Abstract
Purpose
More effective therapy is needed for intrahepatic cholangiocarcinoma (ICC). The encouraging clinical results obtained with checkpoint molecule-specific monoclonal antibodies (mAb) have prompted us to investigate whether this type of immunotherapy may be applicable to ICC. The aims of this study were to determine whether (i) patients mount a T-cell immune response to their ICC, (ii) checkpoint molecules are expressed on both T cells and tumor cells, and (iii) tumor cells are susceptible to recognition by cognate T cells.
Experimental Design
Twenty-seven ICC tumors were analyzed for (i) lymphocyte infiltrate, (ii) HLA class I and HLA class II expression, and (iii) PD-1 and PD-L1 expression by T cells and ICC cells, respectively. The results of this analysis were correlated with the clinicopathologic characteristics of the patients investigated.
Results
Lymphocyte infiltrates were identified in all tumors. PD-L1 expression and HLA class I antigen expression by ICC cells was observed in 8 and 11, respectively, of the 27 tumors analyzed. HLA class I antigen expression correlated with CD8+ T-cell infiltrate. Furthermore, positive HLA class I antigen expression in combination with negative/rare PD-L1 expression was associated with favorable clinical course of the disease.
Conclusions
ICC patients are likely to mount a T-cell immune response against their own tumors. Defects in HLA class I antigen expression in combination with PD-L1 expression by ICC cells provide them with an immune escape mechanism. This mechanism justifies the implementation of immunotherapy with checkpoint molecule-specific mAbs in patients bearing ICC tumors without defects in HLA class I antigen expression.
Introduction
Intrahepatic cholangiocarcinoma (ICC) accounts for 2% to 3% of all gastrointestinal cancers, however, the incidence is rising globally (1). The prognosis of ICC remains very poor, with surgical resection offering the only hope for cure (2). However, even with a complete resection, the majority of patients develop recurrent and metastatic disease, resulting in a 5-year overall survival (OS) rate of 10% to 40% (3). Therefore, novel effective therapies are urgently needed for ICC patients. In recent clinical trials, the administration of monoclonal antibodies (mAb), which inhibit the interaction of immune regulatory checkpoint molecules, such as CTLA-4 and programmed cell death protein 1 (PD-1) with their ligands CD80, CD86, and programmed cell death ligand 1 (PD-L1), has had a major impact on the clinical course of various types of malignancies (4–14). However, the efficacy of this novel immunotherapeutic strategy is limited to less than 30% of patients. These findings have stimulated interest in characterizing the host's immune response, as well as the potential mechanism(s) underlying a tumor's ability to escape the immune attack.
For many malignancies, tumor-infiltrating lymphocytes are evidence that patients are mounting an immune response to their own cancer (15, 16). However, the therapeutic efficacy of this immune response is limited by the multiple escape mechanisms that tumor cells develop to avoid immune recognition and destruction. These mechanisms include (i) inhibition of the activation of cognate cytotoxic T lymphocytes (CTL) due to the interaction of coinhibitory molecules such as CTLA-4 and PD-1 with their own ligands (17) and (ii) defects in HLA class I antigen-processing machinery (APM) components, which result in a defective synthesis and/or expression of HLA class I antigen–tumor antigen-derived peptide complexes. The latter mediate the interaction between tumor cells and cognate CTLs (18, 19). Defects in HLA class I APM components, frequently identified in malignant neoplasms, are associated with a reduced OS in various types of cancer (20, 21). The tumor antigen–specific immune response can also be modulated by the de novo expression of HLA class II antigens by tumor cells, which has been reported in a number of malignancies (20). Tumor cells that express the HLA class II-peptide complex can act as antigen-presenting cells and interact with CD4+helper cells, which can in turn induce growth and differentiation of CD8+CTLs. The HLA class II–peptide complex can however activate subpopulations of CD4+ cells with immunosuppressive properties (Tregs), which can inhibit the tumor antigen–specific T-cell immune response. This double-edged effect of HLA class II–peptide–CD4+ cell interactions could explain why HLA class II expression is correlated with favorable prognosis in some types of tumors, but with a poor prognosis in others (22–25).
Translational Relevance.
The lack of effective therapy for intrahepatic cholangiocarcinoma (ICC) has prompted us to investigate the expression of the checkpoint inhibitors PD-1 and PD-L1 and of HLA class I antigens in this malignancy, as these molecules play a role in the clinical response to immunotherapy with checkpoint inhibitor–specific monoclonal antibodies (mAb). The latter type of immunotherapy is effective in various types of malignancies. Immunostaining with mAbs detected HLA class I defects in 60% of ICC tumors and PD-L1 expression in 30%. Patients bearing tumors with HLA class I defects and PD-L1 expression had a significantly reduced survival. This association is likely to reflect the role of patients' immune response to their tumors. Our findings provide a rationale to implement anti–PD-1/PD-L1–based immunotherapy for the treatment of ICC and to use HLA class I antigen expression to select patients who may benefit from this type of immunotherapy.
