Abstract
Title:
Secukinumab efficacy and safety in Indian patients with moderate-to-severe plaque psoriasis: sub-analysis from FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis), a randomized, placebo-controlled, phase 3 study.
Background:
Evidence has suggested Interleukin (IL)-17A to be an important effector cytokine in the pathogenesis of psoriasis. Here, we report results for an Indian sub-population from a multinational study FIXTURE, designed to assess the safety, tolerability, and long-term efficacy of fully human anti–IL-17A monoclonal antibody secukinumab in patients with moderate-to-severe plaque psoriasis.
Materials and Methods:
In this double-dummy, placebo controlled, 52-weeks phase 3 study FIXTURE, 149 Indian patients were randomized 1:1:1:1 to receive secukinumab at a dose of 300 mg or 150 mg, etanercept, or placebo. The study objective was to show the superiority of secukinumab over placebo at week 12, vis-à-vis proportion of patients achieving a reduction of 75% or more from the baseline in the psoriasis area-and-severity index score (PASI 75) and a score of 0 (clear) or 1 (almost clear) on a 5-point modified investigator's global assessment (IGA mod 2011) (co-primary end points).
Results:
At week 12, 61.0% and 55.9% patients in secukinumab 300 mg and 150 mg groups, respectively, achieved PASI 75 response compared to 20.0% in the etanercept and 7.1% in the placebo groups. Similarly, IGA mod 2011 0 or 1 response was achieved by 43.9% and 20.6% in patients in the secukinumab 300 mg and 150 mg group, respectively, vs. 13.3% in the etanercept and 2.4% in the placebo groups at week 12. Likewise, higher proportions of patients in secukinumab 300 mg (41.5%) and 150 mg (20.6%) group were PASI 90 responders at week 12 than those in the etanercept (10.0%) or placebo (0.0%) groups. The incidences of adverse events (AEs), during the induction period were similar in all the treatment groups. Overall secukinumab was well-tolerated at both doses in the Indian sub-population.
Conclusion:
The results from the Indian sub-population suggest that secukinumab is an efficacious and safe drug for use in moderate-to-severe chronic plaque psoriasis
Keywords: Interleukin (IL)-17A, PASI 75, plaque psoriasis, secukinumab
Introduction
Psoriasis is one of the most common chronic, inflammatory, T-cell-mediated autoimmune diseases caused by inappropriate activation of the cellular immune system.[1,2] In India, the prevalence of psoriasis varies from 0.44 to 2.8%, and it is twice more common in males compared to females.[3] Psoriasis is associated with significant impairment in quality of life, work productivity, and is related with multiple comorbidities adding to the disease burden and complicating the treatment.[4,5] Multiple efforts are now being made to better understand its pathogenesis and to develop target specific treatments.[6,7] More recent an increasing body of evidence suggests that interleukin 17A (IL-17A) is also important in psoriasis pathogenesis, and Th 17/IL-17 axis dysfunction is an important source of inflammation.[5,6] The proinflammatory cytokine IL-17A is not only the primary effector of Th17 cells but is also produced by other cell types in psoriatic lesions, including gamma-delta (γδ) T cells, neutrophils, and possibly mast cells.[8]
IL-17A, the principal effector cytokine of Th17 cells, stimulates keratinocytes to produce chemokines, cytokines, and other proinflammatory mediators, thereby enabling IL-17A to bridge the innate and adaptive immune systems to sustain chronic inflammation, thus possibly acting as a master cytokine in the pathogenesis of psoriasis.[7,9] This underlies the rationale for inhibiting IL-17A signaling as a potential therapeutic approach to disrupt the psoriatic inflammatory loop.[9] Moreover, the molecular features of IL-17 makes it an attractive therapeutic target and specifically as targeted therapy in plaque psoriasis.[6]
Secukinumab is the first IL-17A inhibitor drug approved for the treatment of moderate-to-severe psoriasis in adult patients.[10] Efficacy of secukinumab in the treatment of chronic plaque psoriasis has been demonstrated in dose ranging[11] and a regimen-finding,[12] phase II clinical studies. To further confirm the findings vis-à-vis the crucial role of IL-17A in psoriasis, a phase 3 trial FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis) was conducted to assess the efficacy and safety of secukinumab, at a dose of 300 mg or 150 mg, administered as induction therapy (with assessment at week 12) and maintenance therapy (with assessment at week 52) in patients with moderate-to-severe plaque psoriasis and compared with placebo and etanercept.
Results of the global study (ClinicalTrials.gov number-NCT01358578) have been published previously.[8] Here, we present the efficacy, safety, and tolerability data of secukinumab in Indian patients enrolled in the FIXTURE study. In addition, we compare the trends of the outcomes in the Indian sub-population with those of the global study population.
Materials and Methods
Trial design
Methods, study design, assessments, and objectives for FIXTURE study have been presented previously in detail by Langley et al.[8] This was a multicenter, double-blind, double-dummy, randomized, parallel-group, active and placebo-controlled trial, which was designed to demonstrate the efficacy of subcutaneous secukinumab 12 weeks of treatment, as well as to assess the safety, tolerability, and maintenance of the efficacy at week 52 versus placebo and etanercept in patients with chronic plaque-type psoriasis.
The study consisted of a screening period of 1 to 4 weeks, an induction period of 12 weeks, a 40-weeks maintenance period, and a follow-up period of 8 weeks. Screening for tuberculosis was done specifically using Quantiferon TB Gold test and Chest X-ray. Patients were randomized 1:1:1:1 to receive secukinumab at a dose of 300 mg or 150 mg, etanercept, or placebo. The study was conducted in accordance with the principles of the declaration of Helsinki and in compliance with good clinical practice guidelines.
In India, the study was conducted at 13 centers, and consisted of 149 male and female outpatients (≥18 years old) with moderate-to-severe chronic plaque-type psoriasis diagnosed at least 6 months before randomization; that was poorly controlled with topical treatments, phototherapy, systemic therapy, or a combination of these therapies; with psoriasis area-and-severity index score (PASI) of 12 or greater, and modified investigator's global assessment scale (IGA mod 2011) score of 3 or greater (based on a scale of 0–4); and ≥10% of body surface area (BSA) involvement.
Patients with forms of psoriasis other than chronic plaque-type psoriasis or with drug-induced psoriasis, previous exposure to etanercept or secukinumab or any other biologic drug directly targeting IL-17 or the IL-17 receptor, active ongoing inflammatory diseases other than psoriasis that might confound the evaluation of the benefit of secukinumab therapy, and patients with underlying immune compromising conditions, were excluded from the study.
