Abstract
The human immunodeficiency virus type 1 (HIV-1) Vpr protein is an attractive target for antiretroviral drug development. The conservation both of the structure along virus evolution and the amino acid sequence in viral isolates from patients underlines the importance of Vpr for the establishment and progression of HIV-1 disease. While its contribution to virus replication in dividing and non-dividing cells and to the pathogenesis of HIV-1 in many different cell types, both extracellular and intracellular forms, have been extensively studied, its precise mechanism of action nevertheless remains enigmatic. The present review discusses how the apparently multifaceted interplay between Vpr and host cells may be due to the impairment of basic metabolic pathways. Vpr protein modifies host cell energy metabolism, oxidative status, and proteasome function, all of which are likely conditioned by the concentration and multimerization of the protein. The characterization of Vpr domains along with new laboratory tools for the assessment of their function has become increasingly relevant in recent years. With these advances, it is conceivable that drug discovery efforts involving Vpr-targeted antiretrovirals will experience substantial growth in the coming years.
Keywords: Vpr protein, mechanism of action, pathogenesis, cellular metabolism, antiretroviral target
1. Introduction
The Vpr protein encoded by the human Immunodeficiency virus type 1 (HIV-1), formerly named Viral Protein R, is associated with replication efficiency and cytopathogenicity of the virus [1,2]. Early studies suggested that this 96-amino acid protein was dispensable in vitro for HIV-1 replication and cytopathogenicity in lymphoid cells, and for that reason was considered an “accessory” protein [3]. However, later studies demonstrated the important role that Vpr plays in vivo for virus spread and pathogenesis [4]. Along this line, several studies demonstrated that Vpr increases the rate of viral replication and accelerates the cytopathic effect of the virus on T cells and, more importantly, is essential for viral replication in macrophages [5,6,7]. More detailed analysis clarified that while Vpr was important for virus replication in primary monocytes and differentiated macrophages, it was superfluous for the infection of resting or stimulated peripheral blood mononuclear cells or CD4+ cell lines [8,9]. Follow-up research indicated that Vpr promotes early T cell activation, facilitating productive HIV-1 infection of non-activated T cells [10]. Significant amounts of Vpr protein and anti-Vpr antibodies can be detected in the serum of HIV-1-infected patients [2,4]. Serum Vpr has been linked to the activation of HIV-1 replication in vivo and also with the control of latency [4]. The observed injury in the central nervous system (CNS) of certain HIV infected patients might be related to the neuronal cell death caused by Vpr protein [11,12]. It is important to highlight that Vpr is one of the HIV-1 virion-associated proteins, reaching more than 700 molecules per virion; approximately 1:7 compared with the 5000 Gag molecules estimated by cryo-electron microscopy (EM) and scanning transmission EM [13,14,15]. Once the virion enters the cell, the Vpr protein shuttles between cytoplasm and nucleus to facilitate viral replication and, at late stages in the virus life cycle, newly synthesized Vpr is exported from the cell [16,17]. Two forms of nuclear Vpr have been observed, monomers and very large complexes, 1000× larger than a monomer [17]. Thus, Vpr has been considered as a vehicle to deliver antiviral molecules into virions and also as a potential therapeutic target to block HIV disease [18,19]. Furthermore, the fact that all primate lentiviruses share a vpr gene whose translation product shows highly conserved motifs along the amino acid sequence highlights its fundamental role in primate lentivirus evolution and biology [20,21]. Therefore, the proposed essential role of Vpr for in vivo viral infection and for disease progression is now beyond doubt [9,22].
The absence of an HIV vaccine and the unlikely prospect of its availability in the foreseeable future necessitates the development of an arsenal of anti-HIV drugs to control and treat the infectious disease. Notwithstanding the significant progress achieved, available antiretroviral strategies are not capable of eradicating HIV in treated patients due to viral reservoirs within cells and tissues, emergence of resistant viruses and adverse effects associated with each antiviral drug class. The challenges of improving efficacy and reducing toxicity of current highly active antiretroviral therapy (HAART) demands the exploration of novel targets to develop new drugs to add to those already in use [23]. This review aims to provide a comprehensive update on the state of the art of the multiple molecular mechanisms of HIV-1 Vpr that makes it an attractive candidate for antiretroviral therapy.
2. Origin and Conservation of Vpr Protein from Human Lentiviruses
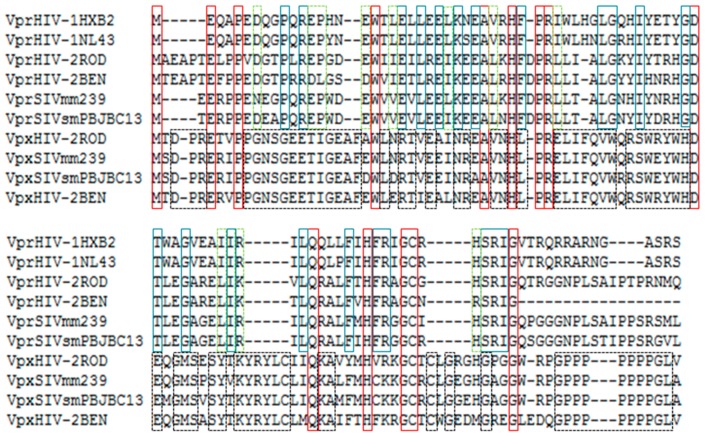
Although HIV-1 vpr is selected against during the establishment of persistent infection in tissue culture, there is a positive selection for Vpr function in vivo in HIV-1 and simian immunodeficiency virus SIVCPZ [24,25]. All primate lentiviruses contain a vpr gene in their genome. HIV-2 and SIVSM genomes additionally carry the vpx gene, which is similar to the unique vpr of SIVAGM [26]. Despite this apparent redundancy, both genes have evolved in HIV-2/SIVSM to produce Vpr and Vpx proteins and each one executes only one of the two functions that are together developed by Vpr HIV-1/SIVCPZ and Vpr SIVAGM proteins [27]. Several residue changes were incorporated into both proteins to be able to interact with cellular factors of new host species, although the similarity in their overall structure is preserved [20,28]. Actually, there is low identity of amino acids between Vpr and Vpx sequences (Figure 1). The structure of HIV-1 Vpr protein is characterized by three well-defined α-helices at amino acid positions, 17–33, 38–50, and 54–77, surrounded by flexible N- and C-terminal domains [29]. Vpr and Vpx proteins from HIV-2 also have three amphipatic α-helices [30] and amino acid identities among the three proteins are particularly concentrated at helix 1 and helix 3.
Figure 1.
Amino acid sequence alignment of Vpr and Vpx proteins from human Immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2), and simian immunodeficiency viruses (SIV) using Fast Fourier Transform (MAFFT) at the EMBL-EBI server (available on 20 October 2016 http://www.ebi.ac.uk). Color code of amino acids within rectangles: red = fully conserved residues in all Vpr and Vpx proteins; blue = fully conserved residues only in Vpr proteins; dotted green = residues with similar properties in Vpr proteins; dotted black = fully conserved residues only in Vpx proteins.
Viruses need to counteract intrinsic antiviral factors that restrict viral replication in host cells and for this purpose HIV and SIV might have acquired accessory proteins along their evolution [31]. The comparison of the antagonism patterns between Vpr, Vpx, and another accessory protein, Vif, from primate lentiviruses toward host antiviral factors such as SAMHD1 and APOBEC3 proteins suggests that the loss of the vpx gene and adaptation to hominids, by reconstruction of the overlapping vif gene, occurred during the passage of SIVs from old world monkeys through chimpanzees [32], subsequently facilitating the adaptation of HIV-1 to humans. Thus, the conservation of Vpr functions together with the genomic adaptations to antagonize the antiviral factors of new host species could have determined the evolution of primate lentiviruses [33].
3. The Role of Vpr Protein in HIV-1 Infection and Disease Progression
One of the outstanding activities performed by Vpr after HIV-1 infection of dividing cells is the blockade of the cell cycle at G2 [25,34,35,36], a phase where the viral long terminal repeat (LTR) promoter is more active [24]. Equally relevant is its activity in non-dividing cells, where its contribution to the nuclear import of the viral preintegration complex (PIC) is critical for virus replication in these cells [9,37]. Other detected activities for Vpr include the regulation of apoptosis and the transcriptional modulation of immune function [38,39,40,41,42]. The relevance of these activities for infection competence is not clear and becomes even more complex when one considers the following issues that could be crucial for in vitro-in vivo extrapolation. Vpr is able to form dimers and even multimers that may determine its functions [42]. While only oligomerizable Vpr incorporates into virus particles and has nuclear transport ability, non-oligomerizable variants retain some activities of the protein, such as inhibition of cellular proliferation and also bystander cell death [42,43,44]. The multiple localization of Vpr protein in infected cells, including inside the nucleus, in mitochondria and dispersed in the cytoplasm, could account for its diverse functions [45,46,47]. It is conceivable that the origin of the protein, released from virions or de novo synthesized, will determine which of the diverse range of activities is prominent when Vpr interacts with each cell type [48,49]. Moreover, differences in the level of its expression might explain the timing of Vpr functionality along the viral replication cycle [50]. The variety of molecular events leading to innate recognition of HIV in different target cells, low permissiveness to infection of primary cells, and defects of cell lines in the release of cytokines to the extracellular milieu are key factors to bear in mind when attempting in vitro assessment of the Vpr modulation of antiviral immune response [51].
3.1. Concurrence and Independence of Cytopathogenic Activities of Vpr
The links between the activities described for Vpr protein have been extensively investigated. The two principal features of HIV-1 Vpr, cell cycle arrest and promotion of macrophage infection, seem to be independent since as shown in other lentiviruses, SIVSM and HIV-2, they are segregated into two proteins, Vpr and Vpx [27,52,53,54]. Nevertheless, several cause-effect relationships have been suggested, especially relating to Vpr-induced G2 arrest with other activities. For example, entry into G2 was proposed to be required for Vpr to induce apoptosis [55]. However, the independence of both Vpr activities was demonstrated with Vpr mutants that do not arrest the cell cycle but retain cell death activity, and vice versa [43,56]. Moreover, results from live imaging suggested that Vpr induces apoptosis in G1 and M phase but fails to induce significant cell death in S or G2 phases [57]. Additionally, the increased expression of HIV-1 LTR in G2-arrested cells might be explained by the cell cycle regulation of some transcription factors [24,58]. Other likely links were proposed for the Vpr-induced nuclear import of the HIV-1 PIC with G2 arrest in CD4+ T cells or with stimulation of virus replication in macrophages [59,60,61]. Conversely, the analysis of Vpr mutants suggested that nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of HIV-1 Vpr [62]. Nonetheless, later findings associated Vpr activities with Vpr interference with the proteasome pathway by interacting with DNA Damage-binding protein 1 (DDB1)-and-Cullin-4-associated Factor 1 (DCAF1) [28]. Thus, the molecular mechanisms of Vpr-induced cell cycle arrest, cell death, nuclear import, and virus activation are still the subject of study. The latest advances in the understanding of Vpr function indicate that some Vpr activities are likely related to the functioning of the cellular machinery and host factors, as will be discussed below.