To the best of our knowledge, only limited information is available regarding a patient's immune response against ICC and its role in the clinical course of the disease. Therefore, the first aim of this study was to test whether ICC patients can mount an immune response against their own tumors, as indicated by the presence of lymphocyte infiltrates. The second aim was to understand possible immune escape mechanisms, such as defects in HLA class I antigen expression and HLA class II de novo expression by tumor cells. The third aim was to evaluate a possible second immune escape mechanism by analyzing the expression of the inhibitory molecule PD-1 and its ligand PD-L1. The interaction between PD-1 and PD-L1 will affect a patient's ability to mount an immune response to his own tumor. The clinical significance of this immumunologic analysis has been assessed by correlating these results with the patients' clinical course of the disease.
Materials and Methods
Tissues
Tumor specimens from primary ICC lesions were obtained from patients who underwent hepatectomies at the Massachusetts General Hospital, between 2004 and 2013. ICC tumors were confirmed histopathologically by a gastrointestinal pathologist (V. Deshpande) according to the AJCC (7th Edition) and the WHO classification systems. Clinicopathologic information available included patient age, gender, margin resection status, histologic grading, tumor stage, tumor size, lymph node involvement, presence of single or multiple lesions, presence of vascular invasion, and overall survival. This study was approved by the institutional review board.
Monoclonal antibodies
The mAb HCA2, which recognizes β2m-free HLA-A (excluding -A24), -B7301, and -G heavy chains (26, 27); mAb HC10, which recognizes β2m-free HLA-A3, -A10, -A28, -A29, -A30, -A31, -A32, -A33, and all β2m-free -HLA-B (excluding -B5702, -B5804, and -B73) and -HLA-C heavy chains (26–28); and mAb LGII-612.14 which recognizes HLA-DR, DQ, and DP β chains were developed and characterized as described (29). PD-L1 antibody clone 22C3 was generated in the Merck Research Laboratories. A list of the other commercially available mAbs is provided in the Supplementary Material.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue sections (4 μm) from ICC tumor samples were used as substrates in immunohisto-chemical (IHC) reactions. IHC staining with HC10, HCA2, LGII-612.14, and anti-nitrotyrosine mAbs was performed as described previously (30). The staining with anti–PD-1 and anti–PD-L1 mAb as well as the slide scoring are described in the Supplementary Material.
Statistical analysis
Statistical analysis was performed with the Stata Statistical Software, Release 13. (StataCorp LP). Correlation of HLA class I antigen, nitrotyrosine, PD-1 and PD-L1 expression with the number of CD8+ and CD4+ cells infiltrating the fibrous septa and tumor lobules of tumor lesions were analyzed by Spearman rank correlation coefficient. Correlation of PD-1 and PD-L1 expression with HLA class I antigen expression was analyzed by Fisher exact test. Differences in the expression levels of variables according to histopathologic and clinical characteristics were analyzed using the Mann–Whitney U test or the Kruskal–Wallis rank test. OS measured as cause-specific survival (CSS) was calculated using the Kaplan–Meier method. Time was defined as the interval between the date of diagnosis to the date of disease-specific death (event) or that of last follow-up visit (censored). A log-rank test was used for screening the possible prognostic factors in relation to the patients' survival. P < 0.05 was considered to be statistically significant. All tests used were two-tailed.
Results
Patients characteristic and tumor specimens
Twenty-seven patients with a median age of 64 years (range, 38–81 years) underwent surgical resection of their ICCs. Table 1 summarizes the patients' clinicopathologic characteristics.
Table 1.
