ABSTRACT
During the past two decades we have seen a phenomenal evolution of bispecific antibodies for therapeutic applications. The ‘zoo’ of bispecific antibodies is populated by many different species, comprising around 100 different formats, including small molecules composed solely of the antigen-binding sites of two antibodies, molecules with an IgG structure, and large complex molecules composed of different antigen-binding moieties often combined with dimerization modules. The application of sophisticated molecular design and genetic engineering has solved many of the technical problems associated with the formation of bispecific antibodies such as stability, solubility and other parameters that confer drug properties. These parameters may be summarized under the term ‘developability’. In addition, different ‘target product profiles’, i.e., desired features of the bispecific antibody to be generated, mandates the need for access to a diverse panel of formats. These may vary in size, arrangement, valencies, flexibility and geometry of their binding modules, as well as in their distribution and pharmacokinetic properties. There is not ‘one best format’ for generating bispecific antibodies, and no single format is suitable for all, or even most of, the desired applications. Instead, the bispecific formats collectively serve as a valuable source of diversity that can be applied to the development of therapeutics for various indications. Here, a comprehensive overview of the different bispecific antibody formats is provided.
KEYWORDS: Appended IgG, bispecific antibodies, Fab, Fc heterodimerization, fusion proteins, immunoglobulin, scFv
Introduction
Bispecific antibodies have become increasingly of interest for diagnostic and therapeutic applications.1-3 While natural antibodies are monospecific, bispecific antibodies recognize two different epitopes either on the same or on different antigens. Bispecific antibodies have a long history,4 starting in the 1960s when antigen-binding fragments (Fabs) from two different polyclonal sera were re-associated into bispecific F(ab')2 molecules.5 Boosted by the hybridoma technology established in 1975,6 it became possible to generate bispecific antibodies of defined specificities by chemical conjugation of two monoclonal antibodies or by fusion of two antibody-producing hybridomas, generating hybrid hybridomas.7,8 The development of methods to produce recombinant antibodies then enabled the generation of bispecific antibodies with defined structure, composition and biochemical, functional, and pharmacological properties.9-12
Applications of bispecific antibodies cover a broad spectrum that includes diagnosis, imaging, prophylaxis and therapy. Initially, therapeutic applications focused mainly on effector cell retargeting for cancer therapy, including T cells, which cannot be recruited to tumor cells by normal antibodies.13,14 However, during the past decade many other therapeutic strategies based on bispecific antibodies have been established. In addition to retargeting of effector molecules, cells and genetic vehicles, dual targeting and pretargeting strategies, half-life extension, and delivery through biological barriers such as the blood-brain barrier have been explored.12,15-19 Bispecific antibodies have been evaluated as potential treatments for a variety of indications, including cancer, chronic inflammatory diseases, autoimmunity, neurodegeneration, bleeding disorders, and infections. The applications of these molecules have been extensively reviewed elsewhere.17,20-28 This review focuses on the various formats and strategies available to generate recombinant bispecific antibodies. Bispecific antibodies are an extremely fast-growing field. With new formats constantly emerging, keeping track is a challenging task. We have endeavored to be comprehensive, but may not have included every format that currently exists.
Classification of bispecific antibodies
Most natural antibodies are bi- or multivalent molecules comprising identical antigen binding sites. The exception are IgG4 molecules which are, due to an instable hinge region, capable of exchanging Fab arms (half-antibody association). This is a random process resulting in bivalent molecules with two different specificities. Bispecific antibodies with defined specificities, however, are artificial molecules, per se not found in nature. They must, therefore, be generated by biochemical, molecular or genetic means. One approach, not explained in detail here, is the chemical conjugation of two different antibodies or antibody fragments and the use of a catalytic antibody to couple reactive bispecific peptides (CovX-Bodies) (Fig. 2, box 1).29-32 Furthermore, by fusing two antibody-producing cells, e.g., hybridomas, a hybrid cell line can be generated that produces two different heavy and two different light chains within the same cell, which results in bispecific IgG molecules (Fig. 2, box 2) as well as non-functional by-products.
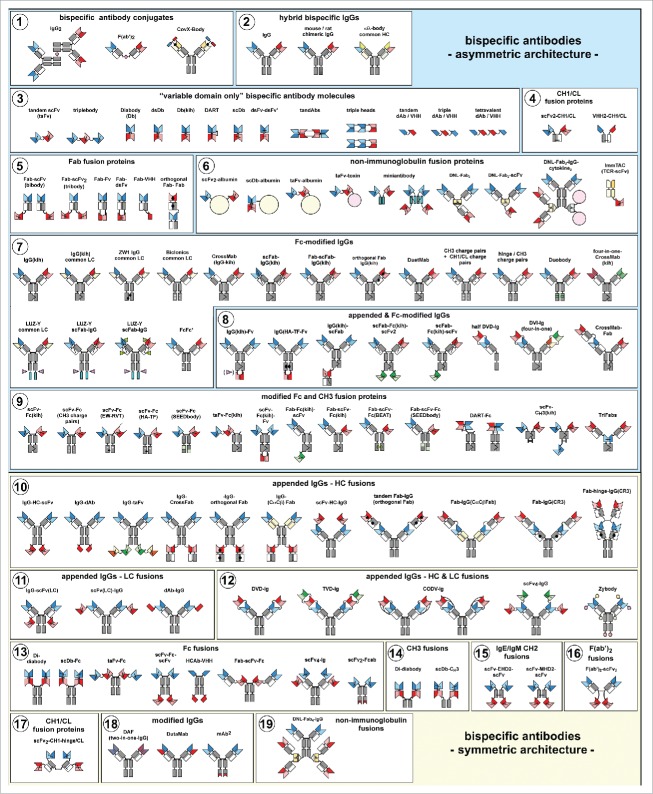
Figure 2.
The zoo of bispecific antibody formats Overview of bispecific antibody formats reduced to practice, grouped into molecules with symmetric or asymmetric architecture.
The generation of bispecific IgG molecules is difficult due to the fact that the antigen-binding sites are build by the variable domains of the light and heavy chain (VL, VH). A bispecific antibody requires two different heavy chains, and two different light chains, and exhibits asymmetry due to the presence of, at least, two different Fv regions. Promiscuous pairing of heavy and light chains of two antibodies expressed in one cell can theoretically result in 16 different combinations (10 different molecules), with only one being bispecific and the remaining pairings resulting in non-functional or monospecific molecules.33 To direct and to force correct assembly of correct binding sites, i.e., heavy and light chains, is one of the challenges of generating bispecific antibodies, and various strategies have been development and established over the past two decades to solve this problem.
Recombinant bispecific antibodies can be classified according to format and composition. A main discrimination is the presence or absence of an Fc region. Bispecific antibodies with no Fc will lack Fc-mediated effector functions, such as antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), complement fixation, and FcRn-mediated recycling, which is responsible for the long half-life of most γ immunoglobulins. Bispecific antibodies that include an Fc region can be further divided into those that exhibit a structure resembling that of an IgG molecule and those that contain additional binding sites, i.e., those with an appended or modified Ig-like structure. The different bispecific antibodies will have either a symmetric or an asymmetric architecture. For example, the majority of bispecific IgG molecules are asymmetric, while IgG fusion proteins often are symmetric in their molecular composition (Fig. 1). A further discriminating feature is the number of binding sites. In the simplest setting, e.g., utilized in IgG molecules, a bispecific antibody contains one binding site for each antigen (1 + 1), i.e., is bivalent. Adding an additional binding site to one of the chains of an IgG results in tetravalent molecules with a 2 + 2 stoichiometry. Other formats allow to generate 1 + 2 or 1 + 3 molecules, having one binding site for one antigen and 2 or 3 binding sites for the other antigen, respectively (Fig. 1). This can be extended by further valencies, but also by implementing further specificities, e.g., to make tri- or tetraspecific molecules. Furthermore, the number of chains needed to produce the bispecific antibody can vary. Thus, bispecific IgGs typically require four different polypeptide chains to be expressed, but a smaller number of chains, i.e., 3, 2 or only a single polypeptide chain, can be used in some formats.
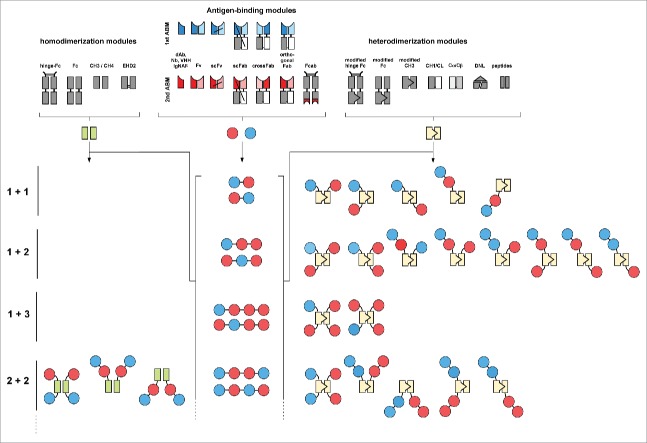
Figure 1.
Building blocks and generation of bispecific antibodies Examples of antigen-binding modules as well as homo- and heterodimierzation modules are shown, which can be used to generate bispecific molecules combining different binding sites within one molecule.
An extensive and versatile toolbox comprising different building blocks, i.e., antigen-binding modules as well as homo- and heterodimerization modules, is now available for the configuration of bispecific antibodies, thus allowing modulation of valency, size, flexibility, and pharmacokinetic and pharmacodynamic properties (Fig. 1).
Fc-less bispecific antibody formats
Tandem single-chain variable fragments (scFv2, taFv) and triplebodies
A minimalistic bispecific antibody is composed of the antigen-binding sites of two antibodies. The single-chain Fv (scFv) format is the most commonly used derivative of the VH and VL domains representing the minimal antigen-binding site of an antibody.34 Due to the single-chain configuration, bispecific antibodies can be build by connecting two scFvs through a linker (connector). Thus, these molecules are bivalent with one valency for each antigen, with a typically size in the range of 50–60 kDa.
The first descriptions of such tandem scFv molecules (taFv, scFv2) (Fig. 2, box 3) date back more than 20 years.35-37 In these studies, two scFvs were either fused by a 27 amino acid helical linker or a flexible linker, e.g., derived from Trichoderma reesi celobiohydrolase I. Since then many other tandem scFv molecules have been generated.10 In principle, the format of taFvs are defined by the arrangement of the VH and VL in the two individual scFvs (i.e., VH-VL or VL-VH), and the length and composition of the connecting linker, which can affect correct folding, stability and antigen-binding of the molecule. Various connecting linkers have been utilized, such as short alanine linkers (Ala3), hydrophilic linkers, e.g., identified by phage display, glycine-serine-rich linkers, linkers adopting a helical conformation, and linkers derived from various immunoglobulin and non-immunoglobulin molecules.38-49 A comparison of a 5 and a 15 aa long glycine-serine linker on the activity of a bispecific tandem scFv directed against CD3 and CD19 did not reveal any differences in in vitro bioactivity, indicating that scFvs with short connecting linkers have sufficient flexibility to direct T cells to tumor cells, i.e., to crosslink to cell surface-displayed antigens.41
The bispecific tandem scFv format has been extensively applied in cancer immunotherapy for the retargeting of T cells to tumor cells or tumor-associated cells of the tumor microenvironment. This format forms the basis of bispecific T-cell engager (BiTE) molecules,50-52 with a first BiTE molecule (Blincyto®, blinatumomab) approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).53-55 Blinatumomab comprises an anti-CD19 scFv in the VL-VH orientation linked through a G4S linker to an anti-CD3 scFv in the VH-VL orientation (DrugBank entry DB09052). Because of the small size, BiTE molecules are rapidly cleared from circulation with a terminal half-life of ∼1.25 h.56 The antibody is thus administered as continuous intravenous infusion at a constant flow rate with a portable pump in repeated four-week cycles.57
Tandem scFv that include an anti-CD16 scFv, i.e., bispecific killer cell engagers (BiKEs) were further developed for retargeting of natural killer (NK) cells. In one study, the anti-CD16 scFv was linked with a 20 amino acid sequence derived from human muscle aldolase (PSGQAGAAASESLFVSNHAY) to an anti-CD133 scFv targeting colorectal cancer cells.49
The modular nature of this approach allows further extension of tandem scFvs, for instance by adding more scFvs. This was applied to generate a trivalent, bispecific molecule (triplebody) exhibiting two binding sites for CD19 and one for CD16 for retargeting of NK cells (Fig. 2, box 3).58 Here, the variable VH and VL domains as well as the three scFv units (all in the VL-VH orientation) were connected with 20 residue (G4S)4 linkers. This approach also allows generation of trispecific molecules, as shown for a trivalent, trispecific triplebody directed against CD16, CD19 and CD33, for dual targeting of two different antigens expressed by mixed lineage leukemia cells.59
Stability of the individual scFv modules is a general concern due to the fact that molecular interactions occur only at the interface between the VH-VL interface without covalent linkage. Stability improvement of tandem scFv was achieved by introducing an interdomain disulfide-bond between the VH and VL domain (e.g., changing residues 44 in VH and 100 in VL to cysteines) generating disulfide-stabilized scFv (dsFv, dsscFv), e.g., in one of the scFv units.60 This was also applied to triplebodies, stabilizing one, two or all scFv units by disulfide bonds.58,59,61 In another approach, hydrogen bonding between VH and VL domains was substituted with electrostatic interaction between residues VH39 and VL38 to favor desired VH-VL association.62
Bispecific single-domain antibody fusion proteins
Rather than connecting antigen-binding sites in a tandem arrangement, single-domain antibodies, such as VH or VL domains, VHH, VNAR and Nanobodies, can be used to make bispecific molecules, and, in general this approach can also be applied to scaffold proteins, an emerging class of antibody-mimetic proteins (Fig. 2, box 3).63 Inclusion of two or more single-domain antibodies will result in bivalent, trivalent, or even multivalent molecules with one or more specificities.64 For example, two VHH domains were fused through a long hinge sequence derived from the upper hinge of the llama IgG2a, selected for its protease resistance and flexibility.65 A flexible linker was also used to combine two variable domains from shark immunoglobulin new antigen receptors (VNAR).66 This linker comprised the native shark IgNAR hinge (PGVQPSP) followed by a flexible GGGGSG sequence. In another study, two human single domain antibodies (dAbs) were combined into bispecific DAbs targeting, for example, two antigens from Candida albicans.67 Tetravalent, bispecific VHH fusion proteins were generated linking four VHH, two directed against TcdA and two against TcdB (both antigens from Clostridium difficile) in the middle of the molecule, by three flexible (G3S)4 linkers.68 This fusion protein showed enhanced neutralizing activity in vitro and in vivo. Nanobodies were combined into trivalent, bispecific molecules, with one site directed against serum albumin for half-life extension and the other two sites against a therapeutic target. One such Nanobody fusion protein targeting the IL-6R is currently in clinical trials.69 Here, the three Nanobody moieties are linked by two flexible GGGGSGGGS linkers. In a similar approach, trivalent bispecific VHH fusion proteins directed against foot-and-mouth virus were generated, with one binding site directed against porcine immunoglobulins, which also increased in vivo half-life of the fusion protein.70
Diabodies and diabody derivatives
Diabodies (Db) are bivalent molecules composed of two chains, each comprising a VH and VL domain, either from the same or from different antibodies.71,72 In the diabody format, the two variable domains are connected by a short linker that is usually 5 residues, e.g., GGGGS. Because the linker length is substantially shorter than that required to allow intrachain assembly of an antigen-binding site, which would result in a scFv, two chains dimerize in a head-to-tail orientation resulting in a compact molecule73 with a molecular mass similar to tandem scFv (∼50 kDa). Expressing two chains within the same cell, with either configuration VHA-VLB and VHB-VLA (A and B representing two different specificities) or VLA-VHB and VLB-VHA, results in bispecific heterodimers with correct pairing of the corresponding variable domains (Fig. 2, box 3). Bispecific diabodies have been developed for retargeting of effector cells, effector molecules and other applications.74-79 A comparative study of anti-epidermal growth factor receptor (EGFR) × anti-insulin-like growth factor receptor bispecific diabodies demonstrated the importance of selecting the optimal VH/VL arrangement and orientation, as only some of the possible combinations retained binding activity for both antigens.80 Besides domain order, the linker length and composition have been addressed for optimization. Studies with an anti-neuraminidase scFv in the VH-VL and VL-VH orientation showed that a linker length of 3–12 residues, composed of glycines and serines, favors assembly as a diabody, while shorter linkers result in tri- and tetrameric assemblies.81,82
One problem with bispecific diabodies is that the two different chains, e.g., VHA-VLB and VHB-VLA, are expressed within one cell, which can also result in the assembly of nonfunctional homodimers with incorrect pairing of the variable domains (VHA/VLB, VHB/VLA). Furthermore, diabodies might be prone to instability due to noncovalent assembly of the two chains.83 A solution is the introduction of an interdomain disulfide bond in one of the VH-VL pairs (dsDb) (Fig. 2, box 3), which also can improve stability.84,85 This has been further modified by introducing disulfide bonds into both VH-VL pairs and, in addition, connecting only one VHA-VLB with a 10 residue linker, while VHB and VLA are expressed as separate polypeptides, thus generating a molecule composed of three polypeptide chains (dsFv-dsFv') (Fig. 2, box 3).86 Alternatively, attempts have been made to remodel the domain interface to favor heterodimerization. For example, a panel of bispecific knob-into-hole diabodies (Fig. 2, box 3) directed against human epidermal growth factor receptor 2 (HER2) and CD3 was generated, carrying mutations at various positions in the VH and VL domains of one binding pair, with some of them showing increased heterodimer formation compared to the parental diabody. However, some of these modifications also reduced antigen binding of the modified VH-VL pair, indicating that modifications have to be carefully selected.85
Another alternative is the conversion of the heterodimeric diabody format into a single-chain format by connecting the first chain (VHA-VLB or VLA-VHB) and the second chain (VHB-VLA or VLB-VHA) by a flexible linker of ∼15 residues (Fig. 2, box 3). The linker in these single-chain diabodies (scDbs) forces correct assembly and improves stability without altering the antigen-binding activity.87,88 ScDb are composed of four variable domains connect by three linkers, two flanking linkers and one middle (internal) linker, the latter one resembling a linker used also in scFvs. Optimized linkers, varying in length and composition, have been selected from phage display libraries.89 Here, a preferred length of 2–6 residues was found for the flanking linkers, and 13 or more residues for the middle linker. Other internal linkers were, for example, derived from a sequence originating from an alpha helical region in the C-terminal domain of the nuclear protein La.90
The scDb format was also used to generate tetravalent bispecific molecules. This can be achieved by shortening the middle linker, resulting in head-to-tail assembly of the scDb polypeptide chains into a dimeric molecule (tandem diabody, TandAb) (Fig. 2, box 3) with a molecular mass of ∼100 kDa, i.e., twice the size of a diabody or scDb.89,91 Efficient production of dimeric molecules was observed using middle linkers between 6 to 12 residues as shown for an anti-C19 × anti-CD3 TandAb.92 This format was further developed to generate a tetravalent, bispecific TandAb for the retargeting of CD16A-positive NK cells to CD30-positive tumor cells. Here, 9-residue linkers composed of three GGS repeats were used for all three linkers.93 This TandAb (AFM13) has been investigated in a clinical Phase 1 study in patients with relapsed or refractory Hodgkin lymphoma where it was well tolerated.94 In another TandAb directed against CD33 and CD3, three different middle linkers (GGSG, GGSGG, GGSGGS) were applied.95
Yet another alternative applied to diabodies is the covalent linkage of the two chains through C-terminal cysteine residues. This was used to generate so-called dual-affinity retargeting (DART) proteins (Fig. 2, box 3). Initial examples included a bispecific DART directed against CD32B and CD16 with the VL domains linked to VH domains through a 8-residue linker (GGGSGGGG) and extension of the first chain at the C-terminus with either the sequence FNRGEC (derived from the IgG1 upper hinge) or LGGC and the second chain with either the sequence VEPKEC (derived from C-terminus of the kappa chain) or LGGC.96 All modifications resulted in stable, disulfide-linked molecules retaining antigen-binding activity. The format was subsequently applied to other specificities, e.g., CD19 × CD3.97,98
Applying the diabody format, attempts to generate trivalent bispecific molecules have also been described. In one approach, an additional VHA domain was fused to the N-terminus of a VLA-VHB chain and coexpressed together with a polypeptide chain composed of VLA-VHA-VHA, using glycine-serine-rich linkers of 16 residues to connect the additional variable domains to the diabody core (Fig. 2, box 3).99 In this study, a trivalent bispecific format with the composition VHA-VHA-VHB and VLB-VLA-VLA, i.e., with all VH domains on one chain and all VL domains on the other, was presented. The C-terminal domain was linked to the first two domains by a 16- or 14-residue linker, respectively, while the first two domains were connected by a GGGGS linker. Both types of triple-head molecules (BS6, BS8) could be produced in soluble and active form in E. coli, with possible applications for pretargeted delivery of radiolabeled bivalent haptens to tumor cells.100
Fab fusion protein
Fc-less bispecific antibodies were obtained using Fabs as the building block to which additional binding units are fused. Fabs are heterodimeric molecules composed of a light chain and a heavy chain fragment (Fd) and can thus be used to generate bivalent, bispecific molecules, but also trivalent, bi- or trispecific fusion proteins, e.g., by fusing a scFv to the C-terminus of either the light chain or Fd (bibody Fab-L-scFv, Fab-H-scFv), or to both chains (tribody, Fab-(scFv)2) (Fig. 2, box 5).101-104 In one example, the Fd chain included the first five amino acids of the upper hinge (EPSGP) and scFv were fused through a DVPSGPG or (G4S)3 linker to the C-terminus of the L and Fd chains.101 This study showed that the VH and VL domains of the Fab greatly enhanced the CH1-CL− mediated heterodimerization and secretion. All forms were fully functional in bispecific binding, and were described as having a low tendency to aggregate and to be stable in physiological conditions. As for other scFv fusion proteins, the linker connecting the scFv to the L chain or Fd and the domain order and linkage within the scFv orders can be adapted to obtain functional molecules and to modulate flexibility. For example, trivalent bispecific Fab-scFv and Fab(scFv)2 fusion proteins were generated targeting CD19 and CD16.105 Here, the scFv was used in the VL-VH orientation, which included interdomain disulfide stabilization, and a GVPGGS linker for fusion to the Fab part. In another example, a disulfide-stabilized scFv was fused through a (G4S)n linker to the Fab to make monovalent cMet binders.106 Fusion of one or two additional binding site(s) to a Fab will result in bi- or trivalent molecules. Extending the Fab moiety to include the hinge region will result in tetravalent F(ab')2-scFv fusion proteins covalently linked at the hinge region with a symmetric architecture (Fig. 2, box 16), as shown for anti-dextran/anti-dansyl bispecific antibodies.107 Generally, this approach can also be used for fusing single-domain antibodies or scaffold proteins to a Fab (Fig. 2, box 5), e.g., as shown for Fab-H-VHH fusion proteins directed against HER2 and CD16.108
Fabs can also be fused at their C-termini via flexible linker peptides (instead of hinge-regions) to a strongly hereodimerizing Fab-like moiety. This generates TriFabs, trivalent, bispecific fusion proteins composed of three units with Fab-functionalities (Fig. 2, box 9).109 TriFabs harbor two regular Fabs fused through flexible 20-residue peptide linkers, (G4S)4, to an asymmetric Fab-like entity as heterodimerization module (stem region). The latter is composed of a VH fused to a CH3 domain with a knob mutation (T366W) and a VL domain fused to a CH3 domain with matching hole mutations (T366S, L368A, Y407V) (see 3.2). To further increase stability, the variable domains of the stem region were stabilized by an interdomain disulfide bond (H44-L100).110,111 The overall structure resembles that of an IgG, with the Fc region substituted by the stem region. The loss of hinge disulfide bonds in TriFabs was further compensated by introducing a disulfide bond between the CH3 domains (S354C-Y349C). Functionality was demonstrated for various bispecific TriFabs, e.g., directed against digoxigenin or biotin in the stem region and CD33, GPC3 or LeY in the outer Fab arms.