3.2. The Influence of Vpr on Disease Progression
Vpr protein likely contributes to disease progression in HIV-1-infected patients in several ways: (1) by inhibiting the proliferation of T cells and inducing cellular differentiation [20,31,63]; (2) by enabling productive infection of primary macrophages and reactivating virus production from latency, which contributes to virus production in the absence of CD4+ T cells and to the establishment of drug resistant reservoirs in patients early in infection [4,6,7,18,64]; (3) by contributing to the bystander cell depletion in lymphoid tissues, peripheral blood, and the CNS [12,38,65]. In this respect, soluble Vpr can activate cells in an autocrine or paracrine manner, and this activation could contribute to immune deficiency in patients [4]. It has been proposed that Vpr intervention enables HIV to circumvent the innate immune sensing of viral infection and to prevent the triggering of an innate immune response [51,66]. However, this notion is currently under debate; a recent study has reported the Vpr-mediated potentiation of HIV-1 sensing in CD4+ T [41]. All of this without forgetting that Vpr can traverse the blood brain barrier as an extracellular soluble protein or as an intracellular protein in lymphocytes and monocytes-macrophages. Additionally, infected brain microvascular endothelial cells and brain resident cells might also release soluble Vpr in the CNS. Once there, extracellular Vpr might directly contribute to the HIV-associated CNS dysfunction or through bystander effects mediated by factors involved in cellular death pathways [46,67].
In macrophages, HIV-1 productive infection is low and infected-cells survive and become viral reservoirs [35,68]. It is proposed that Vpr induces anti-apoptotic pathways in infected macrophages that facilitate viral replication and long-term cell survival. Furthermore, a recent study has shown that, by altering the maturation of their phagosomes Vpr impairs the phagocytic function of macrophages, which in turn could contribute to the establishment of opportunistic infections in HIV-infected patients [69,70].
4. Functional Perturbation of Cells by the HIV-1 Vpr Protein
Vpr protein contributes to the pathogenesis of HIV-1 by direct and indirect disruption of cellular homeostatic mechanisms. In the context of viral infection or on its own, Vpr triggers cell death in several cell types [12,16,38,71,72,73]. Consequently, Vpr is associated with the induction of immune activation by depleting regulatory CD4+ T cells with subsequent massive propagation of CCR5-tropic HIV-1 in vivo [74]. Similarly, by activating Nuclear Factor of Activated T cells (NFAT) in primary T cells, Vpr primes them for productive infection [10]. Moreover, Vpr promotes the release of tumor necrosis factor-alpha by infected lymphocytes [75], and the secretion of this proinflammatory cytokine is associated with rapid disease progression [76,77,78]. Additionally, Vpr induces the expression of other cytokines involved in the regulation of inflammation, such as IL-8, and in the recruitment of immune cells, such as IL-6 [79,80]. By contrast, IL-12, a critical Th1 cytokine secreted by antigen presenting cells, is suppressed by Vpr [81].
It has been demonstrated that purified extracellular Vpr can enter cells, suggesting that circulating Vpr could affect bystander cells in infected patients [18,76]. Extracellular Vpr permeabilizes the cell membrane to calcium and magnesium [82]. Moreover, as a result of Vpr-induced permeabilization of the permeability transition pore complex (PTPC), apoptogenic factors are released from the mitochondrial membrane [83]. Vpr-induced deregulation of genes involved in calcium homeostasis is related to neuronal deregulation and loss [84]. In yeast, Vpr-induced mitochondrial dysfunction transiently produces respiratory deficiency and cells are unable to utilize ethanol or glycerol as the sole carbon source [85]. In addition, cell death induced by Vpr is occasionally independent of caspases, having the hallmarks of necrosis [86].
The molecular dissection of Vpr interactions with cells has been possible through the analysis of cells infected with viruses carrying Vpr variants, Vpr-transfected cells, and from the use of Vpr-derived peptides or vpr-antisense ribonucleotides for expression or inhibition studies, respectively. This, together with various cellular models including yeast, primary cells, established cell lines, and also animal models, has permitted the analysis of the specific cellular mechanisms which are modified by Vpr [82,85,87,88,89,90,91]. Moreover, proteomic analysis of HIV-1 transgenic rat models has identified modifications in the content of cellular proteins involved in these Vpr-targeted pathways [92,93].
5. Molecular Mechanisms That Are Affected by Vpr Interaction with Cellular Factors
Several proteomic analyses have been undertaken in an attempt to unravel the precise mechanism of action of HIV-1 Vpr. Data obtained from these studies revealed Vpr-induced changes to protein mediators and modulators of signaling pathways related to glycolysis and other energy processes, mitochondrial activity, redox homeostasis, cell cycle, cell death, and DNA repair [93,94,95,96]. It is also likely that the mitochondrial dysfunction provoked by Vpr affects proteasomal activity [93,97], which would further impact the regulation of transcription initiation [98]. In some cases there appear to be causal links between the effects of Vpr in host cells, undoubtedly because of the variety of signaling pathways affected by viral protein. In addition to transcription and translation, the turn-over of cellular proteins regulates cellular processes. This section is intended to contextualize the significant changes in the amount/activity of some cellular proteins by Vpr (Table 1).
Table 1.
Cellular proteins modulated by Vpr protein. (+): activation/increase; (−): inhibition/decrease.
| Function | Cellular Protein | Effect | Reference |
|---|---|---|---|
| Mitochondria/reactive oxigen species (ROS) | ANT | + | [83] |
| VDAC | + | [83] | |
| PMCA | − | [138] | |
| GLUD2 | − | [94] | |
| Glucose metabolism | HK1 | + | [94] |
| Pentose phosphate pathway | G6PDH | + | [94] |
| Glycolysis | GAPDH | − | [94] |
| Stress response | ATR | + | [117] |
| Proteasome | DCAF1 | + | [128] |
| Transcription | NFAT | + | [10] |
| NF-ĸB | +/− | [79]/[39] | |
| C/EBP | + | [79] | |
| AP-1 | + | [79] | |
| HIF-1α | + | [114] | |
| HDAC1 | − | [122] | |
| GR | + | [141] | |
| SP1 | + | [143] | |
| DNA metabolism | SLX4 | + | [66] |
| HLTF | − | [59] | |
| UNG2 | − | [148] |
5.1. Energy Pathways, Redox Homeostasis, and Cell Cycle
Molecular biological analyses in a wide variety of virus families suggest that virus production requires glycolysis during later steps in replication [99]. HIV-1 infection of T cells increases glycolysis, whereas infection of macrophages suppresses glycolysis [100]. This cell type-dependent adaptation of glucose metabolism agrees well with the known differences in virus production and cell survival in both cell types. In macrophages, vpr transduction enhances the expression of glucose-6-phosphate dehydrogenase (G6PDH), a pentose phosphate pathway (PPP) enzyme that functions as a sentinel for oxidative stress, while it reduces the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a key glycolytic enzyme [94]. Decreased GAPDH activity by extracellular Vpr is also observed in astrocytes [65].
Besides GAPDH, several key mitochondrial enzymes involved in glutamate metabolism are significantly downregulated by Vpr in macrophages, among them glutamate dehydrogenase 2 (GLUD2), which may contribute to neuronal pathogenesis [94]. Conversely, the survival of HIV-1-infected macrophages has been related to the capacity of Vpr to preserve mitochondrial integrity by increasing the amount of mitochondria-bound hexokinase (HK-1) [101]. In yeast, Vpr triggers the loss of respiratory chain complex I–IV and citrate synthase activities [85]. These data suggest that the G2 growth arrest may be an epiphenomenon of Vpr-induced mitochondrial dysfunction that causes loss of the mitochondrial respiratory function with subsequent ATP deficiency. This suggestion is in agreement with the recent demonstration that glucose restriction provokes transient G2 arrest of yeast [102]. HIV-1-infected individuals display multiple symptoms of redox imbalance and Vpr is one of the viral proteins thought to be involved in these phenomena [56,103,104,105]. This hypothesis was later proven using different experimental approaches. Early studies demonstrated that drugs that replenish intracellular glutathione GSH also counteracted oxidative stress and inhibited HIV replication in models of acute and latent infection [106]. Specific assays using extracellular Vpr protein demonstrated impaired levels of intracellular ATP and GSH in astrocytes [107]. Furthermore, extracellular addition of ATP or GSH and its precursors was sufficient to counter growth arrest by endogenously-produced Vpr in yeasts [108]. These treatments also ameliorated Vpr-induced growth impairment in respiratory-deficient (petite) mutants of Saccharomyces cerevisiae, pointing to a Vpr effect in an energy pathway different than oxidative phosphorylation.
Whereas physiological concentrations of endogenous ATP downregulates proteasome activity, it is rapidly upregulated by reduced ATP [109]. Thus, a Vpr-induced reduction in intracellular ATP might explain the observed decrease of endogenous cellular proteins. HIV-1 infection decreases the abundance of mitochondrial ion channels (VDAC1, VDAC2) and glutathione reductase (GSR), which respectively facilitates the survival of infected cells and protects late stages of virus production [110,111]. Consequently, GSH might exert an antiviral effect by impairing the proper assembly of virus proteins [112]. It should be noted that the functional interplay of peroxisomes with other subcellular organelles, such as mitochondria, is necessary for the regulation of cellular redox metabolism [113].
Hypoxia inducible factor-1 alpha (HIF-1α) has been proposed as the major transcription factor participating in the Vpr-mediated activation of the HIV-1 promoter [114]. The activation of this transcription factor would occur once Vpr activates the oxidative stress pathway [115]. Supporting this model is the finding that the switch from HIV-1 latency to reactivation in infected macrophages is promoted by a marginal increase in glutathione redox potential (EGSH) of about 25 mV [116]. A moderate oxidative shift in EGSH, a consequence of GSH oxidation to glutathione disulfide (GSSG), is detected at early stages of viral replication, but as viral replication increases, higher oxidation and also depletion of GSH leads to a robust oxidative shift in EGSH. Indeed, chronic HIV-1 infection enables a better redox response and tolerance to apoptosis.