Patient and tumor characteristics
| Median age | 64.0 years (38–81) |
| Gender | |
| Female | 14 (51.9%) |
| Male | 13 (48.1%) |
| AJCC stage | |
| I | 8 (29.7%) |
| II | 6 (22.2%) |
| III | 3 (11.1%) |
| IVA | 10 (37.0%) |
| TNM | |
| T1 | 8 (29.6%) |
| T2 | 8 (29.6%) |
| T3 | 10 (37.0%) |
| T4 | 1 (3.7%) |
| N0 | 18 (66.7%) |
| N1 | 9 (33.3%) |
| M1 | 0 (0%) |
| Grade of differentiation | |
| Moderate | 19 (70.4%) |
| Poor | 8 (29.6%) |
| Single lesion | 18 (66.7%) |
| Multiple lesions | 9 (33.3%) |
| No vascular invasion | 16 (59.3%) |
| Vascular invasion | 11 (40.7%) |
| Margin of resection | |
| Negative | 24 (88.9%) |
| Positive | 3 (11.1%) |
| Median follow up | 29.1 months (3.8–105.8) |
| Median OS | 44.6 months (14.9–105.8) |
Lymphocyte infiltrate in primary ICC lesions
To investigate whether ICC patients developed an immune response against their own tumors, we analyzed the presence of CD8+ T cells and CD4+ cells in ICC lesions. Staining with CD8- and CD4-specific mAbs was scored by counting the number of stained infiltrating cells in four high-powered fields of the fibrous septa and tumor lobules of ICC lesions. An immune infiltrate was present in all the tumors analyzed (100.0%). Interestingly, there was a significant difference in the number of CD8+ T lymphocytes in the fibrous septa when compared with that within tumor lobules (Fig. 1A and B). The number of CD8+ T cells ranged between 9.2 and 250.0 (mean n = 103.0 ± 68.1) in fibrous septa, as compared with 0.2 and 33.7 (mean n = 12.0 ± 10.2) in tumor lobules. In every tumor the number of CD8+ T cells was higher in fibrous septa than in the corresponding tumor lobules (Mann–Whitney U test P < 0.00001; Fig. 1A and B).
Figure 1.
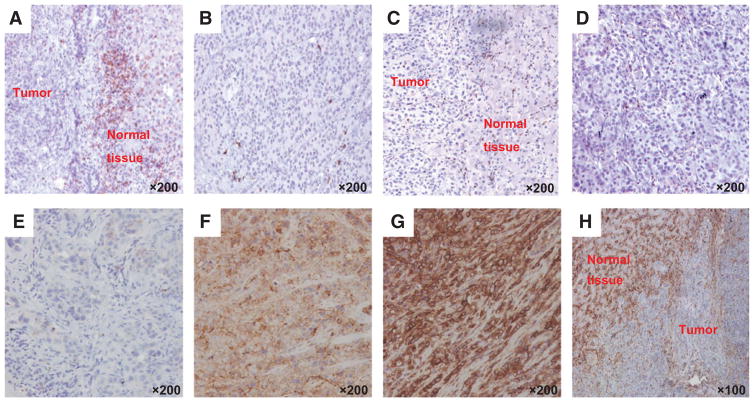
Representative staining patterns of formalin-fixed, paraffin-embedded primary ICC lesions with CD8- (A and B) and CD4- (C and D) specific mAbs in the fibrous septa (A and C) and tumor lobules (B and D) of ICC lesions. E–H, representative staining patterns of formalin-fixed, paraffin-embedded primary ICC lesions with HLA class I antigen-specific mAbs. Tumor tissue sections were IHC stained with a pool of mouse HLA-A–specific mAb HCA2 and HLA-B/C-specific mAb HC10 (ratio, 1:1). The staining with HLA class I antigen–specific mAbs was scored as negative (E), heterogeneous (F), and positive (G). Representative staining of fibrous septa of ICC lesion with HLA class I antigen–specific mAbs is shown (H). Magnification is indicated.
There was a positive association between the two populations (Spearman ρ = 0.7570, P < 0.0001): ICC tumors displaying a higher number of CD8+ T cells in the fibrous septa also had a higher number of CD8+T cells infiltrating the tumor lobules. This association was not found when evaluating CD4+ cells (Fig. 1C and D). Although it did not reach statistical significance, the number of CD4+ cells demonstrated a negative trend of association with the number of CD8+ T cells in the fibrous septa (Spearman ρ = −0.3524, P = 0.0774). This inverse association between CD4+and CD8+infiltrate could be a consequence of the CD4 antigen being expressed by populations of immunosuppressive leukocytes, including regulatory T cells, dendritic cells, monocytes, and macrophages.
Nitrotyrosine expression in primary ICC lesions
We then investigated whether the presence of reactive nitrogen plays a role in the differential distribution of CD8+ T cells within fibrous septa and tumor lobules (31). Staining with the nitrotyrosine-specific mAb showed that the T-lymphocyte distribution mirrored the nitrotyrosine staining (Supplementary Fig. S1). Cancer cell staining intensity for nitrotyrosine negatively correlated with the number of CD8+ T cells both in fibrous septa and in tumor lobules (CD8+ T cells in fibrous septa Spearman ρ = −0.6149, P = 0.0006; CD8+ T cells in tumor lobules Spearman ρ = −0.7410, P = 0.0000). In addition, nitrotyrosine expression was higher in tumor lobules than in fibrous septa; in the latter, more CD8+ T cells were concentrated at the border of the tumor lesion. Therefore, these results suggest that the production of nitrogen reactive species limits the infiltration of CTLs in tumor lobules.