Fab-Fab fusion proteins can be generated by fusing the Fd chain of a first Fab arm to the N-terminus of the Fd chain of a second Fab arm, i.e., generating a polypeptide chain of the composition VHA-CH1-linker-VHB-CH1, and separate expression of the two light chains (Fig. 2, box 5). However, the two light chains chain can pair randomly with both Fd chain, generating a total of four different molecules, only one being bispecific. To direct correct pairing of the cognate light and Fd chains, modifications can be introduced as described in section 3.4. One of these strategies, using orthogonal Fab, has already been applied to generate a bispecific Fab-Fab fusion protein directed against EGFR and CD3 for the retargeting of effector T cells to EGFR-expressing tumor cells. Interestingly, although the protein showed increased thermal stability compared to a tandem scFv, the tandem scFv was more potent in mediating killing of tumor cells, probably by affecting the intercellular distance and formation of immune synapses.112
The Fab can also be used as heterodimerization module to combine VH and VL domains of a second specificity. This was utilized to generate Fab-Fv fusions, by fusing a VH domain to the C-terminus of a Fd chain and a VL domain to the C-terminus of a light chain (Fig. 2, box 5).113 Stabilization of the Fv part was achieved by introducing an interdomain disulfide bond between residues H44-L100 generating a Fab-dsFv molecule. These formats were applied for half-life extension of the Fab moiety, directed against a target antigen, with the Fv moiety binding to serum albumin. The Fab-dsFv was shown to be biophysically stable, retaining affinity for both antigens, and exhibiting pharmacokinetic properties in mice and cynomolgus monkeys similar to a PEGylated Fab'.
Instead of using a Fab, it has been shown that a recombinant T cell receptor (TCR), composed of the extracellular region of the α and β chain resembling a Fab-like structure and capable of binding to MHC-displayed peptides, can be used to generate a bispecific molecule by fusing a scFv, for example, to the β-chain of the TCR through a flexible linker. These bispecific molecules (ImmTACs) (Fig. 2, box 6) can recognize tumor cells through the TCR and are able to retarget effector T cells through a CD3-binding scFv.114,115 Stabilization of the weakly associating α and β chain heterodimer was achieved by introducing a disulfide bond into the Cα - Cβ interface.116
Other Fc-less fusion proteins
Heterodimeric assembly of Fc-less antigen-binding sites, e.g., scFvs, can be achieved through the use of heterodimerizing peptides (miniantibodies) (Fig. 2, box 6). Such heterodimerizing peptides are known from various proteins, e.g., leucine zippers with a coiled coil structure. Heterodimer-forming “zipper” peptides derived form Jun and Fos proteins fused to Fab' molecules or scFv molecules were used to produce bispecific heterodimers, e.g., by reduction, reshuffling, and reoxidation of homerdimeric Jun and Fos fusion protein preparations.117-119 In another approach, two different scFvs were connected by a double helix motif (dhlx) that assembled into a four-helix bundle, thus generating tetravalent, bispecific molecules.120
The IgG CH1 and CL domains were also utilized to form heterodimeric, bispecific scFv fusion proteins (Fig. 2, box 4) shown for a bispecific scFv-CH1/scFv-CL fusion protein directed against EGFR and CD2 and using the human γ1 CH1 and Cκ domains as heterodimerization module.121 The CH1 and CL domains are covalently linked through a naturally occurring disulfide bond, which results in stable heterodimers. The option of varying the length of the flexible linkers connecting the N-terminal scFvs to the constant domains to allow molecules to span distant antigens was discussed. The CH1-CL heterodimerization module was further applied to generated bispecific single-domain antibody fusion proteins (Fig. 2, box 4), e.g., for the retargeting of NK cells to tumor cells through binding to CD16 and carcinoembryonic antigen (CEA).122 Extending the CH1 with the hinge region futher allows homodimerization of two scFv-CH1-hinge/scFv-CL fusion proteins, generating tetravalent bispecific molecules (Fig. 2, box 17). This was applied to bispecific antibodies targeting HIV gp41 and the IgA receptor (CD89) on neutrophils.123 Significant antibody-dependent cell-mediated viral inhibition (ADCVI) was observed for the bivalent scFv-CH1/scFv-CL fusion protein and the tetravalent scFv-CH1-hinge/scFv-CL fusion protein, while a tandem scFv against the same targets was described to be inactive.
The strategies we have described so far utilize direct fusion of different antigen-binding sites or the use of immunoglobulin-derived heterodimerization domains to generate bispecific antibodies. However, non-immunoglobulin heterodimerization modules may also be used to combine different binding sites in a non-covalent or covalent manner. One example is the so-called dock-and-lock method (DNL) utilizing heterodimeric assembly of the regulatory subunit of cAMP-dependent protein kinase (PKA) and the anchoring domains (AD) of A kinase anchor proteins (AKAPs).124-126 Here, an AD domain composed of 23 amino acid residues is fused to a first binding unit, and a 44 amino acid long dimerization and dock domain (DDD) is fused to a second binding unit. This results in assembly of a trimeric complex composed of one AD fusion protein and two DDD fusion proteins, which can be covalently linked through genetically introduced disulfide bonds between the AD and DDD moieties. Feasibility was shown for a bispecific, trivalent DNL-Fab3 protein (Fig. 2, box 6) directed against CEA and histamine-succinyl-glycine (HSG).124 The two fusion proteins (DDD fused through 14 residue linker to the C-terminus of CH1 domain of the anti-CEA Fab and the AD domain fused to the C-terminus of the CH1 domain of the anti-HSP Fab) were expressed separately and then combined, resulting in a bispecific molecule with two binding sites for CEA and one binding site for HSG, with applications for pretargeted radioimmunotherapy.127 The DNL method was further used to generate Fab2-scFv molecules (Fig. 2, box 6), with the scFv fused to an AD domain, and was applied to a variety of other specificities, including CD19, CD20, CD22, HLA-Dr, MUC5AC, Trop-2, and IGF-1R combined with an anti-CD3 antibody for T-cell retargeting, demonstrating the broad feasibility of this approach.128 Furthermore, the DNL method can be used to generate hexavalent, bispecific IgG-Fab4 fusion proteins (see section 4.3 below).
Other examples of non-immunoglobulin heterodimerization modules with possible applications to generate bispecific antibodies include the barnase-barstar system, adapter/docking tag modules based on mutated RNase I fragments, and SNARE modules based on interaction of the three proteins syntaxin, synaptobrevin and SNAP25, which was further modified into a binary system.129-131
Albumin is a plasma protein that lacks any antibody-like effector functions, but exhibits a plasma half-life similar to IgG molecules due to FcRn recycling.132 Fusing two scFvs or bispecific antibody molecules to albumin combines bispecificity with albumin-like properties Fig. 2, box 6). Thus fusion of two different scFvs to human serum albumin (HSA), one at the N-terminus and one at the C-terminus, substantially prolonged the half-life of the scFvs.133 Similarly, fusion of a bispecific tandem scFv or scDb, respectively, with albumin had similar effects (Fig. 2, box 6).133 This approach was adopted to generate scFv-HSA-scFv fusion proteins for dual targeting of HER2 and HER3 using a modified HSA (C34S, N503Q) to achieve greater homogeneity and short connector peptide linkers (AAS, AAAL) inserted at the N- and C-terminus of HSA, respectively, for fusion with the scFv moieties.134 Of course, many other proteins can be used to generate bispecific fusion proteins, such as toxins (Fig. 2, box 6), cytokines, chemokines, or growth factors, thus combining bispecificity, e.g., for dual targeting strategies, with effector functions.135,136
Additional antigen-binding sites grafted onto scFv
A minimalistic bispecific antibody might comprise an antigen-binding site, e.g., a scFv, that is modified to contain a second binding site as an integral part of the scFv. Because the immunoglobulin fold is composed of β-sheets connected by loop structures, attempts were made to graft complementarity-determining regions (CDRs) onto the bottom of an scFv to generate bispecific molecules (χsFv).137 Although this approach was not further developed for scFvs, a related approach was taken to graft an antigen-binding site into the bottom region of CH3 domains (see below section 4.5),138 which might in principle also be applied to other domains, e.g., to the bottom loops of the CH1-CL pair of a Fab.
Bispecific IgGs with asymmetric architecture
All bispecific IgG molecules, i.e., bispecific antibodies indistinguishable in their composition from natural immunoglobulins, are bivalent and possess an asymmetric architecture due to the presence of, at least, different Fv regions. Depending of the method of preparation and origin of heavy and light chains, they may furthermore differ in the constant regions of the heavy or light chain.
Asymmetric IgGs with heavy and light chains from two different antibodies
Fusion of two antibody-producing cell lines, e.g., generating a hybrid-hybridoma (quadroma), allows the combination of the heavy and light chains of two different antibodies.139 The resulting bispecific antibodies thus comprise the heavy and light chain of the first antibody and the heavy and light chain of the second antibody (Fig. 2, box 2). Heavy and light chain constant regions can be of the same isotype, but can also be of different isotype. They can even be from different species, a strategy utilized to generate triomabs.140 Here, a mouse hybridoma is fused with a rat hybridoma, resulting in production of a bispecific, assymmetric hybrid IgG molecule (Fig. 2, box 2). Preferential pairing of light chains with its corresponding heavy chain was described.141 Importantly, the heteromeric Fc part allows fractionated purification by protein A chromatography because of reduced binding, and elution from the column occurs already at a pH of around 5.8.141
Furthermore, cell lines producing two different heavy and light chains can be generated by genetic means. This allows use of heavy and light chains of defined composition, e.g., certain human isotypes, and implementation of mutated sequences. As discussed in the following sections, mutations can be introduced into the heavy chains as well as the light chains to either force correct assembly between the two heavy chains and cognate heavy and light chains, or to facilitate purification of correctly assembled bispecific antibodies.
Bispecific IgGs with an asymmetric Fc region - solving the heavy chain problem
Genetic engineering to force heterodimerization of heavy chains, described in the following section, solves one of the problems of bispecific IgG formation. Heterodimeric heavy chains can still assemble with two different light chains, resulting in four possible combinations, one bispecific molecule, one non-functional combination, and two monospecific molecules. Using this approach, the possible combinations is thus substantially reduced from 10 different molecules to just the 4 remaining combinations (Fig. 3). Heavy chain pairing is mediated by the last domain of the constant region, i.e., CH3 in IgG molecules, which forms high affinity homodimer complexes (KD ∼10 pM). Further interactions reside in the hinge region responsible for covalent linkage of two heavy chains, which form after heavy chain assembly. Interaction in a CH3 homodimer involves ∼16 residues at the CH3-CH3 interface as shown for human γ1 CH3, with a patch formed by 6 residues (T366, L368, F405, Y407, and K409) at the center of the interface strongly contributing to stability.142
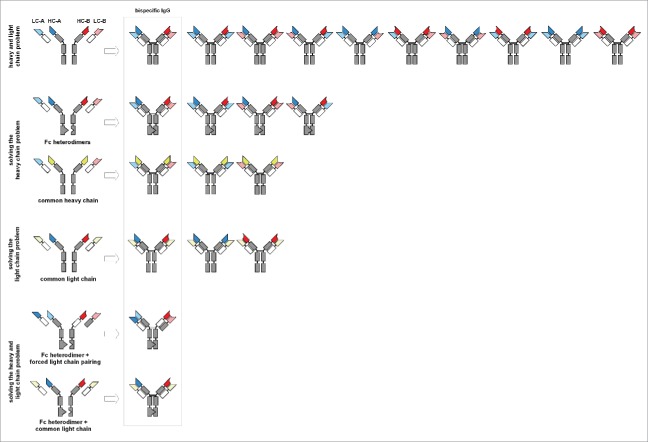
Figure 3.
Combinatorial diversity of bispecific IgGs Overview of possible combinations to arrange heavy and light chains from two different antibodies, including strategies to overcome incorrect heavy chain and heavy-light chain pairing.
Various strategies developed during the past two decades use either steric or electrostatic steering effects, or combination thereof, as well as formation of defined interchain disulfides, to generate a complementary interface favoring heterodimerization over homodimerization (Table 1; Fig. 4).
Table 1.
Fc heterodimerization.
| strategy | CH3 domain 1 | CH3 domain 2 | disulfide bond in CH3 | References |
|---|---|---|---|---|
| knobs-into-holes (Y-T) | T366Y | Y407T | no | 143 |
| knobs-into-holes (CW-CSAV) | S354C, T366W | Y349C, T366S, L368A, Y407V | yes | 145,146 |
| HA-TF | S364H, F405A | Y349T, T394F | 163 | |
| ZW1 (VYAV-VLLW) | T350V, L351Y, F405A, Y407V | T350V, T366L, K392L, T394W | no | 164 |
| CH3 charge pairs (DD-KK) | K392D, K409D | E356K, D399K | no | 166 |
| IgG1 hinge/CH3 charge pairs (EEE-RRR) | IgG1: D221E, P228E, L368E | IgG1: D221R, P228R, K409R | no | 170 |
| IgG2 hinge/CH3 charge pairs (EEE-RRRR) | IgG2: C223E, P228E, L368E | IgG2: C223R, E225R, P228R, K409R | no | 170 |
| EW-RVT | K360E, K409W, | Q347R, D399V, F405T | no | 171 |
| EW-RVTS-S | K360E, K409W, Y349C | Q347R, D399V, F405T, S354C | yes | 172 |
| Biclonic | 366K (+351K) | 351D or E or D at 349, 368, 349, or 349 + 355 | no | 169 |
| DuoBody (L-R) | F405L | K409R | no | 175 |
| SEEDbody | IgG/A chimera | IgG/A chimera | no | 178 |
| BEAT | residues from TCRα interface | residues from TCRβ interface | no | 181 |
| 7.8.60 (DMA-RRVV) | K360D, D399M, Y407A | E345R, Q347R, T366V, K409V | no | 183 |
| 20.8.34 (SYMV-GDQA) | Y349S, K370Y, T366M, K409V | E356G, E357D, S364Q, Y407A | no | 183 |
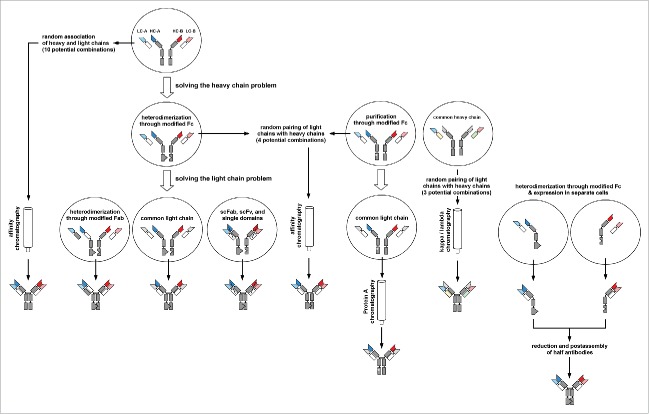
Figure 4.
Strategies to generate bispecific IgGs Overview of strategies to generate bispecific IgG molecules including strategies to force correct assembly of heavy chains and / or heavy-light chains, or utilizing postproduction purification or assembly strategies.
Inspired by the “knobs-into-holes” model proposed by Crick to describe packing of amino acids side chains between two α-helices, Ridgway and coworkers adopted this approach to generate a CH3 interface favoring heterodimeric assembly by replacing small side chains on one CH3 interface with larger ones to generate a knob, and replacing large side chains on the other CH3 domain with smaller ones to generate a hole.143 Testing variants demonstrated a preferential heterodimerization with substitution T366Y in one chain and Y407T on the other chain. These original knobs-into-holes mutations were, for example, used to produce an IgG directed against HER2 and IGF-1R.144 The knobs-into-holes approach was subsequently extended to identify further suitable combinations by phage display.145 These mutations were then used to generate bispecific IgG antibodies (Fig. 2, box 7), testing additional substitutions to allow for disulfide bond formation. One variant showed > 95% heterodimer formation (S354C, T366W / Y349C, T366S, L368A, Y407V) (Table 1). This heterodimeric heavy chain was then applied to construct a bispecific antibody against Mlp and HER3 from scFv using an identical VL domain, thus expressing a common light chain.146 The heteromeric heavy chains produced functional bispecific antibodies, allowed purification by protein A chromatography and retained Fc-mediated effector functions, such as ADCC.
The knobs-into-holes technique was widely adopted, and now forms a versatile basis of producing bispecific IgG molecules,147-151 and derivatives thereof, including trivalent Ig-like antibodies, and bispecific Fc and CH3 fusion proteins (Fig, 2, boxes 7–9).106,152-160 Another example is T-cell retargeting bispecific antibodies. To avoid systemic activation of T cells through bivalent binding to CD3, molecules exhibiting only one binding site for CD3 were designed. This includes scFv-Fc(KIH), with one scFv on each Fc chain, and tandem-scFv-Fc(KIH) (BiTE-KIH), with the tandem scFv fused to one of the Fc chain (Fig. 2, box 9).161 In this study, the CD3 binding moiety was either fused to the knob- or hole-containing Fc chain, KIH or KIHr, respectively. Interestingly, the BiTE-KIHr outperformed the BiTE-KIH in terms of expression titers. However, no differences were observed regarding T-cell activation and tumor cell lysis. In a similar approach, an Fc-KIH was used to generate bivalent, bispecific scFv-Fc fusion proteins directed against CD16 and HER2 for the retargeting of NK cells to tumor cells.155
Monovalent binding can also be essential for antibodies targeting cell surface receptors, such as c-MET, in order to avoid receptor cross-linking and activation. Bispecific antibodies binding monovalently to cell surface receptors, with application for dual targeting and neutralization of two different receptors, were generated by fusing a Fab arm to the N-terminus of an Fc-hole chain and a disulfide-stabilized scFv to the C-terminus of the same Fc chain, and co-expression with an unfused Fc-knob (Fig. 2, box 9).106
Fusion of a VH domain to the C-terminus of one Fc(kih) chain and the VL domain either expressed separately or fused to the C-terminus of the other resulted in a bispecific, trivalent IgG-Fv (mAb-Fv) fusion protein, with the Fv stabilized by a interdomain disulfide bond (Fig. 2, box 8).158 Flexibility of the Fv in the IgG-Fv fusion could be further increased by introducing a proteolytic cleavage site, e.g., for furin or MMP, in the linker connecting the VL domain with the Fc chain (Fig. 2, box 8). After cleavage, this resulted in a bispecific molecule with the C-terminal Fv connected only through the VH domain to the IgG. Similarly, a scFv-Fc-Fv fusion protein was generated that exhibited two binding sites for EGFR (scFv fused to the N-terminus of the Fc chain) and one for LPS (VH fused to the C-terminus of the Fc(knob) and VL fused to the C-terminus of Fc(hole)) (Fig. 2, box 9).162 Further derivatives of bispecific IgG(kih) antibodies include TriMAbs.157 Here, one or two disulfide-stabilized scFvs are fused to one or both Fc(kih) chains resulting in trispecific, trivalent or tetravalent antibodies, respectively (Fig, 2, box 8). This was shown for TriMAbs targeting EGFR, IGF-1R and either cMet or HER3. In this approach, the Fab was composed of a single-chain Fab (see section 3.3) with disulfide-stabilized Fv domains (scFab-Fc(kih)-scFv2, scFab-Fc(kih)-scFv).
Recently, the knobs-into-holes strategy was expanded to other IgG isotypes to generate IgG4 heterodimers, which are per se deficient in Fcγ-mediated effector functions, to produce, for example, bispecific antibodies directed against IL-4 and IL-13.154
Use of structure- and sequence-based approaches to explore energies of paired variant combinations at the interface across the CH3 dimer yielded a HA-TF variant (S364H, F405A / Y349T, 394F) that showed ∼83% heterodimer formation in the context of a bispecific mAb-Fv (IgG-Fv, mAb-Fv) molecule, developed for co-targeting of HER2 (bivalent binding) and CD3 (monovalent binding) (Fig. 2, box 8) (Table 1). Further examples using this CH3 heterodimerization module include mono- and bivalent scFv-Fc fusion proteins.163
Another rational structure-guided approach resulted in a set of mutations that were reported to have a high thermal stability and to form pure heterodimers with no detectable homodimers. The Fc design (ZW1) included T350V, L351Y, F405A, and Y407V substitutions in the first Fc chain and T350V, T366L, K392L, and T394W substitutions in the second Fc chain, used to produce a bispecific IgG comprising a common light chain (Fig. 2, box 7) (Table 1).164,165
While the strategies described above mainly depend on hydrophobic interactions, other approaches utilize electrostatic interactions (steering) to avoid homodimerization of CH3 domains by electrostatic repulsion and to direct heterodimerization by electrostatic attraction. In the wild-type CH3 domains, two charge interactions between K409 and D399 are found at the CH3-CH3 interface. Substituting K409 in one CH3 domain by an aspartate and, in the other CH3 domain, D399 by a lysine was found to favor formation of CH3 heterodimers.166 Introducing further substitutions, e.g., K392D in one chain and E356K in the other chain, resulted in almost exclusive heterodimer formation. Introduction of further charged pairs impaired productivity. This approach, using two charge pair substitutions (K409D, K392D / D399K, E356K; CH3 charge pairs) (Table 1) was applied to generate a bispecific scFv-Fc fusion protein directed against CD3 and TARTK (Fig. 2, box 9), and more recently to generate bispecific IgGs (Fig. 2, box 7) directed against EGFR and HER2 or sclerostin and DKK-1.167,168 This included the introduction of new charge pairs into the Fab arms (see also section 3.4) to direct correct light chain pairing.
Electrostatic steering effects are also used in Biclonics, which are bispecific antibodies utilizing a common light chain and heterodimerizing heavy chains.169 Here, residues in one CH3 (366, 366 + 351) are substituted by a positively charge lysine residue, and one or more residues in the second CH3 (e.g., 349, 351, 355, 368) are substituted by negatively charged glutamic acid or aspartic acid residues (Table 1). One bispecific antibody based on this technology (MCLA-128) directed against HER2 and HER3 is currently in a clinical Phase 1/2 trial.
