Abstract
Current research has demonstrated that mitochondrial morphology, distribution, and function are maintained by the balanced regulation of mitochondrial fission and fusion, and perturbation of the homeostasis between these processes has been related to cell or organ dysfunction and abnormal mitochondrial redistribution. Abnormal mitochondrial fusion induces the fragmentation of mitochondria from a tubular morphology into pieces; in contrast, perturbed mitochondrial fission results in the fusion of adjacent mitochondria. A member of the dynamin family of large GTPases, dynamin-related protein 1 (Drp1), effectively influences cell survival and apoptosis by mediating the mitochondrial fission process in mammals. Drp1-dependent mitochondrial fission is an intricate process regulating both cellular and organ dynamics, including development, apoptosis, acute organ injury, and various diseases. Only after clarification of the regulative mechanisms of this critical protein in vivo and in vitro will it set a milestone for preventing mitochondrial fission related pathological processes and refractory diseases.
Keywords: dynamin-related protein 1, mitochondria, fission, fusion, mammal
1. Introduction
The mitochondrion is the main organelle for producing adenosine triphosphate (ATP) and acts as a regulator in synthesis of metabolites, phospholipids, heme, and intracellular calcium homeostasis [1]. Imbalanced mitochondrial fission and fusion always lead to mitochondrial structural changes and dysfunction, thus it is critical to develop new methods for conserving the balance between mitochondrial fission and fusion in mammals. Abnormal mitochondrial fusion induces the fragmentation of mitochondria from a tubular morphology into pieces; in contrast, perturbed mitochondrial fission results in the fusion of adjacent mitochondria [2,3]. As a main regulator in mitochondrial fission process, Dynamin-related protein 1 (Drp1) in mammals consists of four different domains: the N-terminal GTP-binding, middle, insert B, and C-terminal GTPase effector (GED) domains [4]; insert B (also known as the variable domain) plays a critical role in the regulative process of mitochondrial fission since it binds the target membrane effectively [4]. This functional protein is termed Dnm1p in yeast but Drp3A/B in plants [5,6]. Two morphologically distinct multimers of Drp1 coexist under physiological conditions in vitro and in vivo; dimers reorganize Drp1, thus resulting in remodeling of the mitochondrial membrane, whereas multimers promote Drp1 GTPase activity and induce mitochondrial fission [7]. Intriguingly, loss of Drp1 triggers genome instability and initiates DNA damage response by disrupting the mitochondrial division and distribution [8]; disturbance consequently results in the reduction of mitochondrial membrane potential and the mitochondrial electron transport chain for energy production [9]. This review mainly focuses on the detailed mechanisms and machinery of Drp1-dependent mitochondrial fission, and we can conclude that Drp1-dependent mitochondrial fission is an intricate process for regulating cellular and organ dynamics, including development, apoptosis, acute organ injury, and various diseases in mammals. In this way, it will provide guidance for regulation of mitochondrial fission in various pathological processes and diseases.
2. The Detailed Regulatory Mechanisms of Dynamin-Related Protein 1 (Drp1)-Dependent Mitochondrial Fission
Drp1-dependent mitochondrial fission can be divided into four steps: translocation of Drp1 to the mitochondrial outer membrane (MOM), subsequent higher-order assembly, GTP hydrolysis, and ultimately disassembly [10]. Drp1 binds to receptors in the MOM and forms a functional complex, and a larger oligomer can subsequently be assembled and transported from the cytoplasm to the fission sites [11]. In addition, the endoplasmic reticulum (ER) helps transfer Ca2+ into the mitochondria, resulting in recruitment of Drp1 to the mitochondrial surface [12]. However, reactive oxygen species (ROS) can also be upstream initiators of mitochondrial fission, in which case Drp1 can be activated through Cdk1, PKCδ, and calcineurin-mediated pathways [13]. When Drp1 is activated accompanied with reduced diameters in mitochondria, the mitochondrion divides unevenly and generates two daughter organelles with unequal membrane potential [14], while the lengths of Drp1 helical rings along the mitochondria are not significantly altered [15].
In general, when ER starts to encircle the mitochondria, the mitochondria immediately divide and mitochondrial fission factor (Mff)-dependent Drp1 assembly is subsequently initiated [16]. Although there are various isoforms of Drp1 generated by selective splicing of pre-mRNA transcripts, Mff effectively and differentially regulates the activities of Drp1 in a cooperative GTPase-dependent pathway [11]. Mff knockdown has been shown to induce mitochondrial elongation in mammalian cells, whereas overexpression of Mff recruits Drp1 for mitochondrial fragmentation [17]. Premature self-assembly of Drp1 impairs its interactions with Mff, and the abundance of Drp1–Mff heterodimers is significantly increased after removal of the insert B domain of Drp1 [18]. There are two vital receptors in Drp1-dependent mitochondrial fission: mitochondrial elongation factor 1 (MiD51) binds to an adenosine diphosphate (ADP) co-factor, whereas mitochondrial elongation factor 2 (MiD49) physically recruits Drp1 to the mitochondrial surface [19]. However, MiD51 has been demonstrated to promote the recruitment of Drp1 without regulatory elements, such as Mff, mitochondrial fission 1 (Fis1), and mitofusion 2 (Mfn2) [20]. In addition, MiD49 activity has been demonstrated to be more indispensable than Mff and Fis1 [21]. There is still controversy surrounding the function of human Fis1, which uniformly localizes throughout the MOM and improves the activity of Drp1 by competitively binding to MiD51 [22]. Mai et al. have shown that Fis1 knockdown increases the interconnectivity of mitochondrial tubules, whereas overexpression increases the mitochondrial fragmentation [23]. In contrast, Osellame et al. have demonstrated that Drp1 activity is not influenced by silencing or overexpression of human Fis1 [24].
In addition to the regular process of mitochondrial fission, there are a variety of alternative pathways or elements for regulation of Drp1-dependent fission. GTP hydrolysis and Drp1 activity are restrained in mitochondrial division after Drp1 recognizes the head group of phosphatidic acid and two saturated acyl chains of another phospholipid found in the MOM [25]. Drp1 and cardiolipin cooperatively promote membrane constriction and prime fission through co-localization and self-assembly at the membrane bilayer [26]. Actin filaments are able to bind purified Drp1 and interact with Mff, thereby increasing the GTPase activity, whereas inhibiting actin polymerization significantly reduces Drp1-dependent mitochondrial fission [27]. Inhibition of the receptor-interacting protein kinase 1 decreases the level of c-Jun NH2-terminal kinase activation and effectively abrogates Drp1 translocation [28]. Although ganglioside-induced differentiation-associated protein 1 induces mitochondrial fragmentation, loss of Drp1 effectively rescues the impairment [29]. Through a similar pathway, loss of Drp1 also protects against Tyrphostin A9-induced mitochondrial filament fragmentation [30].
