Abstract
Immunofluorescence (IF) tests have redefined our understanding of many immune-mediated skin diseases, especially autoimmune blistering diseases (AIBDs). Nomenclature of certain AIBDs (for example, linear IgA diseases and IgA pemphigus) has been done based solely on the finding of tissue-bound immunoreactants as detected by IF tests. Direct and indirect are the two major types of IF tests; they are not only useful in the diagnosis but also guide the clinician in the treatment at least in certain AIBDs, as the titer of circulating antibodies as detected by IF reflects the disease activity. In this review, we describe techniques, various types of IF, and its modification.
Keywords: Autoimmune blistering diseases, diagnosis, immunofluorescence tests
Introduction
Immunofluorescence (IF) is a histochemical technique employed to detect antibodies bound to antigens in the tissue or in the circulating body fluids. It acts as a valuable adjunct to clinical and histopathological diagnosis, especially in vesiculobullous and connective tissue disorders.[1] Coons in 1940s was first to apply the IF technique to demonstrate the microorganism in the infected tissue. However, its application in dermatopathology came much later; in 1963, when these techniques were used to demonstrate the deposition of immunoglobulins and complement at the dermoepidermal junction in systemic lupus erythematosus (SLE) (”lupus band test”).[2] A year later, in 1964, Beutner and Jordon using indirect IF (IIF) successfully demonstrated the circulating antibodies in the sera of pemphigus patients.[3] Since then, it has been extensively used to understand and classify various disorders where immune mechanisms play a role. Thus, IF has become an essential investigation in the diagnosis and management of vesiculobullous, autoimmune, and connective tissue disorders.[1]
Types of Immunofluorescence
There are two main types of IF techniques, namely direct IF (DIF) and IIF.[3] Complement binding IIF which was used to diagnose circulating antibodies in pemphigoid gestationis (PG) is obsolete now.
Principle
IF technique involves viewing of antigen–antibody complexes under ultraviolet microscope using corresponding antibodies tagged to a fluorochrome. Fluorochromes are compounds containing electrons which when irradiated with a light of a particular wavelength achieve an unstable higher energetic state. On returning to the ground state as a spontaneous process, they emit light of a longer wavelength.[4] To function as labelers, they must possess chemical groups capable of forming covalent bonds with protein molecules, which emit high fluorescence in visible spectrum. Simple conjugation process, retention of the antibody activity in the labeled protein, and stability of the fluorescent conjugate are prerequisites of an ideal fluorochrome.[5] Fluorochromes, currently in use, are fluorescein isothiocyanate (FITC) which produces apple-green color and tetramethylrhodamine isothiocyanate (TRITC) with a red color of fluorescence.[4]
Direct Immunofluorescence
This is a single-step procedure that demonstrates the antibodies bound in vivo to antigens in the skin or mucosae.[3] A 3–4 mm punch biopsy is optimum for DIF study; to get a maximum yield, it is important to take biopsy from an appropriate site. An ideal site of biopsy in all autoimmune blistering diseases (AIBDs) is the perilesional skin; DIF microscopy may be negative if the biopsy is taken from lesional skin as the in vivo-bound autoantibodies are consumed by the inflammation. In cases of vasculitis, a freshly erupted purpuric spot in the most proximal part of the limb is preferred as IgA deposits may undergo degradation in older lesions. Lesional biopsy is also preferred in cases of discoid lupus erythematosus (DLE), amyloidosis, and lichen planus (LP). In systemic lupus erythematosus (SLE) and other connective tissue diseases, two or three biopsies are taken (lesional/sun exposed and nonlesional/sun protected skin). In porphyria cutanea tarda (PCT), biopsy should be taken preferably from the lesional skin; a second biopsy from the perilesional, normal skin may be considered, especially if the patient has an intact blister.
It is important to avoid contamination of biopsy samples with formalin which render the skin specimen unsuitable for DIF study. Common scenario where formalin contamination of biopsy sample occurs is when two biopsies are planned for routine histopathology and DIF. In a situation like this, the first biopsy is taken for histopathology and the same forceps are used to pick up the second biopsy (for DIF) specimen leading to formalin contamination. Therefore, we advise, when two biopsies are planned, the first biopsy should always be taken for DIF.
