Abstract
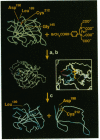
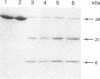
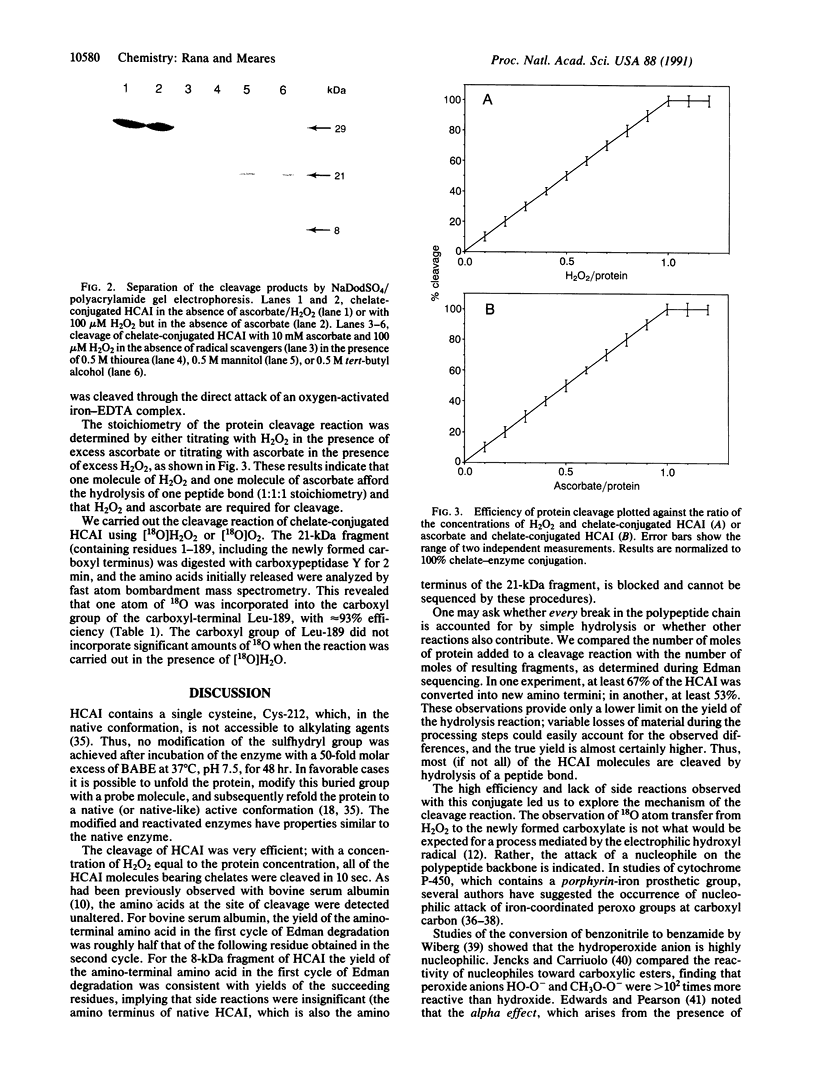
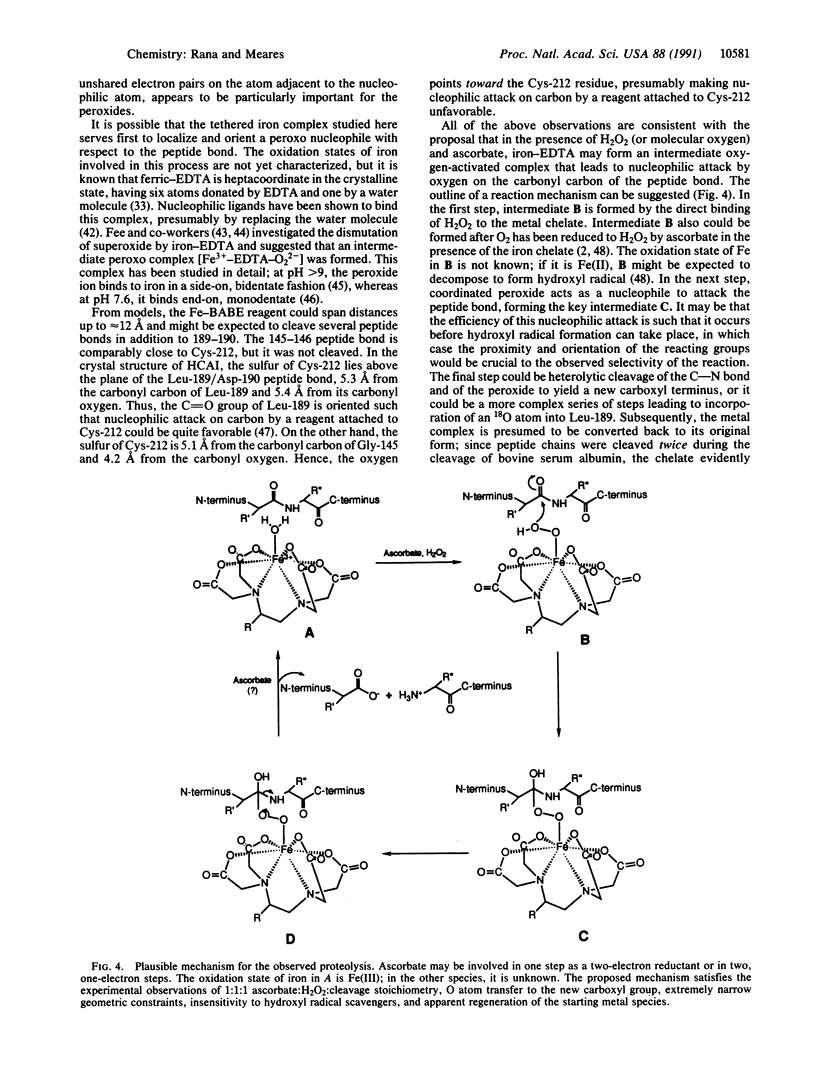
Site-specific cleavage of proteins with metal chelates is an approach for designing artificial proteolytic reagents that are directed by proximity to a peptide bond rather than by an amino acid residue type. In the presence of ascorbate and H2O2, an iron chelate attached to Cys-212 of the enzyme human carbonic anhydrase I quickly cleaved the protein between residues Leu-189 and Asp-190 to produce two discrete fragments. The transfer of an 18O atom from [18O]H2O2 (or [18O]O2) to the carboxyl group of Leu-189 was demonstrated by mass spectrometry. Quantitative experiments revealed that one molecule of H2O2 and one molecule of ascorbate afforded the hydrolysis of one peptide bond (1:1:1 stoichiometry) and that the reaction required ascorbate and H2O2. The process is catalytic, since related experiments on the protein bovine serum albumin revealed two cleavage events for each polypeptide chain cleaved. Hydroxyl radical scavengers had no significant effect. These results may be explained by generation of a highly nucleophilic oxygen species, such as peroxide coordinated to the iron chelate, that attacks a carbonyl carbon nearby.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergenhem N., Carlsson U., Karlsson J. A. Evidence for an initial fast nucleation process in the folding of human carbonic anhydrase I. Int J Pept Protein Res. 1989 Feb;33(2):140–145. doi: 10.1111/j.1399-3011.1989.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Carlsson U., Aasa R., Henderson L. E., Jonsson B. H., Lindskog S. Paramagnetic and fluorescent probes attached to "buried" sulfhydryl groups in human carbonic anhydrases. Application to inhibitor binding, denaturation and refolding. Eur J Biochem. 1975 Mar 3;52(1):25–36. doi: 10.1111/j.1432-1033.1975.tb03969.x. [DOI] [PubMed] [Google Scholar]
- Carlsson U., Henderson L. E., Lindskog S. Denaturation and reactivation of human carbonic anhydrases in guanidine hydrochloride and urea. Biochim Biophys Acta. 1973 Jun 15;310(2):376–387. doi: 10.1016/0005-2795(73)90119-0. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis at the picomole level. Application to the C-terminal sequence analysis of polypeptides. Biochem J. 1981 Dec 1;199(3):547–555. doi: 10.1042/bj1990547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Chemical conversion of a DNA-binding protein into a site-specific nuclease. Science. 