For better clarification of the regulative mechanisms in mitochondrial fission, we need to have a superficial cognition in the opposite direction of Drp1-dependent mitochondrial fission. During the mitochondrial fusion process, the membranes of two adjacent mitochondria fuse through the formation of hetero- and homo-oligomeric protein complexes [31]. Although the MOM is fused independently of the mitochondrial inner membrane (MIM), they together co-regulate the mitochondrial redistribution progress. Mitochondrial fusion proteins including Mfn1 and Mfn2 are located on the MOM, whereas optic atrophy 1 (Opa1) is located on the MIM, and all three proteins cooperatively regulate mitochondrial morphology and function [32]. Even though overexpression of Mfn1 and Mfn2 is not able to restore the mitochondrial fragmentation and reduce cell viability [33], ubiquitylation of Mfn1 plays critical roles in enhancing cell survival rates under stress conditions [34]. Disassembly of Opa1 oligomers induces neither the remodeling of mitochondrial cristae nor the release of cytochrome c [35,36]. However, Opa1 stabilizes mitochondrial structure and increases mitochondrial respiratory efficiency, thereby eliminating mitochondrial dysfunction and cytochrome c release in ischemic environments [37].
3. Post-Translational Modifications of Drp1 in Mitochondrial Fission
Post-translational modifications of Drp1 predominantly include phosphorylation, S-nitrosylation, SUMOylation, ubiquitination, and O-GlcNAcylation [10]. Because Drp1 phosphorylation is initiated at several sites, it takes part in different mechanisms of various pathological processes.
For better understanding post-translational phosphorylation of Drp1 at different sites, the correlated mechanisms in different mammalian cells or tissues for mitochondrial fission are demonstrated in Table 1. In general, phosphorylation at Ser-637 inhibits Drp1 activity while phosphorylation at Ser-616 activates Drp1 activity; some specific upstream kinases also influence Drp1 function [38]. Moreover, phosphorylation of Drp1 at the same residue is likely to have opposite effects on the mitochondrial fission progress; this may depend on external parameters including cell type, age or status, or on internal parameters. For example, Ca2+/calmodulin dependent protein kinase Iα promotes phosphorylation of Drp1 at Ser-637 and increases Drp1 translocation to mitochondria [39]. In contrast, cAMP-dependent protein kinase phosphorylate Drp1 at the same residue effectively inhibits Drp1 activity in a cardiac ischemic injury model [40]. A kinase anchoring protein 1 (AKAP1) promotes phosphorylation of Ser-637, thereby inducing mitochondrial network extension, whereas dephosphorylation of Ser-637 results in programmed necrosis [41,42]. AKAP1 is a scaffold protein that recruits protein kinase A (PKA) and integrates several second messenger cascades to modulate mitochondrial function and associated physiological and pathophysiological outcomes [43]. In addition, Ser-616 can also be phosphorylated by several kinases including the cyclin-dependent kinase (CDK) family [44], ERK1/2 [45], and PKCδ [13]. Adenosine monophosphate-activated protein kinase (AMPK) activation alters the state of Drp1 phosphorylation, and suppresses ER stress and inflammatory cytokine release [46]. PKA suppresses Drp1-dependent mitochondrial fission at different sites including phosphorylation at site Ser-637 or dephosphorylation at site Ser-616 [38,47], while cyclin-B-dependent kinase promoting the phosphorylation of Drp1 at Ser-616 does not directly affect GTPase activity, but facilitates the increasing of mitochondrial fragmentation [48]. The PKA/AKAP1 signaling pathway and protein phosphatase 2A (PP2A)/Bβ2 signaling pathway exert opposite effects on the phosphorylation of Drp1 at the Ser-656 site: PKA/AKAP1 promotes the phosphorylation of Drp1-mediated dendrite occupancy and enhances dendritic outgrowth but paradoxically decreases the synapse number and density, while PP2A/Bβ2 promotes dephosphorylation of Drp1-mediated depolarized mitochondria to cease dendritic outgrowth but augment synapse formation [49]. There are also other phosphorylation sites for regulation of Drp1 activity. Oxidative stress leads to mitochondrial fragmentation by promoting phosphorylation of Ser-579 in human Drp1 isoform 3 [50]; glycogen synthase kinase (GSK)3β-mediated phosphorylation at Ser-693 dramatically decreases GTPase activity but does not affect Drp1 inter- or intra-molecular interactions [51].
Table 1.
Phosphorylated dynamin-related protein 1 (Drp1) at different sites and the correlated regulative ways for mitochondrial fission in mammals in vivo and in vitro.
| Cell Type | Stimuli | Sites | Regulation | Effects | References |
|---|---|---|---|---|---|
| Cardiomyocytes | Anoxia-reoxygenation injury | Ser-616 | Up-regulation of ROS production and mitochondrial fission | [13] | |
| Cardiomyocytes | Anoxia-reoxygenation injury | Ser-637 | Up-regulation of ROS production and mitochondrial fission | [13] | |
| Vascular smooth muscle cell | Glucagon-like peptide-1 | Ser-637 | Stimulation of mitochondrial fusion and inhibition of vascular smooth muscle cell dedifferentiation | [39] | |
| Hippocampal neurons | Ca2+-dependent protein kinase Iα | Ser-600 | Mitochondrial fragmentation | [39] | |
| Mouse podocytes and endothelial cells | Hyperglycemia | Ser-600 | Recruitment of Drp1 | [52] | |
| Human podocytes and endothelial cells | Hyperglycemia | Ser-637 | Recruitment of Drp1 | [52] | |
| STAT2-deficient patient derived fibroblasts | Lentiviral transduction with wild-type STAT2 | Ser-616 | Maintenance of mitochondrial length | [53] | |
| Pulmonary vessels | Cdk1/cyclin B | Ser-616 | Pulmonary arterial remodeling | [54] | |
| Smooth muscle cells | Angiotensin II or hydrogen peroxide | Ser-616 | Proliferation and migration | [55] | |
| Human skeletal muscle cells | Aerobic exercise | Ser-616 | Up-regulation of fat oxidation and insulin sensitivity | [56] | |
| Cardiomyocytes | Pim-1 | Ser-637 | Maintenance of a reticular mitochondrial phenotype under ischemia condition | [57] | |
| Cardiac myocytes | Dominant-negative forkhead box O3a | Ser-637 | Up-regulation of maladaptive cardiac atrophy genes | [58] | |
| Cardiac myocytes | FK506 treatment prior to IR | Ser-637 | Preservation of cardiac function | [59] | |
| Post-mitotic neurons | Cyclin-dependent kinase 5 | Ser-616 | Modulation of mitochondrial morphology | [60] | |
| Neural cells | Mild hypothermia | Ser-616 | Preservation of neural cells integrity | [61] | |
| Neural cells | PTEN-induced putative kinase 1 | Ser-616 | Neuronal survival | [62] | |
| Neuronal cell | Nanoceria | Ser-616 | Reduction of ROS, protein tyrosine nitration, endogenous peroxynitrite and cell death rates | [63] | |
| Hippocampal cells | Wnt-5a | Ser-616 | Up-regulation of intracellular and mitochondrial calcium | [64] | |
| Hippocampal cells | Wnt-5a | Ser-637 | Up-regulation of intracellular and mitochondrial calcium | [64] | |
| HeLa cells | Depletion of death associated protein 3 | Ser-637 | Increased apoptotic sensitivity | [65] | |
| T-cell acute lymphoblastic leukemia cells | Mesenchymal stem cell co-culture | Ser-616 | Maintenance of mitochondrial dynamics, mitochondrial ROS levels, metabolic switching and chemoresistance | [66] |
Down-regulation; , Up-regulation; FK506, tacrolimus; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; IR, ischemia–reperfusion.
After establishing the basic functions of different phosphorylated sites as mentioned above, multiple studies focus on how the phosphorylation of Drp1 at different sites influences related pathological processes in vivo and in vitro. In diabetes animal models or patients, hyperglycemia stimulates the recruitment of Drp1 in podocytes and endothelial cells by directly facilitating the phosphorylation of Drp1 at Ser-600 in mice or Ser-637 in humans [52]. In smooth muscle cell, cdk1/cyclin B mediates the phosphorylation of Ser-616 and leads to pulmonary arterial remodeling [54]; glucagon-like peptide-1 stimulates mitochondrial fusion but decreases vascular smooth muscle cell dedifferentiation through enhanced phosphorylation of Drp1 at Ser-637 site [39]. Dominant-negative forkhead box O3a enhanced phosphorylation of Drp1 at the Ser-637 site and upregulated maladaptive cardiac atrophy genes in cardiac myocytes via modulation of calcium homeostasis-mediated mitochondrial apoptosis and autophagy [58]. In anoxia–reoxygenation injury-induced cardiomyocytes, ROS production, mitochondrial fission, and phosphorylation of Drp1 Ser-616 are increased but are accompanied by the downregulation of phosphorylated Ser-637 [13]. Although angiotensin II or hydrogen peroxide induce smooth muscle cells to proliferate and migrate, decreasing the phosphorylation of Drp1 at Ser-616 neutralizes these effects [55]. Furthermore, Ca2+-dependent protein kinase Iα promotes the phosphorylation of Ser-637 in human Drp1 isoform 1, but also promotes the phosphorylation of Ser-600 in human Drp1 isoform 3, thus consequently inducing mitochondrial fragmentation in cultured hippocampal neurons [39]. Wnt-5a upregulates intracellular and mitochondrial calcium, thus treated hippocampal cells demonstrate increased phosphorylation of Drp1 Ser-616 but decreased phosphorylation of Drp1 at Ser-637 [64]. As a chemical inhibitor of cyclin-dependent kinases, roscovitine increased oligomerization and mitochondrial translocation of Drp1; in contrast, Cyclin-dependent kinase 5 modulates mitochondrial morphology via inhibitory phosphorylation of Drp1 Ser-616 in post-mitotic neurons in vitro [60]. All these regulators help to modulate Drp1 through phosphorylation at different sites and change the status of cells in vitro or tissue function in vivo. As an important step in the regulation of Drp1, SUMOylation stimulate stable binding of Drp1 to the MOM, and its activity is significantly affected [67]. SUMOylation of Drp1 activates the ER-mediated calcium flux, the remodeling of mitochondrial cristae, and the release of cytochrome c [68]. When Drp1 ubiquitylation and proteasomal degradation are inhibited during interphase, synthesis of cytokines is diminished, and unequal mitochondrial distributions occur rapidly [69]. The overexpression of mitochondrial SUMO E3 ligase substantially promotes mitochondrial fission [70], whereas ectopic expression of Sentrin/SUMO-specific protease (SENP)-5 plays opposite roles by promoting the deSUMOylation of Drp1 [71,72]. Furthermore, loss of SENP3 prolongs Drp1 SUMOylation and suppresses cell apoptosis [73].
When the activity of N-acetyl-glucosaminidase is ablated, O-GlcNAcylation of Drp1 at Thr-585 and Thr-586, within the variable domain, is significantly enhanced and consequently increases mitochondrial fragmentation in cardiomyocytes [74]. In addition, S-nitrosylation is activated within the GED domain at Cys644, and then upregulates the production of Drp1 oligomers and GTPase activity in neurons, thereby leading to the pathology of Huntington’s disease [64]. However, this conclusion is controversial, because Bossy et al. have demonstrated that S-nitrosylation of Drp1 exerts no influence on Alzheimer’s disease and does not induce oligomerization of Drp1 [75].
4. Drp1-Dependent Mitochondrial Fission and Development In Vivo and In Vitro
Increasing numbers of studies show that Drp1-dependent mitochondrial fission influences the growth and health of cells, from primordial germ cells to functional terminal cells, in vivo and in vitro (Table 2). Aged mice demonstrate reduced Drp1 activity and defective organelle morphogenesis in oocytes, mainly through impaired Ca2+ signaling and intercellular communication [76]. In the muscle tissue of these mice, although there is no difference in the expression levels of mitochondrial fission and fusion proteins, the Mfn2-to-Drp1 ratio is significantly increased, and the intermyofibrillar mitochondria are longer and more branched [77]. It was also showed that loss of Drp1 induced the death of mice by embryonic day 12.5 [78]; cardiac-specific Drp1 knockout (KO) mice had impaired left ventricular function and died within 13 weeks through suppression of autophagic flux [79]. As a Drp1 peptide inhibitor, P110 is neuroprotective by inhibiting the p53-mediated apoptotic pathways in neurons of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) animal models [80]. Drp1-null mice failed to undergo developmentally regulated apoptosis during neural tube formation in vivo and died at embryonic day 11.5, although without altered intracellular ATP levels in these models [81]. On the other hand, overexpression of Drp1 in a transgenic mouse line results in inhibition of the growth hormone pathway and postnatal muscle growth, as well as reduced mitochondrial DNA (mtDNA) quantity [82].
Table 2.
Drp1-dependent mitochondrial fission and development in vivo and in vitro.
| Basal Background | Treatments for Drp1 | Effects | Targets | Species | References |
|---|---|---|---|---|---|
| In vivo | Loss | Impairment of Ca2+ signaling and intercellular communication | Aged oocytes | Mouse | [76] |
| Unaltered | Significant increase in the Mfn2-to-Drp1 ratio; longer and more branched intermyofibrillar mitochondria | Aged muscles | Mouse | [77] | |
| Loss | Death | Mouse at day 12.5 during embryonic period | Mouse | [78] | |
| Loss | Left ventricular dysfunction and lethal heart defects | Cardiomyocytes | Mouse | [79] | |
| Inhibition | Inhibit the p53 mediated apoptotic pathways | Neurons in MPTP animal model | Mouse | [80] | |
| Cardiac-specific loss | Impair left ventricular function and lead to death within 13 weeks | Cardiomyocytes | Mouse | [79] | |
| Loss | Impair neural tube formation and lead to death at embryonic day 11.5 | Neural cells | Mouse | [81] | |
| Overexpression | Impair postnatal muscle growth and reduce mtDNA quantity and the growth hormone pathway | Muscle | Transgenic mouse line | [82] | |
| In vitro | Loss | Negatively influence terminal differentiation, particularly in the neurogenetic differentiation | ESCs | Mouse | [83] |
| Loss | Augmentation of the cyclin E pool for attenuating cell proliferative rates | Embryonic fibroblasts at low density | Mouse | [84] | |
| Loss | Aberrant cell proliferation | Embryonic fibroblasts at high density | Mouse | [84] | |
| Loss | Impair myogenic differentiation potency | Myogenic precursor cells | Mouse | [85] | |
| Loss | Decrease in aerobic metabolism, calcium flux and proliferation | Ductal smooth muscle cells | Rabbit | [86] | |
| Loss | Increase mitochondrial length and lead to cell death | Cortical neurons | Mouse | [87] |
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; ESCS, embryonic stem cells.
Mitochondria, the dynamic energy powerhouses of the cell, play vital roles in a multitude of cellular processes, including differentiation and cell survival [88]. In addition, growth factor erv1-like (Gfer) helps maintain pluripotency in murine ESCs, and preserves survival ability via the modulation of Drp1 [88]. To further clarify the mechanisms of the Gfer-mediated mitochondrial fission process, Todd et al. studied knockdown Gfer in ESCs and found that the expression levels of pluripotency marker, embryoid body formation, and cell survival all decreased [89]. Although knockdown of Drp1 does not affect mitochondrial function, proliferation ,and pluripotency of ESCs [83], Drp1 knockdown negatively influences terminal differentiation of ESCs, particularly in neurogenetic differentiation by downregulation of pluripotency-associated genes, although it is not critical for mitochondria biogenesis for ESC proliferation [83]. In addition, the loss of Drp1 also augments the cyclin E pool, thus attenuating cell proliferative rates in mouse embryonic fibroblasts (MEFs) seeded at low density. However, these cells exhibit aberrant proliferation when they are seeded at high density [84]. The dominant-negative form also helps to rescue the impaired myogenic differentiation potency in myogenic precursor cells [85]. Through inhibition of cyclin-dependent kinase-mediated Drp1 activation, mdivi-1 decreases aerobic metabolism, calcium flux, and proliferation of ductal smooth muscle cells [86]. Drp1 is demonstrated to be required for cell survival since inhibition of Drp1 significantly increased mitochondrial length and caused cell death in cortical neurons [87].
5. Drp1-Dependent Mitochondrial Fission and Apoptosis
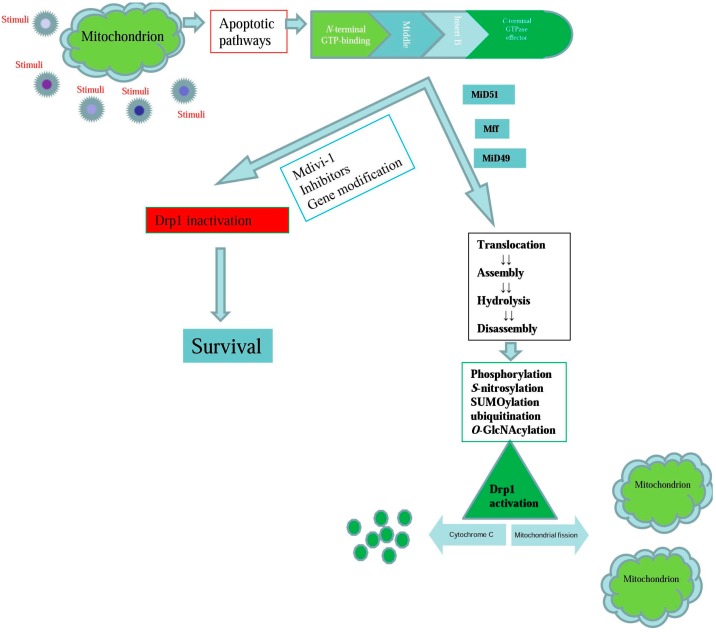
After Drp1-dependent mitochondrial fission is altered directly or indirectly, apoptosis can be altered through different signaling pathways (Figure 1). Various stress conditions induce the translocation of Drp1, thus rapidly resulting in excessive mitochondrial fragmentation and concomitant apoptosis [90]. The apoptosis-related signals are transferred to the ER, and a complex with Bap31 is formed at the interface of the ER and mitochondria [91]. Human Fis1 is also involved in the apoptosis signaling pathway by transporting apoptotic signals to the ER [91]. During apoptosis, the cooperation of Bax and fission-related proteins regulates the mitochondrial fission progress [92]. Mitochondrial fission often occurs adjacent to nucleoids, and the clustering of nucleoids often leads to the formation of enlarged or elongated mitochondria; consequently, cytochrome c release is suppressed under apoptotic stimuli [93]. Despite the above relationship between apoptosis and Drp1-dependent mitochondrial fission, Bik stimulation leads to mobilization of intra-mitochondrial cytochrome c and cooperatively activates Bax independently of Drp1 activity [94].
Figure 1.
Various stress conditions induce the initiation of apoptotic pathways, mitochondrial fragmentation and concomitant apoptosis. Then Drp1-dependent mitochondrial fission is mediated by translocation of Drp1 to the mitochondrial outer membrane (MOM), subsequent higher-order assembly, GTP hydrolysis, and ultimately disassembly. The effective formation of Drp1 is regulated through post-translational modifications, which results in cytochrome c release and mitochondrial fragmentation. However, interferences inhibit the mitochondrial fission pathway and improve survival rates, even under stressful conditions. Mff, mitochondrial fission factor; MiD51, mitochondrial elongation factor 1; MiD49, mitochondrial elongation factor 2.
Since there are various routes to regulate Drp1 activity directly or indirectly, these treatments highly influence the apoptotic outcomes. Pseudo-phosphorylation of Drp1 improves the resistance to various pro-apoptotic elements; in contrast, dephosphorylation of Drp1 significantly increases cell vulnerability [95]. Inhibition of Drp1 also prevents the release of soluble Opa1, thereby affecting mitochondrial cristae remodeling and cytochrome c release [36]. Although mdivi-1 is demonstrated to exert strong cytoprotective effect on various cell types in vivo and in vitro, it also takes part in resisting proliferative and cytotoxic effects in tumors and immortalized cells via reducing cell mitosis and enhancing cell apoptosis [96]; the cytotoxic effect of mdivi-1 is also correlated with the Bax/Bak pathway in MEFs [96]. Mdivi-1 extensively elongates the mitochondria and increases the apoptosis rate, although Drp1 phosphorylation at Ser-637 is gradually decreased in differentiated myoblasts [97]. Because of the essential role of Bax in cytochrome c release, loss of Bax absolutely inhibits Drp1-dependent mitochondrial fission and decreases the apoptosis rate [98]. However, whereas mitofusins and the loss of Drp1 inhibit Bax insertion and activation, they are not able to affect the translocation of Bax to mitochondria [99]. Depletion of death-associated protein 3, which is specifically located in the matrix of mitochondria, dramatically decreases the phosphorylation of Drp1 at Ser-637 and leads to inhibited autophagy and increased apoptotic sensitivity in cells [65]. ABT-737, a non-peptidic Bcl2/X(L) inhibitor, decreases oxygen consumption rates (OCRs) and improves the release of cytochrome c; although Drp1 KO MEFs are less sensitive than wild-type MEFs, the expression of Bax and the related apoptosis rates are unaltered [100]. Astaxanthin markedly promotes myofibroblast apoptosis by upregulating the levels of Drp1 and apoptosis-associated genes, including Bcl2 and p53 [101]. The suppressor of cytokine signaling 6 mediates apoptosis by forming a complex with Drp1 and phosphoglycerate mutase 5 (PGAM5) and attenuating phosphorylation of Drp1 [102]. Both Debcl and Drp1 are downstream of Buffy in the Jun Kinase pathway and trigger an enhanced ROS production and mitochondrial fragmentation during Rbf1-induced apoptosis [103]. Betanodavirus B2 increases the production of hydrogen peroxide (H2O2) and leads to cell death in vitro and in vivo via Drp1 activation [104]. Although Bcl2/adenovirus E1B interacting protein 1 increase Drp1 translocation, this effect is fully abrogated by Bcl2 overexpression [105]. Apoptosis signaling pathways are correlated with the regulation of mitochondrial morphology, and the inhibition or promotion of Drp1 activity effectively influences the effects of apoptotic stimuli in vivo and in vitro.
6. Drp1-Dependent Mitochondrial Fission and Pathological Processes
Because Drp1 participates in various important physiological processes in mammals, the altered expression of this protein significantly affects the results of the related processes. Especially in a variety of ischemia or ischemia-reperfusion (IR) models in vivo and in vitro, for example, inhibition of Drp1 selectively blocks mitophagy without affecting mitochondrial biogenesis, but Drp1-dependent mitophagy is triggered to remove damaged mitochondria during the early phase of ischemic-induced injury [106]. Under inhibition of Drp1 with Drp1-K38A, transduced cardiomyocytes show a significant decrease of OCRs with an inconspicuous alteration of ATP production after IR [107]. In IR-induced cardiomyocytes, mdivi-1 preserves the mitochondrial structure and significantly reduces the myocardial infarction area [54,108]. Tacrolimus (FK506) treatment prior to ischemia-reperfusion (IR) prevented the specific dephosphorylation and preserved cardiac function via reduction of ROS, improvement of left ventricular developed pressure and lowered left ventricular end diastolic pressure in adult rat hearts [59]. Pim-1 activity inhibits Drp1 compartmentalization and protects against the disturbance of mitochondrial morphology by increasing phosphorylation at Ser-637 in response to simulated ischemia cardiomyocytes [109]. Drp1 inhibition by mdivi-1 not only resists IR-induced injury in the heart but also protects other somatic cells from IR injury by blocking the mitochondrial apoptosis pathway [110,111]. Furthermore, necrostatin-1 inhibits cell death in the ischemia-injured rat tubular cell line [112]. Although IR induced injury significantly activates phosphorylated Drp1 at Ser-616, mild hypothermia suppresses the alteration and preserves neural cells integrity for reducing rates of neuronal necrosis and apoptosis [61]. In addition, an agonist of peroxisome proliferator-activated receptor-γ decreases phosphorylation of Drp1 and demonstrates resistance against neuronal IR-induced injury [113]. Through attenuating mitochondrial translocation of Drp1, PTEN-induced novel kinase 1 (PINK1) significantly ameliorates cell death and inhibits the ischemia-induced mitochondrial fission in neurons [62]. Furthermore, mdivi-1 significantly attenuated neurotoxicity and pre-existing deficits in PTEN-induced putative kinase-1 deletion and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse models [114].
After incubation of retinal ganglion (RGC)-5 cells under high hydrostatic pressure for several days in vitro, mitochondrial cristae depletion, ATP reduction and Drp1 translocation are triggered for mitochondrial fission, accompanied by irreversible functional impairments [115]. These alterations have also been observed in glaucomatous mice, whereas the suppression of this progress rescues impaired retinas by maintaining mitochondrial integrity [116]. Cyclosporine A demonstrates nephrotoxicity in renal tubular cells by increasing the levels of fission proteins but decreasing the levels of fusion proteins [117]. Rhabdomyolysis induces kidney dysfunction by upregulating Drp1 activity and ROS production but down-regulating ATP levels, whereas mdivi-1 maintains the function by eliminating release of Bax and cytochrome c [118]. Furthermore, mdivi-1 immediately prevents radiation-induced mitochondrial fragmentation and attenuates induced high apoptosis rate [119]. As an effective inhibitor of Drp1 activity, mdivi-1 has considerable effects in animal models. Although acetaminophen-induced cell death can be suppressed by reduced expression of inhibiting receptor interacting protein kinase 3 and mdivi-1, the protective effects were lost after one day in vivo [120]. Nrf2 KO mice were susceptible to acetaminophen induced liver injury, but mdivi-1 induced these animals to be much more sensitive to acetaminophen [121].
In addition, the nervous system is highly sensitive and significantly alters its mitochondrial function after undergoing acute injury or long-term stimuli. Recent studies state that mutations of Drp1 in human result in disturbance of mitochondrial fission and lead to various diseases. Hyper-phosphorylation of Drp1 at Ser-637 induced abnormal mitochondrial dynamics in somatic cells isolated from hereditary spastic paraplegia patients; this disturbance is mediated by impaired interactions between receptor expression enhancing protein 1(REEP1) and PGAM5 [53]. In autosomal recessive spastic ataxia of Charlevoix Saguenay patients, neurons demonstrate reduced ability to recruit Drp1 and poor mitochondrial health [60]. Drp1 mutation also results in postnatal microcephaly, developmental delay, and pain insensitivity via impairment of mitochondrial fission and mitochondrial respiratory function [63]. In addition, mitochondrial functions are changed in the brains of individuals with specific neurodegenerative disorders. There is still controversy over Drp1-mediated mitochondrial fission in Alzheimer’s disease. Drp1 is termed a crucial factor for mitochondrial dynamics in patients with Alzheimer’s disease [122], and their brain tissues demonstrated elongated interconnected mitochondria; however, this is not associated with altered translocation of Drp1 but with reduced GTPase activity [123]. P110 reduced mitochondrial fragmentation and corrected mitochondrial dysfunction fibroblasts from patients with Hodgkin’s disease and Parkinson’s disease [59,124]. Camptothecins, which specifically target DNA topoisomerase I and serve as anticancer drugs in clinical trials [66], are also demonstrated to decrease the levels of Drp1 and parkin in neurons; overexpression of Drp1 or parkin in neurons improves neuronal viability and maintains mitochondrial morphology, thus promoting resistance to apoptotic stimuli [125]. Furthermore, loss of parkin alone is not sufficient to decrease the mitochondrial connectivity but parkin is effective in the absence of Drp1 [126]. PP2A, a neuron-specific regulatory subunit, activates Drp1 by dephosphorylating Ser-656, which is targeted by the neuroprotective protein kinase, whereas blockage of Drp1 dephosphorylation and mitochondrial fragmentation increase the apoptosis rates of cultured hippocampal neurons [127]. Nanoceria, which acts as an antioxidant by reversibly binding oxygen and shifting between the Ce3+ and Ce4+ forms [128], can not only reduce peroxynitrite induced ROS and protein tyrosine nitration in neurons, but also reduces endogenous peroxynitrite and neuronal cell death rates via inhibition of Drp1 Ser-616 hyperphosphorylation [63]. Most studies have sought to prove that the pathogenesis of nervous system degenerative diseases is closely linked to Drp1-dependent mitochondrial fission, as has been observed in previous acute injury models. Since aging cells are observed in various neurodegenerative diseases, how to inhibit the pathological processes is quite important for treating these diseases. Leucine-rich repeat kinase 2 and Drp1 partially co-localize to induce defects in the mitochondrial dynamics of neurons and consequently lead to Parkinson’s disease [129]. In Alzheimer’s disease, GSK3β increases Drp1-dependent GTPase activity and results in modified neurons being more vulnerable to apoptotic signals; in contrast, blocking GSK3β-induced Drp1 phosphorylation efficiently protects neurons against apoptosis [130]. Partially reduced Drp1 significantly decreases Aβ production and effectively maintains mitochondrial dynamics in Alzheimer’s disease neurons and APP transgenic mice (Tg2576 line) [45]. Lentiviral transduction with wild-type STAT2 rescued the deficiency in STAT2-deficient patient-derived fibroblasts via upregulation of phosphorylation of Drp1 at Ser-616 and maintenance of mitochondrial length [53]. Additionally, Drp1 also plays critical roles in other relatively slowly progressing diseases. For example, airway smooth muscle cells from asthmatic patients exhibited substantial morphological defects and showed increased Drp1 expression [131]. Drp1 protein levels are profoundly diminished in the peripheral blood lymphocytes of systemic lupus erythematosus patients [57]. In diabetes models, inhibition of Drp1 by Drp1-K38A attenuates fatty acid-induced mitochondrial fragmentation in stressed β-cells [132]. Rhein preserves mitochondrial ultrastructure by ablating cellular ROS and decreasing Drp1 activity in hyperglycemia [133]. In addition to these small molecules, aerobic exercise training significantly downregulated the phosphorylation level of Drp1 at Ser-616 and increased fat oxidation and insulin sensitivity in insulin-resistant human skeletal muscle cells [56].
Intriguingly, mitochondrial fission precedes other hallmarks in multiple tumors [134]. Because tumor cells are difficult to selectively clear, and there is high mortality among end-stage cancer patients, there is a great need to find effective and safe methods to save the lives of patients. After Drp1 activity was inhibited in brain-tumor-initiating cells, the tumorigenicity, both in vitro and in vivo, was attenuated through activation of AMPK [135]. Blockage of Drp1 activity also kills thyroid cancer cells and brain-tumor-initiating cells by altering the migration or invasion ability of tumor cells [135,136]. Loss of Drp1 suppresses growth of hepatocellular carcinoma cells through suppression of the p53/p21 and NF-κB/cyclin pathways [137]. In recent years, various protocols for inhibition of Drp1 activity have been applied to the experimental and clinical tests in cancer animal models or patients. Mdivi-1 significantly increases mitochondrial ROS production, mitochondrial mass, and cardiolipin oxidation and it selectively sensitizes cancer cells to apoptosis, providing favorable curative effects [138]. Since cofilin S3E and Drp1 S637D mutants significantly suppressed mitochondrial fission and mitochondria-dependent apoptosis, erucin inhibits tumor growth through mitochondrial translocation of cofilin and Drp1 in a breast cancer cell xenograft mouse model [139]. In addition, Drp1 RNA interference increases the apoptotic rates of human lung and colon cancer cells through inhibition of Drp1-dependent mitochondrial fission [140]. Sodium butyrate significantly reduces the cyclin B1–CDK1 complex and phosphorylates Drp1 and consequently induces apoptosis of human colorectal cancer cells [141]. After knockdown of tumor necrosis factor receptor-associated protein 1, the expression levels of Drp1 and Mff are downregulated, whereas the expression of fusion proteins is not changed in neuroblastoma cells and glioma cells [142]. In addition to these gene modifications in Drp1-related pathways, co-culture with mesenchymal stem cells induces T-cell acute lymphoblastic leukemia cells to keep well-maintained mitochondrial dynamics, mitochondrial ROS levels, and chemoresistance via extracellular signal-regulated kinase activation-mediated phosphorylation of Drp1 at residue Ser-616 [66]. Although altered expression of Drp1 efficiently increases apoptosis in various malignant cells, Drp1-dependent mitochondrial fission alone is not sufficient to cure diffuse large B-cell lymphoma [143]. Currently, the role of Drp1 in malignant tumors is uncertain, and the detailed regulatory mechanisms remain to be determined. Furthermore, new drugs targeting the mitochondrial fission pathway should be developed.
7. Conclusions
As highly dynamic organelles, mitochondria play crucial roles in cell survival as well as cell death by altering their morphology, mass, and function in response to stress and physiological conditions. Although mitochondria are the smallest semi-autonomous system in mammals, mitochondrial morphology, distribution, and function are maintained by the balanced regulation of mitochondrial fission and fusion. Drp1 and its specific receptors (Drp1, Mff, MiD49/51, and human Fis1) cooperatively regulate the fission process. Assembly, hydrolysis, disassembly, and post-translational modifications of Drp1 are also indispensable for the regulation of Drp1 activities in biological dynamics. In addition, because post-translational modifications at different sites may exert effects on cells or tissues considerably differently, Drp1 should be modified at specific sites in order to regulate Drp1 activity. Although mitochondrial fusion is infrequently investigated in recent studies, it has been demonstrated to cooperate with mitochondrial fission machinery in maintaining the health of cells or diminishing apoptosis rates in vivo and in vitro. Interestingly, regulation of this critical protein also takes part in the apoptosis progress of various tumor cells and may greatly improve the survival rates of end-stage cancer patients. Since the composition of the fission apparatus may vary according to disease states, different species, and even different individuals, future studies are necessary to elucidate the complex interplay and coordination between Drp1 and various diseases in human. Developing a better understanding of the mitochondrial fission and fusion machinery should provide an avenue to better address the numerous pathological processes to which mitochondrial homeostasis contributes. Advantage remodeling will be controlled through quantitative balance between mitochondrial fission and fusion processes. According to current studies, several pharmacological agents effectively reverse the impairment of mitochondrial dynamics, but the related pharmacokinetics and toxicology profiles need to be further investigated for clinical usage.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81471794), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (no. 81121002), the Chinese High Tech Research & Development (863) Program (no. SS2013AA020102), and the National Science and Technology Major Project (no. 2012ZX10002004).
Abbreviations
| ATP | Adenosine triphosphate |
| Drp1 | Dynamin-related protein 1 |
| GED | C-terminal GTPase effector |
| MOM | Mitochondrial outer membrane |
| ER | Endoplasmic reticulum |
| ROS | Reactive oxygen species |
| Mff | Mitochondrial fission factor |
| MiD51 | Mitochondrial elongation factor 1 |
| MiD49 | Mitochondrial elongation factor 2 |
| ADP | Adenosine diphosphate |
| Fis1 | Mitochondrial fission 1 |
| Mfn | Mitofusion |
| MIM | Mitochondrial inner membrane |
| Opa1 | Optic atrophy 1 |
| AMPK | Adenosine monophosphate-activated protein kinase |
| PKA | Protein kinase A |
| AKAP1 | A kinase anchoring protein 1 |
| CDK | Cyclin-dependent kinase |
| PP2A | Protein phosphatase 2A |
| GSK | Glycogen synthase kinase |
| FK506 | Tacrolimus |
| PTEN | Phosphatase and tensin homolog |
| SENP | Sentrin/SUMO-specific protease |
| KO | Knockout |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mtDNA | Mitochondrial DNA |
| Gfer | Growth factor erv1-like |
| ESCs | Embryonic stem cells |
| MEFs | Mouse embryonic fibroblasts |
| OCRs | Oxygen consumption rates |
| PGAM5 | Phosphoglycerate mutase 5 |
| H2O2 | Hydrogen peroxide |
| IR | Ischemia–reperfusion |
| PINK1 | PTEN-induced novel kinase 1 |
| RGC | Retinal ganglion cell |
| REEP1 | Receptor expression enhancing protein 1 |
Author Contributions
Chenxia Hu wrote this manuscript. Yong Huang revised this manuscript. Lanjuan Li conceived and designed the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Samanta K., Douglas S., Parekh A.B. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE. 2014;9:e101188. doi: 10.1371/journal.pone.0101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smirnova E., Griparic L., Shurland D.L., van der Bliek A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Chomyn A., Chan D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 4.Chang C.R., Manlandro C.M., Arnoult D., Stadler J., Posey A.E., Hill R.B., Blackstone C. A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. J. Biol. Chem. 2010;285:32494–32503. doi: 10.1074/jbc.M110.142430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper K.F., Khakhina S., Kim S.K., Strich R. Stress-induced nuclear-to-cytoplasmic translocation of cyclin C promotes mitochondrial fission in yeast. Dev. Cell. 2014;28:161–173. doi: 10.1016/j.devcel.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Liu P., Zhang Q., Zhu J., Chen T., Arimura S., Tsutsumi N., Lin J. Phosphorylation and ubiquitination of dynamin-related proteins (AtDRP3A/3B) synergically regulate mitochondrial proliferation during mitosis. Plant. J. 2012;72:43–56. doi: 10.1111/j.1365-313X.2012.05052.x. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald P.J., Stepanyants N., Mehrotra N., Mears J.A., Qi X., Sesaki H., Ramachandran R. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol. Biol. Cell. 2014;25:1905–1915. doi: 10.1091/mbc.E14-02-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian W., Choi S., Gibson G.A., Watkins S.C., Bakkenist C.J., van Houten B. Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J. Cell Sci. 2012;125:5745–5757. doi: 10.1242/jcs.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bras M., Yuste V.J., Roue G., Barbier S., Sancho P., Virely C., Rubio M., Baudet S., Esquerda J.E., Merle-Beral H., et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol. Cell. Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C.R., Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald P.J., Francy C.A., Stepanyants N., Lehman L., Baglio A., Mears J.A., Qi X., Ramachandran R. Distinct splice variants of dynamin-related protein 1 differentially utilize mitochondrial fission factor as an effector of cooperative GTPase activity. J. Biol. Chem. 2016;291:493–507. doi: 10.1074/jbc.M115.680181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz-Sandoval C.G., Hughes S.C., Dacks J.B., Simmen T. Interaction with the effector dynamin-related protein 1 (Drp1) is an ancient function of Rab32 subfamily proteins. Cell. Logist. 2014;4:e986399. doi: 10.4161/21592799.2014.986399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaja I., Bai X., Liu Y., Kikuchi C., Dosenovic S., Yan Y., Canfield S.G., Bosnjak Z.J. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem. Biophys. Res. Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbloom A.B., Lee S.H., To M., Lee A., Shin J.Y., Bustamante C. Optimized two-color super resolution imaging of Drp1 during mitochondrial fission with a slow-switching Dronpa variant. Proc. Natl. Acad. Sci. USA. 2014;111:13093–13098. doi: 10.1073/pnas.1320044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland A.A., Voeltz G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinton R.W., Francy C.A., Ramachandran R., Qi X., Mears J.A. Dynamin-related protein 1 oligomerization in solution impairs functional interactions with membrane-anchored mitochondrial fission factor. J. Biol. Chem. 2016;291:478–492. doi: 10.1074/jbc.M115.680025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loson O.C., Meng S., Ngo H., Liu R., Kaiser J.T., Chan D.C. Crystal structure and functional analysis of MiD49, a receptor for the mitochondrial fission protein Drp1. Protein Sci. 2015;24:386–394. doi: 10.1002/pro.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Liu T., Jin S., Wang X., Qu M., Uhlen P., Tomilin N., Shupliakov O., Lendahl U., Nister M. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 2011;30:2762–2778. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer C.S., Elgass K.D., Parton R.G., Osellame L.D., Stojanovski D., Ryan M.T. Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J. Biol. Chem. 2013;288:27584–27593. doi: 10.1074/jbc.M113.479873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Liu L., Wu S., Xing D. Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis. FASEB J. 2016;30:466–476. doi: 10.1096/fj.15-274258. [DOI] [PubMed] [Google Scholar]
- 23.Mai S., Klinkenberg M., Auburger G., Bereiter-Hahn J., Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- 24.Osellame L.D., Singh A.P., Stroud D.A., Palmer C.S., Stojanovski D., Ramachandran R., Ryan M.T. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J. Cell Sci. 2016;129:2170–2181. doi: 10.1242/jcs.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi Y., Itoh K., Yamada T., Cerveny K.L., Suzuki T.L., Macdonald P., Frohman M.A., Ramachandran R., Iijima M., Sesaki H. Coincident phosphatidic acid interaction restrains Drp1 in mitochondrial division. Mol. Cell. 2016;63:1034–1043. doi: 10.1016/j.molcel.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepanyants N., Macdonald P.J., Francy C.A., Mears J.A., Qi X., Ramachandran R. Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol. Biol. Cell. 2015;26:3104–3116. doi: 10.1091/mbc.E15-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji W.K., Hatch A.L., Merrill R.A., Strack S., Higgs H.N. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dara L., Johnson H., Suda J., Win S., Gaarde W., Han D., Kaplowitz N. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015;62:1847–1857. doi: 10.1002/hep.27939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: New implications for Charcot-Marie-Tooth disease. J. Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.J., Park Y.J., Shin J.H., Kim E.S., Hwang J.J., Jin D.H., Kim J.C., Cho D.H. A receptor tyrosine kinase inhibitor, tyrphostin A9 induces cancer cell death through Drp1 dependent mitochondria fragmentation. Biochem. Biophys. Res. Commun. 2011;408:465–470. doi: 10.1016/j.bbrc.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 31.Anton F., Fres J.M., Schauss A., Pinson B., Praefcke G.J., Langer T., Escobar-Henriques M. Ugo1 and Mdm30 act sequentially during Fzo1-mediated mitochondrial outer membrane fusion. J. Cell Sci. 2011;124:1126–1135. doi: 10.1242/jcs.073080. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C., Shi Z., Zhang L., Zhou Z., Zheng X., Liu G., Bu G., Fraser P.E., Xu H., Zhang Y.W. Appoptosin interacts with mitochondrial outer-membrane fusion proteins and regulates mitochondrial morphology. J. Cell Sci. 2016;129:994–1002. doi: 10.1242/jcs.176792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elwi A.N., Lee B., Meijndert H.C., Braun J.E., Kim S.W. Mitochondrial chaperone DnaJA3 induces Drp1-dependent mitochondrial fragmentation. Int. J. Biochem. Cell Biol. 2012;44:1366–1376. doi: 10.1016/j.biocel.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Park Y.Y., Nguyen O.T., Kang H., Cho H. MARCH5-mediated quality control on acetylated Mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis. 2014;5:e1172. doi: 10.1038/cddis.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M., Chen Z., Wang Y., Tan Z., Zhu C., Li Y., Han Z., Chen L., Gao R., Liu L., et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702. doi: 10.1080/15548627.2016.1151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otera H., Miyata N., Kuge O., Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J. Cell Biol. 2016;212:531–544. doi: 10.1083/jcb.201508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varanita T., Soriano M.E., Romanello V., Zaglia T., Quintana-Cabrera R., Semenzato M., Menabo R., Costa V., Civiletto G., Pesce P., et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes L.C., Di Benedetto G., Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han X.J., Lu Y.F., Li S.A., Kaitsuka T., Sato Y., Tomizawa K., Nairn A.C., Takei K., Matsui H., Matsushita M. CaM kinase I α-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H., Scimia M.C., Wilkinson D., Trelles R.D., Wood M.R., Bowtell D., Dillin A., Mercola M., Ronai Z.A. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol. Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merrill R.A., Dagda R.K., Dickey A.S., Cribbs J.T., Green S.H., Usachev Y.M., Strack S. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol. 2011;9:e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Jiang H., Chen S., Du F., Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Merrill R.A., Strack S. Mitochondria: A kinase anchoring protein 1, a signaling platform for mitochondrial form and function. Int. J. Biochem. Cell Biol. 2014;48:92–96. doi: 10.1016/j.biocel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strack S., Wilson T.J., Cribbs J.T. Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. J. Cell Biol. 2013;201:1037–1051. doi: 10.1083/jcb.201210045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prieto J., Leon M., Ponsoda X., Sendra R., Bort R., Ferrer-Lorente R., Raya A., Lopez-Garcia C., Torres J. Early ERK1/2 activation promotes Drp1-dependent mitochondrial fission necessary for cell reprogramming. Nat. Commun. 2016;7:11124. doi: 10.1038/ncomms11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Wang Y., Wen X., Ma X.N., Chen W., Huang F., Kou J., Qi L.W., Liu B., Liu K. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J. Mol. Cell. Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Rambold A.S., Kostelecky B., Elia N., Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Q., Luo C.L., Tao L.Y. Dynamin-related protein 1 (Drp1) mediating mitophagy contributes to the pathophysiology of nervous system diseases and brain injury. Histol. Histopathol. 2016 doi: 10.14670/HH-11-841. [DOI] [PubMed] [Google Scholar]
- 49.Dickey A.S., Strack S. PKA/AKAP1 and PP2A/Bβ2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi X., Disatnik M.H., Shen N., Sobel R.A., Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Mol. Biol. Cell. 2011;22:256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou C.H., Lin C.C., Yang M.C., Wei C.C., Liao H.D., Lin R.C., Tu W.Y., Kao T.C., Hsu C.M., Cheng J.T., et al. GSK3β-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress. PLoS ONE. 2012;7:e49112. doi: 10.1371/journal.pone.0049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W., Wang Y., Long J., Wang J., Haudek S.B., Overbeek P., Chang B.H., Schumacker P.T., Danesh F.R. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavie J., Serrat R., Bellance N., Courtand G., Dupuy J.W., Tesson C., Coupry I., Brice A., Lacombe D., Durr A., et al. Mitochondrial morphology and cellular distribution are altered in SPG31 patients and are linked to Drp1 hyperphosphorylation. Hum. Mol. Genet. 2016 doi: 10.1093/hmg/ddw425. [DOI] [PubMed] [Google Scholar]
- 54.Marsboom G., Toth P.T., Ryan J.J., Hong Z., Wu X., Fang Y.H., Thenappan T., Piao L., Zhang H.J., Pogoriler J., et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim S., Lee S.Y., Seo H.H., Ham O., Lee C., Park J.H., Lee J., Seung M., Yun I., Han S.M., et al. Regulation of mitochondrial morphology by positive feedback interaction between PKCδ and Drp1 in vascular smooth muscle cell. J. Cell. Biochem. 2015;116:648–660. doi: 10.1002/jcb.25016. [DOI] [PubMed] [Google Scholar]
- 56.Shenouda S.M., Widlansky M.E., Chen K., Xu G., Holbrook M., Tabit C.E., Hamburg N.M., Frame A.A., Caiano T.L., Kluge M.A., et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caza T.N., Fernandez D.R., Talaber G., Oaks Z., Haas M., Madaio M.P., Lai Z.W., Miklossy G., Singh R.R., Chudakov D.M., et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann. Rheum Dis. 2014;73:1888–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch J., Feichtinger R.G., Freisinger P., Pies M., Schrodl F., Iuso A., Sperl W., Mayr J.A., Prokisch H., Haack T.B. Disturbed mitochondrial and peroxisomal dynamics due to loss of MFF causes Leigh-like encephalopathy, optic atrophy and peripheral neuropathy. J. Med. Genet. 2016;53:270–278. doi: 10.1136/jmedgenet-2015-103500. [DOI] [PubMed] [Google Scholar]
- 59.Guo X., Disatnik M.H., Monbureau M., Shamloo M., Mochly-Rosen D., Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J. Clin. Investig. 2013;123:5371–5388. doi: 10.1172/JCI70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradshaw T.Y., Romano L.E., Duncan E.J., Nethisinghe S., Abeti R., Michael G.J., Giunti P., Vermeer S., Chapple J.P. A reduction in Drp1-mediated fission compromises mitochondrial health in autosomal recessive spastic ataxia of Charlevoix Saguenay. Hum. Mol. Genet. 2016;25:3232–3244. doi: 10.1093/hmg/ddw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stauffer S.R. Small molecule inhibition of the Bcl-X(L)-BH3 protein–protein interaction: Proof-of-concept of an in vivo chemopotentiator ABT-737. Curr. Top. Med. Chem. 2007;7:961–965. doi: 10.2174/156802607780906843. [DOI] [PubMed] [Google Scholar]
- 62.Chen S.D., Lin T.K., Yang D.I., Lee S.Y., Shaw F.Z., Liou C.W., Chuang Y.C. Roles of PTEN-induced putative kinase 1 and dynamin-related protein 1 in transient global ischemia-induced hippocampal neuronal injury. Biochem. Biophys. Res. Commun. 2015;460:397–403. doi: 10.1016/j.bbrc.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 63.Sheffer R., Douiev L., Edvardson S., Shaag A., Tamimi K., Soiferman D., Meiner V., Saada A. Postnatal microcephaly and pain insensitivity due to a de novo heterozygous DNM1L mutation causing impaired mitochondrial fission and function. Am. J. Med. Genet. A. 2016;170:1603–1607. doi: 10.1002/ajmg.a.37624. [DOI] [PubMed] [Google Scholar]
- 64.Haun F., Nakamura T., Shiu A.D., Cho D.H., Tsunemi T., Holland E.A., La Spada A.R., Lipton S.A. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid. Redox Signal. 2013;19:1173–1184. doi: 10.1089/ars.2012.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao L., Xian H., Lee K.Y., Xiao B., Wang H., Yu F., Shen H.M., Liou Y.C. Death-associated Protein 3 Regulates Mitochondrial-encoded Protein Synthesis and Mitochondrial Dynamics. J. Biol. Chem. 2015;290:24961–24974. doi: 10.1074/jbc.M115.673343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y.Q., Li W.Q., Morris-Natschke S.L., Qian K., Yang L., Zhu G.X., Wu X.B., Chen A.L., Zhang S.Y., Nan X., et al. Perspectives on biologically active camptothecin derivatives. Med. Res. Rev. 2015;35:753–789. doi: 10.1002/med.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kandimalla R., Reddy P.H. Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim. Biophys. Acta. 2016;1862:814–828. doi: 10.1016/j.bbadis.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prudent J., Zunino R., Sugiura A., Mattie S., Shore G.C., McBride H.M. MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Mol. Cell. 2015;59:941–955. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Horn S.R., Thomenius M.J., Johnson E.S., Freel C.D., Wu J.Q., Coloff J.L., Yang C.S., Tang W., An J., Ilkayeva O.R., et al. Regulation of mitochondrial morphology by APC/CCdh1-mediated control of Drp1 stability. Mol. Biol. Cell. 2011;22:1207–1216. doi: 10.1091/mbc.E10-07-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braschi E., Zunino R., McBride H.M. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E.Y., Zhang Y., Beketaev I., Segura A.M., Yu W., Xi Y., Chang J., Wang J. SENP5, a SUMO isopeptidase, induces apoptosis and cardiomyopathy. J. Mol. Cell. Cardiol. 2015;78:154–164. doi: 10.1016/j.yjmcc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Zunino R., