Abstract
Background
Wound healing in chronic diabetic mellitus is mainly associated with the management of angiogenesis. The angiogenic mechanism of vascular endothelial growth factor (VEGF) has been widely studied in the context of diabetic ulcers. The aim of this study was to investigate the wound-healing potential of curcumol in streptozotocin-induced diabetic rats.
Material/Methods
Sixty male SD (Sprague Dawley) rats were purchased and randomly assigned into four groups: a control group and a model group treated with blank ointment, a high-dose curcumol group, and a low-dose curcumol group. The number of animals in each group was 15. Diabetes was induced by an intraperitoneal injection of streptozotocin. Two cutaneous wounds were incised at the dorsal region of all the experimental animals. Wound healing was assessed for all animal groups by observing the rate of wound closure. The expression of VEGF at the wound sites was studied by immunohistochemical staining to evaluate the vascular endothelial cell reaction. VEGF protein and related mRNA levels were analyzed by Western blotting and RT-PCR (reverse transcription-polymerase chain reaction).
Results
Curcumol treatment significantly increased the rates of wound closure in treated animals, and hence wound healing was drastically enhanced for treatment groups compared to control groups. Histological observations and related mRNA and protein levels showed a higher VEGF expression in the treatment groups.
Conclusions
Our analyses clearly suggested that the observed enhancement in wound healing as a result of curcumol administration was attributable to VEGF-mediated angiogenesis.
MeSH Keywords: Diabetic Foot; Receptors, Vascular Endothelial Growth Factor; Wound Healing
Background
Caring for diabetic wounds remains a major challenge in the medical management of patients, often due to poorer outcomes of treatments, unavailability of standard drugs [1], and longer treatment cycles. The healing of diabetic ulcers is determined by many factors, including blood flow rate and function of neovascularization [2]. It is well known that a normal blood flow will help transport oxygen, nutrients, and other recovery components to promote wound healing [3]. In a study of diabetic mice, Yoon et al. [4] used laser Doppler imaging to investigate wound neogenetic vessels coated with conjugated anti-CD31 (anti-platelet endothelial cell adhesion molecule-1) antibody markers and found that hemoperfusion significantly inhibited molecular microcirculation around injured parts and reduced capillary permeability.
Numerous growth factors provide the necessary cellular and molecular signals for a normal healing process, but these are inadequate in the context of diabetic ulcers. Pharmacological studies related to diabetic wound healing found that neogenetic capillaries could improve microcirculation and provide oxygen and affluent nutrients for tissue repair around regions of wound [5,6]. Wound healing in chronic diabetes mellitus is mainly associated with the management of angiogenesis [7], defined as a phenomenon involving the growth of new blood vessels from pre-existing vessels [8]. The mechanism of action of vascular endothelial growth factor (VEGF) in healing of diabetic ulcers has been widely studied [9], including its mitogenic and angiogenic actions on the endothelial cells through binding to the membrane [9]. VEGF protein tyrosine kinase receptors have also been found to be expressed on endothelial cells, including VEGFR1 (also known as Fms-like tyrosine kinase-1) and VEGFR2 (also known as fetal liver kinase-1 or kinase insert domain-containing receptor) [10–12].
Practitioners of traditional Chinese medicine have developed several formulations to help promote blood circulation to accelerate wound healing. For over a thousand years in China, an essential oil derived from Rhizoma curcumae aeruginosae has been prescribed for removing blood stasis and alleviating pain [13]. Curcumol is one of the major ingredients of this essential oil with the structure of a guaiane-type sesquiterpenoid hemiketal [14]. It possesses anti-tumor [15,16], anti-inflammatory [17], anti-hepatic [18], and antioxidant properties with low cytotoxicity [19]. Our previous study that used curcumol along with a blank control found that curcumol accelerated diabetic wound healing by increasing the synthesis of collagen to form granulation tissues. However, no direct evidence could be drawn from that study on the wound healing capability of curcumol. The aim of our present study was to investigate the action of this compound in diabetic ulcer treatment in vivo.