Association of HLA class I and HLA class II antigen expression in primary ICC lesions with tumor lymphocyte infiltration
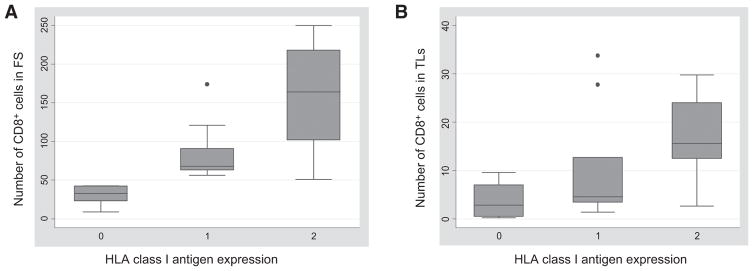
HLA class I antigen expression was downregulated in a total of 16 (59.2%) out of the 27 ICC lesions analyzed. Specifically, HLA class I antigen expression was heterogeneous in 10 (37.0%) lesions and was not detectable in 6 (22.2%). Representative staining patterns of ICC lesions with HLA class I antigen–specific mAbs are shown in Fig. 1E and H. The number of CD8+ T cells within tumor lobules and fibrous septa correlated with HLA class I antigen expression (fibrous septa Spearman ρ = 0.8101, P = 0.0000; tumor lobules Spearman ρ = 0.5679, P = 0.0020; Fig. 2). In contrast, the number of CD4+ cells in fibrous septa and tumor lobules was inversely correlated with HLA class I antigen expression (Spearman ρ = −0.6045, P = 0.0102).
Figure 2.
Correlation between HLA class I antigen expression and number of CD8+ T cells infiltrating the fibrous septa (FS; A) and the tumor lobules (TLs; B) in ICC lesions. Negative, heterogeneous, and positive HLA class I antigen expression is indicated with 0, 1, and 2, respectively. On each box, the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, and outliers are plotted individually.
HLA class II antigens were expressed on ICC cells in 15 (55.5%) of the 27 tumors analyzed; the expression was scored as positive in 4 (14.8%) lesions and heterogeneous in 11 (40.7%). HLA class II antigen expression was associated neither with HLA class I antigen expression nor with CD4+ and CD8+ T-cell infiltration.
HLA class II antigens were expressed on tumor-infiltrating leukocytes both in fibrous septa and within tumor lobules of all the tumors. This expression pattern is compatible with the possibility that tumor-infiltrating lymphocytes are activated and/or that dendritic cells/macrophages are present in the tumor (Supplementary Fig. S2).
PD-1 and PD-L1 expression in primary ICC lesions and their interaction with tumor-infiltrating lymphocytes
Our third aim was to determine whether a patient's immune response is affected by the expression of PD-1 and/or PD-L1 in ICC tumors. All tumors (100.0%) expressed PD-1, except for one which could not be analyzed because of the lack of sufficient tumor tissue in the sample (Fig. 3A–3C). PD-1 was expressed on tumor-infiltrating lymphocytes, but was not detected on ICC cells. Prevalence of PD-1+ cells varied from rare (1) to very high (5). PD-1+ cells had a low-to-moderate prevalence in the majority of the lesions; specifically, the score was low in 11 (42.3%) and moderate in 6 (23.1%) lesions. The prevalence of PD-1+ cells correlated with the degree of lymphocyte infiltration and was higher in fibrous septa than in tumor lobules. A trend was also found between PD-1 expression and the number of CD8+ T cells in fibrous septa (Kruskal—Wallis, P = 0.0832). Interestingly, PD-1 expression was strongly correlated with HLA class II antigen expression (Spearman ρ = 0.6830, P = 0.0001).
Figure 3.
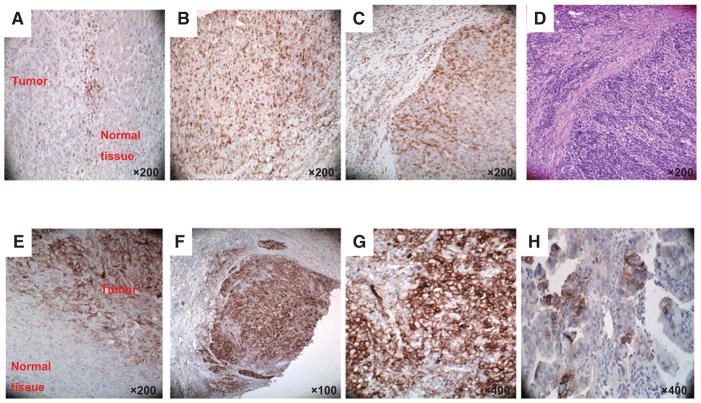
Representative staining patterns of fibrous septa (FS; A) and tumor lobules (TLs; B and C) of formalin-fixed, paraffin-embedded primary ICC lesions with PD-1–specific mAb. H&E staining of tumor tissue section provides orientation (D). Representative tumor cell staining patterns (E, marginal/interface; F and G, diffuse; H, patchy;) of formalin-fixed, paraffin-embedded primary ICC lesions with PD-L1–specific mAb. Magnification is indicated.
PD-L1 was expressed in all the tumors analyzed (100%); however, in only 8 tumors (29.6%) PD-L1 was expressed on cancer cells (Fig. 3E–H). The staining was scored as rare, low, moderate, high, and very high in 1 (12.5%), 2 (25.0%), 2 (25.0%), 2 (25.0%), and 1 (12.5%) lesions, respectively. In these lesions, PD-L1 expression was found on tumor cells as well as on tumor-infiltrating leukocytes and macrophages.
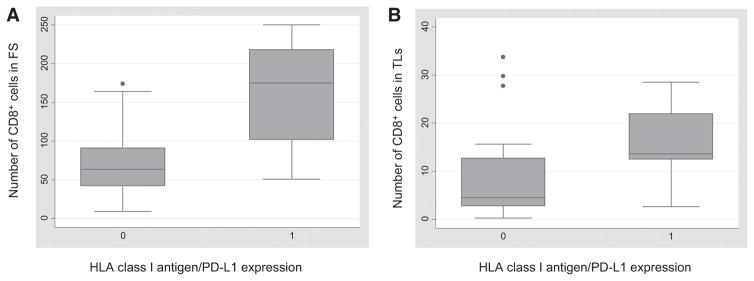
In the remaining 19 ICC lesions, PD-L1 expression was restricted to noncancer cells including tumor-infiltrating leukocytes. In the latter case, PD-L1 was mainly expressed in the fibrous septa. No correlation was identified between PD-L1 expression on ICC cells and the number of infiltrating CD8+ or CD4+ leukocytes. However, PD-L1 expression was correlated with the number of CD8+T cells in fibrous septa (Spearman ρ = 0.5951, P = 0.0011). This correlation was also influenced by HLA class I antigen expression: in tumors with negative/rare PD-L1 expression and positive HLA class I antigen expression (n = 9), the mean number of infiltrating CD8+T cells was significantly higher than in lesions with high PD-L1 expression (PD-L1 score ≥ 2), negative/heterogeneous HLA class I antigen expression or a combination of both (n = 18; mean n = 163.7 ± 66.6 vs. mean n = 73.0 ± 45.7, Wilcoxon rank-sum P = 0.0024; Fig. 4).
Figure 4.
Correlation between HLA class I antigen expression in combination with PD-L1 expression and number of CD8+ T cells infiltrating in ICC lesions. Number of CD8+ T cells infiltrating the fibrous septa (FS; A) and the tumor lobules (TLs; B) in patients with negative/rare PD-L1 and positive HLA class I antigen expression (indicated with 1) was higher than that in patients with high PD-L1 expression (PD-L1 score ≥2), negative/heterogeneous HLA class I antigen expression or a combination of both (indicated with 0). On each box, the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, and outliers are plotted individually.
Correlation of HLA class I antigen, HLA class II antigen, PD-1, and PD-L1 expression and tumor-infiltrating lymphocytes with the staging of ICC lesions and ICC patients' OS
To determine whether the patient population analyzed in this study was representative of the ICC population, standard clinicopathologic characteristics were correlated with patients' OS. In agreement with what has been observed in larger patient populations, T staging (log-rank P = 0.059), single lesions (log-rank P = 0.024), and stage (log-rank P = 0.006) significantly correlated with OS. Therefore, we feel that this cohort of patients is representative of the ICC population.