Preferential heavy chain heterodimerization is also achieved by introducing charge pairs into the hinge region of IgG1 and IgG2 (Fig. 2, box 7).170 For IgG1, these hinge substitutions comprise D221E, P228E in the first hinge and D221R and P228R in the second hinge (Table 1). For IgG2, substitutions comprise C223E and P228E in one hinge region and C223R, E225R, P228R in the other hinge region (Table 1). Here, E225R is able to form an electrostatic interaction with a naturally occurring glutamic acid at position 225, thus only two substitutions are required in the first IgG2 hinge. Combining these mutations with L368E and K409R, respectively, in the CH3 domains forced heterodimeric assembly. Applicability was shown for anti-EGFR x anti-HER2, as well as an anti-CD3 x anti-CD20 bispecific IgG antibodies, utilizing separate expression of the two antibodies and subsequent assembly from half antibodies (see section 3.3).170
In another study, mutations favoring heterodimeric assembly of CH3 domains were identified by, firstly, substituting charged residues around the rim of the preserved hydrophobic core (L351, T366, L368, Y407) with larger or smaller hydrophobic amino acids to replace the symmetric electrostatic interactions with asymmetric hydrophobic ones, and, secondly, substituting amino acids weakly involved in interaction with amino acids carrying charged, long side chains to form asymmetric long-range electrostatic interactions.171 This resulted in a final combination of K360E, K409W in one CH3 with Q347R, D399V, F405T in the other CH3 (EW-RVT) (Table 1). Functionality was demonstrated for a bispecific scFv-Fc heterodimer targeting VEGFR-2 and Met (Fig, 2, box 9). Introducing a disulfide bond into the CH3 domain (Y349C in the first domain and S354C in the second domain) increased heterodimer formation and thermodynamic stability.172 Furthermore, using yeast surface-displayed combinatorial Fc libraries, variants carrying different mutations and exhibiting high heterodimerization yields (80-90%) were selected.173
Based on the observation that IgG4 antibodies are able to exchange their Fab arms, a dynamic process that involves separation of the two heavy chains and reassembly into full IgG4. This process was attributed to IgG4 core hinge sequences in conjugation with residues in the CH3 domain.174 This natural process of Fab arm exchange in IgG4 was adapted to generate stable bispecific IgG1 molecules by controlled Fab arm exchange (cFAE).175 Screening of mutations in the CH3 domain allowing cFAE in the context of a K409R mutation in a corresponding CH3 resulted in the identification of mutation F405L, which allowed efficient exchange of half antibodies of separately expressed antibodies after mixing and mild reduction with β-mercaptoethanol (Table 1).176 Scalability of this process was demonstrated for an anti-EGFR x anti-CD20 bispecific IgG (DuoBody) (Fig. 2, box 7), resulting in > 95% bispecific molecules.177
Complementarity in the CH3 interface allowing for a heterodimeric assembly of Fc chains was developed by designing strand-exchange engineered domain (SEED) heterodimers (Table 1). These SEED CH3 domains are composed of alternating segments derived from human IgA and IgG CH3 sequences (AG SEED CH3 and GA SEED CH3) and were used to generate so-called SEEDbodies.178 Because molecular models suggested that interaction with FcRn is impaired in the AG SEED CH3, residues at the CH2-CH3 junction were returned to IgG sequences. Pharmacokinetic studies confirmed that the half-life of SEEDbodies was comparable to other Fc fusion proteins and IgG1.179 As examples of SEEDbodies, bispecific Fab-scFv-Fc and scFv-Fc fusion protein targeting two different epitopes on EGFR were generated (Fig. 2, box 9).180 This biparatopic antibody demonstrated enhanced activity, similar to the combination of the two parental antibodies.
A further CH3 heterodimerizing interface was generated by mimicking the natural association of the T-cell receptor α and β chains.181 This technology, Bispecific Engagement by Antibodies based on the T cell receptor (BEAT) (Table 1), was applied to generate a Fab/scFv-Fc fusion protein in which light chain mispairing was avoided (Fig. 2, box 9). This approach was used to generate a bispecific antibody directed against CD3 and HER2 for T-cell retargeting.182
Leaver-Fay and coworkers183 applied multistage design (MSD), an approach that designs for multiple protein stages simultaneously, to generate a set of CH3 mutations at the Fc interface. Two sets (7.8.60 and 20.8.34) (Table 1) were used to generate bispecific IgGs derived from pertuzumab (anti-HER2), matuzumab (anti-EGFR), BHA10 (anti-LTβR), and MetMAb (anti-cMet), in combination with orthogonal Fab interface mutations,184 yielding in all cases at least 93% of bispecific antibodies.
Heterodimeric assembly of heavy chains can be also achieved by using a separate heterodimerization module that is subsequently removed from the bispecific antibody. This strategy was applied by employing a leucine zipper structure derived from Acid.p1 (Ap1) and Base.p1 (Bp1) peptides fused to the C-terminus of the two heavy chains.185 This LUZ-Y platform was used to generate monovalent Fab-Fc fusion proteins, but also bispecific IgGs based on a common light chain or scFab arms directed against EGFR and HER3 (Fig. 2, box 7). The introduction of a proteolytic cleavage site between the C-terminus of the Fc chain and the leucine zipper sequences allowes removal of the leucine zipper, yielding bispecific IgG antibody with a natural composition.
Post-assembly approaches to purify bispecific antibodies
While the strategies described above rely on modifications to generate heterodimeric heavy chain pairs at the level of heavy chain assembly, other mutations in the Fc region are intended to facilitate purification of heterodimeric antibodies, i.e., work at the post-assembly level similar to the strategy used for Triomabs (Fig. 4).141 Mutations were introduced into the Fc region to allow fractionated elution by protein A chromatography. For example, substituting H435 with arginine and Y436 with phenylalanine, derived form corresponding IgG3 residues, in the CH3 domain of an IgG1 heavy chain resulted in a Fc chain (Fc*) with ablated Fc-protein A binding (Fig. 2, box 7).186,187 Thus, homodimeric Fc*-Fc* containing antibodies will not bind to the column, while heterodimeric FcFc* comprising bispecific antibodies will have a decreased affinity for protein A. However, protein A can also bind to the VH domain of antibodies derived from VH3 gene segment family, which was shown to interfere with separation of FcFc and FcFc* dimers. This was solved by the use of a protein A-derived Z-domain with negligible binding to variable domains, allowing separation of bispecific antibodies from homodimeric FcFc-comprising antibodies.
Another strategy relying on post-assembly purification steps is the use of a common heavy chain, but different light chains. In this type of molecule, a defined variable domain of the heavy chain is combined with different VL domains to form antigen-binding sites with different specificity. Thus, asymmetry resides solely in the Fab arms. This approach is utilized in κ/λ bodies, which requires expression of 3 polypeptide chains: one heavy chain and two light chains (one kappa and one lambda light chain) (Fig. 2, box 2).188 Binding sites comprising a common VH domain can either be generated using library selections with a generic VH domain, allowing the de novo isolation of two antibodies sharing the same VH, or using the VH from an existing monoclonal antibody, which is then combined with a repertoire of VL domains for selection of an antibody with a second specificity. Importantly, one of the binding site must be a Vκ and the other Vλ. This was shown for a panel of human antigens, demonstrating that this approach can be generalized. The common heavy chain and the two light chains are then expressed in the same cell and bispecific antibodies are purified by three-step chromatography applying: 1) a IgG-CH1 Capture Select or protein A affinity chromatography step, followed by 2) KappaSelect, and 3) LambdaFabSelect affinity chromatography steps. This approach does not require any genetic modifications of heavy and light chains, and thus results in bispecific antibodies with natural sequences. However, yields may be lower than with forced heterodimerization approaches as ‘wrong’ molecules are produced to a significant degree (∼50%) within the producer cell.
Solving the light chain problem in asymmetric antibodies by genetic engineering
While the use of modifications to force heterodimerization of Fc regions solves the heavy chain problem, these approaches still suffer from the light chain problem. Thus, using two different light chains still allows the generation of four different combinations, with only one being bispecific (Fig. 3, 4). Approaches have, therefore, been developed to allow the correct pairing of cognate heavy and light chains (summarized in Table 2) in combination with Fc-modified heavy chains (summarized in Table 1).
Table 2.
Fab arm heterodimerization.
| strategy | VH | CH1 domain | VL | CL domain | References |
|---|---|---|---|---|---|
| CrossMabCH1-CL | — | CL domain | — | CH1 domain | 156 |
| orthogonal Fab VHVRD1CH1CRD2 - VLVRD1CλCRD2 | 39K, 62E | H172A, F174G | 1R, 38D, (36F) | L135Y, S176W | 184 |
| orthogonal Fab VHVRD2CH1wt - VLVRD2Cλwt | 39Y | — | 38R | — | 184 |
| TCR CαCβ | 39K | TCR Cα | 38D | TCR Cβ | 203 |
| CR3 | — | T192E | — | N137K, S114A | 204 |
| MUT4 | — | L143Q, S188V | — | V133T, S176V | 204 |
| DuetMab | — | F126C | — | S121C | 205,206 |
The first approach described involved use of a common light chain.146 This was based on the observation that antibodies isolated from phage display libraries against diverse antigens often use the same VL domain, reflecting the very limited size of the L chain repertoire in the phage library. In combination with the knobs-into-holes modification of the heavy chain, bispecific IgG molecules, e.g., directed against HER3 and Mpl, were generated. Various bispecific IgGs with a common light chain have subsequently been produced using, for example, the knobs-into-holes modification, but also other Fc modifications (Fig. 2, box 7).148,185,187
Although not yet applied to generate bispecific antibodies, surrobodies might be an alternative to the use of a common light chain. Surrobodies are based on Fabs that use a surrogate light chain composed of a λ5 domain fused to the VpreB domain.189,190 Combinatorial libraries based on the surrobody format have been selected against various antigens and, for example, used to generate a dual-acting DR4 and DR5 agonistic antibody. The approach thus should also be applicable to generate bispecific antibodies.191
Several of the Fc modifications described above utilized scFvs fused to the Fc chains to generate bispecific antibodies in order to circumvent the light chain problem.155 Based on the finding that Fabs can be expressed as single-chain derivatives (scFab to connect the C-terminus of the light chain with the N-terminus of the VH domain), full IgG molecules were generated by the expression of a single polypeptide comprising a light chain connected to a heavy chain.192-194 Linkers with a lengths of 30 residues, e.g., (G4S)6, to 38 residues have been utilized, including deletions of the connecting disulfide bond between CH1 and CL. An improved scFab platform was described for disulfide-linked scFab molecules using a linker of 60 flexible residues.195 A (G4S)6 linker was applied to generate a bispecific Fab-Fc fusion protein combined with knobs-into-holes mutations in the Fc region, which was further modified through C-terminal fusion of scFvs to obtain trispecific, trivalent and tetravalent molcules (Fig. 2, box 7, 9), e.g., for targeting EGFR, IGF-1R, and either cMet or HER3.157 This was recently extended to generate tetravalent, tetraspecific IgG-scFv fusion proteins directed against EGFR, IGF-1R, cMet, and HER3 by utilizing either one Fab and one scFab arm, or two scFab with different specificity (Fig. 2, box 8).196 The scFab format was also combined with the LUZ-Y Fc heterodmerization strategy (Fig. 2, box 7). Here, proteolytic cleavage sites were introduced into the Fab linker to allow removal of the linkers from the correctly assembled bispecific IgG molecules.185 Furthermore, scFab were combined with unmodified Fabs to generate bispecific Fab-scFab-Fc fusion proteins in combination with Fc(kih) (OAscFab-IgG format), as shown for a bispecific IgG targeting EGFR and IGF-1R, which could be expressed at high yields.160
The CrossMab technology represents a different approach. Here, in the context of knobs-into-holes heavy chains, either the light chain of one Fab arm is exchanged by the Fd of the corresponding heavy chain (CrossMabFab), or only one pair of the variable (CrossMabVH-VL) or constant domain (CrossMabCH1-CL) of one Fab are is swapped between the light and heavy chain (Table 2).156,197 This results in pairing of the unmodified light chain with the corresponding unmodified heavy chain and pairing of the modified light chain with the corresponding modified heavy chain (Fig. 2, box 7). Exemplified for a bispecific CrossMab directed against VEGF and Ang-2, simultaneous antigen binding with unaltered affinity was demonstrated, with the CrossMabCH1-CL showing a superior side-product profile. In a subsequent study, this antibody (A2V) was able to reprogram tumor-associated macrophages, leading to a prolong survival in a number of extracranial tumor models.198 The antibody (RG7716, RO6867461) is currently in clinical development. In a recent study, the CrossMabCH1-CL format was applied to generate bispecific antibodies directed against the HIV Env protein and CD4/CCR5 for virus neutralization.199 Here, heterogeneity was observed in the original CrossMabs due to incorrect pairing of the unmodified light chain, which could be improved by introducing additional mutations into this chain. The CrossMab approach has developed into a versatile platform technology, allowing not only generation of bivalent, bispecific IgG molecules, but also tri- and tetravalent, bispecific IgG fusion proteins. e.g., by fusing an additional Fab to the N-terminus one of the knobs-into-holes heavy chains, or two CrossMab Fab arms to the C-terminus of homodimerizing heavy chains (Fig. 2, box 1, 8). Many other formats are enabled by the CrossMab technology, including bispecific, trivalent and tetravalent IgG-Fab fusion proteins, e.g., to generate bispecific molecules with one binding site for CD3 and two for a tumor-associated antigen.200,201 The concept was further evolved to generate tetravalent, tetraspecific four-in-1 antibodies by applying a knobs-into-holes Fc region and the CrossMab technology to two-in-1 Fab arms (see below).202
Another solution to the light chain problem is the genetic engineering of the light and heavy chain interface to generate an orthogonal interface that allows a light chain to interact with higher affinity with its cognate heavy chain (Table 2). Here, the interaction between the variable domains (VH-VL pair) and the first constant domains (CH1-CL) is modified. Testing various modifications identified by a multistage design application, a set of mutations was established that favors pairing of the orthogonal Fabs.184 These modification were applied to generate various bispecific IgG molecules, e.g., directed against EGFR x cMET, EGFR x HER2, Axl x cMet, and EGFR x LTβR, or against two epitopes on HER2 by combining the binding site of trastuzumab with that of pertuzumab.184 Here, Fc heterodimerization was achieved through electrostatic steering effects introduced into the CH3 domain.166 For example, a bispecific antibody with orthogonal Fab arms based on pertuzumab (anti-HER2) and matuzumab (anti-EGFR), both with a lambda light chain, were generated by substituting Q39K, R62E, H172A, F174G in the heavy chain and D1R, Q38R, L135Y, S176W in the light chain of pertuzumab (VRD1CRD2 modifications) combined with Q39Y in the heavy chain and Q38R in the light chain of matuzumab (VRD2 modifications), yielding 90% correct light chain assembly (Fig. 2, box 7).
A further attempt to direct light chain pairing with its cognate heavy chain Fd involved substituting the CH1 and CL domains of one Fab arm with the Cα and Cβ domains from the T-cell receptor (TCR).203 This was applied to generate either a Fab-IgG molecule with the Fab arm fused with a (G4S)5 linker to the N-terminus of the heavy chain, or an IgG-Fab molecule molecule with the Fab arm fused with a (G4S)4 linker to the C-terminus of the heavy chain, exemplified for bispecific antibodies derived from trastuzumab and pertuzumab (Fig. 2, box 1). However, probably due to strong VH/VL interactions of the trastuzumab binding site, only a small fraction showed correct Fab arm pairings. This was improved to some extent by introducing an additional mutation in the VL domains (Y36F) to weaken the VH-VL interaction, as well as introducing a charge-charge interaction between the VL and VH domains (VL Q38D, VH Q39K) in the trastuzumab Fv (Table 2).
Paired mutations in the CH1-CL interface were further developed in the context of a tetravalent Fab-IgG fusion protein (see section 4.3).204 Based on three-dimensional (3D) structure modeling and energetic considerations, two modifications were tested. In a first approach (CR3), a pair of interacting polar interface residues were substituted by a pair of neutral and salt bridge-forming residues (T192E in CH1, N137K in CL), complemented by substituting S114 by an alanine to avoid steric clashes with the bigger lysine side chain. In the second appraoch based hydrophobicity-polarity-swap (MUT4), a double mutation was introduced in each chain (L143Q and S188V in CH1, V133T and S176V in CL) (Table 2). In vitro testing showed that superior activity of CR3 due to better stability and highest functional activity of the bispecific antibodies.
In the DuetMab approach, correct Fab arm pairing was achieved by replacing the native disulfide bond in one of the CH1-CL interfaces with an engineered disulfide bond.205,206 A comparison of various mutations identified heavy chain F126C in combination with light chain S121C, which yielded ∼98% bispecific molecules (Table 2). In combination with a knobs-into-holes Fc modification, this was applied to generate various bispecific IgG molecules, e.g., directed against EGFR and HER2 or CD4 and CD70, which could be produced in a highly purified and active form (Fig. 2, box 7).
Solving the light chain problem by post-production assembly from half-antibodies
All of the above described modifications use genetic engineering of the heavy and light to allow correct assembly of the different heavy and light chains expressed in the same cell. However, there is also the possibility of generating bispecific IgG molecules through assembly of two half-antibodies composed of only one heavy chain paired with its cognate light chain (Fig 3, 4). Here, it is sufficient to introduce modifications into the heavy chain Fc to favor heterodimerization or to allow separation by protein A or G chromatography. A proof-of-concept was initially demonstrated for a hingeless bispecific IgG with a knobs-into-holes Fc.153 The two antibodies were separately expressed in E. coli, purified by protein A and additional chromatography steps, then annealing of the two half-antibodies yielded bispecific IgG molecules.
A further development of this approach is the co-culture of two bacterial cells, each expressing a half-antibody. After lysis of the bacterial cells, the half-antibodies assemble into bispecific IgGs, as shown for example for an anti-EGFR x anti MET IgG.149 The ratio of the two different bacterial clones had to be adjusted to yield the optimal amount of each antibody. Thus, a 60:40 ratio of anti-MET:anti-EGFR antibody was found to be optimal. Purification included protein A chromatography followed by hydrophobic interaction chromatography to remove remaining half-antibodies. Importantly, co-culturing removes the need for redox steps. The approach was utilized to generate bispecific IgG directed against IL-13 and IL-4 or against HER2 and CD3.150,154 This neutralizing bispecific antibody does not require ADCC or other Fc-mediated effector functions.
Assembly of half-antibodies was further applied to generate bispecific antibodies carrying charge pairs in the hinge region and the CH3 domain of IgG1 (EEE - RRR) and IgG2 (EEE - RRRR) (Table 1; Fig. 2, box 7).170 Here, the two antibodies were separately expressed in HEK293 and purified by protein A chromatography. The purified antibodies were then subjected to mild reduction to obtain half-antibodies, which were then assembled into bispecific IgGs by mixing equimolar rations incubated at 37 °C overnight in the presence of a mild reducing agent. The use of a mammalian expression system allows production of glycosylated antibodies with unaltered Fc effector functions, which was also applied to produce knobs-into-holes bispecific IgGs.147
Asymmetric Fc and CH3 fusion proteins
The above described Fc modifications further allow generation of bispecific Fc or CH3 fusion proteins, and several examples have been described. Here, different binding modules, such as scFv, Fab and scFab units, have been applied. Fusing an scFv moiety with different specificity to each of the two Fc chains results in a bivalent, bispecific scFv-Fc fusion protein that has IgG-like properties, despite being ∼25 kDa smaller in size. This was utilized for knobs-into-holes Fc regions, heterodimeric Fc regions with charge pairs in the CH3 domain, and EW-RVT modified Fc region described above (Fig. 2, box 9).155,161,166,171 Alternatively, a Fab can be combined with a scFv, e.g., each fused to the N-terminus of one of the heterodimerizing Fc chains, generating a Fab-scFv-Fc fusion protein (Fig. 2, box 9). This approach was demonstrated, for example, applying the BEAT Fc modifications.181 A variation of this approach is the use of the heterodimerizing CH3 domains, for instance shown for bispecific scFv CH3 fusion proteins (Fig. 2, box 9). For example, a bispecific bivalent fusion protein (minibody) was generated fusing an anti-HER2 scFv to the N-terminus of one of the CH3 domains and an anti-CD16 scFv to the other CH3 domain, with the CH3 domains further stabilized by C-terminal disulfide bonds.152 This format was further extended to generate a bispecific trivalent minibody by fusing an additional anti-HER2 scFv to the C-terminus of the first CH3 domain (Fig. 2, box 9).
Furthermore, heterodimeric Fc regions have been employed in which both binding sites were fused to one of the Fc chains and then co-expressed with the corresponding unfused Fc chain (Fig. 2, box 15). For example, a bispecific Fab-Fc-scFv fusion protein was generated by fusing an anti-Met Fab to the N-terminus and an anti-digoxigenin disulfide-stabilized scFv to the C-terminus of the “hole”-containing Fc chain.106 In another study, a bispecific tandem scFv was fused to the N-terminus of one of the Fc chains, resulting also in a bispecific, bivalent fusion protein.161 In another study, a DART-Fc fusion protein was generated with one of the chains of a DART molecule fused to the N-terminus of a knob-containing Fc chain, with co-expression of an unfused hole Fc chain and the second DART chain (Fig. 2, box 9).97 Alternatively, using a knobs-into-holes Fc region, one of the DART chains was fused to the first Fc chain and the second DART chain was fused to the second chain, with two chains then covalently linked by a DART disulfide bond as a substitute of the hinge region.207 These DART-Fc fusion proteins are being developed for T-cell retargeting through a CD3 binding site. To avoid any immune cell activation through the Fc, an effector-deficient Fc was used. These strategies allow the combination of the properties of a bispecific and bivalent antibody moiety with the half-life extension properties of the Fc region.
Bispecific antibodies with a symmetric architecture
Appended IgGs: fusion of scFv
Fusion of an additional binding site to either the heavy or light chain is a simple and straight-forward solution to overcome the random heavy and light chain pairing. It requires, however, that the additional binding site is expressed by a single polypeptide chain, or that the additional binding site is encoded by polypeptide chains that do not interfere with the light chain-heavy chain interaction of the master antibody. ScFv, but also single-domain antibodies and alternative scaffold proteins, are suitable fusion partners. These appended IgG-based molecules have a symmetric architecture and are tetravalent, possessing two binding sites for each antigen, produced by expression of four polypeptide chains (Fig. 1).
This approach was first described in 1997 by Coloma and Morrison107 who fused an anti-dansyl scFv to either the C-terminus of the heavy chain CH3 domain of an anti-dextran antibody or to the C-terminal end of the hinge region. A short G3S linker was used to connect the C-terminus of the heavy chain fragment to the N-terminus of the VH domain of the scFv. Coexpression of the corresponding anti-dextran light chain resulted in an IgG-HC-scFv (CH3-scFv) or F(ab')2-scFv2 (Hinge-scFv) fusion protein comprising four binding sites, two for each antigen (Fig. 2, box 10, 16). The molecular mass of these bispecific fusion proteins is 200 kDa for the IgG-scFv and 150 kDa for the F(ab')2-scFv2. These bispecific antibodies were able to bind to both antigens, although a somewhat reduced affinity for the scFv-encoded specificity was found.
The approach was further extended by fusing an scFv to the N-terminus of a heavy (Fig. 2, box 1) or light chain (scFv-HC-IgG, scFv-LC-IgG) (Fig. 2, box 11), respectively, or to both heavy and light chains (scFv-HC/LC-IgG) (Fig. 2, box 12), the latter being trispecific, as shown for targeting of EphA2, EphA4, and C5a.208 Interchain disulfide-stabilization of the Fv regions improved the stability and reduced the aggregation tendency of such molecules.209 The fusion proteins maintained high expression level, thermostability, and protease resistance. They also maintained Fc effector functions and half-life similar to the parental IgG antibodies. Fusion of an scFv to the C-terminus of an IgG light chain (IgG-LC-scFv) also results in functional bispecific antibodies,210,211 many of which are stable and behave in the same manner as ‘regular’ IgGs. In some instances, a disruption of the disulfide bond between light and heavy chain can be observed.212 Thus, each of the termini of light and heavy chain of an IgG can be utilized to generate appended, bispecific IgG molecules. It was further shown that even trispecific antibodies can be obtained by fusing a tandem scFv to a heavy chain C-terminus or to the N-termini of heavy and light chain (Fig. 2, box 10).208 One can further imagine that multivalent molecules, including tri- or multispecific antibodies, can be produced by fusing scFv molecules of the same or different specificity to the N- or C-terminus of the light or heavy chain of an antibody, although this might affect accessibility of the different antigens within one molecule.
Fusing a scFv to the CH1 of a heavy chain and another scFv to the CL domain of a light chain results in a tetravalent, bispecific antibody (scFv4-Ig) exhibiting four scFv moieties at the N-termini of the constant regions (Fig. 2, box 12). This approach was initially applied to generate a bispecific antibody directed against two epitopes on VEGF receptor and subsequently used by the same group to produce an anti-EGFR x anti-IGF1-R bispecific antibody for dual targeting of tumor cells.213,214
The same format was used to combine EGFR-specific scFv with anti-CD3 scFv for the retargeting of T cells to tumor cells.214 A comparison of this antibody with an equivalent scDb-Fc fusion protein (Fig. 2, box 13) demonstrated the highest cytotoxicity in vitro. However, due to the high molecular mass (∼200 kDa), difficulty with the preparation of sufficient amounts of recombinant protein were reported, and thus the scDb-Fc format was favored.215
A critical issue to be considered for IgG scFv fusion proteins is the linker connecting the scFv moiety to the IgG. The linker has to be stable and ideally flexible, and fusion should not interfere with antigen binding activity of the scFv and the IgG binding site. Fusing the scFv to the C-terminus of either the heavy or light chain keeps the IgG binding site unaffected. However, in this case the connection will be to the N-terminus of the scFv, i.e., close to its antigen-binding site. Typical linkers used for IgG-scFv fusion proteins are composed of 2 or 3 repeats of G4S.216,217 Also other linkers such as a hydrophilic helical linker, e.g., with the sequence SNS(EEAKK)3SNS, have been used to fuse an scFv to the heavy chain C-terminus.104 Short linkers might restrain antigen binding, although recently a three residue linker (GSS) was successfully used to generated an IgG-HC-scFv targeting HER2 and HER3.218
Furthermore, the intrinsic stability of the scFv might affect manufacturability. Therefore, scFvs were optimized for stability by using a longer (20 amino acid residues) linker to connect the VH and VL domain and screening of appropriate positions to introduce an additional disulfide bond between the two domains.110,111,216 Combining the longer linker with a disulfide bond between positions VH44 and VL100 increased the thermal stability of an anti-LTβR scFv by 13 °C, resulting in bispecific anti-TRAILR2 x anti-LTβR IgG-scFv and scFv-IgG fusion proteins with properties desirable for pharmaceutical development.
Disulfide linkage was also applied to generate improved versions of bispecific IgG-scFv of the Morrison format (IgG-HC-scFv) stabilizing an anti-digoxigenin-specific scFv moiety through VH-VL interdomain disulfide bonds (VHCys44 to VLCys100) fused to the C-terminus of the heavy chain or hinge region of antibodies of different specificity, including HER2, IG1-R, CD22, and LeY.217,219 Here, the scFv was connected to the heavy chain by two repeats of G4S. All antibodies retained binding specificity and affinity. The stability of these bispecific antibodies was demonstrated by a lack of aggregation propensity. This approach was subsequently applied to other antibodies, e.g., targeting cMet, HER1, HER2, or HER3.106 The influence of linker length was also studied for bispecific IgG scFv fusion proteins targeting two different epitopes of HIV receptor CCR5.209 Linkers of 3 to 6 G4S repeats were analyzed in non-disulfide-stabilized scFvs. Stable molecules with yields similar to that of the parental IgG could be obtained using scFv with a 30 residue long linker, but also for disulfide-stabilized scFv with a 15 residue linker.
Problems associated with a limited stability of the scFv part were further addressed by selecting scFv with improved stability, e.g., by phage display, early in the engineering process as shown for an anti-IL17A x anti-IL23 bispecific IgG-scFv fusion protein.220 Final selection of the antibodies was based on testing scFv in both orientations (VH-VL and VL-VH) and results from SEC-MALS and differential scanning calorimetry assessing thermal stability and monomeric state. Similarly, stability-improved scFvs were used to generate bispecific antibodies targeting different epitopes on IGF-1R, fusing the scFv to either the N- or C-terminus of an IgG.221
IgG scFv fusion proteins can exert Fc-mediated effector functions, including phagocytosis, ADCC, and complement fixation, depending on the isotype used for construction.222 For some applications, for example dual targeting and neutralization of cellular receptors without destroying the target cell, IgGs with deficient Fc regions might be advantageous. Thus, a fully effectorless Fc, based on a chimeric, aglycosylated IgG4.P/IgG1 constant region, was used to construct a bispecific IgG-HC-scFv fusing a stability-improved scFv against IGF-1R223 to an anti-EGFR IgG.224 Other possibilities include mutated Fc regions225-229 or Fc regions derived from IgG2 and IgG4.230
A variety of different other bispecific IgG-scFv fusion proteins have been generated in the past years with applications in cancer therapy, e.g., for dual targeting approaches,231 delivery of antibodies through the blood-brain-barrier,232 T-cell retargeting,233 and pretargeted radioimmunotherapy.211,234
Appended IgGs: fusion of domain antibodies and scaffold proteins
As an alternative to scFv, single domain antibodies have been utilized to generate appended bispecific IgG molecules. For example, a single variable domain targeting PDGFRα was fused to the N-terminus of the light chain of an anti-VEGFR-2 IgG through a five residue linker (ASTKG), retaining antigen-binding and neutralization activity of both antibodies (Fig. 2, box 11).235 The approach was then extended via fusion of the single variable domain to the C-terminus of the heavy chain (Fig. 2, box 10).236
With various scaffold proteins being developed as alternatives to antibodies, there is ample opportunity to generate appended bispecific IgG-like molecules by fusing these scaffold proteins to the heavy or light chain of an IgG. An example are Fynomers, small 7 kDa globular proteins derived the SH3 domain of human Fyn kinase, which lacks disulfide bonds and is very stable (∼70 °C thermal melting points).237 Fusion of a HER2-specific Fynomer to either the N- or C-terminus of the heavy or light chain of pertuzumab resulted in bispecific molecules (FynomAbs) with potent neutralization of HER2-mediated proliferation and tumor growth. Based on these findings, another FynomAb, COVA322, was generated fusing an anti-IL-17A Fynomer to anti-tumor necrosis factor (TNF) adalimumab (Humira®); COVA322 is currently being evaluated in a Phase 1b/2a study as a for treatment for psoriasis.238
Related to the use of scaffold proteins is the use of specific peptides, which can also be fused to an IgG to generate a bispecific molecule. This approach is utilized in the Zybody technology (Fig. 2, box 12). For example, an Ang2-specific peptide was fused through a six residue Gly-Ser linker to the C-terminus of the heavy chain of an anti-TNF IgG (adalimumab).239 Zybodies with up to five specificities have been described, one specificity coming from the IgG and up to four from fused modular recognition domains, including linear and disulfide-constrained peptides, Affibodies and Knottins, fused to the N- and C-termini of heavy and light chains.240
Appended bispecific antibodies molecules have been further generated by fusing a ligand-binding receptor domain to an IgG molecule. Although not a bispecific antibody in the classical sense, this type of molecule also exhibits two different specificities.241
Appended IgGs: fusion of Fab arms
Fusion of a Fab arm to an IgG, i.e., a tandem arrangement of Fab arms fused to a Fc region, was used to generate tetravalent (Fab)2-Fc fusion proteins by expressing an Fd-extended heavy chain together with a light chain (Tandemabs).242 This format can be applied to generate tetravalent, bispecific (Fab)2-Fc fusion proteins. Here, production of bispecific molecules also faces the light chain problem because only one of the eight possible combinations is bispecific.
Brünker and coworkers243 generated a bispecific IgG-Fab molecule by applying the CrossMab technology to the second Fab fused through a (G4S)4 connector to the C-terminus of an IgG molecule (Fig. 2, box 7). This approach, in which the VH of the fused Fab is linked to a CL domain, and the VL domain to a CH1 domain, was applied to generate a tetravalent, bispecific antibody directed against fibroblast activation protein (FAP), for targeting of activated tumor stroma fibroblasts, and DR5 (TRAIL receptor 2), to induce apoptosis by activation of this death receptor (Fig. 2, box 10). Differences were observed for a VHCL and VLCH1 configuration. The VLCH1 format, with the VL domain fused to the C-terminus of the HC, showed, compared to the parental antibody, reduced binding to FAP, while binding activity was retained in the VHCL format (VH domain fused the HC). In combination with a knobs-into-holes Fc region, this approach can also be applied to generate trivalent, bispecific IgG-Fab fusion proteins.243
The orthogonal Fab design described above184 was applied to generate a tetravalent, bispecific Fab-IgG fusion protein (Fig. 2, box 10).112 Here, the Fd chain of the outer Fab arm is linked to the VH of the heavy chain, i.e., the inner Fab arm using a (G4S)5 linker, and used to produce a bispecific Fab-IgG targeting two epitopes on HER2 (derived from pertuzumab and trastuzumab). Compared to a scFv-IgG fusion protein of the same specificities, superior biophysical properties and unique biological activities were reported.
Fab-IgG fusion proteins were also generated using the charged residue mutations or hydrophobicity-polarity-swap mutations introduced into the CH1-CL interface (see above).204 In this study, the Fd fragment of a first antibody was fused to a hinge sequence (either wild-type or with the two cysteine mutated to serines), then connected by a STPPTPSPSGG linker to the N-terminus of the heavy chain carrying the CR3 or MUT4 mutations in the CH1 domain. The cognate light chain of the inner Fab binding site carried the corresponding mutations. Thus, molecules with heavy chains containing the wild-type hinge sequence were covalently linked by disulfide bonds in the additional hinge between the two Fd sequences (Fig. 2, box 10). Functionality was demonstrated for a tetravalent, bispecific Fab-IgG fusion protein directed against HLA-DR (outer Fab arm) and CD5 (inner Fab arm). Good stability, limited aggregation and in vivo activity was observed for the CD5 x HLA-DR(CR3) molecule. Molecules with a cysteine-free hinge in the inter-Fab region showed a somewhat reduced binding and where slightly less effective, e.g., in complement activation.
As described above, Fabs arm with the Cα and Cβ domains from the TCR were applied to link a second Fab arm either to the N-terminus or the C-terminus of the heavy chain through a (G4S)4, as shown for a bispecific antibodies derived from trastuzumab and pertuzumab (Fig. 2, box 10).203 Additional mutation were introduced into the VL domains (Y36F) to weaken the VH-VL interaction combined with a charge-charge interaction between the VL and VH domains (VL Q38D, VH Q39K) in one of the Fab arms to further facilitate correct pairing of the cognate Fab arms.
The DNL method described above was used to generate hexavalent, bispecific IgG-Fab4 molecules (Fig. 2, box 19), which are produced by mixing DDD2 Fab fusion proteins with a AD2 IgG fusion protein under redox conditions, followed by purification with protein A.244,245 Thus, this approach results in bispecific 2 + 4 molecules, with the two binding sites of the IgG directed against the first antigen and the four binding sites of the Fab arms directed against the second antigen. DDD2 can be fused either to the N-terminus or the C-terminus of the Fab Fc chain.246 Furthermore, the AD2 domain can be fused either to the C-terminus of the heavy chain or the light chain, respectively.131 For HexAbs with the AD2 domain fused to the C-terminus of the heavy chain CH3, a reduced complement activation was observed, while other Fc-mediated effector functions, such as ADCC, were retained. Some of the produced hexavalent antibodies (bsHexAbs), e.g., directed against CD20 and CD22, showed biological activities that were not observed with mixtures of the parental antibodies, resulting in improved apoptosis induction and growth inhibition.247 Despite their large size (∼350 kDa), 2- to 3-fold shorter half-life compared to the parental IgGs were observed in mice, although tissue uptake was similar.244 It was assumed that this result is due to intracellular dissociation of the bsHexAbs.
Appended IgGs: fusion of additional variable heavy and light chain domains
Fusion of an additional VH domain and VL domain of a second specificity to an IgG heavy chain and light chain, respectively, results in a so-called dual-variable-domain antibody (DVD-Ig) (Fig. 2, box 12). Initially shown for a bispecific DVD-Ig targeting IL-12 and IL-18,248 this format has been applied to combine a variety of specificities, including IL-1α and IL-1β,249,250 HIV gp41 and gp120,251 CD20 and CD47,252 EGFR and HER3,253 CD20 and HLA-DR,254 two epitopes on HER2,255 and CEA and DOTA.211 Analyses of a series of DVD-Igs, directed against TNF and an alternate therapeutic target, that varied in the position of the two binding sites (inner and outer position) and the length of connecting linkers, with different linkers (5 to 13 residues) used in the heavy and light chain, has been reported.256 Here, affinities for TNF were generally lower when the binding site was at the inner position. This is likely due to steric hindrance effects, resulting primarily in a reduced association rate. Binding was also influenced by the linkers connecting the two variable domains within one chain. In another study of gp41- and gp120-specific DVD-Igs showed that the inner binding site required at least a linker with a length of 15 amino acids and that linkers adopting a helical structure resulted in more effective DVD-Igs than those based on flexible linkers.251 Using single particle electron microscopy to study a DVD-Ig demonstrated that the outer binding site is highly mobile and can fold out of the plane to allow binding of the inner binding site, which was further confirmed by the 3D structure of a DVD-Ig bound to IL-12 and IL-18.257,258
A further development of the DVD-Ig technology is the introduction of a proteolytic cleavage site, e.g., for a metalloprotease, between the outer and inner VL domains. These activatable DVD-Igs (aDVD-Igs) can target with the outer binding site, e.g., a cellular target and, after cleavage, are able to bind another antigen. This approach was utilized for an aDVD-Ig directed against ICAM and TNF to enhance specificity for sites of inflammation and to reduce specificity for off-target TNF by liberating TNF binding in the inflamed tissue.259
crossover dual-variable domain-Ig-like proteins (CODV-Ig) represent a related format where the two VH and two VL domains are linked in a way that allows crossover pairing of the variable VH-VL domains, which are arranged either (from N- to C-terminus) in the order VHA-VHB and VLB-VLA, or in the order VHB-VHA and VLA-VLB (Fig. 2, box 12).260 Thus, on either the light chain or the heavy chain, the variable domain order is changed, while the other chain serves as a template, resulting in a total of four different types. The CODV-Ig format differs from the DVD-Ig format due to the circular self-contained structure that is generated only in the CODV-Igs. The CODV-Ig format was applied to generate bispecific antibodies directed against IL-4 and IL-13, as well as TNF and IL-12/23.260 Linkers (L1 to L4) of different length, including all-glycine linkers, were used to connect the two variable domains and the inner variable domains to the first constant domains. Although the linker lengths and sequences influenced yields and aggregation propensities, the study showed that almost all analyzed linkers permitted correct Fv and Fc pairing. A compact assembly of the binding sites was revealed by 3D structures of an anti-IL-4 x anti-IL13 CODV-Fab alone and in complex with its antigens.
Modified IgG molecules
Symmetric bispecific IgG molecules, i.e., IgGs that are modified so that they are able to recognize two different antigens, have been obtained by either modifying the existing antigen-binding site formed by the VH and VL domains (two-in-1 and four-in-1 antibodies) or by grafting an additional binding site into the bottom region of the Fc fragment (mAb2).
Engineering the variable domains of an antibody Fv region to target more then one antigen allows generatation of bispecific antibodies without appending its structure. Because the Fv region is formed by two variable domains (VH, VL), it is possible to direct each domain against a different antigen, thus generating tetravalent, bispecific IgGs (two-in-1 antibodies) (Fig. 2, box 18). Such dual-acting Fabs in an IgG directed against HER2 and VEGF were obtained by generating a light chain repertoire of Herceptin® (trastuzumab) by randomizing a subset of solvent exposed VL CDR positions to mimic the natural diversity in amino acid composition and length.261 Selection and further affinity maturation resulted in various IgGs capable of binding to HER2 and VEGF in the low nanomolar range. Crystal structures of a bispecific Fab with either HER2 or VEGF confirmed preferential binding of the VH or VL domain, respectively, although with extensively overlapping paratopes. Neutralization of both antigens was demonstrated in vitro and in vivo for this two-in-1 antibody. Further studies demonstrated increased structural plasticity within the antigen-binding site without increasing the entropic cost.262 This approach was subsequently applied to evolve HER1 x HER3-specific and IL-4 x IL-5-specific two-in-1 antibodies.156,263 The HER1 x HER3-specific two-in-1 antibody exhibits increased receptor neutralization compared to monovalent HER1 and HER3 antibodies and is capable of overcoming acquired resistance to HER1 inhibitors and radiation.264 This antibody (MEHD7945A) has been evaluated in clinical Phase 1 and 2 studies, and was well tolerated with evidence of tumor pharmacodynamic modulation and antitumor activity in squamous cell carcinoma of the head and neck.265 In the two-in-1 format, all six CDRs are modified to recognize both antigens, thus generating overlapping paratopes. An alternative approach is utilized in DutaMab and DutaFab formats (Fig. 2, box 18). Here, paratopes are restricted to three each of the 6 CDRs that make up the antigen-binding site of an Fv. CDRs H1, H3 and L2 make up the binding site for one antigen, CDRs L1, L3 and H2 interact with the other antigen. These two sets of CDRs face toward different ‘sides’ of the Fv. Because of that, DutaFabs and DutaMabs can simultaneously bind two different antigens on one Fab arm (or 2 pairs of different antigens on one IgG), as long as the size or shape of the two antigens do not interfere with each other on the DutaFab/Mab.266
Besides CDR loops of the variable domains, other surface-exposed loop structures might be applicable to generate artificial binding sites. This approach was successfully applied to the IgG1 Fc region. Initially, two loop sequences at the C-terminal region of the CH3 domain were randomized and selected for antigen binding, as shown for HER2, resulting in Fc fragments (Fcab) with nanomolar affinity.267 Importantly, randomization did not affect binding to protein A or CD64 (FcγRI). The stability of the Fcab fragment was further improved by engineering an intradomain disulfide bond268 and by directed evolution using yeast display.269,270 Three loop regions (AB, CD, EF) of the CH3 domain are now used for randomization.138 By combining Fcabs as building blocks with Fab arms of a second specificity, bispecific IgG molecules (mAb2) that maintain all functional properties of an IgG, including its long half-life, can be generated (Fig. 2, box 18).138
Symmetric Fc- and CH3-based bispecific antibodies
In natural antibodies, the Fc region serves as a homodimerization module but it also enables effector functions such ADCC, phagocytosis and complement fixation. Using an Fc region, which might further comprise the hinge region for covalent connection, bispecific antibodies can be generated by fusing either a bispecific antibody module to the N-terminus, or individual antigen-binding moieties, e.g., scFvs, to the N- and C-terminus, generating bispecific, tetravalent molecules (Fig. 2, box 13).
This approach was applied to two scFvs directed against TNF and fluorescein isothiocyanate (FITC) in a proof-of-concept study.271 Here, the first scFv (anti-TNF) was fused to the Fc chain via a modified human IgG1 hinge, while different linkers connecting the second scFv (anti-FITC) to the C-terminus of an Fc region were tested, including a (G4S)3AAA linker, a A4T linker (T(A4T)3AAA), and a CL linker (EGKSSGASGESKVDAAA) (Fig. 2, box 13). All three linkers allowed dual targeting, with similar stability of the scFv-Fc-scFv fusion protein. The A4T linker was then used to generate a bispecific scFv-Fc-scFv fusion protein directed against IT1 and CXCL13. The protein showed mAb-like properties regarding, for example, productivity, stability, and viscosity. The approach was further applied to generate a tetravalent, bispecific antibody directed against LPS and EGFR to target a nanocell to EGFR-expressing tumor cells.162 Here, the two disulfide-stabilized scFvs were connected to the Fc moiety by G4S linkers.
In another approach, scFv molecules arranged in tandem (taFv) with each scFv forming a separate folding and binding unit (see section 2.1) were fused to an Fc region (Fig. 2, box 13). Such a tetravalent, bispecific taFv-Fc fusion protein was generated combining two scFv directed against different epitopes of CD2, which confered potent mitogenic properties inducing proliferation of resting T cells.272 A 24-residue helical linker was used to connect the two scFv moieties. The taFv-Fc format was also used to generate a tetravalent, bispecific Fc fusion protein directed against C5 and cotinine with applications for immunoblotting and immunoprecipitation experiments.273 Here, the two scFvs were connected with a (G3S)3 linker, and the taFv fragment was fused to the hinge of an IgG1 Fc region. The cotinine-binding scFv serves here as a recognition module of a conjugated cotinine, e.g., horseradish peroxidase (HRP). In another study, a taFv-Fc fusion protein directed against HER2 and cotinine was used for ELISA and radioimmunoassays using either HRP-cotinine or a 125I-cotinine-conjugated histidine dipeptide for application in single-photon emission computed tomography imaging of HER2-positive tumors.274 Other examples include taFv-Fc fusion proteins directed against TAG-72 and MUC1 or EGFR and HER2 for dual targeting of tumor-associated antigens.275,276 Other applications include the retargeting of T cells, as shown for an taFv-Fc fusion protein directed against GD2 and CD3, and the target-dependent clustering and activation of death receptors, demonstrated for a taFv-Fc fusion recognizing DR5 (TRAIL receptor 2) and melanoma-associated chondroitin sulfate proteoglycan.60,277
Instead of fusing two scFvs to the N-terminus of an Fc chain, it is also possible to fuse a Fab arm to a scFv-Fc moiety (Fig. 2, box 13). These Fab-scFv-Fc fusion proteins require expression of four polypeptide chain, one encoding the Fc-scFv-Fc part and one encoding the light chain.278 This format was applied to generate a tetravalent, bispecific antibody (BiS4αPa) directed against two different exopolysaccharide antigens (PcrV and Psl) from Pseudomonas aeruginosa for prevention or treatment of P. aeruginosa infections. Here, a scFv was inserted between residue C220 and D221 of the upper hinge flanked by 10-amino acid linkers (G4SG4S). This antibody (MEDI3902) is currently being evaluated in a Phase 2 study (EVADE, NCT02696902) for prevention of nosocomial pneumonia caused by Pseudomonas aeruginosa in mechanically ventilated subjects.279
The bispecific diabody format was used to generate Fc fusion proteins by fusing one of the diabody chains with a covalently linked CH3 domain or a Fc chain (Fig. 2, box 13, 14).280 This resulted in the so-called Di-Diabodies, which are tetravalent, bispecific molecules composed of two chains, a VLB-VHA-CH3 or VLB-VHA-Fc chain, respectively, co-expressed with a second VLA-VHB chain. The approach was used to generate a Di-Diabody directed against EGFR and IGF-1R.281 Although this Di-Diabody showed dual activity in receptor neutralization, a loss of antigen-binding activity was observed in vivo, most likely due to dissociation of the two polypeptide chains in circulation.
ScDb exhibit increased stability due to the covalent linkage of all four variable domains of a bispecific diabody, encoded by a single polypeptide chain. Fusing a scDb to a Fc domain results in tetravalent, bispecific molecules with a size and pharmacokinetic properties similar to IgG (Fig. 2, box 13).282,283
The Fcab format described above was applied to generate bispecific scFv-Fcab fusion proteins encoded by a single polypeptide chain (Fig. 2, box 13).284 Here, two antigen-binding sites reside in the C-terminal region of the Fc fragment and two additional binding sites are formed by the scFv fused to the N-terminus of the Fc chains. This format was used to produce a bispecific antibody directed against HER2 and CD3, with the CD3 binding sites formed by the scFvs. T-cell retargeting and tumor cell killing was demonstrated in vitro and in a xenograft tumor model.
Bispecific antibodies using immunoglobulin-derived homodimerization domains
Various immunoglobulin domains form homodimers, including the last heavy chain domain, i.e., CH3 in IgG, IgA, and IgD, and CH4 in IgM and IgE. Furthermore, the CH2 domain in IgM and IgE acts as a hinge connecting the Fab arm to the Fc region formed by CH3 and CH4. In contrast to the last heavy chain domains, the CH2 domains from IgM and IgE are covalently linked by one or two disulfide bonds, respectively, and comprise furthermore a N-glycosylation site. These domains can be used as building blocks to generate tetravalent, bispecific molecules (Fig. 2, box 14, 15). This was shown, for example, by fusing scFvs to the N- and C-terminus of a heavy chain domain 2 of IgM or IgE,285 or a diabody (DiDiabody), as well as a single-chain diabody (scDb-CH3) to the N-terminus.282
Bispecific antibodies - an industrial perspective
Zoo and playground – do we need so many formats?
The ‘zoo’ of antibody formats depicted in Fig. 2 illustrate the more than 100 different formats that we are aware of that were designed and used to make bispecific antibody derivatives. We aimed to include in this review all experimentally verified formats of conceptually different composition that had been described in the literature as of September 2016, but grant that some may have been overlooked. Antibodies are composed of individual domains, which serve as at least partially interchangeable building blocks for a diversity of compositions. Hence, even more derivatives and creative variations of these formats can be designed, some of which have already been generated.
The tremendous diversity of formats leads to the questions: Do we need this many formats and, if so, why? One reason for the availability of so many different formats may be the robustness of the modular domain ‘toolbox’. In many cases, domains can be assembled in a ‘plug-and-play fashion to generate new molecules. Thus, antibody engineering has been a field that is interesting and rewarding for researchers in the biopharmaceutical industry, as well as academic and government settings. With the experience of more than 2 decades, successes are now almost guaranteed, as long as one follows certain quite intuitive rules. The available (or not) freedom-to-operate (FTO) or intellectual property (IP) space, restrictions on certain formats, and the desire to protect one's own developments may have been additional drivers of the explosion of bispecific antibody formats. These and other factors have initially led to some skepticism regarding the necessity of such diversity. The ‘format zoo’ has now been equated with a playground by quite a number of strategists. We acknowledge that zoos and playgrounds appeal to a similar degree to many young, active, open-minded and explorative humans, and we do not consider this to be something bad!
Reality has meanwhile shown that having a diversity of formats is not just play, but instead a critical driver and necessity for the advancement of bispecific antibody therapeutics: A growing number of genetically engineered bispecific antibodies are now in clinical development, some in advanced stages and some already available as drugs (Table 3).24,286 These bispecific antibodies represent quite different formats, with some being large and containing constant regions and some of small size without constant regions. In fact, most major format clusters are represented in one or more molecules that have advanced into the clinic. It has become clear that, even if there were no FTO or IP restrictions or competitive/strategic challenges, ‘one size fits all’ cannot be applied to the plethora of desired functionalities and applications of bispecific antibodies.
Table 3.
Genetically engineered bispecific antibodies approved or in clinical development.
| Format | Molecule | Targets | MOA | Indication | Status | Developed by |
|---|---|---|---|---|---|---|
| BiTE | Blinatumomab AMG103 MT103 | CD19 + CD3 | T-cell recruitment | Acute lymphoblastic leukemia | Market | Amgen (Micromet) |
| MT111, AMG211, Medi565 | CEA + CD3 | T-cell recruitment | Gastric cancer advanced adenocarcinoma | 1 | Amgen (Micromet) | |
| Pasotuxizumab MT112 BAY2010112 | PSMA + CD3 | T-cell recruitment | Prostate cancer | 1 | Bayer (Micromet) | |
| Solitomab MT110 AMG 110 | EPCAM + CD3 | T-cell recruitment | Colorectal, lung, GI cancers, solid tumors | 1 | Amgen (Micromet) | |
| AMG420, BI 836909 | BCMA + CD3 | T-cell recruitment | Multiple Myeloma | 1 | Boehringer Ingelheim, Amgen (Micromet) | |
| AMG 330 | CD33 + CD3 | T-cell recruitment | Acute myeloid leukemia | 1 | Amgen (Micromet) | |
| Crossmab (-deriva-tives) | Vanucizumab RG7221 (1+1) | Angiopoietin 2 + VEGF | 2-Ligand inactivation | Colorectal cancer | 2* | Roche |
| RG7716 (1+1) | Angiopoietin 2 + VEGF | 2-Ligand inactivation | Wet AMD | 2 | Roche | |
| RG7802 (2+1) | CEA+CD3 | T Cell recruitment | CEA pos. solid tumors | 1 | Roche | |
| RG7386 (2+2) | FAP+DR5 | targeted apoptosis | Solid tumors | 1 | Roche | |
| DVD-Ig | ABT165 | DLL4+VEGF | 2-Ligand inactivation | Adv solid tumors | 1 | Abbvie |
| Remtolumab ABT122 | TNFalpha+IL17 | 2-Ligand inactivation | Psoriatic arthritis | 2* | Abbvie | |
| Lutikizumab ABT981 | IL1a + IL1b | 2-Ligand inactivation | Osteoathritis | 2 | Abbvie | |
| SAR156597 DVD-Ig like TBTI | IL4 + IL13 | 2-Ligand inactivation | Idiopath pulm. Fibrosis | 2 | Sanofi | |
| TandAb | AFM111 | CD19+CD3 | T-cell recruitment | Non Hodkins Lymphoma, Acute lymphoblastic leukemia | 1 | Affimed |
| AFM113 | CD30+CD16A | Immune cell recruitment | Hodkins disease | 2 | Affimed | |
| DART | MGD006 S80880 | CD123 + CD3 | T-cell recruitment | AML | 1 | Macrogenics, Servier |
| MGD007 | GPA33 + CD3 | T-cell recruitment | Colorectal cancer | 1 | Macrogenics, Servier | |
| MGD010 | CD32B + CD79B | B-cell modulation | Immune diseases | 1 | Macrogenics, Takeda | |
| PF06671008 | P cadherin + CD3 | T-cell recruitment | Solid tumors | 1 | Pfizer, Macrogenics | |
| MGD009 | B7H3 + CD3 | T-cell recruitment | Solid Tumors | 1 | Macrogenics | |
| Duvortuxizumab, MGD 011, JNJ64052781 | CD19+CD3 | T-cell recruitment | Solid Tumors | 2 | Macrogenics, Janssen | |
| Bi-nanobody | BI836880 | VEGF+Ang2 | 2-Ligand inactivation | Solid tumors | 1 | Ablynx, Boehringer Ingelheim |
| BI1034020 | beta amyloid 40 + 42 | 2-protein inact | Alzheimers | 1* | Ablynx & Boehringer Ingelheim | |
| ALX0761 | IL17A + IL17F | 2-Ligand inactivation | Inflammatory disease | 1 | Ablynx, Merck Serono | |
| Vobarilizumab ALX0061 | IL6R+albumin | PK-modulated receptor antagonist | Lupus, rheumatoid arthritis | 2 | Abbvie, Ablynx | |
| Ozoralizumab ATN103 | TNF+albumin | PK-modulated ligand antagonist | Rheumatoid arthritis | 2 | Ablynx, Taisho, Eddingpharm | |
| cLC-hetero-H-chain IgG | Emicizumab, ACE910, RG6013 | FXI + FX | 2-Factor dimerization | Hemophilia A | 3 | Chugai, Roche |
| MCLA117 | CLEC12A+CD3 | T-cell recruitment | Acute myeloid leukemia | 1/2 | Merus | |
| MCLA128 | Her2+Her3 | 2-RTK inactivation | Solid tumors | 1/2 | Merus | |
| REGN1979 | CD20+CD3 | T-cell recruitment | B-cell cancer | 1 | Regeneron | |
| Navicixizumab OMP-305B83 | DLL4+VEGF | 2-Ligand inactivation | Solid tumors | 1 | Oncomed, Celgene | |
| ERY974 | GPC3+CD3 | T-cell recruitment | Solid tumors | 1 | Chugai | |
| IgG assembled from half-antibodies bsmAb bsmAb | ZW25 (Azymetrics) | Her2 | RTK-inactivation | Her2 pos. cancer | 1 | Zymeworks |
| RG7828, BTCT 4465A (KiH) | CD20+CD3 | T-cell recruitment | Non-Hodgkin's lymphoma, CLL | 1 | Genentech | |
| JNJ 63709178 Duobody | CD123+CD3 | T-cell recruitment | Acute myeloid leukemia | 1 | Janssen, Genmab | |
| JNJ 61186372, EM1 Duobody | Her1+cMet | 2 RTK inactivation | Non-small-cell lung cancer | 1 | Janssen, Genmab | |
| RG7990 BITS7201A | IL13+IL17 | 2 ligand inactivation | Asthma | 1 | Genentech, Novimmune | |
| RG7992, BFKB8488A | FGFR1+KLB | Hormone (FGF21) mimetic | type 2 diabetes | 1 | Genentech | |
| scFv-Fc-(Fab) -fusions | Xmab14045 Fab-scFv-Fc | CD123+CD3 | T-cell recruitment | Acute myeloid leukemia | 1 | Xencor, Novartis |
| GBR1302 Fab-scFv-Fc | Her2+CD3 | T-cell recruitment | Her2 pos. cancer | 1 | Glenmark | |
| MOR 209, ES414 ScFv2-Fc-scFv2 | PSMA+CD3 | T-cell recruitment | Prostate cancer | 1 | Morphosys, Emergent Biosolutions | |
| Istiratumab MM141, IgG-scFv | IGF1R + Her3 | 2-RTK inactivation | Adv. solid tumors | 2 | Merrimack | |
| IL-17/IL-34 biAb scFv-Fc | IL23 + IL17 | 2-Ligand inactivation | Inflamm. & Autoimm. disease | 1 | BMS (Zymogenetics) | |
| LY3164530 IgG+scFv | Her1 + cMET | 2-RTK inactivation | solid tumor | 1 | Eli Lilly | |
| MM111 scFv2-BSA | Her2 + Her3 | 2-RTK inactivation | Adv. gastric esoph. cancer | 2* | Merrimack | |
| MEDI3902 Fab-scFv-Fc | PsI + PcrV | 2 antigen inactivation | Pneumonia | 2 | Medimmune | |
| MEDI7352 | NGF+TNF | 2 ligand inactivation | Osteoarthritis | 1 | Medimmune | |
| MEDI0700 | BAFF+B7RP1 | 2 ligand inactivation | Lupus | 1 | Medimmune | |
| bsmAb | LY3114062 | TNF+IL17 | 2 ligand inactivation | Rheumatoid arthritis | 1 | Eli Lilly |
| DAF-IgG | Duligotuzumab RG7597 | Her1 + Her3 | 2-RTK inactivation | Head & neck, colorectal cancer | 2 | Genentech |
| D&L Fab3 | TF2 IMP288 | CEACAM5 + hapten | Payload delivery | Colorectal, lung cancer | 1/2 | Immunomedics |
Note: Additional bsAb-related entities in clinical development that are not listed in this table include JNJ 61178104 (undisclosed targets in inflammation, Ph1, Genmab/Janssen-Duobody); VM100 (Ab40&42 ‘bispecific/crossreactive’ normal mAb, Ph1 in macular degeneration, Vision Medicine); MEDI4276 (bi-Her2-tubulysin ADC, Ph1 Medimmune); Bi-EGFR targeted minicell-nanoparticles (EnGene, Ph1 in cancer) and OXS1550/DT2219ARL, a CD19+CD22 targeted truncated diphtheria toxin (Oxis Biotech, Ph1/2 in leukemia and lymphoma) and AMG570, a bispecific peptibody conjugate targeting BAFF and ICOSL (Amgen/AstraZeneca, Ph1) * completed studies to our knowledge.
Format variability is essential to serve diverse bispecific antibody applications
There cannot be ‘one best format’ that is used for most desired molecule combinations because of the diversity of requirements and the variability of desired features of the bispecific antibody to be generated. Different ‘target product profiles’ (i.e., definition of the behavior and functionality of the compound) mandate the need for access to a diverse panel of formats. These may vary in size, arrangement, valencies, flexibility and geometry of their binding modules, as well as in their distribution and pharmacokinetic properties.
To achieve bispecificity, factors that need to be taken into account include the special arrangement or size of the different target antigens, as well as, in some instances, the target densities on cell surfaces. This determines which formats may be applicable to achieve bivalent, trivalent or multivalent binding to one or both antigens in a simultaneous or mutually exclusive manner. Small format variations, such as minor changes in linker length or composition or ‘switch’ of domains, can be crucial determinants for functionality. Some design parameters may be deduced from structural models. In many cases, however, suitable formats must be identified by generating and comparing the functionalities of different formats.
Distribution and pharmacokinetic behavior are other important parameters that are directly influenced by the choice of bispecific antibody formats.287 In cases where a long pharmacokinetic half-life is desired, formats are usually larger than 50 kDa to avoid renal clearance and contain entities that enable FcRn-mediated recycling.288 FcRn-recycling is a feature of Fc-containing bispecific antibodies (as long as the FcRn-binding sites are not mutated). An alternative is the addition of albumin or of other FcRn- or albumin-binding entities.16 Small bispecific antibodies are used for applications that require rapid and effective distribution, or whose levels are desired to be tightly controlled with the option to rapidly reduce the levels (e.g., for safety reasons or pre-targeting purposes).
Identification of bispecific antibody format with desired functionality is just the first step in drug development
Despite the importance of format diversity as a prerequisite for the application of bispecific antibodies for different functions, we want to stress that, as in real life, not all members of a zoo can be easily handled. Some look nice, but are really poorly behaved. For pharmaceutical development, molecules and formats need to be produced in large amounts in a reproducible manner, preferably at high yields with processes that are established or similar to such. The more complex composition (e.g., 3–4 chains of bispecific antibodies in contrast to 2-chain IgGs) does frequently require more extensive optimization of expression systems. It has to be particularly noted that (in contrast to ‘simple‘ molecules), stability of the expression system and yield are not the only factors to be addressed. In fact, composition of the bispecific antibodies and presence or absence of undesired side products can be of equal (or higher) importance.
In addition to being fit for production and upstream/downstream processing, bispecific antibodies need to be well defined, stable and overall ‘well behaved’ to become drugs.289-291 Many of these parameters are addressed under the term ‘developability’.292-296 Bispecific antibodies that fulfill developability criteria would be stable (e.g., against thermal denaturation) with low tendency to aggregate, low tendency to accumulate chemical deviations and (dependent on the mode of application) preferentially able to be formulated at high concentrations without viscosity issues.292-296
Thus, designing, optimizing and characterizing bispecific antibodies with desired specificity and functionality is just the beginning of a long process. Converting such molecules into drugs is a difficult endeavor. That part, however, is the key without which bispecific antibodies would remain only exotic members of a zoo, and not drugs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Janice Reichert for providing a list of bispecific antibodies approved or in clinical trials.
References
- 1.Dhimolea E, Reichert JM. World Bispecific Antibody Summit, September 27-28, 2011, Boston, MA. MAbs 2012; 4:4-13; PMID:22327426; http://dx.doi.org/ 10.4161/mabs.4.1.18821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov 2014; 13:799-801; PMID:25359367; http://dx.doi.org/ 10.1038/nrd4478 [DOI] [PubMed] [Google Scholar]
- 3.Sheridan C. Amgen's bispecific antibody puffs across finish line. Nat Biotechnol 2015; 33:219-21; PMID:25748895; http://dx.doi.org/ 10.1038/nbt0315-219 [DOI] [PubMed] [Google Scholar]
- 4.Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immunity 2012; 12:12; PMID:22896757 [PMC free article] [PubMed] [Google Scholar]
- 5.Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys 1961; 93:460-2; PMID:13729244 [DOI] [PubMed] [Google Scholar]
- 6.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256:495-7; PMID:1172191 [DOI] [PubMed] [Google Scholar]
- 7.Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 1985; 229:81-3; PMID:3925553 [DOI] [PubMed] [Google Scholar]
- 8.Milstein C, Cuello AC. Hybrid hyridomas and their use in immunohistochemistry. Nature 1983; 305:537-40; PMID:6137772 [DOI] [PubMed] [Google Scholar]
- 9.Carter P. Bispecific human IgG by design. J Immunol Methods 2001; 248:7-15; PMID:11223065 [DOI] [PubMed] [Google Scholar]
- 10.Kontermann RE. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol Sin 2005; 26:1-9; PMID:15659107; http://dx.doi.org/ 10.1111/j.1745-7254.2005.00008.x [DOI] [PubMed] [Google Scholar]
- 11.Tiller KE, Thessier PM. Advances in antibody design Annu Rev Biomed Eng 2016; 17:191-216; PMID:26274600; http://dx.doi.org/ 10.1146/annurev-bioeng-071114-040733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhukovsky EA, Morse RJ, Maus MV. Bispecific antibodies and CARs: generalized immunotherapeutics harnessing T cell redirection. Curr Opin Immunol 2016; 40:24-35; PMID:26963133; http://dx.doi.org/ 10.1016/j.coi.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Gast GC, van de Winkel JG, Bast BE. Clinicial perspectives of bispecific antibodies in cancer. Cancer Immunol Immunother 1997; 45:121-3; PMID:9435853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Spries AB, van Ojik HH, van de Winkel JG. Immunotherapeutic perspective for bispecific antibodies. Immunol Today 2000; 21:391-7; PMID:10916142 [DOI] [PubMed] [Google Scholar]
- 15.Lameris R, de Bruin RC, Schneiders FL, van Bergen en Henegouwen PM, Verheul HM, de Gruijl TD, van der Vliet HJ. Bispecific antibody platforms for cancer immunotherapy. Crit Rev Oncol hematol 2014: 92:153-65, ,DOI: 10.1016/j.critrevonc.2014.08.003; PMID:25195094 [DOI] [PubMed] [Google Scholar]
- 16.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol 2011; 22:868-76; PMID:21862310; http://dx.doi.org/ 10.1016/j.copbio.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 17.Kontermann RE. Dual targeting strategies with bispecific antibodies. mAbs 2012; 4:182-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al.. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014; 81:49-60; PMID:24411731; http://dx.doi.org/ 10.1016/j.neuron.2013.10.061 [DOI] [PubMed] [Google Scholar]
- 19.Pardridge WM. Re-engineering therapeutic antibodies for Alzheimer's disease as blood-brain penetrating bi-specific antibodies. Expert Opin Biol Ther 2016; 7:1-14; PMID:27572805; http://dx.doi.org/ 10.1080/14712598.2016.1230195 [DOI] [PubMed] [Google Scholar]
- 20.Kufer P, Lutterbüse R, Baeuerle PA. A revival of bispecific antibodies. Trends Biotechnol 2004; 22:238-44; PMID:15109810; http://dx.doi.org/ 10.1016/j.tibtech.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 21.Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs 2009; 1:539-47; PMID:20073127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs 2010; 24:89-98; PMID:20556545; http://dx.doi.org/ 10.1007/s12033-010-9298-x [DOI] [PubMed] [Google Scholar]
- 23.Byrne H, Conroy PJ, Whisstock JC, O'Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 2013; 31:621-32; PMID:24094861; http://dx.doi.org/ 10.1016/j.tibtech.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontermann R, Brinkmann U. Bispecific antibodies. Drug Discov Today 2015; 20:838-47; PMID:25728220; http://dx.doi.org/ 10.1016/j.drudis.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 25.Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol 2014; 41:653-60; PMID:25440609; http://dx.doi.org/ 10.1053/j.seminoncol.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Stanimirovic D, Kemmerich K, Haqqani AS, Farrington GK. Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv Pharmacol 2014; 71:301-35; PMID:25307221; http://dx.doi.org/ 10.1016/bs.apha.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 27.Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015; 67:95-106; PMID:25637431; http://dx.doi.org/ 10.1016/j.molimm.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 28.Nogami K. Bispecific antibody mimicking factor VIII. Thromb Res 2016; 141 Suppl 2:S34-35; PMID:27207420; http://dx.doi.org/ 10.1016/S0049-3848(16)30361-9 [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, Suresh MR. Bispecific antibodies as novel bioconjugates. Bioconjugat Chem 1998; 9:635-44; PMID:9815155; http://dx.doi.org/ 10.1021/bc980044l [DOI] [PubMed] [Google Scholar]
- 30.Thakur A, Lum LG. Cancer therapy with bispecific antibodies: clinical experience. Curr Opin Mol Ther 2010; 12:340-9; PMID:20521223 [PMC free article] [PubMed] [Google Scholar]
- 31.Ellerman D, Scheer JM. Generation of bispecific antibodies by chemical conjugation In. Bispecific antibodies (ed Kontermann RE.), Springer-Verlag; Berlin Heidelberg, 2011; 47-63; http://dx.doi.org/ 10.1007/978-3-642-20910-9_3 [DOI] [Google Scholar]
- 32.Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, Desharnais J, Hagen C, Levin NJ, Shields MJ, et al.. Chemical generation of bispecific antibodies. Proc Natl Acad Sci USA 2010; 107:22611-6; PMID:21149738; http://dx.doi.org/ 10.1073/pnas.1016478108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer W, Völger HR, Lorenz S, Imhof-Jung S, Regula JT, Klein C, Mølhøj M. Heavy and light chain pairing of bivalent quadroma and knobs-into-holes antibodies analyzed by UHR-ESI-QTOF mass spectrometry. MAbs 2016; 8:49-55; PMID:26496506; http://dx.doi.org/ 10.1080/19420862.2015.1111498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M. ScFv antibody: principles and clinical application. Clin Dev. Immun 2012; 2012:980250; PMID:22474489; http://dx.doi.org/ 10.1155/2012/980250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayden MS, Linsley PS, Gayle MA, Bajorath J, Brady WA, Norris NA, Fell HP, Ledbetter JA, Gilliland LK. Single-chain mono- and bispecific antibody derivatives with novel biological properties and antitumour activity from a COS cell transient expression system. Ther Immunol 1994; 1:3-15; PMID:7584477 [PubMed] [Google Scholar]
- 36.Mallender WD, Voss EW Jr. Construction, expression, and activity of a bivalent bispecific single-chain antibody. J Biol Chem 1994; 269:199-206; PMID:8276795 [PubMed] [Google Scholar]
- 37.Gruber M, Schodin BA, Wilson ER, Kranz DM. Efficient tumor cell lysis mediated by a bispecific single chain antibody expressed in Escherichia coli. J Immunol 1994; 152:5368-74; PMID:8189055 [PubMed] [Google Scholar]
- 38.Brandao JG, Scheper RK, Lougheed SM, Curiel DT, Tillman BW, Gerritsen WR, van den Eertwegh AJ, Pinedo HM, Haisma HJ, de Gruijl TD. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine 2003; 21:2268-72; PMID:12744857 [DOI] [PubMed] [Google Scholar]
- 39.Korn T, Nettelbeck DM, Völkel T, Müller R, Kontermann RE. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: a comparative analysis of bacterially expressed single-chain diabody and tandem scFv. J Gene Med 2004; 6:642-51; PMID:15170735; http://dx.doi.org/ 10.1002/jgm.555 [DOI] [PubMed] [Google Scholar]
- 40.Neri D, Momo M, Prospero T, Winter G. High-affinity antigen binding by chelating recombinant antibodies (CRAbs). J Mol Biol 1995; 246:367-73; PMID:7533215; http://dx.doi.org/ 10.1006/jmbi.1994.0091 [DOI] [PubMed] [Google Scholar]
- 41.Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA 1995; 92:7021-5; PMID:7624362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Jonge J, Brissinck J, Heirman C, Demanet C, Leo O, Moser M, Thielemans K. Production and characterization of bispecific single-chain antibody fragments. Mol Immunol 1995; 32:1405-12; PMID:8643110 [DOI] [PubMed] [Google Scholar]
- 43.Kufer P, Mack M, Gruber R, Lutterbüse R, Zettl F, Rietmühler G. Construction and biological activity of a recombinant bispecific single-chain antibody designed for therapy of minimal residual colorectal cancer. Cancer Immunol Immunother 1997; 45:193-7; PMID:9435872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, Adams GP, Schier R, Marks JD, Weiner LM. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol 2001; 166:6112-7; PMID:11342630 [DOI] [PubMed] [Google Scholar]
- 45.Grosse-Hovest L, Müller S, Minoia R, Wolf E, Zakhartchenko V, Wenigerkind H, Lassnig C, Besenfelder U, Müller M, Lytton SD, et al.. Cloned transgenic farm animals produce a bispecific antibody for T cell-mediated tumor cell killing. Proc Natl Acad Sci USA 2004; 101:6858-63; PMID:15105446; http://dx.doi.org/ 10.1073/pnas.0308487101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren-Heidenreich L, Davol PA, Kouttab NM, Elfenbein GJ, Lum LG. Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overexpressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model. Cancer 2004; 100:1095-103; PMID:14983507; http://dx.doi.org/ 10.1002/cncr.20060 [DOI] [PubMed] [Google Scholar]
- 47.Segal DM, Sconocchia G, Titus JA, Jost CR, Kurucz I. Alternative triggering molecules and singe chain bispecific antibodies. J Hematother 1995; 4:377-82; PMID:8581372; http://dx.doi.org/ 10.1089/scd.1.1995.4.377 [DOI] [PubMed] [Google Scholar]
- 48.Fang M, Zhao R, Yang Z, Zhang Z, Li H, Zhang XT, Lin Q, Huang HL. Characterization of an anti-human ovarian carcinomaxanti-human CD3 bispecific single-chain antibody with an albumin-original interlinker. Gynecol Oncol 2004; 92:135-46; PMID:14751149 [DOI] [PubMed] [Google Scholar]
- 49.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric Bispecific Single Chain Variable Fragments (scFv) Killer Engagers (BiKEs) Enhance NK-cell Activity Against CD133+ Colorectal Cancer Cells. Target Oncol 2016; 11:353-61; PMID:26566946; http://dx.doi.org/ 10.1007/s11523-015-0391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today 2005; 10:1237-44; PMID:16213416; http://dx.doi.org/ 10.1016/S1359-6446(05)03554-3 [DOI] [PubMed] [Google Scholar]
- 51.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009; 69:4941-4; PMID:19509221; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-0547 [DOI] [PubMed] [Google Scholar]
- 52.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol 2015; 93:290-6; PMID:25367186; http://dx.doi.org/ 10.1038/icb.2014.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmcol Ther 2012; 136:334-42; PMID:22940266; http://dx.doi.org/ 10.1016/j.pharmthera.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 54.Stieglmaier J, Benjamin J, Nagorsen D. Utilizing the BiTE (bispecific T-cell engager) platform for immunotherapy of cancer. Expert Opin Biol Ther 2015; 15:1093-9; PMID:25971805; http://dx.doi.org/ 10.1517/14712598.2015.1041373 [DOI] [PubMed] [Google Scholar]
- 55.Newman MJ, Benani DJ. A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract 2016; 22:639-45; PMID:26607163; http://dx.doi.org/ 10.1177/1078155215618770 [DOI] [PubMed] [Google Scholar]
- 56.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, Gökbuget N, Neumann S, Goebeler M, Viardot A, et al.. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 2012; 119:6226-33; PMID:22592608; http://dx.doi.org/ 10.1182/blood-2012-01-400515 [DOI] [PubMed] [Google Scholar]
- 57.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, et al.. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16:57-66; PMID:25524800; http://dx.doi.org/ 10.1016/S1470-2045(14)71170-2 [DOI] [PubMed] [Google Scholar]
- 58.Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, Mentz K, Peipp M, Lang P, Oduncu F, et al.. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J. Immunother 2008; 31:871-84; PMID:18833000 [DOI] [PubMed] [Google Scholar]
- 59.Schubert I, Kellner C, Stein C, Kügler M, Schwenkert M, Saul D, Mentz K, Singer H, Stockmeyer B, Hillen W, et al.. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs 2011; 3:21-30; PMID:21081841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed M, Cheng M, Cheung IY, Cheung NK. Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody. Oncoimmunology 2015; 4:e989776; PMID:26137406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, Schubert I.Singer H, Oduncu F, Stockmeyer B, et al.. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol 2010; 150:574-586; PMID:20636437 [DOI] [PubMed] [Google Scholar]
- 62.Igawa T, Tsunoda H, Kikuchi Y, Yoshida M, Tanaka M, Koga A, Sekimori Y, Orita T, Aso Y, Hattori K, et al.. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng DesSel 2010; 23:667-77; PMID:20576629; http://dx.doi.org/ 10.1093/protein/gzq034 [DOI] [PubMed] [Google Scholar]
- 63.Weidle UH, Auer J, Brinkmann U, Georges G, Tiefenthaler G. The emerging role of new protein scaffold-based agents for treatment of cancer. Cancer Genomics Proteomics 2013; 10:155-68; PMID:23893924 [PubMed] [Google Scholar]
- 64.Saerens D, Ghassebeh GH, Muyldermans S. Single-domain antibodies as building blocks for novel therapeutics. Curr Opin Pharmacol 2008; 8:600-8; PMID:18691671; http://dx.doi.org/ 10.1016/j.coph.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 65.Conrath EK, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem 2001; 276:7346-50; PMID:11053416 [DOI] [PubMed] [Google Scholar]
- 66.Simmons DP, Abregu FA, Krishnan UV, Proll DF, Streltsov VA, Doughty L, Hattarki MK, Nuttall SD. Dimerisation strategies for shark IgNAR single domain antibody fragments. J Immunol Methods 2006; 315:171-84; PMID:16962608; http://dx.doi.org/ 10.1016/j.jim.2006.07.019 [DOI] [PubMed] [Google Scholar]
- 67.De Bernardis F, Liu H, O'Mahony R, La Valle R, Bartollino S, Sandini S, Grant S, Brewis N, Tomlinson I, Basset RC, et al.. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis 2007; 195:149-57; PMID:17152019; http://dx.doi.org/ 10.1086/509891 [DOI] [PubMed] [Google Scholar]
- 68.Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, et al.. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J Infect Dis 2014; 210:964-72; PMID:24683195; http://dx.doi.org/ 10.1093/infdis/jiu196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Roy M, Ververken C, Beirnaert E, Hoefman S, Kolkman J, Vierboom M, Breedveld E, 't Hart B, Poelmans S, Bontinck L, et al.. The preclinical pharmacology of the high affinity anti-IL-6R Nanobody® ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res Ther 2015; 17:135; PMID:25994180; http://dx.doi.org/ 10.1186/s13075-015-0651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harmsen MM, Fijten HP, Dekker A, Eblé PL. Passive immunization of pigs with bispecific llama single-domain antibody fragments against foot-and-mouth disease and porcine immunoglobulin. Vet Microbiol 2008; 132:56-64; PMID:18534789; http://dx.doi.org/ 10.1016/j.vetmic.2008.04.030 [DOI] [PubMed] [Google Scholar]
- 71.Holliger P, Prospero T, Winter G. “Diabodies:” small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 1993; 90:6444-8; PMID:8341653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu C. Diabodies: molecular engineering and therapeutic applications. Drug News Perspect 2009; 22:453-8; PMID:20016854 [PubMed] [Google Scholar]
- 73.Perisic O, Webb PA, Holliger P, Winter G, Williams RL. Crystal structure of a diabody, a bivalent antibody fragment. Structure 1994; 2:1217-26; PMID:7704531 [DOI] [PubMed] [Google Scholar]
- 74.Holliger P, Brissinck J, Williams RL, Thielemans K, Winter G. Specific killing of lymphoma cells by cytotoxic T-cells mediated by a bispecific diabody. Protein Eng 1996; 9:299-305; PMID:8736497 [DOI] [PubMed] [Google Scholar]
- 75.Atwell JL, Pearce LA, Lah M, Gruen LC, Kortt AA, Hudson PJ. Design and expression of a stable bispecific scFv dimer with affinity for both glycophorin and N9 neuraminidase. Mol Immunol 1996; 33:1301-12; PMID:9171890 [DOI] [PubMed] [Google Scholar]
- 76.Kontermann RE, Wing MG, Winter G. Complement recruitment using bispecific diabodies. Nat Biotechnol 1997; 15:629-31; PMID:9219263; http://dx.doi.org/ 10.1038/nbt0797-629 [DOI] [PubMed] [Google Scholar]
- 77.Kontermann RE, Martineau P, Cummings CE, Karpas A, Allen D, Derbyshire E, Winter G. Enzyme immunoassays using bispecific diabodies. Immunotechnology 1997; 3:137-44; PMID:9237098 [DOI] [PubMed] [Google Scholar]
- 78.Cochlovius B, Kipriyanov SM, Stassar MJ, Schuhmacher J, Benner A, Moldenhauer G, Little M. Cure of Burkitt's lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res 2000; 60:4336-41; PMID:10969772 [PubMed] [Google Scholar]
- 79.DeNardo DG, Xiong CY, Shi XB, DeNardo GL, DeNardo SJ. Anti-HLA-DR/anti-DOTA diabody construction in a modular gene design platform: bispecific antibodies for pretargeted radioimmunotherapy. Cancer Biother Radiopharm 2001; 16:525-35; PMID:11789029; http://dx.doi.org/ 10.1089/10849780152752128 [DOI] [PubMed] [Google Scholar]
- 80.Lu D, Jimenez X, Witte L, Zhu Z. The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant human bispecific diabody. Biochem Biophys Res Commun 2004; 318:507-13; PMID:15120630; http://dx.doi.org/ 10.1016/j.bbrc.2004.04.060 [DOI] [PubMed] [Google Scholar]
- 81.Hudson PJ, Kortt AA. High avidity scFv multimers: diabodies and triabodies. J Immunol Meth 1999; 231:177-89; PMID:10648937 [DOI] [PubMed] [Google Scholar]
- 82.Le Gall F, Kipriyanov SM, Moldenhauer G, Little M. Di-, tri- and tetrameric single chain Fv antibody fragments against human CD19: effect of valency on cell binding. FEBS Lett 1999; 453:164-8; PMID:10403395 [DOI] [PubMed] [Google Scholar]
- 83.Kipriyanov SM, Moldenhauer G, Braunagel M, Reusch U, Cochlovius B, Le Gall F, Kouprianova OA, Von der Lieth CW, Little M. Effect of domain order on the activity of bacterially produced bispecific single-chain Fv antibodies. J Mol Biol 2003; 330:99-111; PMID:12818205 [DOI] [PubMed] [Google Scholar]
- 84.Fitzgerald K, Holliger P, Winter G. Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng 1997; 10:1221-5; PMID:9488147 [DOI] [PubMed] [Google Scholar]
- 85.Zhu Z, Presta LG, Zapata G, Carter P. Remodeling domain interfaces to enhance heterodimer formation. Protein Sci 1997; 6:781-8; PMID:9098887; http://dx.doi.org/ 10.1002/pro.5560060404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmiedl A, Breitling F, Dübel S. Expression of a bispecific dsFv-dsFv' antibody fragment in Escherichia coli. Protein Eng 2000; 13:725-34; PMID:11112512 [DOI] [PubMed] [Google Scholar]
- 87.Brüsselbach S, Korn T, Völkel T, Müller R, Kontermann RE. Enzyme recruitment and tumor cell killing in vitro by a secreted bispecific single-chain diabody. Tumor Targeting. 1999; 4:115-23 [Google Scholar]
- 88.Korn T, Müller R, Kontermann RE. Bispecific single-chain diabody-mediated killing of endoglin-positive endothelial cells by cytotoxic T lymphocytes. J Immunother 2004; 27:99-106; PMID:14770081 [DOI] [PubMed] [Google Scholar]
- 89.Völkel T, Korn T, Bach M, Müller R, Kontermann RE. Optimized linker sequences for the expression of monomeric and dimeric bispecific single-chain diabodies. Protein Eng 2001; 14:815-23; PMID:11739902 [DOI] [PubMed] [Google Scholar]
- 90.Stamova S, Cartellieri M, Feldmann A, Arndt C, Koristka S, Bartsch H, Bippes CC, Wehner R, Schmitz M, von Bonin M, et al.. Unexpected recombinations in single chain bispecific anti-CD3-anti-CD33 antibodies can be avoided by a novel linker module. Mol Immunol 2011; 49:474-82; PMID:22014687; http://dx.doi.org/ 10.1016/j.molimm.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 91.Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, von der Lieth CW, Matys ER, Little M. Bispecific tandem diabodies for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol 1999; 293:41-56; PMID:10512714 [DOI] [PubMed] [Google Scholar]
- 92.Le Gall F, Reusch U, Little M, Kipriyanov SM. Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Eng Des Sel 2004; 17:357-66; PMID:15126676; http://dx.doi.org/ 10.1093/protein/gzh039 [DOI] [PubMed] [Google Scholar]
- 93.Reusch U, Burkhardt C, Fucet I, Le Gall F, Le Gall M, Hoffmann K, Knackmuss SH, Kiprijanov S, Little M, Zhukovsky EA. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs 2014; 6:728-39; PMID:24670809; http://dx.doi.org/ 10.4161/mabs.28591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 2015; 125:4024-31; PMID:25887777; http://dx.doi.org/ 10.1182/blood-2014-12-614636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reusch U, Harrington K, Gudgeon C, Fucek I, Ellwanger K, Weichel M, Knackmuss SH, Zhukovsky EA, Fox JA, Kunkel LA, et al.. Characterization of CD33/CD3 Tetravalent Bispecific Tandem Diabodies (TandAbs) for the Treatment of Acute Myeloid Leukemia. Clin Cancer Res 2016; 22(23):5829-38; Epub ahead of print; PMID:27189165; http://dx.doi.org/ 10.1158/1078-0432.CCR-16-0350 [DOI] [PubMed] [Google Scholar]
- 96.Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, Zhang W, Tuaillon N, Rainey J, Barat B, et al.. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 2010; 399:436-449; PMID:20382161; http://dx.doi.org/ 10.1016/j.jmb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 97.Moore P, Chichili GR, Huang L, Li H, Burke S, Chen F, et al.. Preclinical activity and safety of MGD006, a CD123xCD3 bispecific DART molecule for the treatment of hematological malignancies. Eur J Cancer 2014; 50:48 [Google Scholar]
- 98.Brien JD, Sukupolvi-Petty S, Williams KL, Lam CY, Schmid MA, Johnson S, Harris E, Diamond MS. Protection by immunoglobulin dual-affinity retargeting antibodies against dengue virus. J. Virol 2013; 87:7747-53; PMID:23658441; http://dx.doi.org/ 10.1128/JVI.00327-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossi EA, Sharkey RM, McBridge W, Karacay H, Zeng L, Hansen HJ, Goldenberg DM, Chang CH. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res 2003; 9:3886S-96S; PMID:14506187 [PubMed] [Google Scholar]
- 100.Rossi EA, Chang CH, Losman MJ, Sharkey RM, Karacay H, McBridge W, Cardillo TM, Hansen HJ, Qu Z, Horak ID, et al.. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin Cancer Res 2005; 11:7122s-9s; PMID:16203811; http://dx.doi.org/ 10.1158/1078-0432.CCR-1004-0020 [DOI] [PubMed] [Google Scholar]
- 101.Schoonjans R, Willems A, Schoonooghe S, Fiers W, Grooten J, Mertens N. Fab chains as an efficient heterodimerization scaffold for the production of recombinant bispecific and trispecific antibody derivatives. J Immunol 2000; 165:7050-7; PMID:11120833 [DOI] [PubMed] [Google Scholar]
- 102.Schoonjans R, Willems A, Schoonooghe S, Leoen J, Grooten J, Mertenss N. A new model for intermediate molecular weight recombinant bispecific and trispecific antibodies by efficient heterodimerization of single chain variable domains through fusion to a Fab-chain. Biomol Eng 2001; 17:193-202; PMID:11337278 [DOI] [PubMed] [Google Scholar]
- 103.Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Fab-scFv fusion protein: an efficient approach to production of bispecific antibody fragments. J Immunol Methods 2002; 267:213-26; PMID:12165442 [DOI] [PubMed] [Google Scholar]
- 104.Qu Z, Goldenberg DM, Cardillo TM, Shi V, Hansen HJ, Chang CH. Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood 2008; 111:2211-9; PMID:18025153; http://dx.doi.org/ 10.1182/blood-2007-08-110072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kellner C, Bruenke J, Horner H, Schubert J, Schwenkert M, Mentz K, Barbin K, Stein C, Peipp M, Stockmeyer B, et al.. Heterodimeric bispecific antibody-derivatives against CD19 and CD16 induce effective antibody-dependent cellular cytotoxicity against B-lymphoid tumor cells. Cancer Lett 2011; 303:128-39; PMID:21339041; http://dx.doi.org/ 10.1016/j.canlet.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 106.Panke C, Weiniger D, Haas A, Schelter F, Schlothauer T, Bader S, Sircar R, Josel HP, Baer U, Burtscher H, et al.. Quantification of cell surface proteins with bispecific antibodies. Protein Eng Des Sel 2013; 26:645-54; PMID:23960142; http://dx.doi.org/ 10.1093/protein/gzt035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol 1997; 15:159-63; PMID:9035142; http://dx.doi.org/ 10.1038/nbt0297-159 [DOI] [PubMed] [Google Scholar]
- 108.Li A, Xing J, Li L, Zhou C, Dong B, He P, Li Q, Wang Z. A single-domain antibody-linked Fab bispecific antibody Her2-S-Fab has potent cytotoxicity against Her2-expressing tumor cells. AMB Express 2016; 6:32; PMID:27112931; http://dx.doi.org/ 10.1186/s13568-016-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayer K, Baumann AL, Grote M, Seeber S, Kettenberger H, Breuer S, Killian T, Schäfer W, Brinkmann U. TriFabs - Trivalent IgG-Shaped Bispecific Antibody Derivatives: Design, Generation, Characterization and Application for Targeted Payload Delivery. Int J Mol Sci 2015; 16:27497-4507; PMID:26593903; http://dx.doi.org/ 10.3390/ijms161126037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brinkmann U, Reiter Y, Jung SH, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci USA 1993; 90:7538-42; PMID:8356052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reiter Y, Brinkmann U, Lee B, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol 1996; 14:1239-45; PMID:9631086 [DOI] [PubMed] [Google Scholar]
- 112.Wu X, Sereno AJ, Huang F, Lewis SM, Lieu RL, Weldon C, Torres C, Fine C, Batt MA, Fitchett JR, et al.. Fab-based bispecific antibody formats with robust biophysical properties and biological activity. MAbs 2015, 7:470-82; PMID:25774965; http://dx.doi.org/ 10.1080/19420862.2015.1022694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davé E, Adams R, Zaccheo O, Carrington B, Composn JE, Dugdale S, Airey M, Malcolm S, Hailu H, Wild G, et al.. Fab-dsFv: a bispecific antibody format with extended serum half-life through albumin binding. MAbs 2016; 8(7):1319-35; epub ahead of print; PMID:27532598; http://dx.doi.org/ 10.1080/19420862.2016.1210747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bossi G, Buisson S, Oates J, Jakobsen BK, Hassan NJ. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother 2014; 63:437-48; PMID:24531387; http://dx.doi.org/ 10.1007/s00262-014-1525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oates J, Hassan NK, Jakobsen BK. ImmTACs for targeted cancer therapy: Why, what, how, and which. Mol Immunol 2015; 67:67-74; PMID:25708206; http://dx.doi.org/ 10.1016/j.molimm.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 116.Boulter JM, Glick M, Todorov PT, Baston E, Sami M, Rizkallah P, Jakobsen BK. Stable soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng 2003; 16:707-11; PMID:14560057 [DOI] [PubMed] [Google Scholar]
- 117.Kostelny SA, Cole MS, Tso JY. Formation of a bispecific antibody by the use of leucine zippers. J Immunol 1992; 148:1547-53; PMID:1531669 [PubMed] [Google Scholar]
- 118.De Kruif J, Logtenberg T. Leucine zipper dimerized bivalent and bispecific scFv antibodies from a semi-synthetic antibody phage display library. J Biol Chem 1996; 271:7630-34; PMID:8631798 [DOI] [PubMed] [Google Scholar]
- 119.Plückthun A, Pack P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology 1997; 3:83-105; PMID:9237094 [DOI] [PubMed] [Google Scholar]
- 120.Müller KM, Arndt KM, Plückthun A. A dimeric bispecific miniantibody combines two specificities with avidity. FEBS Lett 1998; 432:45-49; PMID:9710248 [DOI] [PubMed] [Google Scholar]
- 121.Müller KM, Arndt KM, Strittmatter W, Plückthun A. The first constant domain (C(H)1 and C(L)) of an antibody used as heterodimerization domain for bispecific miniantibodies. FEBS Lett 1998; 422:259-264; PMID:9490020 [DOI] [PubMed] [Google Scholar]
- 122.Rozan C, Cornillon A, Pétiard C, Chartier M, Behar G, Boix C, Kerfelec B, Robert B, Pèlegrin A, Chames P, et al.. Single-domain antibody-based and linker-free bispecific antibodies targeting FcγRIII induce potent antitumor activity without recruiting regulatory T cells. Mol Cancer Ther 2013; 12:1481-91; PMID:23757164; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-1012 [DOI] [PubMed] [Google Scholar]
- 123.Yu X, Duval M, Gawron M, Posner MR, Cavacini LA. Overcoming the Constraints of Anti-HIV/CD89 Bispecific Antibodies That Limit Viral Inhibition. J Immunol Res 2016; 2016:9425172; PMID:27419146; http://dx.doi.org/ 10.1155/2016/9425172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rossi EA, Goldenberg DM, Cardillo TM, McBridge WJ, Skarkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci USA 2006; 103:68416846; PMID:16636283; http://dx.doi.org/ 10.1073/pnas.0600982103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharkey RM, Karacay H, Litwin S, Rossi EA, McBridge WJ, Chang CH, Goldenberg DM. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res 2008; 68:5282-90; PMID:18593929; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossi EA, Goldenberg DM, Chang CH. Complex and defined biostructures with the dock-and-lock method. Trends Pharmacol Sci 2012; 33:474-81; PMID:22739259; http://dx.doi.org/ 10.1016/j.tips.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 127.Sharkey RM, Rossi EA, McBridge WJ, Chang CH, Goldenberg DM. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med 2010; 40:190-203; PMID:20350628; http://dx.doi.org/ 10.1053/j.semnuclmed.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rossi DL, Rossi EA, Cardillo TM, Goldenberg DM, Chang CH. A new class of bispecific antibodies to redirect T cells for cancer immunotherapy. Mabs 2014; 6:381-91; PMID:24492297; http://dx.doi.org/ 10.4161/mabs.27385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deyev SM, Waibel R, Lebedenko EN, Schubiger AP, Plückthun A. Design of mutlivalent complexes using the barnase*barstar module. Nat Biotechnol 2003; 21:1486-92; PMID:14634668; http://dx.doi.org/ 10.1038/nbt916 [DOI] [PubMed] [Google Scholar]
- 130.Semenyuk EG, Stremovskiy OA, Edelweiss EF, Shirshikova OV, Balandin TG, Buryanov YI, Deyev SM. Expression of single-chain antibody-barstar fusion in plants. Biochimie 2007; 89:31-8; PMID:16938381 [DOI] [PubMed] [Google Scholar]
- 131.Rossi EA, Chang CH, Cardillo TM, Goldenberg DM. Optimization of multivalent bispecific antibodies and immunocytokines with improved in vivo properties. Bioconjug Chem 2012; 24:63-71; PMID:23116517; http://dx.doi.org/ 10.1021/bc300488f [DOI] [PubMed] [Google Scholar]
- 132.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta 2013; 1830:526-5534; PMID:23639804; http://dx.doi.org/ 10.1016/j.bbagen.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 133.Müller D, Karle A, Meissburger B, Höfig I, Stork R, Kontermann RE. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J Biol Chem 2007; 282:12650-60; PMID:17347147; http://dx.doi.org/ 10.1074/jbc.M700820200 [DOI] [PubMed] [Google Scholar]
- 134.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al.. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012; 11:582-93; PMID:22248472; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0820 [DOI] [PubMed] [Google Scholar]
- 135.Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res 2005; 11:3879-88; PMID:15897589; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-2290 [DOI] [PubMed] [Google Scholar]
- 136.Song J, Wang X, Lei C, Piao J, Yin C, Zhang Z, Lin Q, Huang H. Fusion of chemotactic peptide to a single-chain bi-specific antibody (scBsAb) potentiates its cytotoxicity to target tumour cells. Biotechnol Appl Biochem 2006; 45:147-54; PMID:16836487; http://dx.doi.org/ 10.1042/BA20060060 [DOI] [PubMed] [Google Scholar]
- 137.Keck PC, Huston JS. Symmetry of Fv architecture is conducive to grafting a second antibody binding site in the Fv region. Biophys J 1996; 71:2002-11; PMID:8889174; http://dx.doi.org/ 10.1016/S0006-3495(96)79398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lobner E, Traxlmayr MW, Obinger C, Hasenhindl C. Engineered IgG1-Fc - one fragment to bind them all. Immunol Rev 2016; 270:113-31; PMID:26864108; http://dx.doi.org/ 10.1111/imr.12385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suresh MR, Cuello AC, Milstein C. Bispecific monoclonal antibodies from hybrid hybridoma. Methods Enzymol 1986; 121:210-28; PMID:3724461 [DOI] [PubMed] [Google Scholar]
- 140.Chelius D, Ruf P, Gruber P, Plöscher M, Liedtke R, Gansberger E, Hess J, Wasiliu M, Lindhofer H. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs 2010; 2:309-19; PMID:20418662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol 1995; 155:219-25; PMID:7602098 [PubMed] [Google Scholar]
- 142.Dall'Acqua W, Simon AL, Mulkerrin MG, Carter P. Contribution of domain interface residues to the stability of antibody CH3 domain homodimers. Biochemistry 1998; 37:9266-73; PMID:9649307; http://dx.doi.org/ 10.1021/bi980270i [DOI] [PubMed] [Google Scholar]
- 143.Ridgway JB, Presta LG, Carter P. 'Knobs-into-holes' engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617-21; PMID:8844834 [DOI] [PubMed] [Google Scholar]
- 144.Chen C, Zhang Y, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel bispecific antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor. Mol Cancer Ther 2013; 13:90-100; PMID:24227890; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0558 [DOI] [PubMed] [Google Scholar]
- 145.Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol 1997; 270:26-35; PMID:9231898; http://dx.doi.org/ 10.1006/jmbi.1997.1116 [DOI] [PubMed] [Google Scholar]
- 146.Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol 1998; 16:677-681; PMID:9661204; http://dx.doi.org/ 10.1038/nbt0798-677 [DOI] [PubMed] [Google Scholar]
- 147.Shatz W, Chung S, Li B, Marshall B, Tejada M, Phung W, Sandoval W, Kelley RF, Scheer JM. Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. MAbs 2013; 5:872-81; PMID:23995614; http://dx.doi.org/ 10.4161/mabs.26307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sampei Z, Igawa T, Soeda T, Okuyama-Nishida Y, Moriyama C, Wakabayashi T, Tanaka E, Muto A, Kojima T, Kitazawa T, et al.. dentification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One 2013; 8:e57479; PMID:23468998; http://dx.doi.org/ 10.1371/journal.pone.0057479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spiess C, Merchant M, Huang A, Zheng Z, Yang NY, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, et al.. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol 2013; 31:753-8; PMID:23831709; http://dx.doi.org/ 10.1038/nbt.2621 [DOI] [PubMed] [Google Scholar]
- 150.Juntilla TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, et al.. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. 2014; 74:5561-71; PMID:25228655; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3622-T [DOI] [PubMed] [Google Scholar]
- 151.Sun JA, Pickeral J, Liu L, Stanfield-Oakley SA, Lam CY, Garrido C, Pollara J, LaBranche C, Bonsignori M, Moody MA, et al.. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest 2015; PMID:26413868; http://dx.doi.org/ 10.1172/JCI82314125:4077-4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shahied LS, Tang Y, Alpaugh RK, Somer R, Greenspon D, Weiner LM. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J Biol Chem 2004; 279:53907-14; PMID:15471859; http://dx.doi.org/ 10.1074/jbc.M407888200 [DOI] [PubMed] [Google Scholar]
- 153.Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, Lee WP, Zhang J, Sharma N, Lu Y, et al.. Development of two-part strategies to identify a therapeutic bispecific antibody that inhibits IgE receptor signaling. J Biol Chem 2010; 285:20850-9; PMID:20444694; http://dx.doi.org/ 10.1074/jbc.M110.113910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spiess C, Bevers J 3rd, Jackman J, Chiang N, Nakamura G, Dillon M, Liu H, Molina P, Elliott JM, Shatz W, et al.. Development of a human IgG4 bispecific antibody for dual targeting of interleukin-4 (IL-4) and interleukin-13 (IL-13) cytokines. J Biol Chem 2013; 288:26583-93; PMID:23880771; http://dx.doi.org/ 10.1074/jbc.M113.480483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie Z, Guo N, Yu M, Hu M, Shen B. A new format of bispecific antibody: highly efficient heterodimerization, expression and tumor cell lysis. J Immunol Methods 2005; 296:96-101; PMID:15680154; http://dx.doi.org/ 10.1016/j.jim.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 156.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al.. A two-in-one antibody against HER3 and EGFR has superor inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472-86; PMID:22014573; http://dx.doi.org/ 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 157.Castoldi R, Jucknischke U, Pradel LP, Arnold E, Klein C, Scheiblich S. Molecular characterization of novel trispecific ErbB-cMet-IGF1R antibodies and their antigen-binding properties. Protein Eng Des Sel 2012; 25:551-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Metz S, Panke C, Haas AK, Schanzer J, Lau W, Croasdale R, Hoffmann E, Schneider B, Auer J, Gassner C, et al.. Bispecific antibody derivatives with restricted binding functionalities that are activated by proteolytic processing. Protein Eng Des Sel 2012; 25:571-80; PMID:22976197; http://dx.doi.org/ 10.1093/protein/gzs064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J, et al.. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci USA 2013; 110:E2987-2996; PMID:23882082; http://dx.doi.org/ 10.1073/pnas.1302725110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schanzer JM, Wartha K, Croasdale R, Moser S, Künkele KP, Ries C, Scheuer W, Duerr H, Pompiati S, Pollman J, et al.. A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties. J Biol Chem 2014; 289:18693-796; PMID:24841203; http://dx.doi.org/ 10.1074/jbc.M113.528109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu Y, Lee J, Tran C, Heibeck TH, Wang WD, Yang J, Stafford RL, Steiner AR, Sato AK, Hallam TJ, et al.. Production of bispecific antibodies in “knobs-into-holes” using a cell-free expression system. MAbs 2015; 7:231-42; PMID:25427258; http://dx.doi.org/ 10.4161/19420862.2015.989013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Taylor K, Howard CB, Jones ML, Sedliarou I, MacDiarmid J, Brahmbhatt H, Munro TP, Mahler SM. Nanocell targeting using engineered bispecific antibodies. MAbs 2015; 7:53-65; PMID:25523746; http://dx.doi.org/ 10.4161/19420862.2014.985952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moore GL, Bautista C, Pong E, Nguyen DH, Jacinto J, Eivaci A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs 2011; 3:546-557; PMID:22123055; http://dx.doi.org/ 10.4161/mabs.3.6.18123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Von Kreudenstein TS, Escobar-Carbrera E, lario PI, D'Angelo I, Brault K, Kelly J, Durocher Y, Baardsnes J, Woods RJ, Xie MH, et al.. Improving biophysical properties of a bispecific antibody scaffold to aid developability: quality by molecular design. MAbs 2013; 5:646-54; PMID:23924797; http://dx.doi.org/ 10.4161/mabs.25632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Woods RJ, Xie MH, von Kreudenstein TS, Ng GY, Dixit SB. LC-MS characterization and purity assessment of a prototype bispecific antibody. MAbs 2013; 5:711-22; PMID:23884083; http://dx.doi.org/ 10.4161/mabs.25488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al.. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637-46; PMID:20400508; http://dx.doi.org/ 10.1074/jbc.M110.117382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Z, Leng EC, Gunasekaran K, Pentony M, Shen M, Howard M, Stoops J, Manchulenko K, Razinkov V, Liu H, et al.. A novel antibody engineering strategy for making monovalent bispecific heterodimeric IgG antibodies by electrostatic steering mechanism. J Biol Chem 2015; 290:7535-62; PMID:25583986; http://dx.doi.org/ 10.1074/jbc.M114.620260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Florio M, Guaneskaran K, Stolina M, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li C, Sun B, Tan HL, et al.. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 2016; 7:11505; PMID:27230681; http://dx.doi.org/ 10.1038/ncomms11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Geuijen C, Rovers E, Nijhuis R, Visser T, den Blanken-Smit R, Barelink W, et al.. Preclinical activity of MCLA-128, an ADCC enhanced human bispecific IgG1 antibody targeting the HER2:HER3 heterodimer. Journal of Clinical Oncology 2014; 32:suppl:560 [Google Scholar]
- 170.Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al.. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204-19; PMID:22543237; http://dx.doi.org/ 10.1016/j.jmb.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 171.Choi HJ, Kim YJ, Lee S, Kim YS. A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR-2 and Met exhibits potent antitumor activity. Mol Cancer Ther 2013; 12:2748-59; PMID:24132142; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0628 [DOI] [PubMed] [Google Scholar]
- 172.Choi HJ, Seok SH, Kim YJ, Seo Md, Kim YS. Crystal structures of immunoglobulin Fc heterodimers reveal the molecular basis for heterodimer formation. Mol Immunol 2015; 65:377-83; PMID:25743157; http://dx.doi.org/ 10.1016/j.molimm.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 173.Choi HJ, Kim YJ, Choi DK, Kim YS. Engineering of Immunoglobulin Fc Heterodimers Using Yeast Surface-Displayed Combinatorial Fc Library Screening. PLoS One 2015; 10:e0145349; PMID:26675656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinze P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, et al.. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007; 317:1554-7; PMID:17872445; http://dx.doi.org/ 10.1126/science.1144603 [DOI] [PubMed] [Google Scholar]
- 175.Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al.. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci USA 2013; 110:5145-50; PMID:23479652; http://dx.doi.org/ 10.1073/pnas.1220145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Labrijn AF, Meesters JI, Priem P, de Jong RN, van den Bremer ET, van Kampen MD, Gerritsen AF, Schuurman J, Parren PW. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat Protoc 2014; 9:2450-63; PMID:25255089; http://dx.doi.org/ 10.1038/nprot.2014.169 [DOI] [PubMed] [Google Scholar]
- 177.Gramer MJ, van den Bremer ET, van Kampen MD, Kundu A, Kopfmann P, Etter E, Stinehelfer D, Long J, Lannom T, Noordergraaf EH, et al.. Production of stable bispecific IgG1 by controlled Fab-arm exchange: scalability from bench to large-scale manufacturing by application of standard approaches. MAbs 2013, 5:962-73; PMID:23995617; http://dx.doi.org/ 10.4161/mabs.26233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Davis JH, Aperlo C, Kurosawa E, Lan Y, Lo KM, Huston JS. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23:195-202; PMID:20299542; http://dx.doi.org/ 10.1093/protein/gzp094 [DOI] [PubMed] [Google Scholar]
- 179.Muda M, Gross AW, Dawson JP, He C, Kurosawa E, Scheickhardt R, Dugas M, Soloviev M, Bernhardt A, Fischer D, et al.. Therapeutic assessment of SEED: a new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng Des Sel 2011; 24:447-54; PMID:21498564; http://dx.doi.org/ 10.1093/protein/gzq123 [DOI] [PubMed] [Google Scholar]
- 180.Kelton C, Wesolowski JS, Soloviev M, Schweickhardt R, Fischer D, Kurosawa E, McKenna SD, Gross AW. Anti-EGFR biparatopic-SEED antibody has enhanced combination-activity in a single molecule. Arch Biochem Biophys 2012; 526:219-25; PMID:22426455; http://dx.doi.org/ 10.1016/j.abb.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 181.Moretti P, Skegro D, Ollier R, Wassmann P, Aebischer C, Laurent T, et al.. BEAT the bispecific challenge: a novel and efficient platform for the expression of bispecific IgGs. BMC Proceedings 2013; 7(Suppl 6):O9 [Google Scholar]
- 182.Croset A, Macoin J, Ollier R, Pluess M, Delon C, Skegro D, et al.. GBR1302: a BEAT bispecific antibody for the treatment of HER2 positive cancers. Eur J Cancer 2014; 50 Suppl 6:48; http://dx.doi.org/ 10.1016/S0959-8049(14)70265-5 [DOI] [Google Scholar]
- 183.Leaver-Fey A, Froning KJ, Atwell S, Aldaz H, Pustilnik A, Lu F, Huang F, Yuan R, Hassanali S, Chamberlain AK, et al.. Computationally Designed Bispecific Antibodies using Negative State Repertoires. Structure 2016; 24:641-51; PMID:26996964; http://dx.doi.org/ 10.1016/j.str.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, et al.. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol 2014; 32:191-8; PMID:24463572; http://dx.doi.org/ 10.1038/nbt.2797 [DOI] [PubMed] [Google Scholar]
- 185.Wranik BJ, Christensen EL, Schaefer G, Jackman JK, Vendel AC, Eaton D. LUZ-Y, a novel platform for the mammalian cell production of full-length IgG-bispecific antibodies. J Biol Chem 2012; 287:43331-9; PMID:23118228; http://dx.doi.org/ 10.1074/jbc.M112.397869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, et al.. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep 2015; 5:17943; PMID:26659273; http://dx.doi.org/ 10.1038/srep17943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tustian AD, Endicott C, Adams B, Mattila J, Bak H. Development of purification processes for fully human bispecific antibodies based upon modification of protein A binding avidity. MAbs 2016; 8:828-38; PMID:26963837; http://dx.doi.org/ 10.1080/19420862.2016.1160192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fischer N, Elson G, Magistrelli G, Dheilly E, Fougue N, Laurendon A, Gueneau F, Ravn U, Depoisier JF, Moine V, et al.. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat Commun 2015; 6:6613; PMID:25672245; http://dx.doi.org/ 10.1038/ncomms7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu L, Yee H, Chan C, Kashyap AK, Horowitz L, Horowitz M, Bhatt RR, Lerner RA. Combinatorial surrobody libraries. Proc Natl Acad Sci USA 2008; 105:10756-61; PMID:18664586; http://dx.doi.org/ 10.1073/pnas.0805293105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Foreman PK, Gore M, Kobel PA, Xu L, Yee H, Hannum C, Ho H, Wang SM, Tran HV, Horowitz M, et al.. ErbB3 inhibitory surrobodies inhibit tumor cell proliferation in vitro and in vivo. Mol Cancer Ther 2012; 11:1411-20; PMID:22553357; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-0068 [DOI] [PubMed] [Google Scholar]
- 191.Milutinvic S, Kashyap AK, Yanagi T, Wimer C, Zhou S, O'Neill R, Kurtzman AL, Faynboym A, Xu L, Hannum CH, et al.. Dual Agonist Surrobody Simultaneously Activates Death Receptors DR4 and DR5 to Induce Cancer Cell Death. Mol Cancer Ther 2016; 15:114-24; PMID:26516157; http://dx.doi.org/ 10.1158/1535-7163.MCT-15-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee HS, Shu L, De Pascalis R, Giuliano M, Zhu M, Padlan EA, Hand PH, Schlom J, Hong HJ, Kashmiri SV. Generation and characterization of a novel single-gene-encoded single-chain immunoglobulin molecule with antigen binding activity and effector functions. Mol Immunol 1999; 36:61-71; PMID:10369421 [DOI] [PubMed] [Google Scholar]
- 193.Hust M, Jostock T, Menzel C, Voedisch B, Mohr A, Brenneis M, Kirsch MI, Meier D, Dübel S. Single chain Fab (scFab) fragments. BMC Biotechnol 2007; 7:14; PMID:17346344; http://dx.doi.org/ 10.1186/1472-6750-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schirrmann T, Menzel C, Hust M, Prilop J, Jostock T, Dübel S. Oligomeric forms of single chain immunoglobulin (scIgG). MAbs 2010; 2:73-76; PMID:20081378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Koerber JT, Hornsby MJ, Wells JA. An improved single-chain Fab platform for efficient display and recombinant expression. J Mol Biol 2015; 427:576-86; PMID:25481745; http://dx.doi.org/ 10.1016/j.jmb.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Castoldi R, Schanzer J, Panke C, Jucknischke U, Neubert NJ, Croasdale R, Scheuer W, Auer J, Klein C, Niederfellner G, et al.. TetraMabs: simultaneous targeting of four oncogenic receptor tyrosine kinases for tumor growth inhibition in heterogeneous tumor cell populations. Protein Eng Des Sel 2016; PMID:27578890; http://dx.doi.org/ 10.1093/protein/gzw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schazer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 2012; 4:653-663; PMID:22925968; http://dx.doi.org/ 10.4161/mabs.21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al.. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci USA 2016; 113:4476-81; PMID:27044098; http://dx.doi.org/ 10.1073/pnas.1525360113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar MR, Sun M, Seaman MS, Padte NN, et al.. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell 2016; 165:1621-31; PMID:27315479; http://dx.doi.org/ 10.1016/j.cell.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klein C, Schaefer W, Regula JT. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs 2016; 8:1010-20; PMID:27285945; http://dx.doi.org/ 10.1080/19420862.2016.1197457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Quaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, et al.. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin Cancer Res 2016; 22:3286-97; PMID:26861458; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-1696 [DOI] [PubMed] [Google Scholar]
- 202.Hu S, Fu W, Xu W, Yang Y, Cruz M, Berezov SD, Jorissen D, Takeda H, Zhu W. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res 2014; 75:159-70; PMID:25371409; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1670 [DOI] [PubMed] [Google Scholar]
- 203.Wu X, Sereno AJ, Huang F, Zhang K, Batt M, Fitchett JR, He D, Rick HL, Conner EM, Demarest SJ. Protein design of IgG/TCR chimeras for the co-expression of Fab-like moieties within bispecific antibodies. MAbs 2015; 7:364-76; PMID:25611120; http://dx.doi.org/ 10.1080/19420862.2015.1007826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Golay J, Choblet S, Iwaszkiewicz J, Cerutti P, Ozil A, Loisel S, Pugnière M, Ubiali G, Zoete V, Michielin O, et al.. Design and Validation of a Novel Generic Platform for the Production of Tetravalent IgG1-like Bispecific Antibodies. J Immunol 2016; 196:3199-211; PMID:26921308; http://dx.doi.org/ 10.4049/jimmunol.1501592 [DOI] [PubMed] [Google Scholar]
- 205.Mazor Y, Oganesyan V, Yang C, Hansen A, Wang J, Liu H, Sachsenmeier K, Carlson M, Gadre DV, Borrok MJ, et al.. Improving target cell specificity using a novel monovalent bispecific IgG design. MAbs 2015; 7:377-89; PMID:25621507; http://dx.doi.org/ 10.1080/19420862.2015.1007816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mazor Y, Hansen A, Yang C, Chowdhury PS, Wang J, Stephens G, Wu H, Dall'Acqua WF. Insights into the molecular basis of a bispecific antibody's target selectivity. MAbs 2015; 7:461-669; PMID:25730144; http://dx.doi.org/ 10.1080/19420862.2015.1022695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Root AR, Cao W, Li B, LaPan P, Meade C, Sanford J, et al.. Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies 2016; 5:6, doi: 10.3390/antib5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dimasi N, Gao C, Fleming R, Woods RM, Yao XT, Shirinian L, Kiener PA, Wu H. The design and characterization of oligospecific antibodies for simultaneous targeting of multiple disease mediators. J Mol Biol 2009; 393:672-92; PMID:19699208; http://dx.doi.org/ 10.1016/j.jmb.2009.08.032 [DOI] [PubMed] [Google Scholar]
- 209.Schanzer J, Jekle A, Nezu J, Lochner A, Croasdale R, Dioszegi M, Zhang J, Hoffmann E, Dormeyer W, Stracke J, et al.. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob Agents Chemother 2011; 55:2369-78; PMID:21300827; http://dx.doi.org/ 10.1128/AAC.00215-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Croasdale R, Wartha K, Schanzer JM, Kuenkele KP, Ries C, Mayer K. Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Arch Biochem Biophys 2012; 526:206-18; PMID:22464987; http://dx.doi.org/ 10.1016/j.abb.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 211.Yazaki PJ, Lee B, Channappa D, Cheung CW, Crow D, Chea J, Poku E, Li L, Andersen JT, Sandlie I, et al.. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng Des Sel 2013; 26:187-93; PMID:23175797; http://dx.doi.org/ 10.1093/protein/gzs096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, Wittrup KD. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel 2010; 23:221-8; PMID:20019028; http://dx.doi.org/ 10.1093/protein/gzp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zuo Z, Jimenez X, Witte L, Zhu Z. An efficient route to the production of an IgG-like bispecific antibody. Protein Eng 2000; 13:361-7; PMID:10835110 [DOI] [PubMed] [Google Scholar]
- 214.Lu D, Zhang H, Ludwig D, Persaud A, Jimenez X, Burtrum D, Balderes P, Liu M, Bohlen P, Witte L, et al.. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem 2004; 279:2856-65; PMID:14576153; http://dx.doi.org/ 10.1074/jbc.M310132200. [DOI] [PubMed] [Google Scholar]
- 214.Asano R, Watanabe Y, Kawaguchi H, Fukazawa H, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, et al.. Highly effective recombinant format of a humanized IgG-like bispecific antibody for cancer immunotherapy with retargeting of lymphocytes to tumor cells. J Biol Chem 2007; 282:27659-65; PMID:17644522 [DOI] [PubMed] [Google Scholar]
- 215.Watanabe Y, Asano R, Arai K, Shimomura I, Ogata H, Kawaguchi H, Hayashi H, Ohtsuka H, Yoshida H, Katayose Y, et al.. In vitro and in vivo antitumor effects of recombinant bispecific antibodies based on humanized anti-EGFR antibody. Oncol Rep 2011; 26:949-55; PMID:21743971; http://dx.doi.org/ 10.3892/or.2011.1382 [DOI] [PubMed] [Google Scholar]
- 216.Michaelson JS, Demarest SJ, Miller B, Amatucci A, Snyder WB, Wu X, Huang F, Phan S, Gao S, Doern A, et al.. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbR. MAbs 2009; 1:128-41; PMID:20061822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Metz S, Haas AK, Daub K, Croasdale R, Stracke J, Lau W, Georges G, Josel HP, Dziadek S, Hopfner KP, et al.. Bispecific digoxigenin-binding antibodies for targeted payload delivery. Proc Natl Acad Sci USA 2011; 108:8194-9; PMID:21536919; http://dx.doi.org/ 10.1073/pnas.1018565108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kang JC, Poovassery JS, Bansal P, You S, Manjarres IM, Ober RJ, Ward ES. Engineering multivalent antibodies to target heregulin-induced HER3 signaling in breast cancer cells. MAbs 2014; 6:340-53; PMID:24492289; http://dx.doi.org/ 10.4161/mabs.27658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Grote M, Haas AK, Klein C, Schaefer W, Brinkmann U. Bispecific antibody derivatives based on full-length IgG formats. Methods Mol Biol 2012; 901:247-63; PMID:22723106; http://dx.doi.org/ 10.1007/978-1-61779-931-0_16 [DOI] [PubMed] [Google Scholar]
- 220.Mabry R, Lewis KE, Moore M, McKernan PA, Bukowski TR, Bontadelli K, Brender T, Okada S, Lum K, West J, et al.. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng Des Sel 2009; 23:115-27; PMID:20022918; http://dx.doi.org/ 10.1093/protein/gzp073 [DOI] [PubMed] [Google Scholar]
- 221.Dong J, Sereno A, Snyder WB, Miller BR, Tamraz S, Doern A, Favis M, Wu X, Tran H, Langley E, et al.. Stable IgG-like bispecific antibodies directed toward the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem 2011; 286:47034717; PMID:21123183; http://dx.doi.org/ 10.1074/jbc.M110.184317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotpyes: from structure to effector functions. Front Immunol 2014; 5:520; PMID:25368619; http://dx.doi.org/ 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, Croner LJ, Wang N, Amatucci A, Michaelson JS, et al.. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel 2010; 23:549-57; PMID:20457695; http://dx.doi.org/ 10.1093/protein/gzq028 [DOI] [PubMed] [Google Scholar]
- 224.Dong J, Sereno A, Aivazian D, Langley E, Miller BR, Snyder WB, Chan E, Cantele M, Morena R, Joseph IB, et al.. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 2011b; 3:273-88; PMID:21393993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. 1999; 29:2613-24; PMID:10458776 [DOI] [PubMed] [Google Scholar]
- 226.Wines BD, Powell MS, Parren PW, Barnes N, Hogarth PM. The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors Fc gamma RI and Fc gamma RIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol 2000; 164:5313-8; PMID:10799893 [DOI] [PubMed] [Google Scholar]
- 227.Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64:700-4; PMID:18560159; http://dx.doi.org/ 10.1107/S0907444908007877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 2013; 65:114-26; PMID:23872058; http://dx.doi.org/ 10.1016/j.ymeth.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 229.Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006; 58:640-56; PMID:16904789; http://dx.doi.org/ 10.1016/j.addr.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 230.An Z, Forrest G, Moore R, Cukan M, Haytko P, Vitelli S, Zhao JZ, Lu P, Hua J, Gibson CR, et al.. IgG2m4, an engineered antibody isotype with reduced Fc function. MAbs 2009; 1:572-9; PMID:20073128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Poovassery JS, Kang JC, Kim D, Ober RJ, Ward ES. Antibody targeting of HER2/HER3 signaling overcomes heregulin-induced resistance to PI3K inhibition in prostate cancer. Int J Cancer 2015; 137:267-77; PMID:25471734; http://dx.doi.org/ 10.1002/ijc.29378 [DOI] [PubMed] [Google Scholar]
- 232.Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardidge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm 2010; 7:237-44; PMID:19921848; http://dx.doi.org/ 10.1021/mp900235k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lu CY, Chen GJ, Tai PH, Yang YC, Hsu YS, Chang M, Hsu CL. Tetravalent anti-CD20/CD3 bispecific antibody for the treatment of B cell lymphoma. Biochem Biophys Res Commun 2016; 473:808-813; PMID:27040766; http://dx.doi.org/ 10.1016/j.bbrc.2016.03.124 [DOI] [PubMed] [Google Scholar]
- 234.Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther 2014; 13:1803-12; PMID:24944121; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shen J, Vil MD, Jimenez X, Iacolina M, Zhang H, Zhu Z. Single variable domain-IgG fusion. A novel recombinant approach to Fc domain-containing bispecific antibodies. J Biol Chem 2006; 281:10706-14; PMID:16481314; http://dx.doi.org/ 10.1074/jbc.M513415200 [DOI] [PubMed] [Google Scholar]
- 236.Shen J, Vil MD, Jimenez X, Zhang H, Iacolina M, Mangalampalli V, Balderes P, Ludwig DL, Zhu Z. Single variable domain antibody as a versatile building block for the construction of IgG-like bispecific antibodies. J Immunol Methods 2007; 318:65-74; PMID:17126853; http://dx.doi.org/ 10.1016/j.jim.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 237.Brack S, Attinger-Toller I, Schade B, Mourlane F, Klupsch K, Woods R, Hachemi H, von der Bey U, Koenig-Friedrich S, Bertschinger J, et al.. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol Cancer Ther 2014; 13:2030-9; PMID:24994770; http://dx.doi.org/ 10.1158/1535-7163.MCT-14-0046-T [DOI] [PubMed] [Google Scholar]
- 238.Silacci M, Lembke W, Woods R, Attinger-Toller I, Baenziger-Tobler N, Batey S, Santimaria R, von der Bey U, Koenig-Friedrich S, Zha W, et al.. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs 2016; 8:141-149; PMID:26390837; http://dx.doi.org/ 10.1080/19420862.2015.1093266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kanakaraj P, Puffer BA, Yao XT, Kankanala S, Boyd E, Shah RR, Wang G, Patel D, Krishnamurthy R, Kaithamana S, et al.. Simultaneous targeting of TNF and Ang2 with a novel bispecific antibody enhances efficacy in an in vivo model of arthritis. MAbs 2012; 4:600-613; PMID:22864384; http://dx.doi.org/ 10.4161/mabs.21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.LaFleur DW, Abramyan D, Kanakaraj P, Smith RG, Shah RR, Wang G, Yao XT, Kankanala S, Boyd E, Zaritskaya L, et al.. Monoclonal antibody therapeutics with up to five specificities: functional enhancement through fusion of target-specific peptides. MAbs 2013; 5:208-218; PMID:23575268; http://dx.doi.org/ 10.4161/mabs.23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shen Y, Zeng L, Novosyadlyy R, Forest A, Zhu A, Korytko A, Zhang H, Eastman SW, Topper M, Hindi S, et al.. A bi-functional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation. MAbs 2015; 7:931-45; PMID:26073904; http://dx.doi.org/ 10.1080/19420862.2015.1055442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Miller K, Meng G, Liu J, Hurst A, Hsei V, Wong WL, Ekert R, Lawrence D, Sherwood S, DeForge L, et al.. Design, construction, and in vitro analysis of multivalent antibodies. J Immunol 2003; 170:4854-61; PMID:12728922 [DOI] [PubMed] [Google Scholar]
- 243.Brünker P, Wartha K, Friess T, Grau-Richards S, Waldhauer I, Koller CF, Weiser B, Majety M, Runza V, Niu H, et al.. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis. Mol Cancer Ther 2016; 15:946-57; PMID:27037412; http://dx.doi.org/ 10.1158/1535-7163.MCT-15-0647 [DOI] [PubMed] [Google Scholar]
- 244.Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics: 1. Properties of anti-CD20/CD22 antibodies in lymphoma. Blood 2009; 113:6161-71; PMID:19372261; http://dx.doi.org/ 10.1182/blood-2008-10-187138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N, Furman RR, Chang CH. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood 2012; 119:3767-2778; PMID:22271448; http://dx.doi.org/ 10.1182/blood-2011-09-381988 [DOI] [PubMed] [Google Scholar]
- 246.Rossi EA, Goldenberg DM, Chang CH. The dock-and-lock method combines recombinant engineering with site-specific covalent conjugation to generate multifunctional structures. Bioconjug Chem 2012; 23:309-23; PMID:22168393; http://dx.doi.org/ 10.1021/bc2004999 [DOI] [PubMed] [Google Scholar]
- 247.Gupta P, Goldenberg DM, Rossi EA, Chang CH. Multiple signaling pathways induced by hexavalent, monospecific, anti-CD20 and hexavalent, bispecific, anti-CD20/CD22 humanized antibodies correlate with enhanced toxicity to B-cell lymphomas and leukemias. Blood 2010; 116:3258-67; PMID:20628151; http://dx.doi.org/ 10.1182/blood-2010-03-276857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al.. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290-7; PMID:17934452; http://dx.doi.org/ 10.1038/nbt1345 [DOI] [PubMed] [Google Scholar]
- 249.Wu C, Ying H, Bose S, Miller R, Medina L, Santora L, Ghayur T. Molecular construction and optimization of anti-human IL-1alpha/beta dual variable domain immunoglobulin (DVD-Ig) molecules. MAbs 2009; 1:339-47; PMID:20068402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lacy SE, Wu C, Ambrosi DJ, Hsieh CM, Bose S, Miller R, Conlon DM, Tarcsa E, Chari R, Ghayur T, et al.. Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-Ig(TM)) molecule that specifically and potently neutralizes both IL-1α and IL-1β. MAbs 2015; 7:605-19; PMID:25764208; http://dx.doi.org/ 10.1080/19420862.2015.1026501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Craig RB, Summa CM, Corti M, Pincus SH. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS One 2012; 7:e46778; PMID:23056448; http://dx.doi.org/ 10.1371/journal.pone.0046778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, Wang L, Weiskopf K, Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs 2015; 7:946-56; PMID:26083076; http://dx.doi.org/ 10.1080/19420862.2015.1062192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gu J, Yang J, Chang Q, Liu Z, Ghayur T, Gu J. Identification of anti-EGFR and anti-ErbB3 dual variable domains immunoglobulin (DVD-Ig) proteins with unique activities. PLoS One 2015; 20:e0124135; PMID:25997020; http://dx.doi.org/ 10.1371/journal.pone.0124135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zeng J, Liu R, Wang J, Fang Y. A bispecific antibody directly induces lymphoma cell death by simultaneously targeting CD20 and HLA-DR. J Cancer Res Clin Oncol 2015; 141:1899-907; PMID:25773122; http://dx.doi.org/ 10.1007/s00432-015-1949-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gu J, Yang J, Chang Q, Lu X, Wang J, Chen M, Ghayur T, Gu J. Identification of anti-ErbB2 dual variable domain immunoglobulin (DVD-Ig™) proteins with unique activities. PLoS One 2014; 9:e97292; PMID:24824849; http://dx.doi.org/ 10.1371/journal.pone.0097292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.DiGiammarino EL, Harlan JE, Walter KA, Ladror US, Edalji RP, Hutchings CW, Lake MR, Greischar AJ, Liu J, Ghayur T, et al.. Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs 2011; 3:487-494; PMID:21814039; http://dx.doi.org/ 10.4161/mabs.3.5.16326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Correia I, Sung J, Burton R, Jakob CG, Carragher B, Ghayur T, Radziejewski C. The structure of dual-variable-domain immunoglobulin molecules alone and bound to antigen. MAbs 2013; 5:364-72; PMID:23572180; http://dx.doi.org/ 10.4161/mabs.24258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jacob CG, Edalji R, Judge RA, diGiammarino E, Li Y, Gu J, Ghayur T. Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig™) molecule. MAbs 2013; 5:358-63; PMID:23549062; http://dx.doi.org/ 10.4161/mabs.23977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Onuoha SC, Ferrari M, Sblattero D, Pfizalis C. Rational design of antirheumatoic prodrugs specific for sites of inflammation. Arthritis Rheumatol 2015; 67:2661-72; PMID:26097196; http://dx.doi.org/ 10.1002/art.39232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Steinmetz A, Vallée F, Beil C, Lange C, Baurin N, Beninga J, Capdevila C, Corvey C, Dupuy A, Ferrari P, et al.. CODV-Ig, a universal bispecific tetravalent and multifunctional immunoglobulin format for medical applications. MAbs 2016; 8:867-78; PMID:26984268; http://dx.doi.org/ 10.1080/19420862.2016.1162932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, et al.. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 2009; 323:1610-4; PMID:19299620; http://dx.doi.org/ 10.1126/science.1165480 [DOI] [PubMed] [Google Scholar]
- 262.Bostrom J, Haber L, Koenig P, Kelley RF, Fuh G. High affinity antigen recognition of the dual specific variants of herceptin is entropy-driven in spite of structural plasticity. PLoS One 2011; 6:e17887; PMID:21526167; http://dx.doi.org/ 10.1371/journal.pone.0017887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lee CV, Koenig P, Fuh G. A two-in-one antibody engineered from a humanized interleukin 4 antibody through mutation in heavy chain complementarity-determining regions. MAbs 2014; 6:622-7; PMID:24618680; http://dx.doi.org/ 10.4161/mabs.28483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, Sliwkowski MX, Harari PM. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2012; 73:824-33; PMID:23172311; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Juric D, Dienstmann R, Cervantes A, Hidalgo M, Messerschmith W, Blumenschein GR Jr, Tabernero J, Roda D, Calles A, Jimeno A, et al.. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin Cancer Res 2015; 21:2462-70; PMID:26034219; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Beckmann R. Dual targeting. 2012; WO2012163520 A1 [Google Scholar]
- 267.Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschläger M, Antes B, Ettl K, Kainer M, Weberhofer G, Wiederkum S, et al.. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel 2010; 23:289-297; PMID:20150180; http://dx.doi.org/ 10.1093/protein/gzq005 [DOI] [PubMed] [Google Scholar]
- 268.Wozniak-Knopp G, Stadlmann J, Rüker F. Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds. PLoS One 2012; 7:e30083; PMID:22272277; http://dx.doi.org/ 10.1371/journal.pone.0030083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Traxlmayr MW, Faissner M, Stadlmayr G, Hasenhindl C, Antes B, Rüker F, Obinger C. Directed evolution of stabilized IgG1-Fc scaffolds by application of strong heat shock to libraries displayed on yeast. Biochim Biophys Acta 2012; 1824:542-9; PMID:22285845; http://dx.doi.org/ 10.1016/j.bbapap.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Traxlmayr MW, Lobner E, Antes B, Kainer M, Wiederkum S, Hasenhindl C, Stadlmayr G, Rüker F, Woisetschläger M, Moulder K, et al.. Directed evolution of Her2/neu-binding IgG1-Fc for improved stability and resistance to aggregation by using yeast surface display. Protein Eng Des Sel 2013; 26:255-65; PMID:23267121; http://dx.doi.org/ 10.1093/protein/gzs102 [DOI] [PubMed] [Google Scholar]
- 271.Fennell BJ, McDonnell B, Tam AS, Chang L, Steven J, Broadbent ID, Gao H, Kieras E, Alley J, Luxenberg D, et al.. CDR-restricted engineering of native human scFvs creates highly stable and soluble bifunctional antibodies for subcutaneous delivery. MAbs 2013; 5:882-95; PMID:23995618; http://dx.doi.org/ 10.4161/mabs.26201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Connelly RJ, Hayden MS, Scholler JK, Tsu TT, Dupont B, Ledbetter JA, Kanner SB. Mitogenic properties of a bispecific single-chain Fv-Ig fusion generated from CD2-specific mAb to distinct epitopes. Int Immunol 1998; 10:1863-72; PMID:9885907 [DOI] [PubMed] [Google Scholar]
- 273.Kim H, Park S, Lee HK, Chung J. Application of bispecific antibody against antigen and hapten for immunodetection and immunopurification. Exp Mol Med 2013; 45:e43; PMID:24071736; http://dx.doi.org/ 10.1038/emm.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yoon S, Kim YH, Kang SH, Kim SK, Lee HK, Kim H, Chung J, Kim IH. Bispecific Her2 × cotinine antibody in combination with cotinine-(histidine)2-iodine for the pre-targeting of Her2-positive breast cancer xenografts. J Cancer Res Clin Oncol 2014; 140:227-33; PMID:24292501; http://dx.doi.org/ 10.1007/s00432-013-1548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Natsume A, Wakitani M, Yamne-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, Satoh M, Shitara K. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region. J Biochem 2006; 140:359-68; PMID:16861252; http://dx.doi.org/ 10.1093/jb/mvj157 [DOI] [PubMed] [Google Scholar]
- 276.Wu SC, Chen YJ, Wang HC, Chou MY, Chang TY, Yuan SS, Chen CY, Hou MF, Hsu JT, Wang YM. Bispecific Antibody Conjugated Manganese-Based Magnetic Engineered Iron Oxide for Imaging of HER2/neu- and EGFR-Expressing Tumors. Theranostics 2016; 6:118-30; PMID:26722378; http://dx.doi.org/ 10.7150/thno.13069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.He Y, Hendriks D, van Ginkel R, Samplonius D, Bremer E, Helfrich W. Melanoma-Directed Activation of Apoptosis Using a Bispecific Antibody Directed at MCSP and TRAIL Receptor-2/Death Receptor-5. J Invest Dermatol 2016; 136:541-4; PMID:26967487; http://dx.doi.org/ 10.1016/j.jid.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 278.DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, Bonnell J, Fleming R, Bezabeh B, Dimasi N, et al.. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med 2014; 6:262ra155; PMID:25391481; http://dx.doi.org/ 10.1126/scitranslmed.3009655 [DOI] [PubMed] [Google Scholar]
- 279.François B, Chastre J, Eggiman P, Laterre PF, Torres A, Sanchez M, Esser MT, Bishop B, Bonten M, Goosens H, Jafri HS. The SAATELLITE and EVADE clinical studies within the COMBACTE Consortium: A public-private collaborative effort in designing and performing clinical trials for novel antibacterial drugs to prevent nosocomial pneumonia. Clin Infect Dis 2016; 63 Suppl 2:S46-51. doi: 10.1093/cid/ciw245 [DOI] [PubMed] [Google Scholar]
- 280.Lu D, Jimenez X, Zhang H, Atkins A, Brennan L, Balderes P, Bohlen P, Witte L, Zhu Z. Di-diabody: a novel tetravalent bispecific antibody molecule by design. J Immunol Methods 2003; 279:219-32; PMID:12969563 [DOI] [PubMed] [Google Scholar]
- 281.Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D, et al.. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem 2005; 280:19665-72; PMID:15757893; http://dx.doi.org/ 10.1074/jbc.M500815200 [DOI] [PubMed] [Google Scholar]
- 282.Alt M, Müller R, Kontermann RE. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region. FEBS Lett 1999; 454:90-4; PMID:10413102 [DOI] [PubMed] [Google Scholar]
- 283.Unverdorben F, Richter F, Hutt M, Seifert O, Malinge P, Fischer N, Kontermann RE. Pharmacokinetic properties of IgG and various Fc fusion proteins in mice. MAbs 2016; 8:120-8; PMID:26514880; http://dx.doi.org/ 10.1080/19420862.2015.1113360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang L, He Y, Zhang G, Ma J, Liu C, He W, Wang W, Han H, Boruah BM, Gao B. Retargeting T cells for HER2-positive tumor killing by a bispecific Fv-Fc antibody. PLoS One 2013; 8:e75589. PMID:24086580; http://dx.doi.org/ 10.1371/journal.pone.0075589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Seifert O, Plappert A, Heidel N, Fellermeier S, Messerschmidt SK, Richter F, Kontermann RE. The IgM CH2 domain as covalently linked homodimerization module for the generation of fusion proteins with dual specificity. Protein Eng Des Sel 2012; 25:603-12; PMID:22988132; http://dx.doi.org/ 10.1093/protein/gzs059 [DOI] [PubMed] [Google Scholar]
- 286.Schuurman J, Parren PW. Editorial overview: special section: New concepts in antibody therapeutics: What's in store for antibody therapy. Curr Opin Immunol 2016; 40:VII-XIII; PMID:27083411; http://dx.doi.org/ 10.1016/j.coi.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 287.Deng R, Jin F, Prabhu S, Iyer S. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin Drug Metab Toxicol 2012; 8:141-60; PMID:22248267; http://dx.doi.org/ 10.1517/17425255.2012.643868 [DOI] [PubMed] [Google Scholar]
- 288.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivry and therapy. Adv Drug Deliv Rev 2015; 91:109-24; PMID:25703189; http://dx.doi.org/ 10.1016/j.addr.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jarasch A, Koll H, Regula JT, Bader M, Papadimitriou A, Kettenberger H. Developability assessment during the selection of novel therpaeutic antibodies. J Pharm Sci 2015; 104:1885-98; PMID:25821140; http://dx.doi.org/ 10.1002/jps.24430 [DOI] [PubMed] [Google Scholar]
- 290.Razinkov VI, Treuheit MJ, Becker GW. Accelerated formulation development of monoclonal antibodies (mAbs) an mAb-based modalities: a review of methods and tools. J Biomol Screen 2015; 20:468-83; PMID:25576149; http://dx.doi.org/ 10.1177/1087057114565593 [DOI] [PubMed] [Google Scholar]
- 291.Mould DR, Meibohm B. Drug development of therapeutic monoclonal antibodies. BioDrugs 2016; 30:275-93; PMID:27342605; http://dx.doi.org/ 10.1007/s40259-016-0181-6 [DOI] [PubMed] [Google Scholar]
- 292.Dashivets T, Stracke J, Dengl S, Knaupp A, Pollmann J, Buchner J, Schlothauer T. Oxidation in the complementarity-determining regions differentially influences the properties of therapeutic antibodies. MAbs 2016; PMID:27612038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Alt N, Zhang TY, Motchnik P, Taticek R, Quarmby V, Schlothauer T, Beck H, Emrich T, Harris RJ. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 2016; 44:291-305; http://dx.doi.org/ 10.1016/j.biologicals.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 294.Haberger M, Heidenreich AK, Schlothauer T, Hook M, Gassner J, Bomans K, Yegres M, Zwick A, Zimmermann B, Wegele H, et al.. Functional assessment of antibody oxidation by native mass spectrometry. MAbs 2015; 7:891-900; PMID:26000623; http://dx.doi.org/ 10.1080/19420862.2015.1052199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Haberger M, Bomans K, Diepold K, Hook M, Gassner J, Schlothauer T, Zwick A, Spick C, Kepert JF, Hienz B, et al.. Assessment of chemical modifications of sites in the CDRs of recombinant antibodies: Susceptibility vs. functionality of critical quality attributes. MAbs 2014; 6:327-39; PMID:2444108; http://dx.doi.org/ 10.4161/mabs.27876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Walters BT, Jensen PF, Larraillet V, Lin K, Patapoff T, Schlothauer T, Rand KD, Zhang J. Conformational destabilization of immunoglobulin G increases the low pH binding affinity with the neonatal Fc receptor. J Biol Chem 2016; 291:1817-25; PMID:26627822; http://dx.doi.org/ 10.1074/jbc.M115.691568 [DOI] [PMC free article] [PubMed] [Google Scholar]