Transportation of the Biopsy Sample
Skin biopsy sample should be transported to the laboratory in phosphate-buffered saline (PBS). If the facility for IF is not available locally, biopsy sample can be transported to the test center in Michel's medium (MM). This transport medium contains ammonium sulfate, N-ethylmaleimide, potassium citrate buffer, magnesium sulfate, and distilled water.[5] It probably preserves immunoantigenicity of the specimen by its ability to precipitate macromolecules while inhibiting proteolytic enzymes.[6] Immunoreactants may be demonstrable by DIF even at 6 months, indicating the reliability of this medium in long-term preservations of skin biopsies.[7] Recently, normal saline is also shown as a useful transport medium if the samples can be shipped to the IF laboratory within 24 h.[8]
Biopsy specimen received in MM is washed in PBS, preferably in a rotator at 4°C. It is then oriented and embedded in optimal cutting temperature compound and snap frozen. This can be done by dipping it in n-hexane solution which is kept inside a thermos flask containing liquid nitrogen until the edges of biopsy specimen are frozen, and the central parts remain fluid. Sections of 4–6 µm thickness are then cut using a cryostat and taken off the cryostat onto the adhesive slides. In our department, special type of adhesive slides are used as shown in Figure 1. Two frozen sections are taken in each panel, and there are five such panels each for anti-IgG, anti-IgM, anti-IgA, anti-C3, and anti-fibrinogen. Sections are then air dried and washed in PBS to remove any unbound proteins. It is then treated with adequately diluted FITC-labeled conjugates (IgG, IgM, IgA, C3, and fibrin) and incubated for 1 h in a moist chamber at room temperature. Alternatively, slides can be coated with poly-L-lysine to improve the adhesive property. The sections are then washed in PBS (three washes of 10 min each) and mounted in buffered glycerol and examined under fluorescent microscope.
Figure 1.
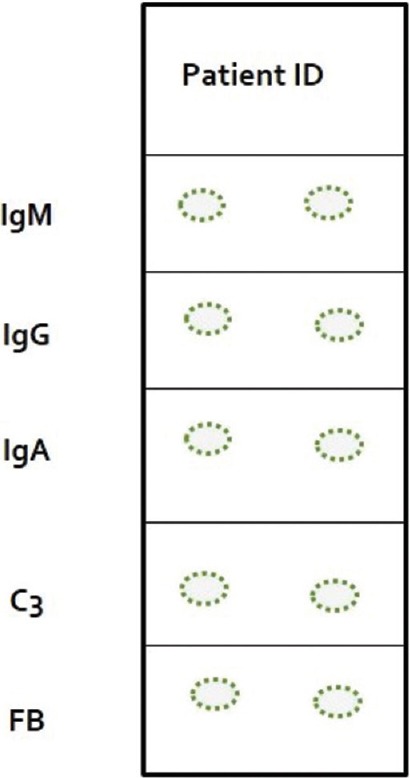
Schematic diagram of a special type of adhesive slide used in our department (Procured from Henley, UK). Each panel in the slide contains two frozen sections of patient's skin and are stained with different fluorescein isothiocyanate conjugates
Direct Immunofluorescence of Hair
Outer root sheath of anagen hair is structurally analogous to epidermal keratinocytes; hence, pemphigus-specific fluorescence pattern can be demonstrated in the plucked hair. Here, the hair is plucked using rubber-tipped artery forceps and approximately five anagen hairs are chosen. They are initially washed with PBS for 10 min following which they are incubated with the fluorescent-labeled conjugates for 1 h. At the end of this process, they are once again washed in PBS before examining under fluorescent microscope. This technique may be used in patients who do not give consent for biopsy.[9]
Interpretation of Direct Immunofluorescence
The DIF test is analyzed based on the following four parameters:
The primary site of immune deposits
The type of immune deposit
The number of immune deposits, if multiple to identify the most intense deposits
Sites of deposition other than the primary.
With the help of above parameters, a patterned approach can result in the most accurate diagnosis, especially in AIBDs [Figure 2].
Figure 2.

Algorithmic approach based on the immunofluorescence findings in AIBDs. (Abbreviations: ICS - Intercellular space, BMZ - basement membrane zone, PV - pemphigus vulgaris, PF - Pemphigus foliaceus, PNP - paraneoplastic pemphigus, PE - pemphigus erythematosus, LAD - linear IgA disease, DH - dermatitis herpetiformis, EBA - epidermolysis bullosa acquisita, b-SLE - bullous systemic lupus erythematosus, Lam 332 MMP - laminin-332 mucous membrane pemphigoid, p-200 - p200 pemphigoid)
Intercellular Space Staining
Autoantibodies in pemphigus are directed against desmosomal proteins, namely desmoglein 1 and 3 (Dsg 1 and 3) which are responsible for cell-to-cell adhesion in the epidermis.
IgG staining in intercellular space staining
This pattern is seen in all types of pemphigus except IgA pemphigus. The staining pattern is identical in pemphigus vulgaris (PV) and pemphigus foliaceus (PF), but at times, the fluorescence may be localized to or more intense along the upper layers of epidermis in PF.[10] C3 deposition follows the same pattern as IgG, but it is less intensely stained compared to IgG and usually detected in patients with active disease.[10] This pattern of deposition of IgG and C3 in intercellular space staining (ICS) has been referred to as “chicken-wire” or “fish-net” appearance.[11] [Figure 3]. The sensitivity of DIF is around 90%–100% in patients with active disease.
