Abstract
Brain circuitry underlying cognition, emotion, and perception is abnormal in schizophrenia. There is considerable evidence that the neuropathology of schizophrenia includes the thalamus, a key hub of cortical-subcortical circuitry and an important regulator of cortical activity. However, the thalamus is a heterogeneous structure composed of several nuclei with distinct inputs and cortical connections. Limitations of conventional neuroimaging methods and conflicting findings from post-mortem investigations have made it difficult to determine if thalamic pathology in schizophrenia is widespread or limited to specific thalamocortical circuits. Resting-state fMRI has proven invaluable for understanding the large-scale functional organization of the brain and investigating neural circuitry relevant to psychiatric disorders. This article summarizes resting-state fMRI investigations of thalamocortical functional connectivity in schizophrenia. Particular attention is paid to the course, diagnostic specificity, and clinical correlates of thalamocortical network dysfunction.
1.0. Introduction
There is considerable evidence that the thalamus is abnormal in schizophrenia (Sim et al., 2006; Glahn et al., 2008; Cronenwett and Csernansky, 2010; Pergola et al., 2015). The heterogeneous structure of the thalamus and widespread connectivity with the cortex has made it difficult to determine if thalamocortical network pathology in schizophrenia is constrained to specific thalamo-cortical circuits or widespread. Connectivity-based neuroimaging methods, including resting-state fMRI, are critical tools for mapping functional brain networks and investigating circuit-level pathologies in psychiatry and neurological disorders.
This review summarizes resting-state fMRI studies of thalamocortical functional connectivity in schizophrenia. We begin with a brief overview of the structure and function of the thalamus, and conclude with a discussion of the limitations of the existing literature and significant knowledge gaps that need to be addressed.
1.1. Organization and function of the thalamus
The thalamus is a heterogeneous structure composed of several nuclei; each of which has its own distinct inputs and cortical outputs. Thalamocortical networks are arranged topographically; specific nuclei project to and receive input from largely non-overlapping cortical areas (Jones, 2007). Thalamic nuclei can be divided into two categories based on their inputs; first order (FO) and higher order (HO) nuclei (Guillery, 1995). FO nuclei receive input from peripheral sensory organs and subcortical structures, and send projections to primary sensory and motor cortical areas. They include the lateral geniculate nucleus (LGN: visual), medial geniculate nucleus (MGN: auditory), ventral posteromedial/lateral nucleus (VPM/VPL: somatosensory), and ventral lateral nuclei (VL: motor). Sensory and motor information is relayed to layer 4 of primary sensory and motor cortical areas, which, in turn, project back to the same thalamic nucleus they receive input from. In contrast to FO nuclei, HO nuclei, including the mediodorsal (MD) nucleus and pulvinar, receive most of their input directly from the cortex; cortical layer 5 of the PFC and posterior parietal association area, respectively. Inputs arriving from sensory organs/subcortical areas, or cortical layer 5 in the case of HO nuclei, provide the driving excitatory input to the thalamus. Conversely, reciprocal cortical-thalamic projections originating from cortical layer 6 inhibit thalamic activity via excitation of GABAergic neurons in the reticular nucleus, a thin sheet of neurons that envelops the thalamus. This arrangement allows the cortex to modulate, or gate, incoming sensory/cortical information. HO nuclei also play a prominent role in regulating cortical activity and coordinating activity between cortical regions given that the driving input to these nuclei originates from the cortex itself and thalamocortical projections are more diffuse than FO relay networks (Sherman, 2016).
Functionally, the thalamus has historically been viewed largely as a relay station that transmits information from peripheral sensory organs to the cortex, but performs little information processing itself. Accumulating evidence indicates this view is not entirely accurately (Sherman, 2016). For instance, the LGN, the most studied nucleus of the thalamus, plays an important role in perception and cognition, far beyond that of a relay nuclei, and is considered an early gatekeeper in the control of visual attention and awareness (Kastner et al., 2006). Specifically, human functonal imaging studies have shown that attention effects in the LGN are larger than in the striate cortex, suggesting that the LGN not only relays information, but also influences cortical input (O’Connor et al., 2002).