Material and Methods
Drug
Curcumol (purity >99.9%) was obtained from Chinese Materials Research Center (Beijing, China). This compound was then mixed homogeneously with blank ointment (containing white Vaseline, lanolin, and liquid paraffin at the ratio of 10: 1: 1, mixing with stirring at 60°C for 2 hours) at the concentrations of 20% (w/w).
Ethical statement
The animal handling protocol was approved by the Institutional Animal Care and Utilization Committee of the Experimental Animal Center of Zhejiang Academy of Medical Science (Approval No. SCXK (Zhe) 2014-0001).
Experimental animals
Sixty pathogen-free male SD rats weighing 200–220 g were purchased from Zhejiang Academy of Medical Science. All experimental animals were given standard diet and filter-sterilized water and housed in isolated cages under a controlled environment (humidity 50%–70%; 12 hour light/dark cycle; 22±2°C). A previously established procedure for chemically inducing type I diabetic mellitus in rats was employed in this study [20]. After an overnight fast, all animals other than those in the control group (n=15) received an intraperitoneal injection of streptozotocin (55 mg/kg; Sigma Chemical Company, St. Louis, USA). Rats with plasma glucose levels >15.5 mmol/L were considered diabetic. Subsequently, two circular wounds of diameter 1.8 cm were incised on both sides of the back flank skin of all rats. The rats were divided into four groups. While group 1 (control group) and group 2 (model group) rats were dressed with a thin layer of blank ointment twice daily, group 3 and group 4 animals were treated topically with a thin layer of ointment containing 0.5 and 1 mg/kg curcumol, respectively, twice daily.
Evaluation of wound healing
Wound closure status was evaluated using a digital camera on days 14 and 28 after treatment. The area of wound left unhealed was measured using ImageJ software.
Histopathological assay
At 14 and 28 days of treatment, five rats were randomly chosen from each group and sacrificed by an intraperitoneal injection of a pentobarbital overdose. The incision area and adjacent normal skin was excised for each sacrificed animal. A portion of the excised samples were fixed with paraformaldehyde (4%) and embedded in paraffin for future use.
Skin tissues were sectioned (4 μm). For each wound, two serial sections were placed on a slide, deparaffinized, and then rehydrated for histopathological examination by VEGF staining. The VEGF antibody (dilution 1: 100) turned the color of the granulation tissue of capillary endothelial cells to appear brown. Immunohistochemical staining was performed according to standard procedures. The wound sections were photographed using a Nikon Eclipse E400 light microscope (Japan) equipped with a camera.
Analysis of protein levels by Western blotting
Wound tissues (1 g) were lysed in 1 mL RIPA and proteinase inhibitor. The mixture was incubated on ice for 30 minutes and centrifuged at 12,000 g for 10 minutes. Protein concentration was determined using Bradford protein assay kit (Beyotime, CA, USA). Equal amounts of protein (100.0 μg) were subjected to Western blot analysis. The membranes were incubated with primary antibodies anti-β-actin (dilution 1: 1000) and anti-VEGF (dilution 1: 800) followed by the addition of secondary antibodies (dilution 1: 1,000) conjugated to horseradish peroxidase (HRP). Protein bands were visualized using a HRP Western blotting detection system according to the manufacturer’s protocol. The same membrane was stripped and re-blotted in an antibody specific to β-actin. VEGF concentrations were normalized by β-actin.
RNA extraction and RT-qPCR
VEGFR1 and VEGFR2 expressions were examined by quantitative real-time PCR (qRT-PCR). Total RNA was extracted from plasma samples using an extraction kit (Invitrogen, USA) according to the manufacturer’s instructions, and literature reports [21]. Subsequently, total RNA was reverse-transcribed into cDNA and used for the PCR template. The specific primer sequences and product sizes are listed in Table 1. The PCR thermal cycle conditions were as follows: stage 1 predegeneration at 95°C for 30 seconds; stage 2 denaturation at 40 cycles of 5 seconds at 95°C, and annealing and extension at 30 seconds at 60 °C; stage 3 melt curve at 95 °C for 15 seconds, 60°C for 60 seconds, and 95°C for 15 seconds. The mRNA abundance was normalized to GAPDH levels and expressed as a percentage of the vehicle control (100%) for statistical analysis. Three independent experiments were performed.