Figure 3.
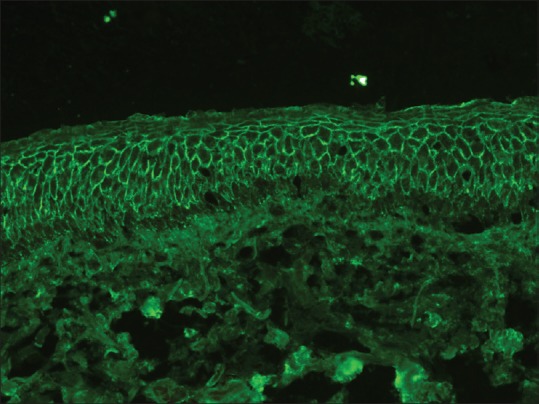
Intercellular staining of the epidermis with IgG in pemphigus (fluorescein isothiocyanate, ×200)
IgA staining in intercellular space staining
It is characteristically seen in IgA pemphigus; two types of IgA pemphigus have been recognized - subcorneal pustular dermatoses (SPD) type and intraepidermal neutrophilic (IEN) type.[10] In SPD type, IgA deposition is seen predominantly in the upper epidermal layers, whereas in IEN type, it is seen throughout the epidermis or restricted to the lower epidermis.[12]
Intercellular and basement membrane zone staining
This type of dual staining of epidermal ICS and basement membrane zone (BMZ) occurs in two conditions, namely, pemphigus erythematosus (PE) and paraneoplastic pemphigus (PNP).[10] PE is a variant of PF characterized by immunopathological coexistence of PF and lupus erythematosus (LE).[13] DIF in PE reveals ICS in a “fish-net” pattern; in addition, there is granular BMZ staining with IgG resembling “lupus band.” Occasionally, these patients may have circulating antinuclear antibodies (ANA) in their blood.
PNP, on the other hand, is characterized by autoantibodies against desmosomal (Dsg 1 and 3, desmoplakin, envoplakin, and periplakin) as well as BMZ protein (bullous pemphigoid [BP] 180). Accordingly, DIF in PNP reveals ICS and linear BMZ staining with IgG and C3. Intercellular staining in PNP tends to be weak, diffuse, and nonspecific, while deposition along BMZ is almost identical to that of BP [Figure 4].[10]
Figure 4.
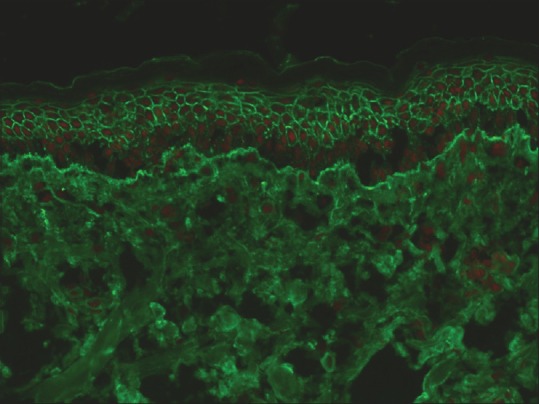
Both intercellular and basement membrane zone staining with IgG in a patient with paraneoplastic pemphigus (fluorescein isothiocyanate, ×200)
Basement Membrane Zone Staining
Deposition of immunoreactants at the dermoepidermal junction occurs in a diverse group of conditions such as subepidermal AIBDs (sAIBDs) and connective tissue diseases such as LE and LP.
Linear basement membrane zone staining
The deposition of IgG, C3, or both in a linear fashion along the BMZ [Figure 5] is seen in BP, mucous membrane pemphigoid (MMP), PG, epidermolysis bullosa aquistia (EBA), bullous SLE, and recently described anti-p200 pemphigoid.[14,15,16,17,18,19] Relative intensity of staining with IgG and C3 may sometimes help to provisionally subcategorize these conditions. For example, a more intense staining of BMZ with C3 when compared to IgG indicates the diagnosis of pemphigoid group of disorders (BP, MMP, and PG); vice versa holds good for EBA. However, this pattern may not always be discernable under microscopy; moreover, the cause of this variation in intensity of immune reactants is also not fully understood.[20,21,22] PG is characterized by an exclusive staining of BMZ with C3. On the other hand, presence of multiple deposits at BMZ strongly suggests the diagnosis of EBA or bullous SLE. In EBA, intense IgG deposition is almost always present.[17] The intensity of C3 deposits is less than IgG. IgM is found in half of the cases, and IgA is found in two-third cases of EBA. In the absence of clinical history, it may be impossible to differentiate between EBA from bullous SLE as DIF features may be similar in these conditions.
Figure 5.