Results
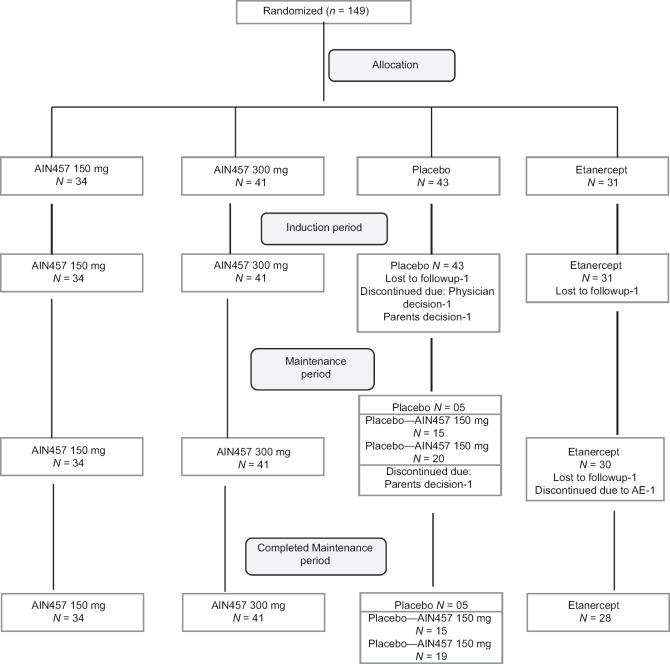
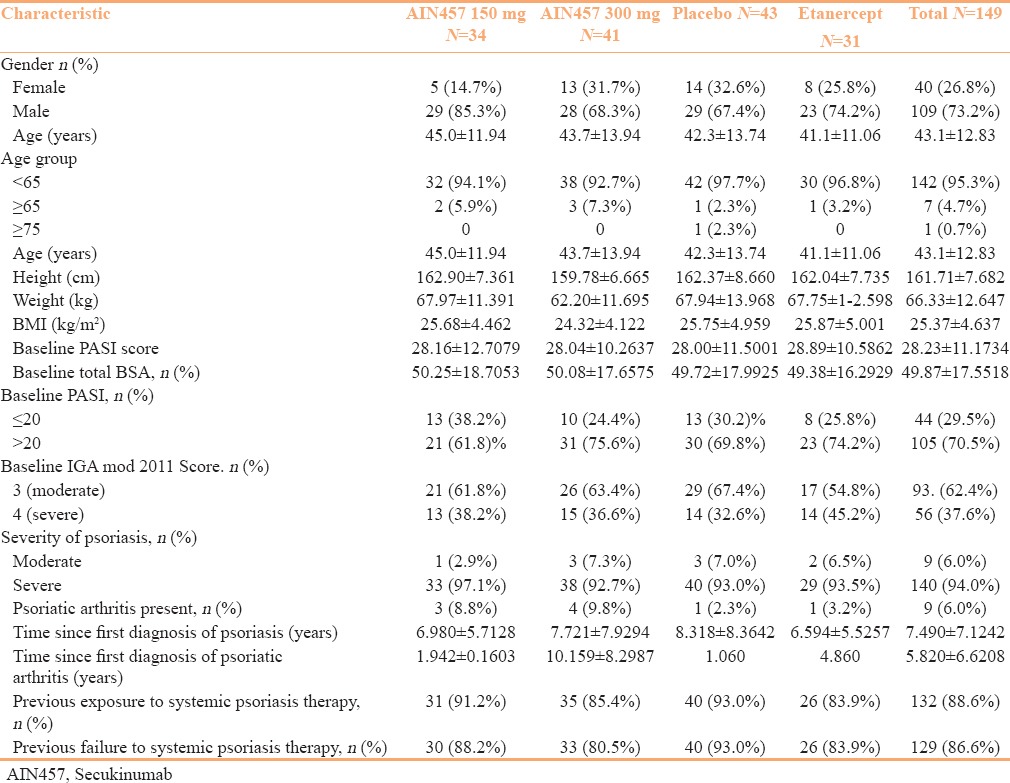
In the global study 1306 patients underwent randomization, of whom 1233 (94.4%) completed the induction period and 1100 (84.2%) completed the maintenance period.[8] For the Indian sub-population, a total of 149 patients were recruited from 13 sites and randomized to 4 treatment groups in the induction period: secukinumab 300 mg (n = 41), secukinumab 150 mg (n = 34), placebo (n = 43), or etanercept (n = 31). Of 149 patients enrolled, 145 (97.3%) patients completed the induction period. The patient flow through consecutive study visits is presented in Figure 1. The most common reason for premature discontinuation during the induction period was loss to follow-up, 2 (1.3%) patients (1 each in the placebo and etanercept groups). In the placebo group, 1 (2.3%) patient each discontinued due to physician and patient/guardian decision, respectively. None of the patients randomized to any secukinumab arms (150 mg and 300 mg) discontinued during the entire period of the study. Overall, 145 patients entered the maintenance period and 142 (97.9%) completed the maintenance period. All 149 patients enrolled in the study were included in all analysis sets. Baseline characteristics of the Indian sub-population are presented in Table 1.
Figure 1.
Patient flow through consecutive study visits
Table 1.
Demographic and Baseline Clinical Characteristics of the Indian sub-population
Primary efficacy end points
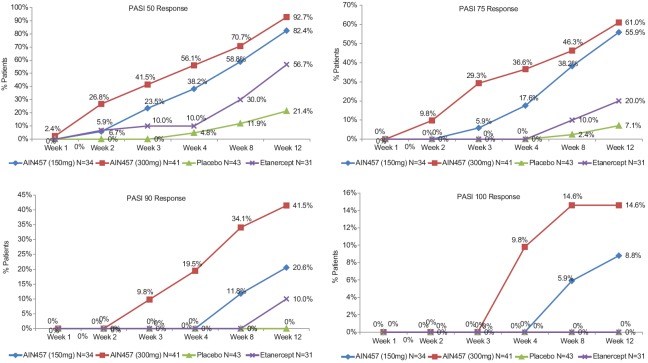
For the Indian sub-population, no hypothesis testing of the co-primary efficacy results was performed. However, PASI 75 response (primary end point) and IGA mod 2011 0 or 1 response (co-primary end point) at week 12 was met by more patients in the secukinumab 300 mg and 150 mg groups than by the patients in the placebo and etanercept groups [Table 2, Figures 2 and 3]. In addition, patients receiving secukinumab 300 mg had numerically superior response rate than patients receiving secukinumab 150 mg.