Early studies associated the Vpr-induced accumulation of cells in G2 phase with the accumulation of the hyperphosphorylated form of the cyclin-dependent kinase cell division control 2 (CDC2) [34,35]. Consequently, Vpr would prevent the required activation of p34cdc2/cyclin B complex for entry into M phase. Later studies found that Vpr activates the stress-induced kinase ataxia telangiectaxia and Rad3-related (ATR), which results in phosphorylation of various substrates including Chk1 [117]. While previous evidence suggested that Vpr-induced G2 arrest does not use classical checkpoint pathways [118,119], subsequent studies demonstrated that Vpr directly binds to chromatin and the ATR stress signaling pathway then becomes activated [120]. The mechanism by which Vpr protein activates this DNA repair response is, however, not clear since ATR responds to a broad spectrum of DNA damage [121]. Alternative models to explain Vpr-mediated G2 arrest rely on the proteasome-mediated downregulation of several cellular factors. Among them are the structure-specific endonuclease regulator SLX4, histone deacetylases (HDAC), the DNA replication factor minichromosome maintenance 10 (MCM10), and also unknown factors [66,122,123,124,125,126].
5.2. Proteasomal Activity and Cell Death
The identification of an endogenous cellular protein that binds to Vpr, VprBP/DCAF1, provided a crucial clue to elucidate the molecular mechanisms of Vpr-induced cell cycle arrest [127,128]. The discovery of the interaction between Vpr and DCAF1 gave rise to alternative models to explain the potential depletion of cellular factors that could be required for cell cycle progression [123,124,125,126,129,130]. DCAF1 is an element of the E3 ubiquitin ligase complex. The DCAF1-DDB1-Cul4 E3 ubiquitin ligase complex is involved in the facilitation of macrophage infection and Vpr-mediated protein degradation. Thus, the hijacking of host DCAF1-CUL4 E3 ubiquitin ligase by Vpr enables targeting of the endonuclease complex MUS81 structure specific endonuclease subunit/essential meiotic structure-specific endonuclease 1 (MUS81/EME1) for degradation via the proteasome and also the activation of SLX4 endonuclease complex that promotes G2/M arrest and escape from innate immune sensing [66,131]. In addition, Vpr-induced acceleration of DCAF1 turnover protects viral envelope (Env) protein from lysosomal degradation and enhances virion production in macrophages [132]. Indeed, Vpr and DCAF1 were found to be necessary for efficient cell-to-cell spread of HIV-1 from macrophages to CD4+ T lymphocytes [133]. The Vpr-induced mitochondrial dysfunction might affect proteasomal activity and vice versa [109]. Ubiquitin-proteasome system and mitochondria are involved in the cellular response to oxidative stress and intracellular variation of ATP levels. Several authors have proposed that Vpr has a dual pro-apoptotic or anti-apoptotic role on programmed cell death that is dependent on its intracellular level, the stage of the infection, and also the cell type [39,50,133,134]. Vpr permeabilizes mitochondrial membranes through a specific interaction with the PTPC via interaction with the adenine nucleotide translocator (ANT) that is located in the inner mitochondrial membrane [83]. This interaction results in the permeabilization of the inner mitochondrial membrane. The subsequent swelling of the mitochondrial matrix might result in impairment of the outer mitochondrial membrane [135,136]. Thus, it has been proposed that Vpr induces a mitochondria-dependent apoptotic pathway in T cells and primary mononuclear cells [137]. In neurons, an alternative mechanism has been proposed where the uptake of extracellular Vpr permeabilizes the plasma membrane by downregulating the plasma membrane Ca2+ ATPase (PMCA) [138]. As a consequence, Vpr triggers an increase of intracellular Ca2+ levels, leading to ROS production and impairing signaling in neurons. Conversely, Vpr can regulate in transfected T cells the oncogene bcl-2, which is endowed with anti-apoptotic activity and downmodulates the pro-apoptotic factor bax, protecting the mitochondrial outer membrane from permeabilization [139]. In this manner, and at early stages of infection, low levels of endogenous Vpr may protect T lymphocytes from death, contributing to the virus dispersion. In addition, the alternative anti-apoptotic role that ATR plays in the mitochondria might contribute to the regulation of apoptosis by Vpr [140].
5.3. Transcriptional Regulation
Early observations revealed that extracellular Vpr was capable of reactivating HIV-1 virus from latency [4,18]. Direct interaction of Vpr with the glucocorticoid receptor (GR) and/or other components of the glucocorticoid-induced transcription initiation complex would signal the transactivation of HIV LTR [141,142]. Further investigation showed that this was due to its ability to transactivate several promoters, among them the viral LTR [5,143,144,145]. According to a recent study, this transactivation capacity occurs via the depletion of histone deacetylase (HDAC) 1 by Vpr. In T cells Vpr increases the basal ubiquitination of HDAC1 and HDAC3 by 2.2- and 3.4-fold, respectively [122]. In infected macrophages, Vpr induced depletion of HDAC1 on specific chromatin regions was associated with hyperacetylation of histones and consequently the activation of the viral promoter [126]. Hence, Vpr may enable the virus to overcome latent infection in primary macrophages. In resting CD4+ cells, virion encapsidated Vpr activates NFAT through Ca2+ influx and the nuclear import of this transcription factor [10]. Modification of the regulation of several transcription factors, such as NF-κB, AP-1, and C/EBP-delta by Vpr could be the cause of the observed Vpr–induced impairment of cytokines and GR signaling in a broad range of cell types [79,80,81].
5.4. DNA Repair Mechanisms
Curiously, the HIV-1 Vpr directs two repair enzymes, helicase-like transcription factor (HLTF) and uracil DNA glycosylase (UNG2), for proteasome-dependent degradation, while the HIV-2 Vpr targets the dNTPase SAM and HD domain containing deoxynucleoside triphosphate triphosphohydrolase 1 (SAMHD1) [53]. Thus, both Vpr proteins reprogram CRL4 (DCAF1) E3 ligase to remove key enzymes involved in three DNA repair pathways, although each protein uses a different strategy to achieve this [53]. HIV-1 Vpr interacts with UNG2, a nuclear DNA repair enzyme that excises uracil from DNA containing miss-incorporated deoxyuridine triphosphate (dUTP), leading to its degradation [146,147,148]. The association of Vpr with the DDB1-containing E3 ligase complex mediates the degradation of UNG2 [149]. The relevance of UNG2 counteraction by Vpr is controversial. On the one hand, Vpr recruits UNG2 into HIV-1 particles in producer cells and consequently modulates virus mutation rate [88,90]. On the other hand, retroviral DNA uraciliation requires exceptional conditions in the host cell in order to be relevant as a barrier to HIV-1 infection. These requirements are low dUTPase levels, high dUTP levels, and abundant nuclear hUNG2; however, not all of these conditions coincide in macrophages or CD4+ T cells [150]. Concerning the HIV-1 Vpr-mediated downregulation of the DNA translocase, HLTF, a recent study demonstrated that this occurs independently of cell cycle stage [96]. As far as is known, the depletion of this DNA translocase occurs in a DCAF1-dependent manner in T cells and macrophages.
6. Amino Acid Residues Contributing to Vpr Functions
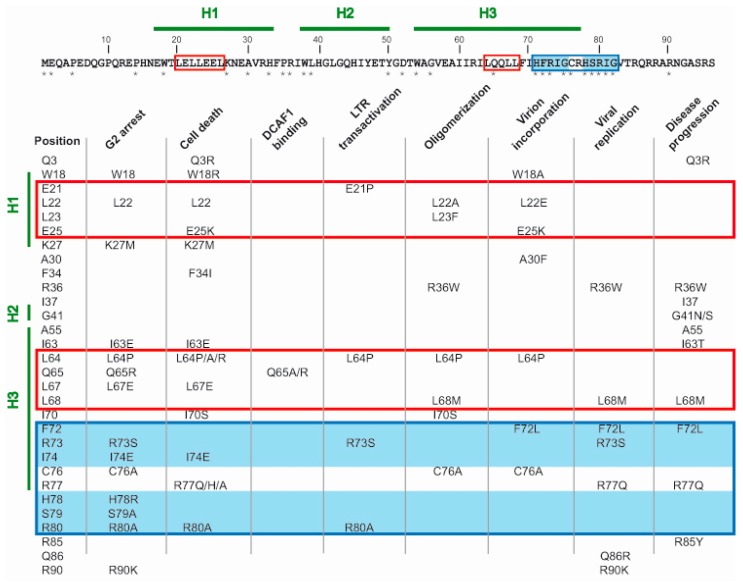
The comparison of amino acid sequence between Vpr proteins from different HIV-1 subtypes reveals a high degree of conservation [151]. Several approaches have been undertaken to determine the relationships between sequence amino acid positions and functionality of Vpr. Analysis of Vpr from cultured and natural HIV-1 variants together with site-directed mutagenesis studies have suggested specific domains and residues in the protein sequence that are associated with virus cytopathogenicity and with disease progression (Figure 2).
Figure 2.
Summary of single sequence polymorphisms that have been associated with alteration in any of the activities of Vpr. The HXB2 Vpr represents the consensus sequence. * symbols indicate positions which have fully conserved residues in HIV-1 Vpr isolates from different M subtypes. H1, H2, and H3 refer to the three α-helices. Relevant residues are outlined in rectangles with the following color code: red = leucine-rich domain, blue = mitochondrial membrane permeabilization (MMP)-inducing sequence. The two HF/SRIG motifs are shadowed in blue.
The N-terminal 42 amino acids of Vpr constitute the oligomerization domain of the protein [42]. This domain includes helix 1 and several residues including Q3, W18, L22, L23, K27, and F34, which have been associated with cytopathicity functions of Vpr [10,56,87,152,153,154]. The C-terminal moiety (Vpr 52–96) binds to ANT and can induce apoptosis [135]. Two highly conserved leucine-rich domains are located within helix 1 (20–26) and helix 3 (64–68). The first domain is likely involved in the interactions of multimerization and, as a result, virion incorporation, while the second domain binds heterologous proteins such as DCAF1 and GR, which then become coactivated [127,132,155,156]. Non-conservative mutations of L64 enhance the pro-apoptotic activity of Vpr, but in a subtype-dependent manner [157]. Helix 3 contains several hydrophobic amino acids including I63, L67, I70, and I74 that enable nuclear localization, cell cycle arrest, and oligomerization of the viral protein [43]. The protein folds around a hydrophobic core defined by leucine, isoleucine, valine, and aromatic residues located in helix 1, 2, and 3 [29]. The mitochondrial membrane permeabilization-inducing activity of Vpr (MMP) resides within a 12-amino acid moiety (71–82) [135]. This moiety contains two H(F/S)RIG motifs (at 71–75 and 78–82); the conservation of these two motifs correlates with HIV pathogenicity [158]. Located between the two motifs is a very well conserved cysteine residue (C76), which is critical for oligomerization and incorporation into HIV-1 virions, while the H(F/S)RIG motifs are necessary for G2 arrest and/or cell death [10,87,154,158,159,160,161]. Additionally, arginine residues R73, R77, R80, and R90 are strongly conserved, and their mutation reduces virus replication and Vpr-induced activities such as apoptosis, LTR activation, IL-12 suppression, and cell cycle arrest [10,87,130,162,163,164,165,166]. The phosphorylation of S79, but not of S94, and S96, is crucial for the cell cycle arrest, although all three serines can be phosphorylated [154]. Mutations at positions 3, 36, 37, 41, 55, 63, 68, 72, and 77 have been associated with variations in the disease progression or the degree of neurocognitive deficit in patients. The simultaneous presence of A55 and T63 in patient-derived Vpr sequences has been associated with lower plasma viral load and higher CD4 count compared with those that express either single or none of these residues [167]. The impact on the neurocognitive function of patients has been associated with the presence of G41N and A55 (detrimental effect) or I37 and S41 (beneficial effect) [168]. Mutations that have been associated with long-term non-progressor (LTNP) patients are Q3R, Q65R, F72L, and R77Q [153,161,162,163,169]. The R77Q mutation is less frequent among patients with progressive disease (36%) than in LTNP patients (about 80%) and shows poor replication [162,163,170]. Nevertheless, the use of this mutation as a marker of slow disease progression is in disagreement with the lack of correlation of this mutation with the course of disease in progressor patients that were receiving therapy [171,172]. Conversely, rapid progression of disease has been associated with R36W, L68M, and R85Y [162,173].