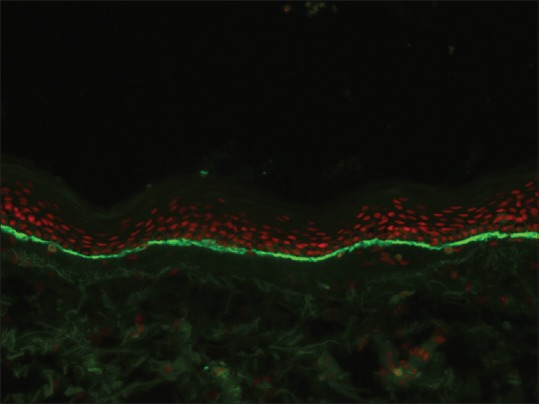
Linear basement membrane zone staining with C3 in bullous pemphigoid (fluorescein isothiocyanate, ×200)
An exclusive linear deposition IgA along the BMZ is a pathognomonic feature of linear IgA disease (LAD) [Figure 6]. Occasionally, C3 deposition may be seen, but it is less intense when compared to IgA.[23]
Figure 6.
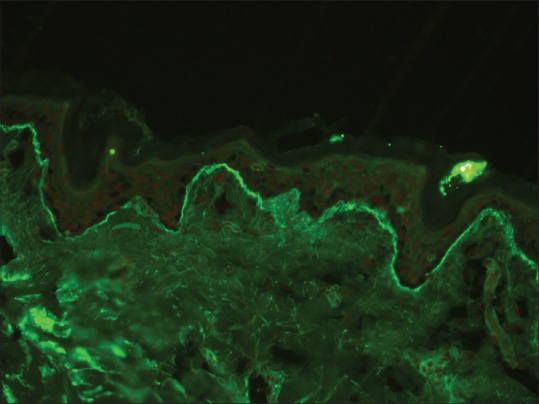
Linear basement membrane zone staining with IgA in linear IgA disease (fluorescein isothiocyanate, ×200)
Granular basement membrane zone staining
SLE demonstrates the deposition of immunoreactants in a granular pattern along the BMZ both in the lesional skin and sun protected, nonlesional skin (”the lupus band test”). It is usually seen with IgM but may be seen with other immunoreactants as well. In fact, presence of 3 or more immunoreactants deposition in BMZ is highly suggestive of SLE. In addition, colloid bodies (staining frequently with IgM) in the papillary dermis and epidermal nuclear staining with IgG (epidermal “ANA”) may be seen in SLE. DLE, on the other hand, reveals BMZ staining with these immunoreactants which may be more homogeneous and thick, especially when the biopsy is taken from the well-established lesion.
Ragged or Shaggy Basement Membrane Zone Staining
Ragged or shaggy BMZ staining with fibrinogen is characteristically seen in LP. In addition, there will be clusters of colloid bodies staining mainly with IgM and occasionally with other immunoreactants may be seen in the upper dermis. Although DIF is not generally advised in LP, it may be of particular help in certain situations such as LP–LE overlap and mucosal LP, where it helps to differentiate it from other conditions that presents with mucosal erosions such as PV and MMP.[10]
Basement Membrane Zone and Blood Vessel Wall Staining
DIF microscopy in porphyrias (PCT, pseudo–PCT, and erythropoietic protoporphyria) is characterized by a homogeneous deposition of IgG, IgA, and less frequently C3 along the BMZ as well as within superficial blood vessel walls. The density of these reactants is quite considerable and extends onto the surrounding dermis in EPP.[10]
Papillary Dermal Staining
Granular deposits of IgA in the papillary dermis are diagnostic of dermatitis herpetiformis (DH) [Figure 7]. Less frequently, a similar pattern may be seen with other immunoreactants (C3 and fibrinogen).[24] Occasionally, a fibrillar pattern of IgA deposition along BMZ may be seen, especially in atypical cases of DH.[25] Patients showing this pattern of immunoglobulin deposition may lack the circulating anti-transglutaminase and antiendomysial antibodies.[26]
Figure 7.
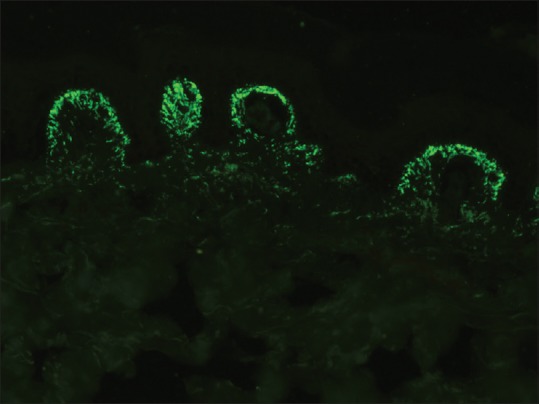
Granular staining of dermal papillae with IgA in dermatitis herpetiformis (fluorescein isothiocyanate, ×400)
Exclusive Blood Vessel Wall Staining
This feature is characteristically seen in cutaneous small vessel vasculitis (CSVV). The site of immune deposits in CSVV is within the walls of postcapillary venules in the superficial dermis.[10] The most frequent immune deposits are C3 and fibrinogen. Henoch–Schönlein purpura, a form of CSVV that is seen commonly in children, is characterized by the presence of granular staining of blood vessel wall with IgA with or without the presence of other immunoreactants [Figure 8].