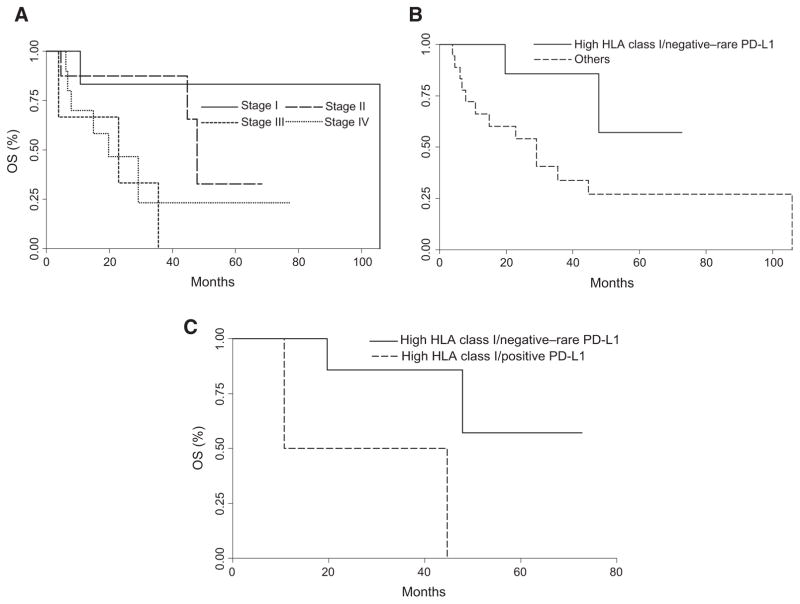
Next, we assessed the clinical relevance of the immunophenotypic staining of the ICC tumors by correlating these data with the clinicopathologic characteristics of the patient population (Supplementary Table S1). A negative correlation was identified between HLA class I antigen expression and tumor stage (Fisher exact = 0.029). ICC tumors with downregulation of their HLA class I antigen expression presented with a more advanced stage than tumors with high HLA class I antigen expression levels. T staging was positively correlated with both margins of resection (Spearman ρ = 0.5239, P = 0.0050) and PD-L1 expression by tumor cells (Fisher exact = 0.016). Tumors with higher T stages expressed more PD-L1 than early T stage ICCs. The number of CD8+T cells and CD4+leukocytes, HLA class I antigen expression by ICC cells, and PD-1 and PD-L1 expression in the tumor microenvironment were not associated with patients' survival. In contrast, positive HLA class I antigen expression in combination with negative/rare PD-L1 expression (n = 9) in ICC tumors was associated with a significantly longer patient survival (log-rank P = 0.0472; Fig. 5) as compared with high PD-L1 expression (PD-L1 score ≥ 2), negative/heterogeneous HLA class I antigen expression or a combination of both (n = 18). When HLA class I antigen expression was stratified in negative/heterogeneous or positive tumors, patients with HLA class I antigen–positive and PD-L1–negative tumors (n = 9) had a significantly longer survival than patients who expressed both HLA class I antigens and PD-L1 (n = 2, log-rank P = 0.0306; Fig. 5).
Figure 5.
Association of HLA class I antigen and PD-L1 expression in primary ICC lesions with OS in patients with ICC. A, the OS of patients with lesions grouped based on AJCC stage system was compared using the Kaplan–Meier method. Stage group of ICC lesions is indicated. Differences in patients' survival were analyzed using a log-rank test. B, the OS of patients with lesions stained with a positive HLA class I antigen expression in combination with negative/rare PD-L1 expression and that of lesions with high PD-L1 expression (PD-L1 score ≥ 2), negative/heterogeneous HLA class I antigen expression or a combination of both were compared using the Kaplan–Meier method. Differences in patients' survival were analyzed using a log-rank test. C, patients were stratified on HLA class I antigen expression as those with positive or negative/heterogeneous ICC. Then the OS of patients with lesions stained with high PD-L1 expression (PD-L1 score ≥2) was compared with that of patients with negative/rare PD-L1 expression using the Kaplan–Meier method. Differences in patients' survival were analyzed using a log-rank test.
Discussion
The current study has provided for the first time evidence that ICC patients mount a T-cell immune response against their own tumors, as lymphocyte infiltrates are present in all the tumors analyzed. Furthermore, lymphocytes bear an activated phenotype as they express HLA class II antigens. Lymphocytes appear to impose a selective pressure on tumor cell populations, as HLA class I antigen defects have been found in more than 50% of the tumors analyzed. These defects reflect the outgrowth of tumor cells that escape from recognition and destruction by the host immune system because of abnormalities in HLA class I antigens. The latter may cause a defective expression of HLA class I antigen–tumor antigen–derived peptide complexes that mediate the interaction of tumor cells with cognate CTLs. The molecular mechanism(s) underlying the defects in HLA class I antigen expression in ICC tumors are not known and could not be analyzed in the current study because ICC cell lines with abnormalities in HLA class I antigen expression are not available to us. It is likely that defects in the expression and/or function of APM components play a role in the HLA class I antigen downregulation we have described, as IHC analysis of a limited number of ICC tumors with mAbs detected downregulation of several APM components in at least 50% of the tumors analyzed (Supplementary Fig. S3).