That different Vpr variants emerge during in vitro infection and different Vpr subtypes from isolates show different pro-apoptotic potential might explain the diversity of HIV-1 pathogenesis [157]. Indeed, the conservation of functional domains and low heterogeneity of the vpr gene in mother-infant pair isolates suggests the important role played by Vpr in vertical transmission [174]. Curiously, among the subtypes of HIV-1 Vpr, the subtype C consensus sequence does not function in apoptosis induction as effectively as that from subtype B, but some natural C variants are able to induce cell cycle arrest and apoptosis at a similar rate to subtype B [175]. These observations would support the need for caution when choosing the HIV-1 clone as a reference sequence in drug discovery studies, in order to ensure potential effectiveness of any Vpr-targeted inhibitor in developing countries. Moreover, in some cases single Vpr mutations that are associated with rapid disease progression, do not correlate with the expected Vpr functioning profile [162]. Thus, it is possible that several residues in the Vpr sequence and even Vpr itself, as well as other HIV-1 proteins, make a concomitant contribution to the disease progression.
7. Progress in Searching for Vpr-Targeted Drugs
The so-called group of accessory proteins from HIV-1, which includes Nef, Vif, Vpu, and Vpr, has long been considered a promising target for developing therapeutic strategies against acquired immunodeficiency syndrome (AIDS) [176]. The development of effective antiretroviral drugs specifically targeted to Nef, Vif, or Vpu proteins has been attempted using in vitro-selected compounds, although none of these have yet progressed to final approval [177,178,179,180]. Among the accessory proteins, Vpr ranks second only to Nef in terms of the attention generated to halt virus spread. Notably, Vpr packaged into naturally non-infectious virions or into virions deactivated by reverse transcriptase or protease inhibitors retains the capacity of cell cycle arrest [181]. Its determinant role in acutely and latently infected macrophages distinguishes Vpr for HIV eradication by antiretroviral therapy [182,183]. Indeed, soon after the first description of Vpr protein it was shown that antisense phosphorothioate oligodeoxynucleotides complementary to the vpr mRNA could inhibit HIV-1 replication in primary human macrophages [6]. Attempts to exploit Vpr activity during viral replication for the development of antiretroviral strategies are many and varied. Early studies explored this protein as a vehicle to target foreign proteins to HIV virions given its packaging into virion particle [19,184]. Investigations on targeting other Vpr activities showed that methylxanthines, such as pentoxifylline and caffeine, inhibited Vpr-induced cell cycle arrest but not Vpr-induced apoptosis [72,185]. Several natural products have also been screened for anti-Vpr activity. As a result, the anthraquinone damnacanthal from the root of Morinda citrifolia was reported as an inhibitor of Vpr-induced cell death [186]. Furthermore, several isopimarane diterpenoids extracted from the medicinal herb Kaempferia pulchra were recently reported to exhibit potent anti-Vpr activity [187]. In addition, Vpr-induced transcription from HIV-LTR and the Vpr-induced cell cycle abnormality can be inhibited by the naturally derived anti-HIV flavonoid quercetin [188]. Fumagillin, a natural yeast product and a known inhibitor of angiogenesis, not only reversed the growth inhibitory activity of Vpr in yeast and human cells, but also inhibited Vpr-dependent viral gene expression upon infection of human macrophages [189]. Other studies, which focused on the interaction of Vpr with the GR, described the anti-Vpr activity of mifepristone (RU486) and also neutralizing antibodies to Vpr [81,190,191]. Despite their high cytotoxicity, two PTPC inhibitors, cyclosporin A and bongkrekic acid, can specifically block Vpr-induced mitochondrial damage [83]. It was also reported that di-tryptophan-containing peptides inhibit HIV-1 Vpr-mediated apoptosis and G2 arrest in HIV-1-producing CD4+ T cell lines by interacting with Vpr and interfering with critical protein interactions [192]. Hematoxylin blocks virus replication by targeting the nuclear import of HIV-1 promoted by Vpr protein but does not block Vpr-induced G2 cell cycle arrest [193]. Moreover, a stable hematoxylin derivative strongly binds to the third α-helix of Vpr and inhibits HIV-1 replication in macrophages [194]. Screening of a chemical compound array identified SIP-1 (spirooxindole), which binds to Vpr and displays anti-HIV activity in macrophages by a yet unknown mechanism [195]. The 3-phenyl coumarin-based compound, vipirinin, was reported to inhibit the cell cycle arrest activity of Vpr in yeast and Vpr-dependent viral infection of human macrophages [196]. Recently, the caspase-1 inhibitor VX-765 was reported to improve neurobehavioral deficits observed in HIV-1 Vpr transgenic mice [197]. Finally, the use of compounds that minimize redox imbalance or increase intracellular ATP stores protects from Vpr-induced cytopathogenicity and consequently could prevent the neuropathogenesis associated with HIV-1 disease [107,108,198,199].
8. Conclusions and Perspectives
The current arsenal of antiretroviral agents is abundant but still insufficient. The development of novel targets to direct new antiretroviral drugs is necessary to combat residual problems of resistance, toxicity, and persistence of latent virus reservoirs in HAART regimens. The development of therapeutic drugs directed towards the multifaceted target Vpr might contribute to reduce these problems. Recent advances in the characterization of its interaction with host cells points to Vpr as having a changeable behavior depending on its multimerization status, protein concentration, and cell type infected. None of the Vpr-targeted inhibitors of HIV-1 infection are yet sufficiently potent to enter into clinical trials. The approach used in studies searching for Vpr-targeted antiretrovirals has focused on blocking one specific activity of Vpr during the virus life cycle, such as cell cycle arrest, apoptosis, or nuclear import. However, it is possible that a different approach will be required to efficiently block this multifaceted viral protein. The protection from energetic deficit and antioxidant production nullifying Vpr-induced pathogenesis represents a promising strategy in drug discovery of Vpr inhibitors. Several cellular models and transgenic animals are available for screening this Vpr-induced inhibition. Following the nuclear magnetic resonance (NMR) resolution of the Vpr structure, it is now possible to characterize the molecular interactions that Vpr might establish. Hence, a structure-based approach for drug design will also be very useful in the development of Vpr as a novel target for antiretroviral therapy. Along this line, molecular docking methodologies should be promoted in the rational design of new Vpr-targeted drugs.
Acknowledgments
This work was supported by grant PI08/0912 from Acción Estratégica en Salud from Instituto de Salud Carlos III. The author apologizes to the many authors whose work was not cited due to space limitations. The helpful and constructive comments of anonymous reviewers are acknowledged. The author wishes to thank Kenneth McCreath for editorial support.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Ogawa K., Shibata R., Kiyomasu T., Higuchi I., Kishida Y., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus Vpr open reading frame. J. Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong-Staal F., Chanda P.K., Ghrayeb J. Human immunodeficiency virus: The eighth gene. AIDS Res. Hum. Retrovir. 1987;3:33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- 3.Dedera D., Hu W., Vander H.N., Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J. Virol. 1989;63:3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D.N., Refaeli Y., MacGregor R.R., Weiner D.B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen E.A., Terwilliger E.F., Jalinoos Y., Proulx J., Sodroski J.G., Haseltine W.A. Identification of HIV-1 Vpr product and function. J. Acquir. Immune Defic. Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 6.Balotta C., Lusso P., Crowley R., Gallo R.C., Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J. Virol. 1993;67:4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori N., Michaels F., Fargnoli K., Marcon L., Gallo R.C., Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc. Natl. Acad. Sci. USA. 1990;87:8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 9.Heinzinger N.K., Bukinsky M.I., Haggerty S.A., Ragland A.M., Kewalramani V., Lee M.A., Gendelman H.E., Ratner L., Stevenson M., Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohne K., Businger R., van Nuffel A., Bolduan S., Koppensteiner H., Baeyens A., Vermeire J., Malatinkova E., Verhasselt B., Schindler M. Virion encapsidated HIV-1 Vpr induces NFAT to prime non-activated T cells for productive infection. Open Biol. 2016;6:160046. doi: 10.1098/rsob.160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel C.A., Mukhtar M., Pomerantz R.J. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 2000;74:9717–9726. doi: 10.1128/JVI.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piller S.C., Jans P., Gage P.W., Jans D.A. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: Implications for AIDS pathology. Proc. Natl. Acad. Sci. USA. 1998;95:4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggs J.A., Simon M.N., Gross I., Krausslich H.G., Fuller S.D., Vogt V.M., Johnson M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004;11:672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 14.Cohen E.A., Dehni G., Sodroski J.G., Haseltine W.A. Human immunodeficiency virus Vpr product is a virion-associated regulatory protein. J. Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller B., Tessmer U., Schubert U., Krausslich H.G. Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells. J. Virol. 2000;74:9727–9731. doi: 10.1128/JVI.74.20.9727-9731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y.L., Spearman P., Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai T.M., Marin M., Sood C., Shi J., Nawaz F., Aiken C., Melikyan G.B. Fluorescent protein-tagged Vpr dissociates from HIV-1 core after viral fusion and rapidly enters the cell nucleus. Retrovirology. 2015;12:88. doi: 10.1186/s12977-015-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D.N., Refaeli Y., Weiner D.B. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J. Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Liu H., Xiao H., Kim J., Seshaiah P., Natsoulis G., Boeke J.D., Hahn B.H., Kappes J.C. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J. Virol. 