Not surprisingly, given that their inputs arise from association cortical areas, HO nuclei are critical for cognitive functioning. Lesions to the MD nucleus profoundly impair cognitive functioning, especially executive cognitive abilities and memory, in humans (Kubat-Silman et al., 2002; Dagenbach et al., 2001; Van der Werf et al., 2003) as well as animals (Floresco et al., 1999; Mair, 1994). Like the PFC, the MD nucleus also exhibits sustained WM delay-related activity (Watanabe and Funahashi, 2012). A recent meta-analysis of over 190 functional neuroimaging studies concluded that the thalamus is part of a superordinate prefrontal-cingulo-parietal “executive control” network that supports WM and executive functions, including cognitive flexibility, initiation, and inhibition (Niendam et al., 2012).
2.0. Thalamocortical Pathology in Schizophrenia: Contributions from Resting-state fMRI
In vivo human neuroimaging investigations have revealed a number of thalamic abnormalities in schizophrenia, including reduced volume (McCarley et al., 1999; Shenton et al., 2001; Glahn et al., 2008), altered activity during cognition (Minzenberg et al., 2009), and diminished expression of neurochemical markers of neuronal integrity, such as N-acetyl aspartate (NAA) (Kraguljac et al., 2012) Given the centrality of cognitive impairment, thalamocortical models of schizophrenia emphasize dysfunction of the MD nucleus and, to a lesser extent, the pulvinar (Andreasen et al., 1998; Jones, 1997; Swerdlow, 2010; Cronenwett and Csernansky, 2010). However, evidence for differential involvement of specific nuclei is inconsistent. Postmortem findings are mixed; some studies have found reduced volume and/or neuron number in the MD nucleus (Pakkenberg, 1990; Pakkenberg, 1992b; Pakkenberg, 1992a; Popken et al., 2000; Young et al., 2000; Danos et al., 2005) and pulvinar (Byne et al., 2002), but others have not (Cullen et al., 2003; Dorph-Petersen et al., 2004; Kreczmanski et al., 2007). Decreased somal volume of deep cortical layer 3/4 PFC pyramidal neurons, the primary targets of thalamocortical projection neurons, indirectly implicates the MD thalamus-PFC pathway (Lewis et al., 2001; Pierri et al., 2001). A handful of neuroimaging investigations have found abnormalities in the structure and function of the MD nucleus and pulvinar (Hazlett et al., 2004; Hazlett et al., 1999; Mitelman et al., 2006; Mitelman et al., 2005; Buchmann et al., 2014). However, the strikingly small number of such studies relative to the broader schizophrenia neuroimaging literature highlights the difficulty in reliably identifying specific thalamic nuclei and associated cortical connections using conventional neuroimaging methods. Consequently, while evidence of thalamic dysfunction is abundant; the anatomical specificity of thalamus abnormalities remains unclear.
Innovative connectivity-based neuroimaging approaches have proven useful for mapping specific thalamocortical networks. Using probabilistic diffusion tensor imaging (DTI) tractography, Behrens and colleagues (2003) showed that it is possible to map thalamocortical networks in vivo in humans. In their approach, the cortex is partitioned into large regions-of-interest (ROIs) (e.g. prefrontal cortex, occipital lobe, etc.) that correspond to the major anatomical connections of specific thalamic nuclei (e.g. MD nucleus, LGN). The cortical ROIs are then used to map connectivity between cortex and thalamus and indirectly identify specific thalamic nuclei based on their differential patterns of cortical connectivity. The validity of this approach is supported by the strong correspondence between connectivity-based in vivo DTI segmentation of the thalamus and post-mortem histology (Johansen-Berg et al., 2005). This same approach has been applied to resting-state fMRI to show that different cortical regions are also functionally connected to distinct, largely non-overlapping regions of the thalamus (Zhang et al., 2008).
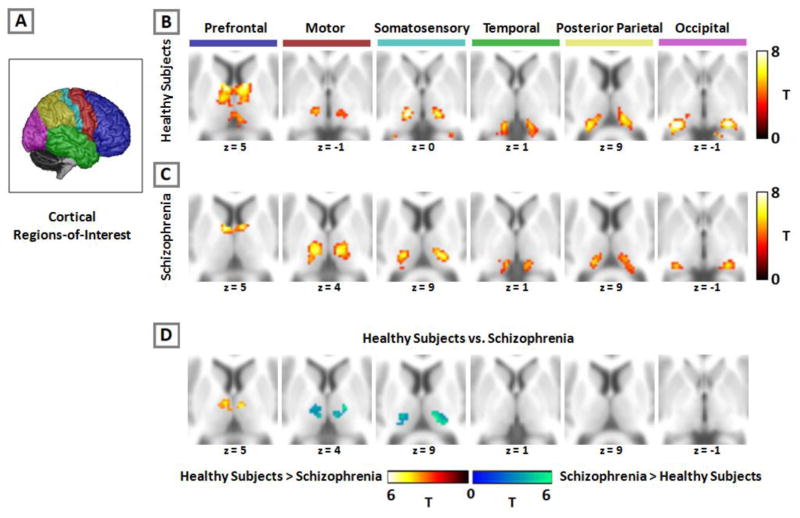
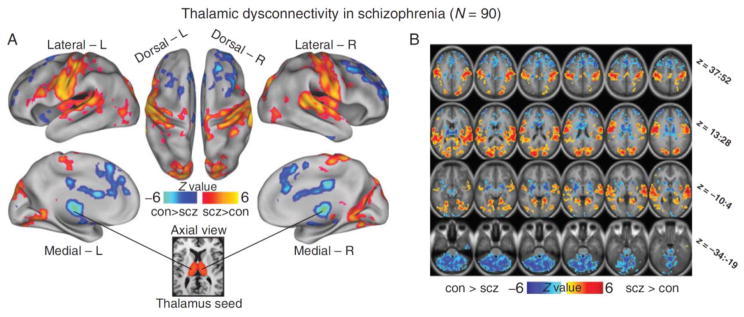
Woodward et al., (2012) subsequently used this method to investigate the anatomical specificity of thalamocortical network dysfunction in schizophrenia (see Figure 1). Replicating findings from an earlier, small study of 11 patients (Welsh et al., 2010), Woodward et al. (2012) found reduced PFC-thalamic connectivity in schizophrenia. The reduction was most pronounced in dorsal medial regions of the thalamus. Unexpectedly, they also found that thalamic connectivity with motor and somatosensory cortical areas was actually increased in schizophrenia. Using large cortical ROIs to map connectivity within the thalamus allows for the examination of multiple thalamocortical networks; however, this approach has limited spatial specificity at the level of cortex and rest of the brain. To overcome this limitation, Anticevic at al., (2014) used a complimentary approach in which the whole thalamus, rather than large cortical ROIs, is used as a seed. They replicated the combination of reduced PFC connectivity and increased somatomotor cortex connectivity in two independent cohorts of schizophrenia patients (see Figure 2). Specifically, they found that thalamic connectivity with medial and lateral prefrontal areas was reduced, while thalamic connectivity with somatomotor cortical areas was increased. Interestingly, Anticevic et al., (2014) also found that thalamic hyper-connectivity extended to other sensory cortical areas, including superior temporal gyrus and occipital lobe suggesting that sensory systems in general are over-connected in schizophrenia.
Figure 1.
Functional connectivity of cortical regions with the thalamus in healthy individuals and schizophrenia. Using the cortical regions-of-interest (ROI) approach, the cortex is partitioned into six, non-overlapping ROIs which are used as seeds in a functional connectivity analysis (panel A). Activity in each cortical ROI correlates with distinct areas of the thalamus in both healthy subjects (panel B) and patients with schizophrenia (panel C). Compared to healthy subjects, prefrontal connectivity is reduced and somatomotor connectivity increased in schizophrenia (panel D). Figure modified from Woodward et al., (2012).
Figure 2.
Dysconnectivity of the thalamus in schizophrenia. Functional connectivity of the thalamus with the rest of the brain is altered in schizophrenia (Panel A). Individuals with schizophrenia exhibit reduced thalamic connectivity with areas of the prefrontal cortex (blue) and increased connectivity with sensory and motor areas (red). Thalamus seed is shown in the bottom inset. Axial slices with corresponding Z-coordinate ranges are presented in Panel B. Abbreviations: con=healthy controls; L=left; R=right; scz=schizophrenia. Figure modified from Anticevic et al., (2013).