1987 Sep 4;237(4819):1197–1201. doi: 10.1126/science.2820056. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Barbier C., Chassignol M., Thuong N. T., Hélène C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of iron in oxygen radical reactions. Methods Enzymol. 1984;105:47–56. doi: 10.1016/s0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Notstrand B., Fridborg K., Lövgren S., Ohlsson A., Petef M. Crystal structure of human erythrocyte carbonic anhydrase B. Three-dimensional structure at a nominal 2.2-A resolution. Proc Natl Acad Sci U S A. 1975 Jan;72(1):51–55. doi: 10.1073/pnas.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham J. A., Cech T. R. Defining the inside and outside of a catalytic RNA molecule. Science. 1989 Jul 21;245(4915):276–282. doi: 10.1126/science.2501870. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Meares C. F., McCall M. J., Reardan D. T., Goodwin D. A., Diamanti C. I., McTigue M. Conjugation of antibodies with bifunctional chelating agents: isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal Biochem. 1984 Oct;142(1):68–78. doi: 10.1016/0003-2697(84)90517-7. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Mukkala V. M., Mikola H., Hemmilä I. The synthesis and use of activated N-benzyl derivatives of diethylenetriaminetetraacetic acids: alternative reagents for labeling of antibodies with metal ions. Anal Biochem. 1989 Feb 1;176(2):319–325. doi: 10.1016/0003-2697(89)90316-3. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stackhouse T. M., Meares C. F. Photoaffinity labeling of Escherichia coli RNA polymerase/poly[d(A-T)] transcription complexes by nascent RNA. Biochemistry. 1988 Apr 19;27(8):3038–3045. doi: 10.1021/bi00408a056. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective oxidation of the exonuclease domain of bacteriophage T7 DNA polymerase. J Biol Chem. 1987 Nov 15;262(32):15330–15333. [PubMed] [Google Scholar]
- Tolan D. R., Lambert J. M., Boileau G., Fanning T. G., Kenny J. W., Vassos A., Traut R. R. Radioiodination of microgram quantities of ribosomal proteins from polyacrylamide gels. Anal Biochem. 1980 Mar 15;103(1):101–109. doi: 10.1016/0003-2697(80)90243-2. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]