9. doi: 10.1002/cncr.21350. [DOI] [PubMed] [Google Scholar]
- 5.Wakely PE., Jr The diagnosis of non-Hodgkin lymphoma using fine-needle aspiration cytopathology: a work in progress. Cancer Cytopathol. 2010;118:238–243. doi: 10.1002/cncy.20106. [DOI] [PubMed] [Google Scholar]
- 6.Zeppa P, Marino G, Troncone G, et al. Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: a critical review of 307 cases with technical suggestions. Cancer. 2004;102:55–65. doi: 10.1002/cncr.11903. [DOI] [PubMed] [Google Scholar]
- 7.Zeppa P, Vigliar E, Cozzolino I, et al. Fine needle aspiration cytology and flow cytometry immunophenotyping of non-Hodgkin lymphoma: can we do better? Cytopathology. 2010;21:300–310. doi: 10.1111/j.1365-2303.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Zeppa P, Sosa Fernandez LV, Cozzolino I, et al. Immunoglobulin heavy-chain fluorescence in situ hybridization–chromogenic in situ hybridization DNA probe split signal in the clonality assessment of lymphoproliferative processes on cytological samples. Cancer Cytopathol. 2012;120:390–400. doi: 10.1002/cncy.21203. [DOI] [PubMed] [Google Scholar]
- 9.Ferrone CR, Ting DT, Shahid M, et al. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA–enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol. doi: 10.1245/s10434-014-4247-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahid M, Mubeen A, Tse J, et al. Branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation: evaluation of manual and automated in situ hybridization platforms. Am J Surg Pathol. 2015;39:25–34. doi: 10.1097/PAS.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minca EC, Wang H, Wang Z, et al. Detection of immunoglobulin light-chain restriction in cutaneous B-cell lymphomas by ultrasensitive bright-field mRNA in situ hybridization. J Cutan Pathol. 2015;42:82–89. doi: 10.1111/cup.12415. [DOI] [PubMed] [Google Scholar]
- 12.Tubbs RR, Wang H, Wang Z, et al. Ultrasensitive RNA in situ hybridization for detection of restricted clonal expression of low-abundance immunoglobulin light chain mRNA in B-cell lymphoproliferative disorders. Am J Clin Pathol. 2013;140:736–746. doi: 10.1309/AJCPJTWK07FSABRJ. [DOI] [PubMed] [Google Scholar]
- 13.Panomics. [Accessed August 2015];User manual for ViewRNA™eZ assay. http://www.panomics.com/downloads/15891_RevC%20140910_ViewRNA%20eZ%20Assay%20Manual_Compressed.pdf.
- 14.Bagwell CB, Hill BL, Wood BL, et al. Human B-cell and progenitor stages as determined by probability state modeling of multidimensional cytometry data. Cytometry B Clin Cytom. 2015;88:214–226. doi: 10.1002/cyto.b.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caraway NP. Strategies to diagnose lymphoproliferative disorders by fine-needle aspiration by using ancillary studies. Cancer. 2005;105:432–442. doi: 10.1002/cncr.21452. [DOI] [PubMed] [Google Scholar]
- 16.Dong HY, Harris NL, Preffer FI, Pitman MB. Fine-needle aspiration biopsy in the diagnosis and classification of primary and recurrent lymphoma: a retrospective analysis of the utility of cytomorphology and flow cytometry. Mod Pathol. 2001;14:472–481. doi: 10.1038/modpathol.3880336. [DOI] [PubMed] [Google Scholar]
- 17.Levasseur SD, Wittenberg LA, White VA. Vitreoretinal lymphoma: a 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol. 2013;131:50–55. doi: 10.1001/jamaophthalmol.2013.569. [DOI] [PubMed] [Google Scholar]
- 18.Kussick SJ, Kalnoski M, Braziel RM, Wood BL. Prominent clonal B-cell populations identified by flow cytometry in histologically reactive lymphoid proliferations. Am J Clin Pathol. 2004;121:464–472. doi: 10.1309/4EJ8-T3R2-ERKQ-61WH. [DOI] [PubMed] [Google Scholar]
- 19.Nam-Cha SH, San-Millan B, Mollejo M, et al. Light-chain-restricted germinal centres in reactive lymphadenitis: report of eight cases. Histopathology. 2008;52:436–444. doi: 10.1111/j.1365-2559.2008.02965.x. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Caraway N, Gu J, et al. Evaluation of interphase fluorescence in situ hybridization for the t(14;18)(q32;q21) translocation in the diagnosis of follicular lymphoma on fine-needle aspirates: a comparison with flow cytometry immunophenotyping. Cancer. 2003;99:385–393. doi: 10.1002/cncr.11787. [DOI] [PubMed] [Google Scholar]