Defects in HLA class I antigen expression are not the only escape mechanism utilized by ICC cells to avoid destruction by host immune cells. Our study identified at least two additional distinct potential mechanisms that may be utilized by ICC cells. One is represented by PD-L1 expression, which we found to be expressed on ICC cells in about 30% of the tumors analyzed. This frequency is similar to that described in breast cancer, but lower than that observed in melanoma and ovarian cancer (32–34). By interacting with its receptor, PD-L1 may coinhibit the activation of CTLs. In addition, reactive nitrogen species (RNS), which are secreted by ICC cells, as shown by the presence of nitrated tyrosines, may inhibit migration of CTLs to the tumor lobules (31). This mechanism could explain the uneven distribution of T-cell infiltration in tumor lobules and fibrous septa, as we have found an inverse relationship between nitrotyrosine expression and degree of T-cell infiltration.
To the best of our knowledge, this study is the only one to have analyzed HLA class I antigen expression in ICC tumors, and to have shown that defects in HLA class I antigen expression are present also in this type of tumor. The frequency of HLA class I antigen defects in ICC appears to be similar to that which has been described in breast cancer and prostate cancer, but higher than that described in melanoma and renal cell carcinoma (20). It is of interest that the gene products of the HLA-A locus appear to be less expressed than those of the HLA-B and C loci, provided that this difference does not reflect the different characteristics of the antibodies utilized for staining. On the other hand, HLA class II antigen expression has already been studied by Chou and colleagues, and their results are similar to ours in terms of frequency of HLA class II antigen expression (35). The frequency of HLA class II antigen expression in ICC tumors is similar to that described in adenocarcinoma of the lung and colon cancer, but lower than that observed in renal cell carcinoma (20). The frequency of PD-L1 expression in the ICC tumors we analyzed is significantly lower than that reported by Ye and colleagues in 31 tumors (100.0%) and by Suleiman and colleagues in 37 tumors (94.6%; refs. 36, 37). This difference may reflect the different characteristics of the PD-L1–specific mAbs used in the two published studies and in ours.
As observed in other types of malignancies, HLA class I antigen expression was associated with CD8+ T-lymphocyte infiltrate. Whether this association reflects the attraction of cognate CTLs to tumor sites expressing the targeted HLA class I antigen–tumor antigen–derived peptide complexes or the induction or upregulation of HLA class I antigen by IFNγ secreted by tumor-infiltrating lymphocytes remains to be determined. The latter cytokine is likely to play a role also in the expression of PD-L1 by tumor cells; however, IFNγ does not appear to be the only player in inducing PD-L1 expression as this molecule was not detected on malignant cells in a number of tumors with a high lymphocyte infiltrate.
Neither HLA class I antigens nor PD-L1 or PD-1 alone were associated with the clinical course of the disease. However, analysis of HLA class I antigen and PD-L1 expression was found to be statistically correlated with patient outcome. This finding is not unique of ICC, as it has recently been described also in patients with hepatocellular carcinoma (HCC; ref. 38). These results suggest that the failure of patients' immune system to control tumor growth may reflect not only defects in the effector phase of the immune response, but also loss of susceptibility of targeted tumor cells to immune destruction.
Given the paucity of effective therapies for the treatment of ICC and the recent major successes of immunotherapy with checkpoint molecule–specific mAbs in various types of malignancies, our results deserve some comments given their relevance for the identification of potential prognostic biomarkers and for the optimization of the rational design of immunotherapeutic strategies in this type of malignancy. First, HLA class I antigen expression in combination with PD-L1 expression by tumor cells may provide useful information about the prognosis in ICC patients, although this possibility has to be substantiated in a prospective study that enrolls a much larger number of patients than that investigated in the present retrospective study. Second, ICC patients may benefit from immunotherapeutic strategies utilizing PD-1- and PD-L1–specific mAbs as these molecules are expressed in ICC lesions. Third, HLA class I antigen expression by tumor cells needs to be taken into account when selecting ICC patients who may be eligible for treatment with checkpoint molecule–specific mAbs; in our study, this corresponded to approximately 20% of the patients. Finally, strategies to increase HLA class I antigen expression by ICC cells may improve the efficacy of checkpoint molecule-specific mAb-based immunotherapy, as the number of tumor-infiltrating lymphocytes in tumors expressing HLA class I antigens is higher than that in tumors with downregulated HLA class I antigen expression.
Acknowledgments
The authors thank Ms. Ann S. Adams for her expert editorial assistance.
Grant Support
This work was supported by a grant from the Cholangiocarcinoma Foundation, a grant from the Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, the Public Health Service (PHS) grant R21 CA 164756, awarded by the NCI and generous donations from patients and their families affected by chlolangiocarcinoma. F. Sabbatino is the recipient of a Post-Doctoral Fellowship awarded by the Fondazione Umberto Veronesi. V. Villani is the recipient of a Research Fellowship from the Centro per la Comunicazione e la Ricerca of the Collegio Ghislieri of Pavia.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: F. Sabbatino, V. Villani, L. Cai, A.X. Zhu, S. Ferrone
Development of methodology: F. Sabbatino, V. Deshpande, L. Cai, S. Nota, Y. Wang
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): F. Sabbatino, V. Villani, J.H. Yearley, V. Deshpande, I.T. Kostantinidis, C. Moon, S. Nota, Y. Wang, A. Al-Sukaini, A.X. Zhu, D.T. Ting
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): F. Sabbatino, V. Villani, J.H. Yearley, V. Deshpande, L. Cai, I.T. Kostantinidis, S. Nota, T.S. Hong, C.R. Ferrone
Writing, review, and/or revision of the manuscript: F. Sabbatino, V. Villani, J.H. Yearley, V. Deshpande, I.T. Kostantinidis, C. Moon, S. Nota, A. Al-Sukaini, A.X. Zhu, L. Goyal, N. Bardeesy, T.S. Hong, K.K. Tanabe, K.D. Lillemoe, S. Ferrone, C.R. Ferrone
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.H. Yearley, V. Deshpande, L. Cai, C. Moon
Study supervision: S. Ferrone, C.R. Ferrone
Other (contributed clinical material; discussed results and implications of findings): C. Fernandez-del Castillo
References
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Yachimski P, Pratt DS. Cholangiocarcinoma: natural history, treatment, and strategies for surveillance in high-risk patients. J Clin Gastroenterol. 2008;42:178–90. doi: 10.1097/MCG.0b013e31806daf89. [DOI] [PubMed] [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 4.Chouaib S. At the crossroads of cancer. Bull Cancer. 2013;100:569–74. doi: 10.1684/bdc.2013.1754. [DOI] [PubMed] [Google Scholar]
- 5.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol. 2013;31:4199–206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–22. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 15.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31:4252–9. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–7. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 17.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–46. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 19.Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- 20.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–85. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabbatino F, Schwab JH, Ferrone S, Ferrone CR. Evolution of studies of HLA class I antigen processing machinery (APM) components in malignant cells. Clin Transpl. 2013:453–63. [PubMed] [Google Scholar]
- 22.Esteban F, Ruiz-Cabello F, Concha A, Perez-Ayala M, Sanchez-Rozas JA, Garrido F. HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin Exp Metastasis. 1990;8:319–28. doi: 10.1007/BF01810678. [DOI] [PubMed] [Google Scholar]
- 23.Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, et al. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014;16:31–42. doi: 10.1593/neo.131568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, et al. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res. 1997;7:313–21. doi: 10.1097/00008390-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Evaluation of HLA-DR expression and T-lymphocyte infiltration in osteosarcoma. Pathol Res Pract. 1998;194:679–84. doi: 10.1016/S0344-0338(98)80126-X. [DOI] [PubMed] [Google Scholar]
- 26.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–306. [PubMed] [Google Scholar]
- 27.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–88. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 28.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–26. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 29.Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612. 14 reacting with formalin fixed tissues. J Immunol Methods. 1993;161:239–56. doi: 10.1016/0022-1759(93)90300-v. [DOI] [PubMed] [Google Scholar]
- 30.Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S. Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res. 2003;9:4043–51. [PubMed] [Google Scholar]
- 31.Kasic T, Colombo P, Soldani C, Wang CM, Miranda E, Roncalli M, et al. Modulation of human T-cell functions by reactive nitrogen species. Eur J Immunol. 2011;41:1843–9. doi: 10.1002/eji.201040868. [DOI] [PubMed] [Google Scholar]
- 32.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 34.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou FF, Sheen-Chen SM, Chen CL, Chen YS, Chen MC. Prognostic factors of resectable intrahepatic cholangiocarcinoma. J Surg Oncol. 1995;59:40–4. doi: 10.1002/jso.2930590111. [DOI] [PubMed] [Google Scholar]
- 36.Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–4. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 37.Suleiman Y, Coppola D, Zibadi S, Dalia S, Juan TH, Lee JK, et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) and expression of PD-L1 in cholangiocarcinoma. Gastrointestinal Cancers Symposium J Clin Oncol. 2015;33:2015. (suppl 3; abstr 294) [Google Scholar]
- 38.Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2015;50:65–75. doi: 10.1007/s00535-014-0933-3. [DOI] [PubMed] [Google Scholar]