Figure 8.
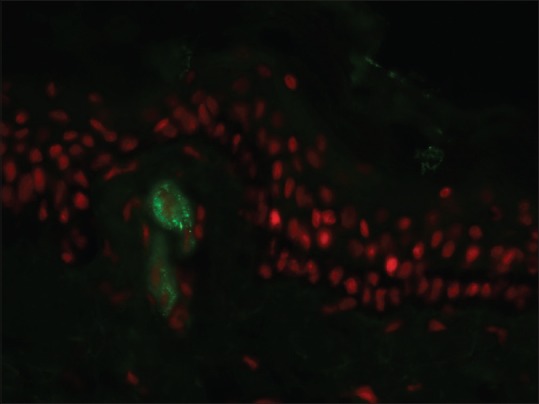
Granular blood vessel wall staining with IgA Henoch–Schönlein purpura (fluorescein isothiocyanate, ×400)
Indirect Immunofluorescence
This test is carried out to detect the circulating autoantibodies in patient's serum.[3] In addition to diagnosis, IIF titers may correlate with the disease severity and hence predicts the prognosis and helps to monitor the response to therapy.
It is a two-step procedure; in the first step of IIF, serial dilution of patient's sera is incubated with frozen sections of a suitable substrate. The second step of this technique is similar to DIF and involves staining of frozen sections with FITC conjugated IgG ± IgA. Sensitivity of IIF is generally low when compared to DIF and depends on the substrate used. Monkey esophagus is considered as an ideal substrate for PV, normal human skin (NHS) for PF whereas rat bladder epithelium is used for PNP.[2,27] For all sAIBDs, salt-split skin is the ideal substrate.
Salt-split Technique
This technique was first introduced by Gammon et al. to distinguish between sAIBDs with similar DIF findings.[28] This technique involves artificially splitting the skin at the level of lamina lucida by incubating it in 1 M solution of sodium chloride for 24 h. There are two types of salt-split technique (SST) - direct and indirect. Direct SST employs patient's skin biopsy specimen, either freshly obtained or the one which was initially used for routine DIF. Indirect SST uses NHS as a substrate and is usually preferred over direct SST. BMZ staining in the split skin may take either a “roof” pattern (band is seen toward the epidermal side of the split) or “floor” pattern (band is seen on the dermal side of the split) or a “combined” pattern (BMZ deposits on either side of the split) [Figure 9]. In BP, antibodies tend to bind to the “roof” in 70% of the cases and in the remaining 30% of cases it reveals “combined” pattern, whereas EBA (antibodies to type VII collagen) characteristically shows a “floor” pattern on indirect SST. Other conditions that may also show “floor” pattern of staining of antibodies are antilaminin-332 subtype of MMP, anti-p200 pemphigoid, and bullous SLE.
Figure 9.
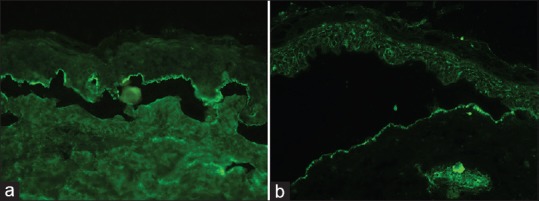
Salt-spit technique showing IgG staining on the epidermal side of the split skin in bullous pemphigoid (a) and staining with IgG on the dermal side in epidermolysis bullosa acquisita (b) (fluorescein isothiocyanate, ×200)
Antigen identification using epidermolysis bullosa skin
In sAIBD, autoantibodies are produced against the constituents of BMZ; the same molecule may be deficient in hereditary epidermolysis bullosa (EB). Using a panel of EB skin (with a known deficient BMZ protein) as a substrate and serum of patients with sAIBDs, one can detect the target antigens in certain sAIBDs using a modified IIF. This technique may especially be useful to differentiate “floor” binding sAIBDs such as EBA, p200 pemphigoid, and laminin-332 pemphigoid. Serum of patients with EBA will have antibodies against collagen type VII; this protein is deficient in recessive dystrophic EB (RDEB) skin. Hence, EBA patient's serum does not show BMZ band in RDEB skin, whereas it shows band in other types of EB skin, thus confirming the diagnosis of EBA. The advantage of the antigen-identification technique is that it is relatively simple to perform compared with other tests directed at antigen detection such as immunoblotting. However, a major limitation of the technique is the availability of suitable skin substrates from patients with EB.[29]
Serrated pattern analysis
Serration pattern analysis by routine DIF has been shown to be a useful technique to distinguish BP from EBA. EBA typically demonstrates the “u-serrated” pattern, whereas BP shows “n-serrated” pattern. The significance of this test lies in the fact that only 40%–60% of serum of patients with EBA shows circulating antibodies; remaining (nearly 50%) patients may reveal a negative IIF leading to difficulty in distinguishing this subgroup of patients from BP. Studying the serration pattern of BMZ deposits by DIF microscopy may help to distinguish these two conditions. However, this technique requires great precision, for example, thinner frozen sections and higher magnification (≥600-fold) and is difficult to carry out in the routine laboratory.[30,31]
Use of BIOCHIP mosaic slides
BIOCHIP mosaic slides (Euroimmun, Lubeck, Germany) has been found to be useful in screening for autoantibodies in patients with autoimmune blistering diseases (AIBDs). These ready-to-use slides are available commercially and contain six different substrates (monkey esophagus, primate salt-split skin, recombinant BP180 NC16A, membrane-bound Dsg1 ectodomain, Dsg3 ectodomain, and the C-terminal globular domain of BP230) in a miniature field. Technically, this is a modified IIF, wherein serum from patients with suspected AIBD is added to these slides and examined under fluorescence microscopy. The advantage of this technique is that it is a useful tool to screen autoantibodies in AIBDs as well as to identify the target antigen. This technique avoids the need to take frozen sections of a suitable substrate. Thus, BIOCHIP mosaic is a simple, standardized, and readily available novel tool which will further facilitate the diagnosis of AIBDs. Validation of the BIOCHIP showed high specificity and high sensitivity for PV, PF, and BP.[32]
Antigen Mapping
It is a modified IF technique employed to distinguish various major types of hereditary EB. Biopsy for antigen mapping is ideally taken from an artificially induced blister. This can be achieved by mechanically rubbing the skin with an eraser till faint erythema develops. Shave biopsy if preferred over the punch biopsy as traction of punch may dislodge the epidermis, especially in severe forms of EB. Frozen sections of patient's skin are then stained with commercially available monoclonal antibodies directed against different antigenic components of BMZ/epidermis such as keratin 5/14 (K5/14), laminin-332, type VII collagen, and type IV collagen. Staining pattern in the patient's skin is compared with NHS which is used as a control. Alternatively, staining with monoclonal antibodies with respect to the artificial cleft in the frozen section also enables one to subclassify EB. For example, staining with all the three antigens (laminin-332, type VII collagen, and type IV collagen) is seen on the “floor” of the artificially induced blister in EB simplex, whereas in dystrophic EB, staining with laminin-332 and type IV collagen is seen on the “roof.”[33]
Pitfalls of Immunofluorescence Technique
Although uncommon, both false-negative and false-positive results can occur in DIF microscopy. False-negative reactions usually occur due to technical reasons such as formalin contamination of specimen and improper transport medium or delay in shipping the sample to the laboratory. One prerequisite of IF microscopy is to keep the biopsy sample in a moist environment. Sometimes, the transport medium may leak out due to faulty closure of the lid of an aliquot, and skin biopsy reaches the laboratory in a dry state; immunoreactants in such biopsies would have undergone degradation leading onto negative result. Occasionally, biopsy sample may be devoid of epithelium - this happens when the sample is taken from the blisters or in mucosal biopsies (especially the gingival biopsy) making them suboptimal for the IF study. Recently, false-negative DIF microscopy has been reported in a case of drug-induced LAD; however, a repeat biopsy revealed the presence of linear IgA band in this patient.[34] This signifies the importance of reviewing the slide, re-sectioning the biopsy, and if necessary repeating the biopsy in clinically suspected cases of AIBD where initial DIF is negative.
On the other hand, nonspecific staining in the epidermis or BMZ may occasionally mimic the specific staining pattern leading onto diagnostic dilemma, especially to inexperienced eyes. Pemphigus-like pattern may be seen due to crushing and freezing artifacts. Nonspecific granular BMZ staining has been reported in diverse conditions such as bullous mastocytosis (with IgM) and elastosis perforans serpiginosa (with IgG).[35,36] It might either be due to intense inflammation along the BMZ or nonspecific binding to the altered elastic fibers. Biopsy from the sun-exposed skin may show granular IgM staining along the BMZ resembling that of lupus band. Biopsy from the lower leg near the ankle may exhibit staining around the blood vessel wall, especially with fibrinogen. This may be confused with vasculitis; to avoid this, it is ideal to take biopsy from the most proximal part of the lower limb in a suspected case of vasculitis.
Conclusion
IF techniques and its modifications have helped us to understand the immunopathology in various dermatological diseases. IF is an extremely useful and has remained gold standard even more than 50 years after its introduction in the diagnosis of AIBDs. It not only clinches the diagnosis in these conditions but also provides a platform based on which further testing can be planned. An algorithmic approach should be adopted while interpreting the IF findings in AIBDs. In many other conditions such as connective tissue disease and vasculitis, it supplements the clinical and histopathological diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vassileva S. Immunofluorescence in dermatology. Int J Dermatol. 1993;32:153–61. doi: 10.1111/j.1365-4362.1993.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 2.Chhabra S, Minz RW, Saikia B. Immunofluorescence in dermatology. Indian J Dermatol Venereol Leprol. 2012;78:677–91. doi: 10.4103/0378-6323.102355. [DOI] [PubMed] [Google Scholar]
- 3.Mohan KH, Pai S, Rao R, Sripathi H, Prabhu S. Techniques of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol. 2008;74:415–9. doi: 10.4103/0378-6323.42898. [DOI] [PubMed] [Google Scholar]
- 4.Sawant P, Kshar A, Byakodi R, Paranjpe A. Immunofluorescence in oral mucosal diseases – A review. Oral Surg Oral Med Oral Radiol. 2014;2:6–10. [Google Scholar]
- 5.Aoki V, Sousa JX, Jr, Fukumori LM, Périgo AM, Freitas EL, Oliveira ZN. Direct and indirect immunofluorescence. An Bras Dermatol. 2010;85:490–500. doi: 10.1590/s0365-05962010000400010. [DOI] [PubMed] [Google Scholar]
- 6.Michel B, Milner Y, David K. Preservation of tissue-fixed immunoglobulins in skin biopsies of patients with lupus erythematosus and bullous diseases – Preliminary report. J Invest Dermatol. 1972;59:449–52. doi: 10.1111/1523-1747.ep12627611. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan Jones SA, Salas J, McGrath JA, Palmer I, Bhogal GS, Black MM. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel's medium. Dermatology. 1994;189(Suppl 1):131–2. doi: 10.1159/000246955. [DOI] [PubMed] [Google Scholar]
- 8.Vodegel RM, de Jong MC, Meijer HJ, Weytingh MB, Pas HH, Jonkman MF. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10. doi: 10.1186/1471-5945-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao R, Dasari K, Shenoi S, Balachandran C. Demonstration of pemphigus-specific immunofluorescence pattern by direct immunofluorescence of plucked hair. Int J Dermatol. 2009;48:1187–9. doi: 10.1111/j.1365-4632.2009.04153.x. [DOI] [PubMed] [Google Scholar]
- 10.Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803–22. doi: 10.1067/mjd.2001.117518. [DOI] [PubMed] [Google Scholar]
- 11.Rani Z, Hussain I. Immunofluorescence in immunobullous diseases. J Pak Assoc Dermatol. 2003;13:76–88. [Google Scholar]
- 12.Neethu KC, Rao R, Balachandran C, Pai S. Juvenile IgA pemphigus: A case report and review of literature. Indian J Dermatol Venereol Leprol. 2016;82:439–42. doi: 10.4103/0378-6323.181467. [DOI] [PubMed] [Google Scholar]
- 13.Chorzelski T, Jablonska S, Blaszczyk M. Immunopathological investigations in the Senear-Usher syndrome (coexistence of pemphigus and lupus erythematosus) Br J Dermatol. 1968;80:211–7. doi: 10.1111/j.1365-2133.1968.tb11961.x. [DOI] [PubMed] [Google Scholar]
- 14.Gammon WR. In: The immunopathology of bullous pemphigoid antibodies. Immunopathology of the Skin. 3rd ed. Beutner EH, Chorzelski TP, Kumar V, editors. New York: John Wiley & Sons; 1987. pp. 322–36. [Google Scholar]
- 15.Bean SF. Cicatricial pemphigoid. Immunofluorescent studies. Arch Dermatol. 1974;110:552–5. [PubMed] [Google Scholar]
- 16.Harrington CI, Bleehen SS. Herpes gestationis: Immunopathological and ultrastructural studies. Br J Dermatol. 1979;100:389–99. doi: 10.1111/j.1365-2133.1979.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 17.Woodley DT, Gammon WR, Briggaman RA. In: Epidermolysis bullosa acquisita. Immunologic Diseases of the Skin. Jordon RE, editor. Norwalk, CT: Appleton & Lange; 1991. pp. 321–33. [Google Scholar]
- 18.Olansky AJ, Briggaman RA, Gammon WR, Kelly TF, Sams WM., Jr Bullous systemic lupus erythematosus. J Am Acad Dermatol. 1982;7:511–20. doi: 10.1016/s0190-9622(82)70134-3. [DOI] [PubMed] [Google Scholar]
- 19.Goletz S, Hashimoto T, Zillikens D, Schmidt E. Anti-p200 pemphigoid. J Am Acad Dermatol. 2014;71:185–91. doi: 10.1016/j.jaad.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Gammon WR, Inman AO, 3rd, Wheeler CE., Jr Differences in complement-dependent chemotactic activity generated by bullous pemphigoid and epidermolysis bullosa acquisita immune complexes: Demonstration by leukocytic attachment and organ culture methods. J Invest Dermatol. 1984;83:57–61. doi: 10.1111/1523-1747.ep12261694. [DOI] [PubMed] [Google Scholar]
- 21.Gammon WR, Lewis DM, Carlo JR, Sams WM, Jr, Wheeler CE., Jr Pemphigoid antibody mediated attachment of peripheral blood leukocytes at the dermal-epidermal junction of human skin. J Invest Dermatol. 1980;75:334–9. doi: 10.1111/1523-1747.ep12531082. [DOI] [PubMed] [Google Scholar]
- 22.Gammon WR, Merritt CC, Lewis DM, Sams WM, Jr, Wheeler CE, Jr, Carlo JR. Functional evidence for complement-activating immune complexes in the skin of patients with bullous pemphigoid. J Invest Dermatol. 1982;78:52–7. doi: 10.1111/1523-1747.ep12497912. [DOI] [PubMed] [Google Scholar]
- 23.Chorzelski TP, Jablonska S, Beutner EH, Wilson BD. In: Linear IgA bullous dermatosis. Immunopathology of the Skin. 3rd ed. Beutner EH, Chorzelski TP, Kumar V, editors. New York: John Wiley & Sons; 1987. pp. 407–20. [Google Scholar]
- 24.Leonard JN, Haffenden GP, Fry L. In: Dermatitis herpetiformis. Immunopathology of the Skin. 3rd ed. Beutner EH, Chorzelski TP, Kumar V, editors. New York: John Wiley & Sons; 1987. pp. 433–53. [Google Scholar]
- 25.Vaughan-Jones SA, Bhogal BS, Black MM. Fibrillar IgA deposition may be associated with atypical dermatitis herpetiformis – A report of two cases. J Eur Acad Dermatol Venereol. 1996;7:270–8. [Google Scholar]
- 26.Ko CJ, Colegio OR, Moss JE, McNiff JM. Fibrillar IgA deposition in dermatitis herpetiformis – An underreported pattern with potential clinical significance. J Cutan Pathol. 2010;37:475–7. doi: 10.1111/j.1600-0560.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 27.Beutner EH, Chorzelski TP, Jablonska S. Immunofluorescence tests. Clinical significance of sera and skin in bullous diseases. Int J Dermatol. 1985;24:405–21. doi: 10.1111/j.1365-4362.1985.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 28.Gammon WR, Briggaman RA, Inman AO, 3rd, Queen LL, Wheeler CE. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol. 1984;82:139–44. doi: 10.1111/1523-1747.ep12259692. [DOI] [PubMed] [Google Scholar]
- 29.Rao R, Bhogal B, Groves R. Antigen identification using skin deficient in basement-membrane protein: A novel tool for the diagnosis of subepidermal immunobullous diseases. Clin Exp Dermatol. 2013;38:289–94. doi: 10.1111/ced.12115. [DOI] [PubMed] [Google Scholar]
- 30.Vodegel RM, Jonkman MF, Pas HH, de Jong MC. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112–8. doi: 10.1111/j.1365-2133.2004.06006.x. [DOI] [PubMed] [Google Scholar]
- 31.Buijsrogge JJ, Diercks GF, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165:92–8. doi: 10.1111/j.1365-2133.2011.10346.x. [DOI] [PubMed] [Google Scholar]
- 32.van Beek N, Rentzsch K, Probst C, Komorowski L, Kasperkiewicz M, Fechner K, et al. Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49. doi: 10.1186/1750-1172-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao R, Mellerio J, Bhogal BS, Groves R. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692–7. doi: 10.4103/0378-6323.102358. [DOI] [PubMed] [Google Scholar]
- 34.Winn AE, Spillane EL, Peterson DJ, Sperling LC, Meyerle JH. False-negative direct immunofluorescence testing in vancomycin-induced linear IgA bullous dermatosis: A diagnostic pitfall. J Cutan Pathol. 2016;43:802–4. doi: 10.1111/cup.12742. [DOI] [PubMed] [Google Scholar]
- 35.Kumudhini S, Rao R, Salgaonkar G, Shetty S, Pai S. Granular IgM Deposition at basement membrane zone in an infant with diffuse cutaneous mastocytosis. Indian J Dermatol. 2016;61:581. doi: 10.4103/0019-5154.190134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramírez-Bellver JL, Bernárdez C, Macías E, Moya L, Molina-Ruiz AM, Cannata Ortiz P, et al. Dermoscopy and direct immunofluorescence findings of elastosis perforans serpiginosa. Clin Exp Dermatol. 2016;41:667–70. doi: 10.1111/ced.12882. [DOI] [PubMed] [Google Scholar]