Since the initial studies by Welsh et al., (2010), Woodward et al., (2012), and Anticevic et al., (2014), several other groups have found abnormal thalamic functional connectivity in schizophrenia (Klingner et al., 2014; Tu et al., 2015; Lerman-Sinkoff and Barch, 2016; Atluri et al., 2015; Wang et al., 2015). In almost all cases, the combined pattern of thalamic under-connectivity with PFC and over-connectivity with sensory and motor areas was replicated. Notably, a recent multi-site investigation of 415 patients and 405 healthy subjects found that the combination of increased somatomotor connectivity and reduced PFC connectivity was the most significant abnormality in schizophrenia and was reliably detected in the 5 case-control cohorts included in the study (Cheng et al., 2015). The consistency of findings across studies that used different methods suggests that thalamocortical functional dysconnectivity is a core neurobiological abnormality in schizophrenia. However, numerous questions remain to be addressed. When does thalamocortical dysconnectivity emerge? Is it progressive? Is thalamocortical dysconnectivity specific to schizophrenia, or is it present in other psychotic disorders? What are the clinical and cognitive correlates of thalamocortical dysconnectivity? What are the potential mechanisms underlying thalamocortical network dysfunction? Progress towards answering these questions, and remaining challenges, is discussed in the following sections.
2.1. Course of Thalamocortical Network Dysfunction in Schizophrenia
So far, the vast majority of investigations examined patients regardless of illness stage. Moreover, no longitudinal investigations have been carried out. It is not known when in the course of the illness thalamocortical network dysconnectivity emerges and if the abnormalities worsen over time. Our group and others have hypothesized that thalamic connectivity disturbances in schizophrenia, and functional dysconnectivity more broadly, is a consequence of abnormal brain maturation (Woodward et al., 2011; Woodward et al., 2012; Satterthwaite and Baker, 2015). In the case of thalamocortical network dysfunction, we have speculated that the combination of reduced PFC-thalamic connectivity and somatomotor hyper-connectivity is due to atypical neurodevelopment during the transition from childhood to adolescence when thalamocortical functional connectivity undergoes significant changes. In normal development, the changes are characterized by marked strengthening of PFC connectivity and a sharpening of somatomotor connectivity (Fair et al., 2010). Thus, diminished PFC-thalamic connectivity and increased somatomotor connectivity may result from disruption of normal thalamocortical development. If correct, the abnormalities observed in chronic patients should be present, possibly in attenuated form, at the earliest stages of the illness, perhaps even before the onset of a full-blown psychotic illness.
Two recent investigations lend support to the dysmaturation hypothesis of thalamocortical dysconnectivity. First, a recent investigation found similar patterns of thalamocortical connectivity disturbances in chronic and early-stage psychosis patients (Woodward and Heckers, 2015). Specifically, thalamic connectivity with key regions of the fronto-parietal or ‘executive control’ network, which includes dorsolateral and medial PFC, inferior parietal lobe, and cerebellum, was reduced in both chronic and early stage patients. Additionally, thalamic over-connectivity with motor cortex observed in chronic patients was also present in early-stage patients. The second investigation, by Anticevic and colleagues, examined baseline RS-fMRI data from 243 clinical high-risk (CHR) individuals and 154 healthy control subjects included in the North America Prodromal Longitudinal Study (NAPLS) (Anticevic et al., 2015). They found that the pattern of reduced thalamic-PFC connectivity and sensory/motor-thalamic hyper-connectivity was present in CHR individuals. Critically, this pattern was more pronounced in the subset of 21 CHR individuals that went on to develop a full-blown psychotic illness. Both results should be interpreted cautiously until they are replicated in larger samples. However, the findings suggest that thalamocortical dysconnectivity emerges very early or even prior to the onset of florid psychosis and may predict conversion to illness. Conservatively, it seems unlikely that thalamocortical network abnormalities are wholly attributable to factors associated with having a chronic psychotic illness, such as long-term antipsychotic treatment and increased substance use.
2.2. Diagnostic Specificity of Thalamocortical Dysconnectivity
The majority of RS-fMRI studies of thalamic connectivity in psychotic disorders have focused on schizophrenia spectrum illnesses. Phenotypes that cut-across diagnoses, including cognitive impairment, suggest that brain connectivity disturbances may extend to other psychotic disorders, primarily bipolar disorder. The few studies that are available suggest this may the case. For example, the combination of PFC-thalamic under-connectivity and somatomotor-thalamic over-connectivity present in schizophrenia has also been detected in bipolar disorder, but in attenuated form (Woodward and Heckers, 2015; Anticevic et al., 2014). Sample size limitations, patient heterogeneity (e.g. inclusion of both psychotic and non-psychotic bipolar patients), and medication differences between diagnostic groups makes it difficult to draw definitive conclusions from the limited data. More work is required to determine the extent of phenotypic overlap between schizophrenia and bipolar disorder when it comes to thalamocortical connectivity disturbances. The issue is further complicated by evidence that the trajectory of cognitive impairment may differ between schizophrenia and bipolar disorder. Based largely on cross-sectional studies, it has been proposed that, in contrast to schizophrenia which is typically characterized as a static neurodevelopmental disorder, bipolar disorder may be a neuroprogressive illness defined by relatively normal pre-morbid functioning and progressive cognitive decline following illness onset (Lewandowski et al., 2011; Woodward, 2016). As such, it will be important for future neuroimaging investigations comparing schizophrenia and bipolar disorder to control for illness stage.
2.3. Clinical Correlates of Thalamocortical Dysconnectivity in Schizophrenia
Many of the studies reviewed above examined the relationship between thalamocortical dysconnectivity and clinical symptoms of psychosis, typically summary scores from commonly used clinical scales such as the Positive and Negative Syndrome Scales (PANSS: Kay et al., 1987). Findings are mixed. Several studies did not find any significant relationship (e.g. Woodward et al., 2012; Woodward and Heckers, 2015). Among the handful that did, the consistency and direction of the correlations varies across studies. Anticevic et al., (2014) found that mean connectivity extracted from sensory-motor regions that demonstrated over-connectivity with the thalamus in schizophrenia correlated positively with PANSS total score, but not positive or negative syndrome scores. Thalamocortical hypo-connectivity was unrelated to clinical symptoms. Cheng et al. (2015) found similar correlations between somatomotor-thalamic over-connectivity and clinical symptoms; although the association was only detected for negative symptoms. However, in contrast to Anticevic et al. (2014), frontal-thalamic hypo-connectivity was related to negative symptoms (Cheng et al., 2015). The findings from Anticevic et al.’s (2015) investigation suggest thalamic dysconnectivity is related to psychosis symptoms in CHR individuals and the general population. However, the pattern of results differs from those found in patients. In contrast to their earlier study in patients, they found that greater thalamic hypo-connectivity was related to Scale of Prodromal Symptoms (SOPS) total scores across all subjects and CHR individuals specifically. Similarly, the positive association between hyper-connectivity and symptoms detected in their prior investigation of patients was not present in CHR individuals. The small magnitude of the correlations, which generally fall below r=.30, heterogeneity of patient samples and imaging methods, and the post-hoc nature of the analyses may account for the inconsistent findings across studies.
It’s also possible that relatively small, inconsistent correlations may have to do with the fidelity, or lack thereof, of clinical rating scales for investigating brain-behavior relationships. Complex clinical phenomena assessed via coarse, subjective rating scales may not directly map on to specific brain circuits. Measures that capture core cognitive and perceptual processes underlying clinical phenomena may be more useful for uncovering the behavioral and cognitive consequences of thalamocortical dysconnectivity. For instance, it has been hypothesized that some of the symptoms of psychosis, hallucinations and delusions specifically, result from a defect in the efferent copy and corollary discharge systems (Frith and Done, 1989; Frith, 1987). In brief, these two systems operate together to predict and suppress sensory input generated from self-initiated actions. For example, during self-vocalization, an efferent copy of the motor command is sent to the auditory cortex which results in a corollary discharge that suppresses perception thereby insuring that sensory experiences related to self-generated vocalization are not misattributed to an outside source (Ford and Mathalon, 2012). There is considerable evidence from behavioral and neurophysiological studies supporting efferent copy/corollary discharge dysfunction in schizophrenia across a variety of motor/sensory modalities (e.g. Thakkar et al., 2015; Ford et al., 2014; Ford and Mathalon, 2012). However, evidence for an association between corollary discharge and specific symptoms, such as auditory hallucinations, is mixed (Rosler et al., 2015; Ford and Mathalon, 2012). The neural mechanisms underlying efferent copy and corollary discharge are incompletely understood; however, human (Ostendorf et al., 2013) and non-human primate (Cavanaugh et al., 2016; Sommer and Wurtz, 2008) studies have linked the thalamus, especially the mediodorsal nucleus, to corollary discharge. To date, no study has examined the relationship between corollary discharge and thalamocortical functional connectivity, but this may be a fruitful avenue for future research.
As reviewed earlier, lesion and functional imaging studies in humans have repeatedly implicated the thalamus in executive cognitive abilities, including working memory (Niendam et al., 2012). A recent investigation in rodents directly linked thalamocortical functional connectivity to disturbances in working memory (Parnaudeau et al., 2013). Although the neuropsychological correlates of altered thalamic connectivity have not been thoroughly investigated, Woodward and Heckers (Woodward and Heckers, 2015) found that connectivity of the dorsal region of the thalamus with fronto-parietal regions was weakly correlated with overall cognitive functioning across a combined sample of psychosis patients and healthy individuals. Interestingly, this relationship was strongest for a test of memory which is consistent with findings from human and animal lesions studies linking the mediodorsal nucleus to memory (Mitchell and Chakraborty, 2013).
2.4. Mechanisms of Thalamocortical Dysconnectivity in Schizophrenia
Multiple lines of evidence support a neural basis for functional connectivity measured at rest (Karbasforoushan and Woodward, 2012). Electrical cortical recording and stimulation studies have found that the patterns of brain activity elicited by cortical stimulation correspond to functional connectivity networks (He et al., 2008; Mitchell et al., 2013). Functional networks are supported by white matter connections; severing the corpus callosum for example virtually eliminates inter-hemispheric functional connectivity (Johnston et al., 2008). While not as extensively studied as functional connectivity, a recent diffusion tensor imaging investigation found reduced PFC-thalamic connectivity and increased somatosensory-thalamic connectivity in schizophrenia suggesting a structural basis for abnormal functional connectivity (Marenco et al., 2012). However, regions that are not directly connected by white matter tracts can be functionally connected (Adachi et al., 2011; Honey et al., 2010; Honey et al., 2009), which is advantageous for investigating extended, functional networks (Lee et al., 2013). For example, in contrast to individuals that undergo resection of the corpus callosum, individuals with developmental agenesis of the corpus callosum (CCA) demonstrate robust inter-hemispheric functional connectivity (Tyszka et al., 2011; Owen et al., 2013; Tovar-Moll et al., 2014), presumably due to neuroplastic changes including enlargement of the anterior commissure and the development of probst and sigmoidal bundles (Jakab et al., 2015; Fischer et al., 1992; Tovar-Moll et al., 2007; Barr and Corballis, 2002). This dramatic example indicate that while white matter connectivity may provide the ‘scaffolding’ for functional connectivity, functional networks are not constrained to structural connections and are inherently plastic.
Functional networks are also sensitive to pharmacological manipulations. Notably, ketamine, an NMDA receptor antagonist that elicits many of features of psychosis in healthy individuals including cognitive impairment, produces some of the same changes in thalamocortical connectivity observed in schizophrenia. Specifically, Hoflich et al. (2015) found that ketamine increases thalamic connectivity with the somatosensory cortex in healthy individuals. This finding supports a role for NMDA receptor function in thalamocortical connectivity and is consistent with the NMDA receptor hypo-function model of schizophrenia (Javitt et al., 2012).
3.0. Conclusions
Altered resting-state functional connectivity between cortex and thalamus is a consistent finding in schizophrenia. The alterations are characterized by reduced prefrontal-thalamic connectivity and increased motor and somatosensory connectivity with the thalamus. This pattern of disturbed thalamocortical connectivity has been detected in chronic patients, early stage patients, and high-risk individuals.
Despite the consistency of findings across studies, a number of critical knowledge gaps remain. Diagnostic specificity has not been adequately addressed. There are also technical challenges that need to be overcome. The findings from connectivity-based imaging studies reviewed here are consistent with selective dysfunction of specific thalamic nuclei; however, confirmation of the anatomical specificity will require imaging techniques capable of resolving and reliably identifying specific thalamic nuclei. Newly developed sequences have produced promising results, but have yet to be applied to schizophrenia (Tourdias et al., 2014). There are data suggesting bipolar patients also exhibit atypical thalamocortical connectivity, but studies with larger sample sizes are needed. Thalamocortical dysconnectivity is associated with conversion to psychosis in high-risk individuals, but its usefulness as a predictive biomarker remains to be confirmed. The role of thalamocortical dysconnectivity in the manifestation of clinical and cognitive symptoms is poorly understood, as are the biological mechanisms underlying atypical functional connectivity. Progress in these areas will require not just additional studies in patients, but also a better understanding of the role of thalamocortical connectivity in normal cognition and sensory and motor functioning, particularly HO order nuclei such as the MD nucleus.
Acknowledgments
Role of Funding Source: This research was supported by the NIMH (R01-MH102266 awarded to NDW) and the Jack Martin, MD., Research Professorship in Psychopharmacology (held by NDW). The funding sources did not have any role in writing the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Disclosures: No commercial support was received for the preparation of this manuscript. The authors have no conflicts of interest to report.
Contributors: MGC and NDW co-wrote the final draft of the manuscript. Author NDW provided funding for the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. Functional Connectivity between Anatomically Unconnected Areas Is Shaped by Collective Network-Level Effects in the Macaque Cortex. Cereb Cortex. 2011;22:1586–1592. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TG, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry. 2015;72:882–891. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri G, Steinbach M, Lim KO, Kumar V, MacDonald A., III Connectivity cluster analysis for discovering discriminative subnetworks in schizophrenia. Hum Brain Mapp. 2015;36:756–767. doi: 10.1002/hbm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Corballis MC. The role of the anterior commissure in callosal agenesis. Neuropsychology. 2002;16:459–471. [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, Tononi G, Ferrarelli F. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage. 2014;102(Pt 2):540–547. doi: 10.1016/j.neuroimage.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Berman RA, Joiner WM, Wurtz RH. Saccadic Corollary Discharge Underlies Stable Visual Perception. J Neurosci. 2016;36:31–42. doi: 10.1523/JNEUROSCI.2054-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Palaniyappan L, Li M, Kendrick KM, Zhang J, Luo Q, Liu Z, Yu R, Deng W, Wang Q, Ma X, Guo W, Francis S, Liddle P, Mayer AR, Schumann G, Li T, Feng J. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. Npj Schizophrenia. 2015;1:15016. doi: 10.1038/npjschz.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Parkinson N, Craven R, Crow TJ, Esiri MM, Harrison PJ. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr Res. 2003;60:157–166. doi: 10.1016/s0920-9964(02)00297-9. [DOI] [PubMed] [Google Scholar]
- Dagenbach D, Kubat-Silman AK, Absher JR. Human verbal working memory impairments associated with thalamic damage. Int J Neurosci. 2001;111:67–87. doi: 10.3109/00207450108986553. [DOI] [PubMed] [Google Scholar]
- Danos P, Schmidt A, Baumann B, Bernstein HG, Northoff G, Stauch R, Krell D, Bogerts B. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res. 2005;140:281–289. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Sun Z, Sampson AR, Lewis DA. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol. 2004;472:449–462. doi: 10.1002/cne.20055. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Arch Neurol. 1992;49:271–277. doi: 10.1001/archneur.1992.00530270085023. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;19:11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Anticipating the future: automatic prediction failures in schizophrenia. Int J Psychophysiol. 2012;83:232–239. doi: 10.1016/j.ijpsycho.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Mathalon DH. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40:804–812. doi: 10.1093/schbul/sbt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19:359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry. 1999;156:1190–1199. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W. Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am J Psychiatry. 2004;161:305–314. doi: 10.1176/appi.ajp.161.2.305. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Windischberger C, Saria A, Kasper S, Winkler D, Lanzenberger R. Ketamine-Induced Modulation of the Thalamo-Cortical Network in Healthy Volunteers As a Model for Schizophrenia. Int J Neuropsychopharmacol. 2015;18:1–11. doi: 10.1093/ijnp/pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52:766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Jakab A, Kasprian G, Schwartz E, Gruber GM, Mitter C, Prayer D, Schopf V, Langs G. Disrupted developmental organization of the structural connectome in fetuses with corpus callosum agenesis. Neuroimage. 2015;111:277–288. doi: 10.1016/j.neuroimage.2015.02.038. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, Shimony JS, Snyder AZ, Raichle ME. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus University Press; Cambridge, UK: 2007. [Google Scholar]
- Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12:2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- Kastner S, Schneider KA, Wunderlich K. Beyond a relay nucleus: neuroimaging views on the human LGN. Prog Brain Res. 2006;155:125–143. doi: 10.1016/S0079-6123(06)55008-3. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, Nenadic I. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:111–119. doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den HJ, Lowman D, Lahti AC. Neurometabolites in schizophrenia and bipolar disorder - A systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- Kubat-Silman AK, Dagenbach D, Absher JR. Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cogn. 2002;50:178–193. doi: 10.1016/s0278-2626(02)00502-x. [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman-Sinkoff DB, Barch DM. Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage: Clinical. 2016;10:96–106. doi: 10.1016/j.nicl.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Mair RG. On the role of thalamic pathology in diencephalic amnesia. Rev Neurosci. 1994;5:105–140. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, Verchinski BA, Barnett AS, Dickinson D, Apud JA, Callicott JH, Meyer-Lindenberg A, Weinberger DR. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37:499–507. doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci. 2013;7:1–37. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TJ, Hacker CD, Breshears JD, Szrama NP, Sharma M, Bundy DT, Pahwa M, Corbetta M, Snyder AZ, Shimony JS, Leuthardt EC. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery. 2013;73:969–982. doi: 10.1227/NEU.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann’s areas of the left hemisphere in schizophrenia. Am J Psychiatry. 2005;162:1733–1735. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Correlations between volumes of the pulvinar, centromedian, and mediodorsal nuclei and cortical Brodmann’s areas in schizophrenia. Neurosci Lett. 2006;392:16–21. doi: 10.1016/j.neulet.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Liebermann D, Ploner CJ. A role of the human thalamus in predicting the perceptual consequences of eye movements. Front Syst Neurosci. 2013;7:1–12. doi: 10.3389/fnsys.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Li YO, Yang FG, Shetty C, Bukshpun P, Vora S, Wakahiro M, Hinkley LB, Nagarajan SS, Sherr EH, Mukherjee P. Resting-state networks and the functional connectome of the human brain in agenesis of the corpus callosum. Brain Connect. 2013;3:547–562. doi: 10.1089/brain.2013.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Stereological quantitation of human brains from normal and schizophrenic individuals. Acta Neurol Scand Suppl. 1992a;137:20–33. doi: 10.1111/j.1600-0404.1992.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res. 1992b;7:95–100. doi: 10.1016/0920-9964(92)90038-7. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. doi: 10.1016/j.neubiorev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler L, Rolfs M, van der Stigchel S, Neggers SF, Cahn W, Kahn RS, Thakkar KN. Failure to use corollary discharge to remap visual target locations is associated with psychotic symptom severity in schizophrenia. J Neurophysiol. 2015;114:1129–1136. doi: 10.1152/jn.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;16:533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- Sim K, Cullen T, Ongur D, Heckers S. Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm. 2006;113:907–928. doi: 10.1007/s00702-005-0363-8. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Visual perception and corollary discharge. Perception. 2008;37:408–418. doi: 10.1068/p5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. Curr Top Behav Neurosci. 2010;4:555–583. doi: 10.1007/7854_2010_48. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Heckers S, Park S. Disrupted Saccadic Corollary Discharge in Schizophrenia. J Neurosci. 2015;35:9935–9945. doi: 10.1523/JNEUROSCI.0473-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. Neuroimage. 2014;84:534–545. doi: 10.1016/j.neuroimage.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R. Neuroplasticity in human callosal dysgenesis: a diffusion tensor imaging study. Cereb Cortex. 2007;17:531–541. doi: 10.1093/cercor/bhj178. [DOI] [PubMed] [Google Scholar]
- Tovar-Moll F, Monteiro M, Andrade J, Bramati IE, Vianna-Barbosa R, Marins T, Rodrigues E, Dantas N, Behrens TE, de Oliveira-Souza R, Moll J, Lent R. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci U S A. 2014;111:7843–7848. doi: 10.1073/pnas.1400806111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PC, Lee YC, Chen YS, Hsu JW, Li CT, Su TP. Network-specific cortico-thalamic dysconnection in schizophrenia revealed by intrinsic functional connectivity analyses. Schizophr Res. 2015;166:137–143. doi: 10.1016/j.schres.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Adolphs R, Paul LK. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31:15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Wang HL, Rau CL, Li YM, Chen YP, Yu R. Disrupted thalamic resting-state functional networks in schizophrenia. Front Behav Neurosci. 2015;9:1–9. doi: 10.3389/fnbeh.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Funahashi S. Thalamic mediodorsal nucleus and working memory. Neurosci Biobehav Rev. 2012;36:134–142. doi: 10.1016/j.neubiorev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND. The course of neuropsychological impairment and brain structure abnormalities in psychotic disorders. Neurosci Res. 2016;102:39–46. doi: 10.1016/j.neures.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biol Psychiatry. 2015;79:1016–1025. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47:944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]