Table 1.
Specific primer sequences for RT-PCR.
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| VEGFR1 | TTTAAAAGGCACCCAGCACAT | TTACTCACCATTTCAGGCAAAGAC |
| VEGFR2 | GGCCCAATAATCAGAGTGGCA | TGTCATTTCCGATCACTTTTGGA |
| GAPDH | GGGAAACTGTGGCGTGAT | AAAGGTGGAGGAGTGGGT |
Statistical analysis
All results were expressed as mean ±SD. The means were compared using analysis of variance (ANOVA) followed by Scheffe’s test. Data analysis was performed using SPSS 19.0. A p value <0.05 was considered statistically significant.
Results
Macroscopic analysis
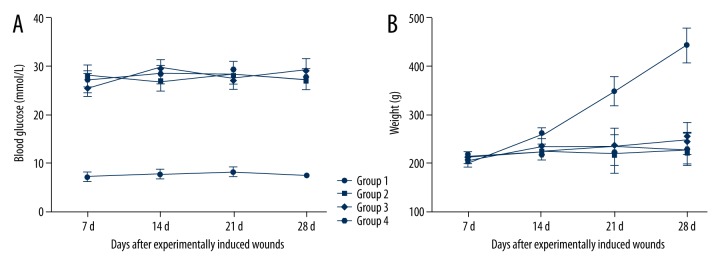
Blood glucose levels and weight of each rat were measured on 7, 14, 21, and 28 days after wound incision. The body weights were significantly higher for the control groups compared to the diabetic groups for the study period (Figure 1B). All the diabetic groups had glucose levels ≥15.5 mmol/L compared to the control group (Figure 1A). However, there was no significant difference in glucose levels among the diabetic groups during the period of study.
Figure 1.
(A, B) Blood glucose and body weights of different groups during the study period.
Wound closure
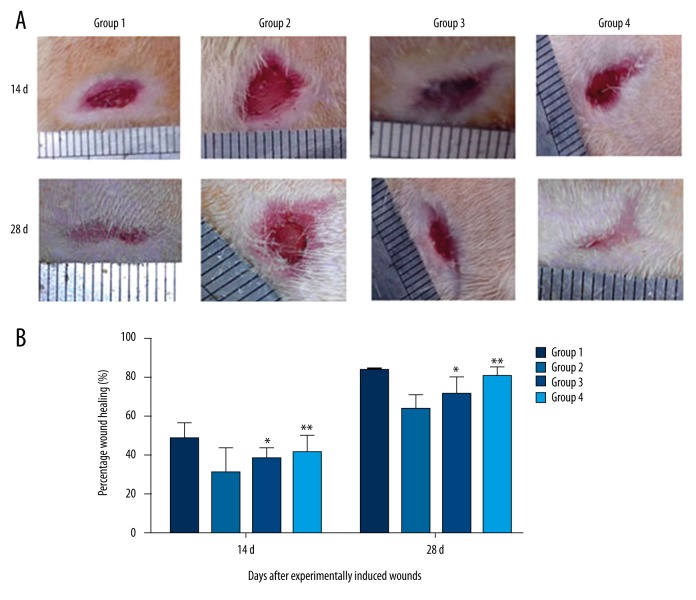
At 14 and 28 days of wound incision, all rats were photographed and the percentage of wound closure was evaluated using ImageJ software [22] and computed using the formula from literature, stated as percentage of wound closure=[(Area on 1 day – Area of X days)/Area on 1 day]×100% [23]. Wound closure (in percentage) was presented as mean ±SD and analyzed using one-way ANOVA (Figure 2). Accelerated wound closure was observed in the group that received curcumol ointment as compared to the model group (p<0.05). Diabetic rats that received a high dose of curcumol ointment showed a higher rate of wound closure compared to the low-dose group.
Figure 2.
(A, B) Wound closure of different groups (* p<0.05 compared to model group; ** p<0.01 compared to model group).
Histopathological evaluation
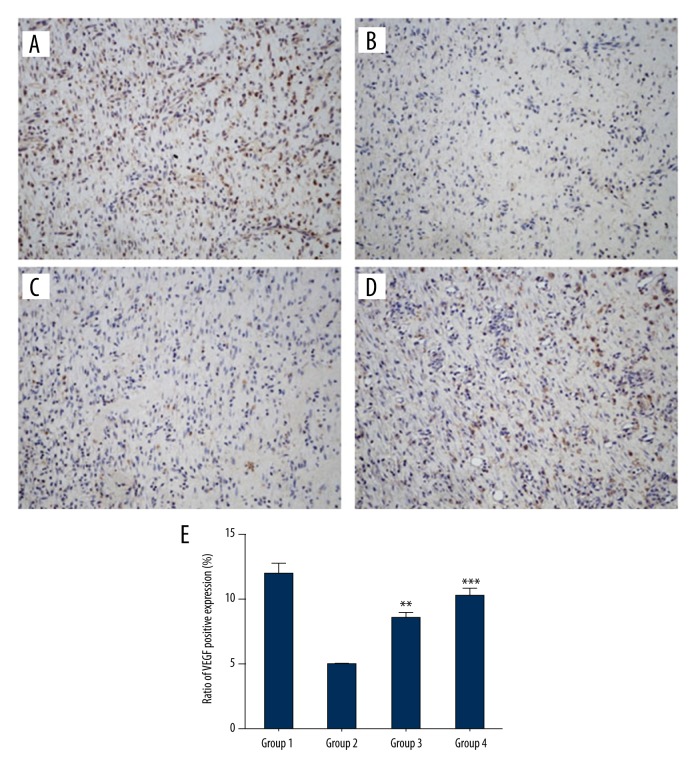
In the results of immunohistochemistry, VEGF stained in brown (positive). The expression of VEGF was significantly enhanced in the vehicle-treated normal rats (Figure 3A) compared to the diabetic groups (Figure 3B) at day 14. Figure 3 shows that the expression of VEGF was higher in both curcumol-treated groups (Figure 3C, 3D) compared to the model group. In addition, one-way ANOVA showed that the expression of VEGF was significantly different between the groups (p<0.05) (Figure 3E).
Figure 3.
VEGF immunohistochemistry: Effect of curcumol treatment on angiogenesis as shown by the brownish appearance of vascular endothelial cell marker on day 14: (A) group 1; (B) group 2; (C) group 3; (D) group 4. Magnification 200×. (E) Ratio of VEGF positive expression (%). (** p<0.01 compared to model group; *** p<0.001 compared to model group).
Curcumol-mediated activation of VEGF
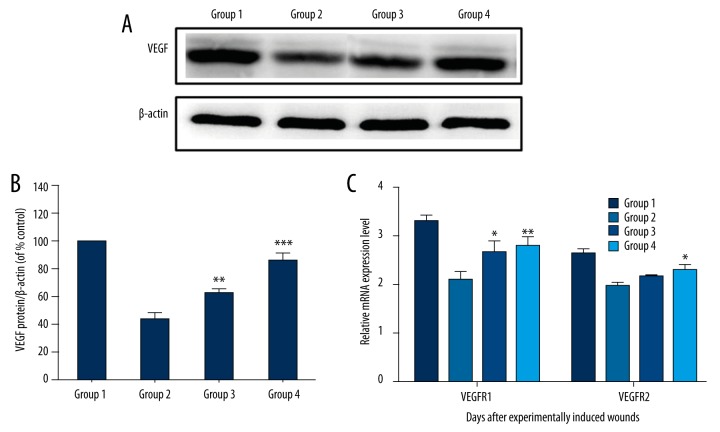
Western blot analysis revealed that curcumol upregulated the expression of VEGF (Figure 4A, 4B), suggesting that it promoted angiogenesis via the VEGF signaling pathway. In addition, curcumol increased VEGFR1 and VEGFR2 mRNA levels significantly compared to the model group.
Figure 4.
(A,B) VEGF expression as measured by Western blot analysis and quantified by densitometry, normalized by β-actin levels, and expressed as a percentage of the model group. (C) qRT-PCR of VEGFR1 and VEGFR2. (* p<0.05 compared to model group; ** p<0.01 compared to model group; *** p<0.001 compared to model group).
Effects of VEGFR1 and VEGFR2 mRNA expression
We studied the angiogenic mechanism of growth factors VEGFR1 and VEGFR2, which are well-characterized angiogenic markers. As shown in Figure 4C, VEGFR1 expression was significantly enhanced in curcumol-treated groups compared to the model group. In addition, curcumol increased VEGFR2 expression in curcumol-treated groups as compared to the model group (p<0.05). Our results showed evidence of positive effects of curcumol on VEGFR1 and VEGFR2 expression and that such stimulation effects showed a relationship with the extension of dosage.
Discussion
Complications arising from diabetes mellitus, a common endocrine disease, place a significant burden on public health [24]. Clinical trials have indicated that diabetic wound healing occurs in three phases: the inflammatory phase through compromising the immune system; the proliferative phrase through fibroblasts proliferation, collagen deposition, and formation of new angiogenesis; and the remodeling phase involving recognition and restoration of tissue structural integrity [25–27]. Delayed wound healing in a hyperglycemic state may be due to several reasons, including presence of infections, immunity suppression, local ischemia, and oxidative stress [28,29]. In addition, angiogenesis plays a major role in diabetic ulcer healing [30]. For skin tissues, angiogenesis is important for the maintenance of tissue health and quicker wound healing. Impairment in the formation of new blood vessels retards the healing process and induces ulceration [31]. Numerous growth factors and cytokines are involved in regulating the angiogenesis [31–33]. The most potent agent is VEGF [34]. This mediator initiates wound healing and promotes the expansion of the vascular network [35,36]. It is well documented that diabetes mellitus causes an increased expression of VEGF in numerous tissues as a response to both hyperglycemia and tissue ischemia [37].
Collectively, impairment of diabetes wound healing has been proposed to be mostly related to improper angiogenic response and a number of biochemical abnormalities. These pathogenic features observed clinically in diabetic patients can be investigated in diabetic rat models. In this study, we tested and compared the effects of curcumol on the rates of wound enclosure in four groups. The time profile of ulcer area determination was according to previous wound healing investigations (usually on day 7 or 14) [38–40]. In our study, we chose day 14 as the first testing point. Our previous histological studies observed more capillaries in the granulation area as well as granulation maturation in the curcumol-treated group after 14 days of treatment. Accelerated wound closure was observed in the group that received curcumol treatment compared to vehicle-treated diabetic rats. Based on the quantification of VEGF and its associated protein, our study demonstrated that curcumol enhanced angiogenesis, resulting in better wound healing. Considering the possibility that curcumol may modulate inflammatory responses, one limitation of the present study was that we did not consider measuring inflammatory-related molecules in this wound healing study.
Conclusions
In this study, we monitored the percentage of wound closure for the four experimental groups. We measured the VEGF protein and the mRNA levels of the receptors of this protein by Western blotting and qRT-PCR. Western blot analysis showed that VEGF levels have increased for both high-dose and low-dose curcuma groups, consistent with their VEGFR1 and VEGFR2 mRNA expression levels.
Our study found that curcumol characteristically promotes angiogenesis of diabetic skin ulcers. This preliminary mechanism of action of curcumol is VEGF-mediated, as demonstrated by our immunohistochemical analyses. In light of these findings, this study demonstrated that curcumol can be a prospective compound for effective treatment of diabetic ulcers, which has been a common complaint among diabetic patients necessitating frequent clinical visits.
Footnotes
Source of support: This work was supported by Zhejiang Province’s 151 Talent Project, TCM Science and Technology Plan of Zhejiang Province (2016ZB002) and Medicine and Health Science Fund of Zhejiang Province (2017KY266)
Conflict of Interests
All authors declare no conflict of interest in relation to this study.
References
- 1.Boulton AJ, Vileikyte L, Ragnarsontennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA. 2009;106(32):13505–10. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20(5):517–27. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Yoon C, Jung HM, Lee S, et al. Sonoporation of the minicircle-VEGF 165, for wound healing of diabetic mice. Pharm Res. 2009;26(4):794–801. doi: 10.1007/s11095-008-9778-x. [DOI] [PubMed] [Google Scholar]
- 5.Gabbay IE, Gabbay M, Gabbay U. Diabetic foot cellular hypoxia may be due to capillary shunting – A novel hypothesis. Med Hypotheses. 2013;82(1):57–59. doi: 10.1016/j.mehy.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11(18):2301–9. doi: 10.2174/1381612054367328. [DOI] [PubMed] [Google Scholar]
- 7.Drela E, Ruszkowska B, Kulwas A, et al. Angiogenesis in diabetic foot syndrome. Adv Clin Exp Med. 2011;20(3):243–48. [Google Scholar]
- 8.Tellechea A, Kafanas A, Leal EC, et al. Increased skin inflammation and blood vessel density in human and experimental diabetes. Int J Low Extr Wound. 2013;12(1):4–11. doi: 10.1177/1534734612474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulaki V, Qin W, Joussen AM, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109(6):805–15. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funahashi Y, Shawber CJ, Vorontchikhina M, et al. Notch regulates the angiogenic response via induction of VEGFR-1. Vascular Cell. 2010;2(1):1–10. doi: 10.1186/2040-2384-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law AY, Wong CK. Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol Cell Endocrinol. 2013;374(1–2):73–81. doi: 10.1016/j.mce.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Neiva KG, Lingen MW, et al. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17(3):499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Fang Y, Wang Y, et al. Inhibitory effect of curcumol on Jak2-STAT signal pathway molecules of fibroblast-like synoviocytes in patients with rheumatoid arthritis. Evid Based Complement Alternat Med. 2012;2012(18):746426. doi: 10.1155/2012/746426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ping G, YueWu W, BiXia W, et al. Synthesis, anti-tumor activity, and structure – activity relationships of curcumol derivatives. J Asian Nat Prod Res. 2014;16(1):53–58. doi: 10.1080/10286020.2013.857660. [DOI] [PubMed] [Google Scholar]
- 15.Lichuni XU, Bian K, Liu Z, et al. The inhibitory effect of the curcumol on women cancer cells and synthesis of RNA. Tumor. 2005;25:570–72. [Google Scholar]
- 16.Zhang W, Wang Z, Chen T. Curcumol induces apoptosis via caspases-independent mitochondrial pathway in human lung adenocarcinoma ASTC-a-1 cells. Med Oncol. 2011;28(1):307–14. doi: 10.1007/s12032-010-9431-5. [DOI] [PubMed] [Google Scholar]
- 17.Han MK, Kim JS, Park BH, et al. NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial fibroblasts by hypoxia and reoxygenation: potential role in rheumatoid arthritis. J Leukocyte Biol. 2003;73(4):525–29. doi: 10.1189/jlb.0502256. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Li ZS, Jiang FS, et al. Effects of different ingredients of zedoary on gene expression of HSC-T6 cells. Word J Gastroenterol. 2005;11(43):6780–86. doi: 10.3748/wjg.v11.i43.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngamwongsatit P, Banada PP, Panbangred W, et al. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of Toxigenic bacillus, species using CHO cell line. J Microbiol Meth. 2008;73(3):211–15. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Karim SSA, Karim QA. Anti-diabetic effects of aqueous ethanolic extract of Hibiscus Rosasinensis L. on streptozotocin-induced diabetic rats and the possible morphologic changes in the liver and kidney. Int J Pharmacol. 2011;7(3):363–69. [Google Scholar]
- 21.Roybal JD, Zang Y, Ahn YH, et al. miR-200 inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol Cancer Res. 2011;9(1):25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Watban FA, Zhang XY, Andres BL. Low-level laser therapy enhances wound healing in diabetic rats: A comparison of different lasers. Photomed Laser Surg. 2007;25(2):72–77. doi: 10.1089/pho.2006.1094. [DOI] [PubMed] [Google Scholar]
- 23.Byrnes KR, Barna L, Chenault VM, et al. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed Laser Surg. 2004;22(4):281–90. doi: 10.1089/pho.2004.22.281. [DOI] [PubMed] [Google Scholar]
- 24.Babaei S, Bayat M, Nouruzian M, et al. Pentoxifylline improves cutaneous wound healing in streptozotcin-induced diabetic rats. Eur J Pharmacol. 2013;700(1–3):165–72. doi: 10.1016/j.ejphar.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Park NY, Lim Y. Short term supplementation of dietary antioxidants selectively regulates the inflammatory responses during early cutaneous wound healing in diabetic mice. Nutr Metab (Lond) 2010;8(3):1325–26. doi: 10.1186/1743-7075-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baum CL, Arpey CJ. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Der Surg. 2005;31(6):674–86. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 27.Singer AJ, Clark RA. Cutaneous wound healing. J Burn Care Res. 2007;28(4):738–46. doi: 10.1097/BCR.0B013E318093E44C. [DOI] [PubMed] [Google Scholar]
- 28.Laitiff AA, Teoh SL, Das S. Wound healing in diabetes mellitus: Traditional treatment modalities. Clin Ter. 2010;161(4):359–64. [PubMed] [Google Scholar]
- 29.Miquel J, Bernd A, Sempere JM, et al. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. Arch Gerontol Geriatr. 2002;34(1):37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 30.Lau TW, Lam FFY, Lau KM, et al. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J Ethnopharmacol. 2009;123(1):155–62. doi: 10.1016/j.jep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Bodnar RJ. Chemokine regulation of angiogenesis during wound healing. Adv Skin Wound Care. 2015;4(11):641–50. doi: 10.1089/wound.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Bio. 2011;12(9):551–64. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin LC, Kumar P, Palmer JA, et al. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Brit J Dermatol. 2011;165(6):1223–35. doi: 10.1111/j.1365-2133.2011.10599.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramya, Kumar S. Expression of VEGF in periodontal tissues of type II diabetes mellitus patients with chronic periodontitis – an immunohistochemical study. J Clin Diagn Res. 2014;8(8):ZC01–3. doi: 10.7860/JCDR/2014/7772.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucarini G, Zizzi A, Aspriello SD, et al. Involvement of vascular endothelial growth factor, CD44 and CD133 in periodontal disease and diabetes: An immunohistochemical study. J Periodontol. 2009;36(1):3–10. doi: 10.1111/j.1600-051X.2008.01338.x. [DOI] [PubMed] [Google Scholar]
- 36.Artese L, Piattelli A, de Gouveia Cardoso LA, et al. Immunoexpression of angiogenesis, nitric oxide synthase, and proliferation markers in gingival samples of patients with aggressive and chronic periodontitis. J Periodontol. 2010;81(5):718–26. doi: 10.1902/jop.2010.090524. [DOI] [PubMed] [Google Scholar]
- 37.Mealey BL, Oates TW. AAP-commissioned review-diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 38.Fahey T, Sadaty A, Barber A, et al. Diabetes impairs the late inflammatory response to wound healing. J Surg Res. 1991;50(4):308–13. doi: 10.1016/0022-4804(91)90196-s. [DOI] [PubMed] [Google Scholar]
- 39.Rendell MS, Milliken BK, Finnegan MF, et al. The skin blood flow response in wound healing. Microvasc Res. 1997;53(53):222–34. doi: 10.1006/mvre.1997.2008. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno K, Yamamura K, Yano K, et al. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A. 2003;64(1):177–81. doi: 10.1002/jbm.a.10396. [DOI] [PubMed] [Google Scholar]