Table 2.
Efficacy End Points in FIXTURE-Indian subgroup
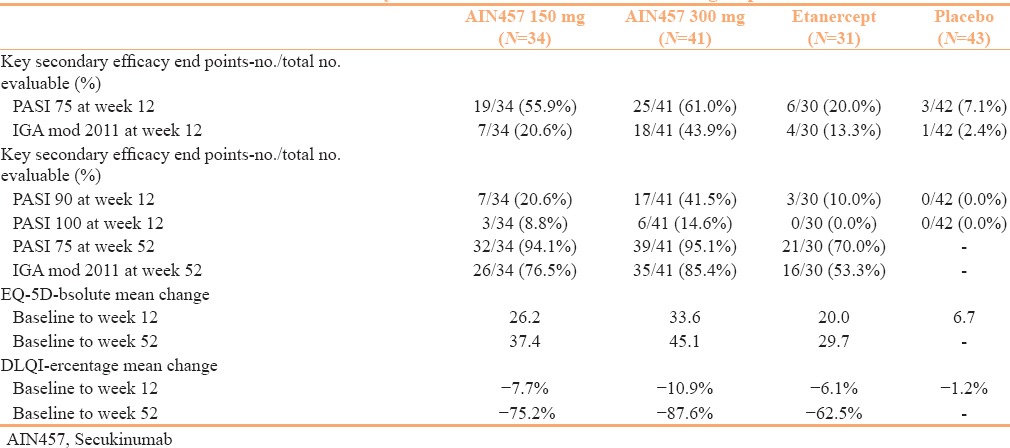
Figure 2.
The results in the efficacy of response and safety of two fixed secukinumab regimens in FIXTURE study. The PASI 50, PASI 75, PASI 90, and PASI 100 responses indicate reductions from baseline to week 12 in the PASI score of 75% or more, 90% or more, and 100%, respectively. PASI 50, 75, 90, and 100 response from baseline to week 12 in Indian sub-population
Figure 3.
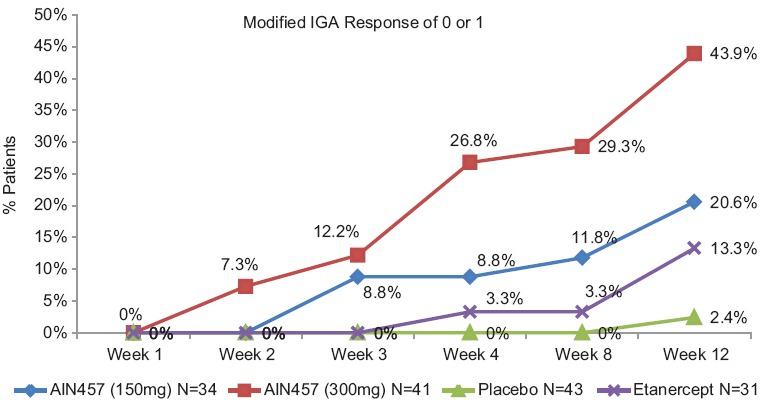
IGA mod 2011 0 or 1 response from baseline to week 12 in Indian sub-population
Sensitivity analyses of PASI 75 and IGA mod 2011 0 or 1 response at week 12 in the Indian sub-population showed that higher response rates were observed in both secukinumab dose groups than in the placebo and the etanercept group (58.5% in the secukinumab 300 mg group and 50.0% in the secukinumab 150 mg group vs. 7.0% in the placebo group and 19.4% in the etanercept group for PASI 75; and 41.5% and 17.6% vs. 2.3% and 12.9%, respectively, for IGA mod 2011 0 or 1).
Secondary efficacy end-points
For the Indian sub-population, no hypothesis testing for secondary efficacy parameters was performed at week 12. Nevertheless, secukinumab showed numerically greater response than etanercept and placebo with respect to all key secondary end points. Higher proportions of patients in both secukinumab dose groups were PASI 90 responders at week 12 than those in the placebo and etanercept groups [Table 2 and Figure 2].
Sensitivity analysis of the number and percentage of patients with PASI 90 response at week 12 showed that higher PASI 90 response rates were observed in both secukinumab groups than in the placebo and etanercept groups (39.0% for the secukinumab 300 mg group and 17.6% for the secukinumab 150 mg group vs. 0.0% for the placebo group and 9.7% for the etanercept group).