1995;69:3389–3398. doi: 10.1128/jvi.69.6.3389-3398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stivahtis G.L., Soares M.A., Vodicka M.A., Hahn B.H., Emerman M. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J. Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tristem M., Marshall C., Karpas A., Hill F. Evolution of the primate lentiviruses: Evidence from Vpx and Vpr. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang S.M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Muller J., Muller-Hermelink H., Fuchs D., Wachter H., Daniel M.M. Importance of Vpr for infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman T.L., Buckheit R.W. The continuing evolution of HIV-1 therapy: Identification and development of novel antiretroviral agents targeting viral and cellular targets. Mol. Biol. Int. 2012;2012:401965. doi: 10.1155/2012/401965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh W.C., Rogel M.E., Kinsey C.M., Michael S.F., Fultz P.N., Nowak M.A., Hahn B.H., Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat. Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 25.Rogel M.E., Wu L.I., Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp P.M., Bailes E., Stevenson M., Emerman M., Hahn B.H. Gene acquisition in HIV and SIV. Nature. 1996;383:586–587. doi: 10.1038/383586a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher T.M., Brichacek B., Sharova N., Newman M.A., Stivahtis G., Sharp P.M., Emerman M., Hahn B.H., Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) EMBO J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y., Zhou X., Barnes C.O., DeLucia M., Cohen A.E., Gronenborn A.M., Ahn J., Calero G. The DDB1-DCAF1-Vpr-UNG2 crystal structure reveals how HIV-1 Vpr steers human UNG2 toward destruction. Nat. Struct. Mol. Biol. 2016;23:933–940. doi: 10.1038/nsmb.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morellet N., Bouaziz S., Petitjean P., Roques B.P. NMR structure of the HIV-1 regulatory protein Vpr. J. Mol. Biol. 2003;327:215–227. doi: 10.1016/S0022-2836(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 30.Khamsri B., Murao F., Yoshida A., Sakurai A., Uchiyama T., Shirai H., Matsuo Y., Fujita M., Adachi A. Comparative study on the structure and cytopathogenic activity of HIV Vpr/Vpx proteins. Microbes Infect. 2006;8:10–15. doi: 10.1016/j.micinf.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Yan N., Chen Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etienne L., Hahn B.H., Sharp P.M., Matsen F.A., Emerman M. Gene loss and adaptation to hominids underlie the ancient origin of HIV-1. Cell Host Microbe. 2013;14:85–92. doi: 10.1016/j.chom.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai Y., Doi N., Miyazaki Y., Adachi A., Nomaguchi M. Phylogenetic insights into the functional relationship between primate lentiviral reverse transcriptase and accessory proteins Vpx/Vpr. Front. Microbiol. 2016;7:1655. doi: 10.3389/fmicb.2016.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J., Choe S., Walker R., di M.P., Morgan D.O., Landau N.R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jowett J.B., Planelles V., Poon B., Shah N.P., Chen M.L., Chen I.S. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Re F., Braaten D., Franke E.K., Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popov S., Rexach M., Ratner L., Blobel G., Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 38.Arunagiri C., Macreadie I., Hewish D., Azad A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2:69–76. doi: 10.1023/A:1026487609215. [DOI] [PubMed] [Google Scholar]
- 39.Ayyavoo V., Mahboubi A., Mahalingam S., Ramalingam R., Kudchodkar S., Williams W.V., Green D.R., Weiner D.B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat. Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 40.Majumder B., Venkatachari N.J., Srinivasan A., Ayyavoo V. HIV-1 mediated immune pathogenesis: Spotlight on the role of viral protein R (Vpr) Curr. HIV Res. 2009;7:169–177. doi: 10.2174/157016209787581445. [DOI] [PubMed] [Google Scholar]
- 41.Vermeire J., Roesch F., Sauter D., Rua R., Hotter D., van Nuffel A., Vanderstraeten H., Naessens E., Iannucci V., Landi A., et al. HIV triggers a cGAS-dependent, Vpu- and Vpr-regulated type I interferon response in CD4+ T cells. Cell Rep. 2016;17:413–424. doi: 10.1016/j.celrep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L.J., Wang L., Mukherjee S., Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J. Biol. Chem. 1994;269:32131–32137. [PubMed] [Google Scholar]
- 43.Bolton D.L., Lenardo M.J. Vpr cytopathicity independent of G2/M cell cycle arrest in HIV-1-infected CD4+ T cells. J. Virol. 2007;81:8878–8890. doi: 10.1128/JVI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatachari N.J., Walker L.A., Tastan O., Le T., Dempsey T.M., Li Y., Yanamala N., Srinivasan A., Klein-Seetharaman J., Montelaro R.C., et al. Human immunodeficiency virus type 1 Vpr: Oligomerization is an essential feature for its incorporation into virus particles. Virol. J. 2010;7:119. doi: 10.1186/1743-422X-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di M.P., Choe S., Ebright M., Knoblauch R., Landau N.R. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrucci A., Nonnemacher M.R., Wigdahl B. Human immunodeficiency virus viral protein R as an extracellular protein in neuropathogenesis. Adv. Virus Res. 2011;81:165–199. doi: 10.1016/B978-0-12-385885-6.00010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C.Y., Chiang S.F., Lin T.Y., Chiou S.H., Chow K.C. HIV-1 Vpr triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction. PLoS ONE. 2012;7:e33657. doi: 10.1371/journal.pone.0033657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Silva S., Planelles V., Wu L. Differential effects of Vpr on single-cycle and spreading HIV-1 infections in CD4+ T cells and dendritic cells. PLoS ONE. 2012;7:e35385. doi: 10.1371/journal.pone.0035385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hrimech M., Yao X.J., Bachand F., Rougeau N., Cohen E.A. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 1999;73:4101–4109. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conti L., Matarrese P., Varano B., Gauzzi M.C., Sato A., Malorni W., Belardelli F., Gessani S. Dual role of the HIV-1 Vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 2000;165:3293–3300. doi: 10.4049/jimmunol.165.6.3293. [DOI] [PubMed] [Google Scholar]
- 51.Trotard M., Tsopoulidis N., Tibroni N., Willemsen J., Binder M., Ruggieri A., Fackler O.T. Sensing of HIV-1 infection in Tzm-bl cells with reconstituted expression of STING. J. Virol. 2015;90:2064–2076. doi: 10.1128/JVI.02966-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goujon C., Riviere L., Jarrosson-Wuilleme L., Bernaud J., Rigal D., Darlix J.L., Cimarelli A. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology. 2007;4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hrecka K., Hao C., Shun M.C., Kaur S., Swanson S.K., Florens L., Washburn M.P., Skowronski J. HIV-1 and HIV-2 exhibit divergent interactions with HLTF and UNG2 DNA repair proteins. Proc. Natl. Acad. Sci. USA. 2016;113:E3921–E3930. doi: 10.1073/pnas.1605023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharova N., Wu Y., Zhu X., Stranska R., Kaushik R., Sharkey M., Stevenson M. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4:e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersen J.L., Dehart J.L., Zimmerman E.S., Ardon O., Kim B., Jacquot G., Benichou S., Planelles V. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2006;2:e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stromajer-Racz T., Gazdag Z., Belagyi J., Vagvolgyi C., Zhao R.Y., Pesti M. Oxidative stress induced by HIV-1 F34IVpr in Schizosaccharomyces pombe is one of its multiple functions. Exp. Mol. Pathol. 2010;88:38–44. doi: 10.1016/j.yexmp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Murakami T., Aida Y. Visualizing Vpr-induced G2 arrest and apoptosis. PLoS ONE. 2014;9:e86840. doi: 10.1371/journal.pone.0086840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felzien L.K., Woffendin C., Hottiger M.O., Subbramanian R.A., Cohen E.A., Nabel G.J. HIV transcriptional activation by the accessory protein, Vpr, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vodicka M.A., Koepp D.M., Silver P.A., Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacquot G., Le R.E., David A., Mazzolini J., Bouchet J., Bouaziz S., Niedergang F., Pancino G., Benichou S. Localization of HIV-1 Vpr to the nuclear envelope: Impact on Vpr functions and virus replication in macrophages. Retrovirology. 2007;4:84. doi: 10.1186/1742-4690-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iijima S., Nitahara-Kasahara Y., Kimata K., Zhong Z.W., Kamata M., Isogai M., Miwa M., Tsunetsugu-Yokota Y., Aida Y. Nuclear localization of Vpr is crucial for the efficient replication of HIV-1 in primary CD4+ T cells. Virology. 2004;327:249–261. doi: 10.1016/j.virol.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Mahalingam S., Ayyavoo V., Patel M., Kieber-Emmons T., Weiner D.B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J. Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy D.N., Fernandes L.S., Williams W.V., Weiner D.B. Induction of cell differentiation by human immunodeficiency virus 1 Vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-Y. [DOI] [PubMed] [Google Scholar]
- 64.Igarashi T., Brown C.R., Endo Y., Buckler-White A., Plishka R., Bischofberger N., Hirsch V., Martin M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA. 2001;98:658–663. doi: 10.1073/pnas.98.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrucci A., Nonnemacher M.R., Wigdahl B. Extracellular HIV-1 viral protein R affects astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. J. Neurovirol. 2013;19:239–253. doi: 10.1007/s13365-013-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laguette N., Bregnard C., Hue P., Basbous J., Yatim A., Larroque M., Kirchhoff F., Constantinou A., Sobhian B., Benkirane M. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell. 2014;156:134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Lindl K.A., Marks D.R., Kolson D.L., Jordan-Sciutto K.L. HIV-associated neurocognitive disorder: Pathogenesis and therapeutic opportunities. J. Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cosenza M.A., Zhao M.L., Lee S.C. HIV-1 expression protects macrophages and microglia from apoptotic death. Neuropathol. Appl. Neurobiol. 2004;30:478–490. doi: 10.1111/j.1365-2990.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 69.Dumas A., Le-Bury G., Marie-Anais F., Herit F., Mazzolini J., Guilbert T., Bourdoncle P., Russell D.G., Benichou S., Zahraoui A., et al. The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J. Cell Biol. 2015;211:359–372. doi: 10.1083/jcb.201503124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jambo K.C., Banda D.H., Kankwatira A.M., Sukumar N., Allain T.J., Heyderman R.S., Russell D.G., Mwandumba H.C. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenkins Y., Sanchez P.V., Meyer B.E., Malim M.H. Nuclear export of human immunodeficiency virus type 1 Vpr is not required for virion packaging. J. Virol. 2001;75:8348–8352. doi: 10.1128/JVI.75.17.8348-8352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart S.A., Poon B., Jowett J.B., Chen I.S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y., Elder R.T., Chen M., Cao J. Fission yeast expression vectors adapted for positive identification of gene insertion and green fluorescent protein fusion. Biotechniques. 1998;25:438–440. doi: 10.2144/98253st06. [DOI] [PubMed] [Google Scholar]
- 74.Sato K., Misawa N., Iwami S., Satou Y., Matsuoka M., Ishizaka Y., Ito M., Aihara K., An D.S., Koyanagi Y. HIV-1 Vpr accelerates viral replication during acute infection by exploitation of proliferating CD4+ T cells in vivo. PLoS Pathog. 2013;9:e1003812. doi: 10.1371/journal.ppat.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roesch F., Richard L., Rua R., Porrot F., Casartelli N., Schwartz O. Vpr enhances tumor necrosis factor production by HIV-1-infected T cells. J. Virol. 2015;89:12118–12130. doi: 10.1128/JVI.02098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aukrust P., Liabakk N.B., Muller F., Lien E., Espevik T., Froland S.S. Serum levels of tumor necrosis factor-alpha (TNFα) and soluble TNF receptors in human immunodeficiency virus type 1 infection-correlations to clinical, immunologic, and virologic parameters. J. Infect. Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 77.Vaidya S.A., Korner C., Sirignano M.N., Amero M., Bazner S., Rychert J., Allen T.M., Rosenberg E.S., Bosch R.J., Altfeld M. Tumor necrosis factor alpha is associated with viral control and early disease progression in patients with HIV type 1 infection. J. Infect. Dis. 2014;210:1042–1046. doi: 10.1093/infdis/jiu206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts L., Passmore J.A., Williamson C., Little F., Bebell L.M., Mlisana K., Burgers W.A., van Loggerenberg F., Walzl G., Djoba Siawaya J.F., et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gangwani M.R., Kumar A. Multiple protein kinases via activation of transcription factors NF-κB, AP-1 and C/EBP-γ regulate the IL-6/IL-8 production by HIV-1 Vpr in astrocytes. PLoS ONE. 2015;10:e0135633. doi: 10.1371/journal.pone.0135633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roux P., Alfieri C., Hrimech M., Cohen E.A., Tanner J.E. Activation of transcription factors NF-κB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J. Virol. 2000;74:4658–4665. doi: 10.1128/JVI.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mirani M., Elenkov I., Volpi S., Hiroi N., Chrousos G.P., Kino T. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: Potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J. Immunol. 2002;169:6361–6368. doi: 10.4049/jimmunol.169.11.6361. [DOI] [PubMed] [Google Scholar]
- 82.Macreadie I.G., Arunagiri C.K., Hewish D.R., White J.F., Azad A.A. Extracellular addition of a domain of HIV-1 Vpr containing the amino acid sequence motif H(S/F)RIG causes cell membrane permeabilization and death. Mol. Microbiol. 1996;19:1185–1192. doi: 10.1111/j.1365-2958.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 83.Jacotot E., Ravagnan L., Loeffler M., Ferri K.F., Vieira H.L., Zamzami N., Costantini P., Druillennec S., Hoebeke J., Briand J.P., et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mukerjee R., Chang J.R., del V.L., Bagashev A., Gayed M.M., Lyde R.B., Hawkins B.J., Brailoiu E., Cohen E., Power C., et al. Deregulation of microRNAs by HIV-1 Vpr protein leads to the development of neurocognitive disorders. J. Biol. Chem. 2011;286:34976–34985. doi: 10.1074/jbc.M111.241547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macreadie I.G., Thorburn D.R., Kirby D.M., Castelli L.A., de Rozario N.L., Azad A.A. HIV-1 protein Vpr causes gross mitochondrial dysfunction in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:145–149. doi: 10.1016/S0014-5793(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 86.Bolton D.L., Hahn B.I., Park E.A., Lehnhoff L.L., Hornung F., Lenardo M.J. Death of CD4+ T cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J. Virol. 2002;76:5094–5107. doi: 10.1128/JVI.76.10.5094-5107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen M., Elder R.T., Yu M., O’Gorman M.G., Selig L., Benarous R., Yamamoto A., Zhao Y. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 1999;73:3236–3245. doi: 10.1128/jvi.73.4.3236-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen R., Le R.E., Kearney J.A., Mansky L.M., Benichou S. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J. Biol. Chem. 2004;279:28419–28425. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- 89.Huang M.B., Weeks O., Zhao L.J., Saltarelli M., Bond V.C. Effects of extracellular human immunodeficiency virus type 1 Vpr protein in primary rat cortical cell cultures. J. Neurovirol. 2000;6:202–220. doi: 10.3109/13550280009015823. [DOI] [PubMed] [Google Scholar]
- 90.Mansky L.M., Preveral S., Selig L., Benarous R., Benichou S. The interaction of Vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 in vivo mutation rate. J. Virol. 2000;74:7039–7047. doi: 10.1128/JVI.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamada E., Yoshikawa R., Nakano Y., Misawa N., Koyanagi Y., Sato K. Impacts of humanized mouse models on the investigation of HIV-1 infection: Illuminating the roles of viral accessory proteins in vivo. Viruses. 2015;7:1373–1390. doi: 10.3390/v7031373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reid W., Sadowska M., Denaro F., Rao S., Foulke J., Jr., Hayes N., Jones O., Doodnauth D., Davis H., Sill A., et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Villeneuve L.M., Purnell P.R., Stauch K.L., Callen S.E., Buch S.J., Fox H.S. HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J. Neurovirol. 2016;22:564–574. doi: 10.1007/s13365-016-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barrero C.A., Datta P.K., Sen S., Deshmane S., Amini S., Khalili K., Merali S. HIV-1 Vpr modulates macrophage metabolic pathways: A SILAC-based quantitative analysis. PLoS ONE. 2013;8:e68376. doi: 10.1371/journal.pone.0068376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He F., Zeng Y., Wu X., Ji Y., He X., Andrus T., Zhu T., Wang T. Endogenous HIV-1 Vpr-mediated apoptosis and proteome alteration of human T cell leukemia virus-1 transformed C8166 cells. Apoptosis. 2009;14:1212–1226. doi: 10.1007/s10495-009-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lahouassa H., Blondot M.L., Chauveau L., Chougui G., Morel M., Leduc M., Guillonneau F., Ramirez B.C., Schwartz O., Margottin-Goguet F. HIV-1 Vpr degrades the HLTF DNA translocase in T cells and macrophages. Proc. Natl. Acad. Sci. USA. 2016;113:5311–5316. doi: 10.1073/pnas.1600485113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ross J.M., Olson L., Coppotelli G. Mitochondrial and ubiquitin proteasome system dysfunction in ageing and disease: two sides of the same coin? Int. J. Mol. Sci. 2015;16:19458–19476. doi: 10.3390/ijms160819458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Durairaj G., Kaiser P. The 26S proteasome and initiation of gene transcription. Biomolecules. 2014;4:827–847. doi: 10.3390/biom4030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hollenbaugh J.A., Munger J., Kim B. Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology. 2011;415:153–159. doi: 10.1016/j.virol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sen S., Kaminiski R., Deshmane S., Langford D., Khalili K., Amini S., Datta P.K. Role of hexokinase-1 in the survival of HIV-1-infected macrophages. Cell Cycle. 2015;14:980–989. doi: 10.1080/15384101.2015.1006971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masuda F., Ishii M., Mori A., Uehara L., Yanagida M., Takeda K., Saitoh S. Glucose restriction induces transient G2 cell cycle arrest extending cellular chronological lifespan. Sci. Rep. 2016;6:19629. doi: 10.1038/srep19629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antal J., Pesti M. The dose-dependent H2O2 stress response promotes increased survival for Schizosaccharomyces pombe cells expressing HIV-1 Vpr. Folia Microbiol. 2006;51:406–412. doi: 10.1007/BF02931584. [DOI] [PubMed] [Google Scholar]
- 104.Baruchel S., Wainberg M.A. The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J. Leukoc. Biol. 1992;52:111–114. doi: 10.1002/jlb.52.1.111. [DOI] [PubMed] [Google Scholar]
- 105.Porter K.M., Sutliff R.L. HIV-1, reactive oxygen species, and vascular complications. Free Radic. Biol. Med. 2012;53:143–159. doi: 10.1016/j.freeradbiomed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Staal F.J., Ela S.W., Roederer M., Anderson M.T., Herzenberg L.A., Herzenberg L.A. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992;339:909–912. doi: 10.1016/0140-6736(92)90939-Z. [DOI] [PubMed] [Google Scholar]
- 107.Ferrucci A., Nonnemacher M.R., Cohen E.A., Wigdahl B. Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir. Virus Res. 2012;167:358–369. doi: 10.1016/j.virusres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monroy N., Herrero L., Carrasco L., Gonzalez M.E. Influence of glutathione availability on cell damage induced by human immunodeficiency virus type 1 viral protein R. Virus Res. 2016;213:116–123. doi: 10.1016/j.virusres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 109.Huang H., Zhang X., Li S., Liu N., Lian W., McDowell E., Zhou P., Zhao C., Guo H., Zhang C., et al. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010;20:1372–1385. doi: 10.1038/cr.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan E.Y., Sutton J.N., Jacobs J.M., Bondarenko A., Smith R.D., Katze M.G. Dynamic host energetics and cytoskeletal proteomes in human immunodeficiency virus type 1-infected human primary CD4 cells: Analysis by multiplexed label-free mass spectrometry. J. Virol. 2009;83:9283–9295. doi: 10.1128/JVI.00814-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palamara A.T., Perno C.F., Aquaro S., Bue M.C., Dini L., Garaci E. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Res. Hum. Retrovir. 1996;12:1537–1541. doi: 10.1089/aid.1996.12.1537. [DOI] [PubMed] [Google Scholar]
- 112.Garaci E., Palamara A.T., Ciriolo M.R., D’Agostini C., Abdel-Latif M.S., Aquaro S., Lafavia E., Rotilio G. Intracellular GSH content and HIV replication in human macrophages. J. Leukoc. Biol. 1997;62:54–59. doi: 10.1002/jlb.62.1.54. [DOI] [PubMed] [Google Scholar]
- 113.Nordgren M., Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie. 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 114.Deshmane S.L., Amini S., Sen S., Khalili K., Sawaya B.E. Regulation of the HIV-1 promoter by HIF-1α and Vpr proteins. Virol. J. 2011;8:477. doi: 10.1186/1743-422X-8-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deshmane S.L., Mukerjee R., Fan S., del Valle L., Michiels C., Sweet T., Rom I., Khalili K., Rappaport J., Amini S., et al. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia inducible factor 1 alpha expression. J. Biol. Chem. 2009;284:11364–11373. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhaskar A., Munshi M., Khan S.Z., Fatima S., Arya R., Jameel S., Singh A. Measuring glutathione redox potential of HIV-1-infected macrophages. J. Biol. Chem. 2015;290:1020–1038. doi: 10.1074/jbc.M114.588913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roshal M., Kim B., Zhu Y., Nghiem P., Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J. Biol. Chem. 2003;278:25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 118.Bartz S.R., Rogel M.E., Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elder R.T., Benko Z., Zhao Y. HIV-1 VPR modulates cell cycle G2/M transition through an alternative cellular mechanism other than the classic mitotic checkpoints. Front. Biosci. 2002;7:349–357. doi: 10.2741/elder. [DOI] [PubMed] [Google Scholar]
- 120.Lai M., Zimmerman E.S., Planelles V., Chen J. Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo. J. Virol. 2005;79:15443–15451. doi: 10.1128/JVI.79.24.15443-15451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marechal A., Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013;5:a012716. doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romani B., Baygloo N.S., Hamidi-Fard M., Aghasadeghi M.R., Allahbakhshi E. HIV-1 Vpr protein induces proteasomal degradation of chromatin-associated class I HDACs to overcome latent infection of macrophages. J. Biol. Chem. 2016;291:2696–2711. doi: 10.1074/jbc.M115.689018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dehart J.L., Zimmerman E.S., Ardon O., Monteiro-Filho C.M., Arganaraz E.R., Planelles V. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol. J. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hrecka K., Gierszewska M., Srivastava S., Kozaczkiewicz L., Swanson S.K., Florens L., Washburn M.P., Skowronski J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl. Acad. Sci. USA. 2007;104:11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Rouzic E., Belaidouni N., Estrabaud E., Morel M., Rain J.C., Transy C., Margottin-Goguet F. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle. 2007;6:182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- 126.Romani B., Shaykh B.N., Aghasadeghi M.R., Allahbakhshi E. HIV-1 Vpr protein enhances proteasomal degradation of MCM10 DNA replication factor through the Cul4-DDB1[VprBP] E3 ubiquitin ligase to induce G2/M cell cycle arrest. J. Biol. Chem. 2015;290:17380–17389. doi: 10.1074/jbc.M115.641522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao L.J., Mukherjee S., Narayan O. Biochemical mechanism of HIV-1 Vpr function. Specific interaction with a cellular protein. J. Biol. Chem. 1994;269:15577–15582. [PubMed] [Google Scholar]
- 128.Zhang S., Feng Y., Narayan O., Zhao L.J. Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein–protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001;263:131–140. doi: 10.1016/S0378-1119(00)00583-7. [DOI] [PubMed] [Google Scholar]
- 129.Wen X., Duus K.M., Friedrich T.D., de Noronha C.M. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and cullin4A containing ubiquitin ligase complex using VPRBP/DCAF1 as an adaptor. J. Biol. Chem. 2007;282:27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 130.Belzile J.P., Duisit G., Rougeau N., Mercier J., Finzi A., Cohen E.A. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 2007;3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou X., DeLucia M., Ahn J. SLX4-SLX1 orotein-independent down-regulation of MUS81-EME1 protein by HIV-1 viral protein R (Vpr) J. Biol. Chem. 2016;291:16936–16947. doi: 10.1074/jbc.M116.721183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mashiba M., Collins D.R., Terry V.H., Collins K.L. Vpr overcomes macrophage-specific restriction of HIV-1 Env expression and virion production. Cell Host Microbe. 2014;16:722–735. doi: 10.1016/j.chom.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fukumori T., Akari H., Iida S., Hata S., Kagawa S., Aida Y., Koyama A.H., Adachi A. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 1998;432:17–20. doi: 10.1016/S0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- 134.Zhu Y., Roshal M., Li F., Blackett J., Planelles V. Upregulation of survivin by HIV-1 Vpr. Apoptosis. 2003;8:71–79. doi: 10.1023/A:1021653119934. [DOI] [PubMed] [Google Scholar]
- 135.Jacotot E., Ferri K.F., El Hamel C., Brenner C., Druillennec S., Hoebeke J., Rustin P., Metivier D., Lenoir C., Geuskens M., et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vieira H.L., Haouzi D., El H.C., Jacotot E., Belzacq A.S., Brenner C., Kroemer G. Permeabilization of the mitochondrial inner membrane during apoptosis: Impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7:1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- 137.Muthumani K., Hwang D.S., Desai B.M., Zhang D., Dayes N., Green D.R., Weiner D.B. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 2002;277:37820–37831. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- 138.Rom I., Deshmane S.L., Mukerjee R., Khalili K., Amini S., Sawaya B.E. HIV-1 Vpr deregulates calcium secretion in neural cells. Brain Res. 2009;1275:81–86. doi: 10.1016/j.brainres.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Conti L., Rainaldi G., Matarrese P., Varano B., Rivabene R., Columba S., Sato A., Belardelli F., Malorni W., Gessani S. The HIV-1 Vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: Possible implications for the pathogenesis of AIDS. J. Exp. Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hilton B.A., Li Z., Musich P.R., Wang H., Cartwright B.M., Serrano M., Zhou X.Z., Lu K.P., Zou Y. ATR plays a direct antiapoptotic role at mitochondria, which is regulated by prolyl isomerase Pin1. Mol. Cell. 2015;60:35–46. doi: 10.1016/j.molcel.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kino T., Gragerov A., Kopp J.B., Stauber R.H., Pavlakis G.N., Chrousos G.P. The HIV-1 virion-associated protein Vpr is a coactivator of the human glucocorticoid receptor. J. Exp. Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vanitharani R., Mahalingam S., Rafaeli Y., Singh S.P., Srinivasan A., Weiner D.B., Ayyavoo V. HIV-1 Vpr transactivates LTR-directed expression through sequences present within −278 to −176 and increases virus replication in vitro. Virology. 2001;289:334–342. doi: 10.1006/viro.2001.1153. [DOI] [PubMed] [Google Scholar]
- 143.Wang L., Mukherjee S., Jia F., Narayan O., Zhao L.J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J. Biol. Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 144.Agostini I., Navarro J.M., Rey F., Bouhamdan M., Spire B., Vigne R., Sire J. The human immunodeficiency virus type 1 Vpr transactivator: Cooperation with promoter-bound activator domains and binding to TFIIB. J. Mol. Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 145.Sawaya B.E., Khalili K., Mercer W.E., Denisova L., Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem. 1998;273:20052–20057. doi: 10.1074/jbc.273.32.20052. [DOI] [PubMed] [Google Scholar]
- 146.Selig L., Benichou S., Rogel M.E., Wu L.I., Vodicka M.A., Sire J., Benarous R., Emerman M. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J. Virol. 1997;71:4842–4846. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bouhamdan M., Benichou S., Rey F., Navarro J.M., Agostini I., Spire B., Camonis J., Slupphaug G., Vigne R., Benarous R., et al. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J. Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schrofelbauer B., Yu Q., Zeitlin S.G., Landau N.R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 2005;79:10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schrofelbauer B., Hakata Y., Landau N.R. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc. Natl. Acad. Sci. USA. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weil A.F., Ghosh D., Zhou Y., Seiple L., McMahon M.A., Spivak A.M., Siliciano R.F., Stivers J.T. Uracil DNA glycosylase initiates degradation of HIV-1 cDNA containing misincorporated dUTP and prevents viral integration. Proc. Natl. Acad. Sci. USA. 2013;110:E448–E457. doi: 10.1073/pnas.1219702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Los Alamos HIV Sequence Database and Analysis Staff . HIV Sequence Compendium 2016. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; Los Alamos, NM, USA: [(accessed on 20 October 2016)]. Available online: https://www.hiv.lanl.gov/content/sequence/HIV/COMPENDIUM/2016compendium.html. [Google Scholar]
- 152.Barnitz R.A., Chaigne-Delalande B., Bolton D.L., Lenardo M.J. Exposed hydrophobic residues in human immunodeficiency virus type 1 Vpr helix-1 are important for cell cycle arrest and cell death. PLoS ONE. 2011;6:e24924. doi: 10.1371/journal.pone.0024924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Somasundaran M., Sharkey M., Brichacek B., Luzuriaga K., Emerman M., Sullivan J.L., Stevenson M. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc. Natl. Acad. Sci. USA. 2002;99:9503–9508. doi: 10.1073/pnas.142313699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou Y., Ratner L. Phosphorylation of human immunodeficiency virus type 1 Vpr regulates cell cycle arrest. J. Virol. 2000;74:6520–6527. doi: 10.1128/JVI.74.14.6520-6527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sherman M.P., de Noronha C.M., Pearce D., Greene W.C. Human immunodeficiency virus type 1 Vpr contains two leucine-rich helices that mediate glucocorticoid receptor coactivation independently of its effects on G(2) cell cycle arrest. J. Virol. 2000;74:8159–8165. doi: 10.1128/JVI.74.17.8159-8165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yao X.J., Subbramanian R.A., Rougeau N., Boisvert F., Bergeron D., Cohen E.A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: Role of a predicted N-terminal α-helical structure in Vpr nuclear localization and virion incorporation. J. Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jian H., Zhao L.J. Pro-apoptotic activity of HIV-1 auxiliary regulatory protein Vpr is subtype-dependent and potently enhanced by nonconservative changes of the leucine residue at position 64. J. Biol. Chem. 2003;278:44326–44330. doi: 10.1074/jbc.C300378200. [DOI] [PubMed] [Google Scholar]
- 158.Macreadie I.G., Castelli L.A., Hewish D.R., Kirkpatrick A., Ward A.C., Azad A.A. A domain of human immunodeficiency virus type 1 Vpr containing repeated H(S/F)RIG amino acid motifs causes cell growth arrest and structural defects. Proc. Natl. Acad. Sci. USA. 1995;92:2770–2774. doi: 10.1073/pnas.92.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maudet C., Bertrand M., Le R.E., Lahouassa H., Ayinde D., Nisole S., Goujon C., Cimarelli A., Margottin-Goguet F., Transy C. Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways. J. Biol. Chem. 2011;286:23742–23752. doi: 10.1074/jbc.M111.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paxton W., Connor R.I., Landau N.R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: Requirement for the p6 region of gag and mutational analysis. J. Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caly L., Saksena N.K., Piller S.C., Jans D.A. Impaired nuclear import and viral incorporation of Vpr derived from a HIV long-term non-progressor. Retrovirology. 2008;5:67. doi: 10.1186/1742-4690-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hadi K., Walker L.A., Guha D., Murali R., Watkins S.C., Tarwater P., Srinivasan A., Ayyavoo V. Human immunodeficiency virus type 1 Vpr polymorphisms associated with progressor and nonprogressor individuals alter Vpr-associated functions. J. Gen. Virol. 2014;95:700–711. doi: 10.1099/vir.0.059576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lum J.J., Cohen O.J., Nie Z., Weaver J.G., Gomez T.S., Yao X.J., Lynch D., Pilon A.A., Hawley N., Kim J.E., et al. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 2003;111:1547–1554. doi: 10.1172/JCI16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sawaya B.E., Khalili K., Rappaport J., Serio D., Chen W., Srinivasan A., Amini S. Suppression of HIV-1 transcription and replication by a Vpr mutant. Gene Ther. 1999;6:947–950. doi: 10.1038/sj.gt.3300907. [DOI] [PubMed] [Google Scholar]
- 165.Tcherepanova I., Starr A., Lackford B., Adams M.D., Routy J.P., Boulassel M.R., Calderhead D., Healey D., Nicolette C. The immunosuppressive properties of the HIV Vpr protein are linked to a single highly conserved residue, R90. PLoS ONE. 2009;4:e5853. doi: 10.1371/journal.pone.0005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tzitzivacos D.B., Tiemessen C.T., Stevens W.S., Papathanasopoulos M.A. Viral genetic determinants of nonprogressive HIV type 1 subtype C infection in antiretroviral drug-naive children. AIDS Res. Hum. Retrovir. 2009;25:1141–1148. doi: 10.1089/aid.2009.0080. [DOI] [PubMed] [Google Scholar]
- 167.Kamori D., Hasan Z., Ohashi J., Kawana-Tachikawa A., Gatanaga H., Oka S., Ueno T. Identification of two unique naturally occurring Vpr sequence polymorphisms associated with clinical parameters in HIV-1 chronic infection. J. Med. Virol. 2017;89:123–129. doi: 10.1002/jmv.24612. [DOI] [PubMed] [Google Scholar]
- 168.Dampier W., Antell G.C., Aiamkitsumrit B., Nonnemacher M.R., Jacobson J.M., Pirrone V., Zhong W., Kercher K., Passic S., Williams J.W., et al. Specific amino acids in HIV-1 Vpr are significantly associated with differences in patient neurocognitive status. J. Neurovirol. 2016 doi: 10.1007/s13365-016-0462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Collins D.R., Lubow J., Lukic Z., Mashiba M., Collins K.L. Vpr promotes macrophage-dependent HIV-1 infection of CD4+ T lymphocytes. PLoS Pathog. 2015;11:e1005054. doi: 10.1371/journal.ppat.1005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mologni D., Citterio P., Menzaghi B., Zanone P.B., Riva C., Broggini V., Sinicco A., Milazzo L., Adorni F., Rusconi S., et al. Vpr and HIV-1 disease progression: R77Q mutation is associated with long-term control of HIV-1 infection in different groups of patients. AIDS. 2006;20:567–574. doi: 10.1097/01.aids.0000210611.60459.0e. [DOI] [PubMed] [Google Scholar]
- 171.Cavert W., Webb C.H., Balfour H.H. Alterations in the C-terminal region of the HIV-1 accessory gene vpr do not confer clinical advantage to subjects receiving nucleoside antiretroviral therapy. J. Infect. Dis. 2004;189:2181–2184. doi: 10.1086/420788. [DOI] [PubMed] [Google Scholar]
- 172.Chui C., Cheung P.K., Brumme C.J., Mo T., Brumme Z.L., Montaner J.S., Badley A.D., Harrigan P.R. HIV VprR77Q mutation does not influence clinical response of individuals initiating highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 2006;22:615–618. doi: 10.1089/aid.2006.22.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jacquot G., Le R.E., Maidou-Peindara P., Maizy M., Lefrere J.J., Daneluzzi V., Monteiro-Filho C.M., Hong D., Planelles V., Morand-Joubert L., et al. Characterization of the molecular determinants of primary HIV-1 Vpr proteins: Impact of the Q65R and R77Q substitutions on Vpr functions. PLoS ONE. 2009;4:e7514. doi: 10.1371/journal.pone.0007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yedavalli V.R., Chappey C., Ahmad N. Maintenance of an intact human immunodeficiency virus type 1 vpr gene following mother-to-infant transmission. J. Virol. 1998;72:6937–6943. doi: 10.1128/jvi.72.8.6937-6943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Romani B., Glashoff R.H., Engelbrecht S. Functional integrity of naturally occurring mutants of HIV-1 subtype C Vpr. Virus Res. 2010;153:288–298. doi: 10.1016/j.virusres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 176.Miller R.H., Sarver N. HIV accessory proteins as therapeutic targets. Nat. Med. 1997;3:389–394. doi: 10.1038/nm0497-389. [DOI] [PubMed] [Google Scholar]
- 177.Gonzalez M.E. Vpu protein: The viroporin encoded by HIV-1. Viruses. 2015;7:4352–4368. doi: 10.3390/v7082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ran X., Ao Z., Yao X. Apobec3G-based strategies to defeat HIV infection. Curr. HIV Res. 2016;14:217–224. doi: 10.2174/1570162X14999160224100541. [DOI] [PubMed] [Google Scholar]
- 179.Smithgall T.E., Thomas G. Small molecule inhibitors of the HIV-1 virulence factor, Nef. Drug Discov. Today Technol. 2013;10:523–529. doi: 10.1016/j.ddtec.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weydert C., Rijck J.D., Christ F., Debyser Z. Targeting virus-host interactions of HIV replication. Curr. Top. Med. Chem. 2016;16:1167–1190. doi: 10.2174/1568026615666150901115106. [DOI] [PubMed] [Google Scholar]
- 181.Poon B., Grovit-Ferbas K., Stewart S.A., Chen I.S. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science. 1998;281:266–269. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- 182.Gavegnano C., Schinazi R.F. Antiretroviral therapy in macrophages: Implication for HIV eradication. Antivir. Chem. Chemother. 2009;20:63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumar A., Herbein G. The macrophage: A therapeutic target in HIV-1 infection. Mol. Cell Ther. 2014;2:10. doi: 10.1186/2052-8426-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Serio D., Rizvi T.A., Cartas M., Kalyanaraman V.S., Weber I.T., Koprowski H., Srinivasan A. Development of a novel anti-HIV-1 agent from within: Effect of chimeric Vpr-containing protease cleavage site residues on virus replication. Proc. Natl. Acad. Sci. USA. 1997;94:3346–3351. doi: 10.1073/pnas.94.7.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Poon B., Jowett J.B., Stewart S.A., Armstrong R.W., Rishton G.M., Chen I.S. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J. Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kamata M., Wu R.P., An D.S., Saxe J.P., Damoiseaux R., Phelps M.E., Huang J., Chen I.S. Cell-based chemical genetic screen identifies damnacanthal as an inhibitor of HIV-1 Vpr induced cell death. Biochem. Biophys. Res. Commun. 2006;348:1101–1106. doi: 10.1016/j.bbrc.2006.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Win N.N., Ito T., Matsui T., Aimaiti S., Kodama T., Ngwe H., Okamoto Y., Tanaka M., Asakawa Y., Abe I., et al. Isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorg. Med. Chem. Lett. 2016;26:1789–1793. doi: 10.1016/j.bmcl.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 188.Shimura M., Zhou Y., Asada Y., Yoshikawa T., Hatake K., Takaku F., Ishizaka Y. Inhibition of Vpr-induced cell cycle abnormality by quercetin: A novel strategy for searching compounds targeting Vpr. Biochem. Biophys. Res. Commun. 1999;261:308–316. doi: 10.1006/bbrc.1999.0994. [DOI] [PubMed] [Google Scholar]
- 189.Watanabe N., Nishihara Y., Yamaguchi T., Koito A., Miyoshi H., Kakeya H., Osada H. Fumagillin suppresses HIV-1 infection of macrophages through the inhibition of Vpr activity. FEBS Lett. 2006;580:2598–2602. doi: 10.1016/j.febslet.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 190.Kino T., Kopp J.B., Chrousos G.P. Glucocorticoids suppress human immunodeficiency virus type-1 long terminal repeat activity in a cell type-specific, glucocorticoid receptor-mediated fashion: Direct protective effects at variance with clinical phenomenology. J. Steroid Biochem. Mol. Biol. 2000;75:283–290. doi: 10.1016/S0960-0760(00)00187-4. [DOI] [PubMed] [Google Scholar]
- 191.Refaeli Y., Levy D.N., Weiner D.B. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc. Natl. Acad. Sci. USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yao X.J., Lemay J., Rougeau N., Clement M., Kurtz S., Belhumeur P., Cohen E.A. Genetic selection of peptide inhibitors of human immunodeficiency virus type 1 Vpr. J. Biol. Chem. 2002;277:48816–48826. doi: 10.1074/jbc.M207982200. [DOI] [PubMed] [Google Scholar]
- 193.Suzuki T., Yamamoto N., Nonaka M., Hashimoto Y., Matsuda G., Takeshima S.N., Matsuyama M., Igarashi T., Miura T., Tanaka R., et al. Inhibition of human immunodeficiency virus type 1 (HIV-1) nuclear import via Vpr-importin alpha interactions as a novel HIV-1 therapy. Biochem. Biophys. Res. Commun. 2009;380:838–843. doi: 10.1016/j.bbrc.2009.01.180. [DOI] [PubMed] [Google Scholar]
- 194.Hagiwara K., Ishii H., Murakami T., Takeshima S.N., Chutiwitoonchai N., Kodama E.N., Kawaji K., Kondoh Y., Honda K., Osada H., et al. Synthesis of a Vpr-binding derivative for use as a novel HIV-1 inhibitor. PLoS ONE. 2015;10:e0145573. doi: 10.1371/journal.pone.0145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hagiwara K., Murakami T., Xue G., Shimizu Y., Takeda E., Hashimoto Y., Honda K., Kondoh Y., Osada H., Tsunetsugu-Yokota Y., et al. Identification of a novel Vpr-binding compound that inhibits HIV-1 multiplication in macrophages by chemical array. Biochem. Biophys. Res. Commun. 2010;403:40–45. doi: 10.1016/j.bbrc.2010.10.107. [DOI] [PubMed] [Google Scholar]
- 196.Ong E.B., Watanabe N., Saito A., Futamura Y., Abd El Galil K.H., Koito A., Najimudin N., Osada H. Vipirinin, a coumarin-based HIV-1 Vpr inhibitor, interacts with a hydrophobic region of Vpr. J. Biol. Chem. 2011;286:14049–14056. doi: 10.1074/jbc.M110.185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mamik M.K., Hui E., Branton W.G., McKenzie B.A., Chisholm J., Cohen E.A., Power C. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: Implications for HIV-1 associated neuroinflammation. J. Neuroimmune Pharmacol. 2016 doi: 10.1007/s11481-016-9708-3. [DOI] [PubMed] [Google Scholar]
- 198.Kitayama H., Miura Y., Ando Y., Hoshino S., Ishizaka Y., Koyanagi Y. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J. Virol. 2008;82:2528–2542. doi: 10.1128/JVI.02094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.James T., Nonnemacher M.R., Wigdahl B., Krebs F.C. Defining the roles for Vpr in HIV-1-associated neuropathogenesis. J. Neurovirol. 2016;22:403–415. doi: 10.1007/s13365-016-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]