In the Indian sub-population, time courses for PASI 50, PASI 90, and PASI 100 during the induction period and the maintenance periods was generally very similar to the time course for PASI 75, and were consistent with the trends observed in the global population. Constant with the trends for the global population, the proportion of responders increased continuously during the induction period in the secukinumab 300 mg and secukinumab 150 mg groups, with a higher response rate in the higher dose group. Response rates at week 12 is summarized by treatment group in Fgure 2. Speed of onset of efficacy, as assessed by PASI 75, was faster for both secukinumab groups than for the compared groups at week 4; PASI 75 response rate was 36.6% (300 mg) and 17.6% (150 mg) for secukinumab compared to 0.0% for etanercept and 0.0% for placebo [Figure 2].
During the induction period, the mean PASI score continuously decreased in all treatment groups, but to a higher extent in the secukinumab dose groups (reaching absolute changes of –22.53 points for secukinumab 300 mg and −20.18 points for secukinumab 150 mg between baseline and week 12) than in the etanercept group (−12.81 points at week 12) and the placebo group (−4.20 points at week 12). These trends were similar to the global population. Similarly, during the maintenance period, mean absolute changes from baseline in mean PASI score reduced promptly from approximately −22.5 to −26 points in the secukinumab 300 mg, −20 to −26 points in the secukinumab 150 mg group, and −13 to −25 points in the etanercept group from week 12 to weeks 20–24; the scores were then sustained throughout the maintenance period of 52 weeks.
PASI 50, PASI 75, PASI 90, and IGA mod 2011 0 or 1 responses at week 52 in all patients, regardless of response at week 12, are presented in Figure 4. Importantly, the percentages of responders were higher in the secukinumab groups than in the etanercept group for all parameters.
Figure 4.
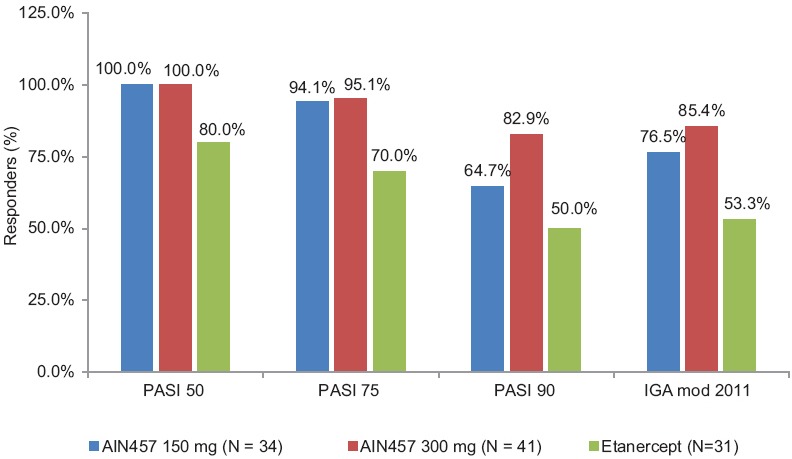
PASI 50, 75, 90, and IGA mod 2011 0 or 1 response at week 52 in Indian sub-population
More patients in the secukinumab 300 mg group (22.0%) than in any other treatment group (8.8% secukinumab 150 mg, 0.0% etanercept, and 0.0% placebo) achieved IGA mod 2011 category “clear” as their best category up to week 12. Throughout the maintenance period, the IGA mod 2011 category that most patients achieved as their best category was “clear” for the secukinumab 300 mg group (63.4%), “almost clear” for the secukinumab 150 mg group (70.6%), and “almost clear” for the etanercept group (46.7%).
EQ-5D health assessment score (ranging from 0 (worst possible health state) to 100 (best possible health state)) in the Indian sub-population increased continuously during the induction period (baseline to Week 12) in both the secukinumab dose groups and in the etanercept group, but only marginally in the placebo group [Table 2]. Improvement EQ-5D score in all active treatment groups was sustained pos-induction period, and improved further throughout the maintenance period at week 52 [Table 2].
Similar to the global population, the DLQI (Dermatology Life Quality Index) total score improved (decreased) continuously during the induction period in all active treatment groups in the Indian sub-population. The percentage mean reduction in DLQI score at week 12 was more prominent in both the secukinumab dose groups than in the etanercept or placebo group [Table 2]. Mean absolute (and relative) changes from baseline were highest in the secukinumab 300 mg group, followed by the secukinumab 150 mg group and the etanercept group, at each assessed time point of the study.
The proportion of patients with DLQI response (DLQI score of 0 or 1) during the maintenance period was higher for secukinumab 300 mg and secukinumab 150 mg compared to etanercept and placebo after 24 weeks. Response rates at week 52 were 60.0% for placebo-secukinumab 300 mg, 70.7% for secukinumab 300 mg, 52.9% for secukinumab 150 mg, 60.0% for placebo-secukinumab 150 mg, and 43.3% for etanercept. The improvements achieved for the mean DLQI total score during the maintenance period were sustained in both the secukinumab dose groups and in the etanercept group throughout the maintenance period.
Safety
The incidences of adverse events (AEs) in the Indian sub-population during the induction period were similar in all the treatment groups. In any secukinumab dose group, 20.0% (15/75) of patients reported AEs, with slightly lower percentages of patients in the etanercept and placebo groups, at 16.1% (5/31) and 14.0% (6/43), respectively. The incidences of AEs reported by the Indian sub-population were much lower than those reported by the global population.[8]
During the induction period, diarrhea (4.0%), asthenia (2.7%), and pyrexia (2.7%) were the most common AEs in the secukinumab groups in the Indian sub-population. During the induction period, compared to any secukinumab groups, higher percentage of patients in the etanercept group reported gastrointestinal disorders (5.3% vs. 6.5%), as well as general disorders and administration site conditions (5.3% vs. 6.5%), infections and infestations (4.0% vs. 9.7%), skin and subcutaneous tissue disorders (4.0% vs. 6.5%), and metabolism and nutrition disorders (1.3% vs. 6.5%).
During the entire treatment period, the frequency of treatment-emergent AEs was higher in secukinumab dose groups (53.6%), followed by 41.9% and 20.9% in etanercept and placebo groups, respectively. The 5 most commonly reported AEs during the entire treatment period for the the secukinumab dose groups were diarrhea (8.2%), pyrexia (8.2%), headache (7.3%), nasopharyngitis (5.5%), and decreased appetite (4.5%). Notably, pyrexia occurred in 22.6% of patients in the etanercept group, which was higher than that in any secukinumab group (8.2%) and the placebo group (0.0%). There were no other meaningful differences among treatment groups.
Preclinical data and observations in humans with genetic defects affecting the Th17 pathway suggests that blockade of IL-17 might lead to an increased risk for fungal infections. IL-17 is considered relevant to the mucosal defense to Candida, typically resulting in oropharyngeal forms of Candida infections.[13] Though more frequent with secukinumab 300 mg,[13] in Indian sub-population, 1 (2.0%) incidence of intertriginous candida infection was reported in secukinumab 150 mg group during the entire study period. However, candida infection was responsive to standard treatment and did not necessitate discontinuation of the study medication.
In the Indian sub-population, the incidence of serious AEs (SAEs) across treatment groups during the entire treatment period was similar for the any secukinumab dose group (5 patients, 4.5%) than for the etanercept group (1 patient, 3.2%). SAEs were more frequent in the secukinumab 150 mg roup (4 patients, 8.2%) than in the secukinumab 300 mg group (1 patient, 1.6%). No patients in any secukinumab group discontinued the study prematurely due to AEs, whereas 1 patient (3.2%) in the etanercept group discontinued due to AEs. There were no deaths reported during the study treatment period in the Indian sub-population.
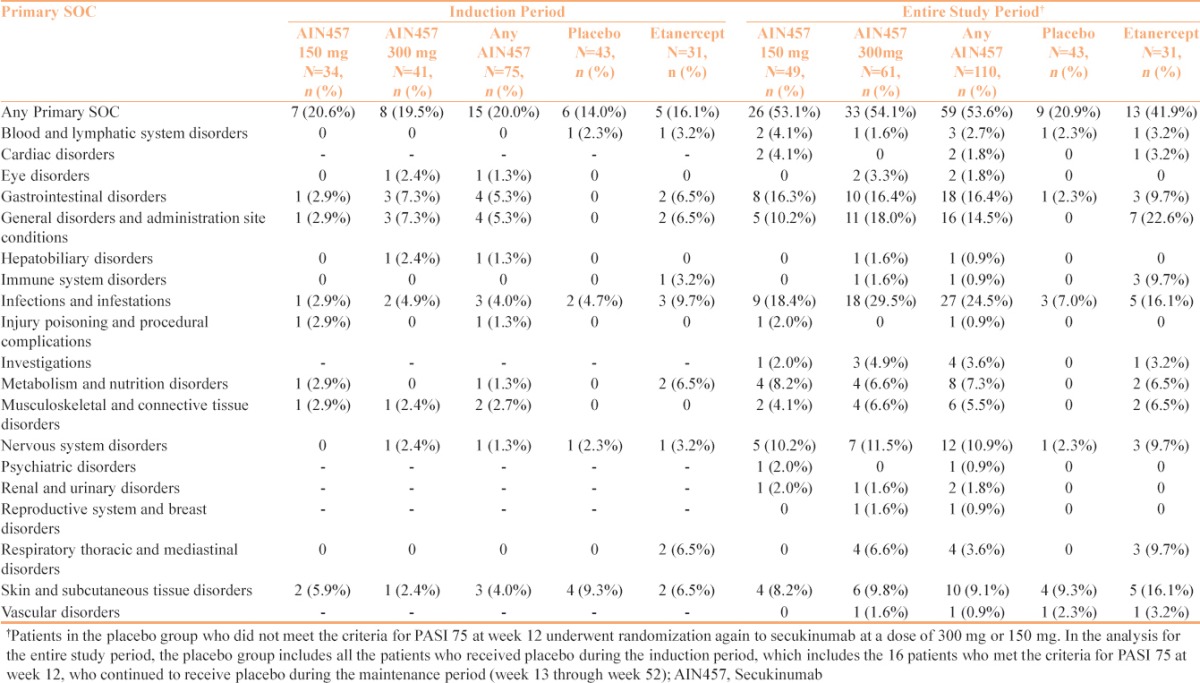
Overall, secukinumab was well-tolerated at both doses in the Indian sub-population, and the safety results in the Indian patients were similar to those observed in the global population. For the Indian sub-population, the treatment-emergent AEs by primary system organ class (SOC) for the induction and the entire treatment period are summarized in Table 3.
Table 3.
Adverse Events during the Induction Period and the Entire 52 weeks' Study Period in FIXTURE, Indian subgroup.
Discussion
Secukinumab is first fully human monoclonal immunoglobulin G1κ antibody targeting human interleukin-17A, an “important cytokine” that mediates the pathogenesis of psoriasis. Secukinumab is the first biologic treatment to be approved in the European Union for the first-line systemic treatment of moderate-to-severe plaque psoriasis.[14]
In the Indian population, secukinumab was more efficacious compared to etanercept and placebo, with respect to the co-primary efficacy end points and all key secondary end points. Secukinumab was associated with greater PASI 75 rates and higher rates of IGA mod 2011 0 or 1 responses over the comparators at week 12 [Table 2]. These results were comparable to the responses for the global study population.[8] For the key secondary efficacy objectives, secukinumab 300 mg and 150 mg both yielded higher response rates than etanercept or placebo in PASI 90 (which indicates clear or almost clear skin) and PASI 100 (complete clearance) response at week 12 [Table 2].
Responses at week 12 were sustained up to week 52 of secukinumab treatment in the Indian sub-population with slightly better efficacy, as compared to the global population. In the Indian population, maintenance of the PASI 75 response at week 52 for patients who were PASI 75 responders at week 12 was similar in secukinumab 300 and 150 mg groups (96.0% and 100.0%, respectively). Moreover, maintenance of IGA mod 2011 0 or 1 response at week 52 was also high and similar in both secukinumab 300 and 150 mg groups (83.3% and 85.7%, respectively). In the global population, 82.2% and 84.3% of patients who achieved PASI 75 at week 12 in the secukinumab 150 mg and 300 mg groups, respectively, maintained PASI 75 at 52 weeks. Whereas 67.7% and 79.7% of patients who achieved IGA mod 2011 0 or 1 response at week 12 in the secukinumab 150 mg and 300 mg groups, respectively, maintained this response at 52 weeks. Therefore, the efficacy appeared slightly better in the Indian sub-population.
Consistent with the global population, EQ-5D and DLQI scores improved continuously during the induction period and improved further throughout the maintenance period at week 52 in the Indian sub-population [Table 2].
Secukinumab was largely well-tolerated at both the doses in the Indian sub-population and the safety results in the Indian patients were similar to those observed in the global population. There were no noticeable differences in AEs reported between the secukinumab groups. In addition, in the Indian sub-population, there were no treatment-emergent AEs in any of the secukinumab groups leading to discontinuation from study treatment. A limitation of this Indian sub-population analysis is small population size, which may not have been sufficient to detect rare AEs. More studies with adequate sample size are required in the Indian psoriasis population.
The findings of the sub-analysis of Indian population in the FIXTURE study demonstrated that secukinumab, a novel, selective anti-IL-17A targeted therapy, improves symptoms rapidly and significantly. The results showed a trend that the secukinumab 300 mg was relatively better than the secukinumab 150 mg in the Indian patients with moderate to severe psoriasis during the entire study duration. Secukinumab provided faster onset of action and sustained higher clinical efficacy, compared to etanercept. The results also demonstrated that secukinumab was well-tolerated, suggesting that the targeted blockade of IL-17A does not result in any notable off-target AEs.
In conclusion, secukinumab 300 mg and 150 mg administered subcutaneously over a period of 52 weeks in the Indian population were superior to placebo and etanercept in treating moderate-to-severe plaque psoriasis. In addition to this, no major safety concerns were identified with secukinumab treatment in this study, demonstrating that the drug was well-tolerated in the Indian population.
Financial support and sponsorship
This study was supported by Novartis Pharmaceuticals and designed by the scientific steering committee and Novartis Pharmaceuticals personnel. Novartis Pharmaceuticals also conducted the data analyses.
Conflicts of interest
Ramesh Bhat has received research grants from Novartis Pharmaceuticals. Amit Thavkar is an employee of Novartis India Limited.
Acknowledgement
The authors would like to thank the investigators who participated in the study; Dr. Sacchidanand Aradhya, Dr. Maragondanahalli G. Gopal, Dr. Pratap D V S, Dr. Mir Mubashir, Dr. Srinivas Putta, Dr. Rachita Dhurat, Dr. B. V. Rama Chandra, Dr. Sushil Pande, Dr. D.G. Saple, and Dr. B Leelavathy. The authors would also like to acknowledge the MIS team at medONE Pharma Solutions, New Delhi, India, for assistance in the preparation of this manuscript.
References
- 1.Flatz L, Conrad C. Role of T-cell-mediated inflammation in psoriasis: Pathogenesis and targeted therapy. Psoriasis Targets Ther. 2013;3:1–10. [Google Scholar]
- 2.Krueger JG, Bowcock A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl 2):ii30–6. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dogra S, Yadav S. Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595–601. doi: 10.4103/0378-6323.72443. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: Findings from the National Psoriasis Foundation survey data 2003–2011. PLoS One. 2012;7:e52935. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girolomoni G, Mrowietz U, Paul C. Psoriasis: Rationale for targeting interleukin-17. Br J Dermatol. 2012;167:717–24. doi: 10.1111/j.1365-2133.2012.11099.x. [DOI] [PubMed] [Google Scholar]
- 6.Gooderham M, Posso-De Los Rios CJ, Rubio-Gomez GA, Papp K. Interleukin-17 (IL-17) inhibitors in the treatment of plaque psoriasis: A review. Skin Therapy Lett. 2015;20:1–5. [PubMed] [Google Scholar]
- 7.Das RP, Jain AK, Ramesh V. Current concepts in the pathogenesis of psoriasis. Indian J Dermatol. 2009;54:7–12. doi: 10.4103/0019-5154.48977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffths CE, Papp K, et al. Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 9.Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141–50. doi: 10.1016/j.jaad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Prescribing information: SCAPHO® (Secukinumab 150 mg Powder for solution for injection), Novartis Healthcare Private Limited, India. Issued on 22 July 2015 based on IPL dated 22 October 2013. referred on 25th December 2015. [Google Scholar]
- 11.Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412–21. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
- 12.Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402–11. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- 13.Secukinumab (AIN457), Advisory Committee Briefing Material: Available For Public Release. [Last accessed on 25 December 2015]. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM419023.pdf .
- 14.Garnock-Jones KP. Secukinumab: A Review in Moderate to Severe Plaque Psoriasis. Am J Clin Dermatol. 2015;16:323–30. doi: 10.1007/s40257-015-0143-7. [DOI] [PubMed] [Google Scholar]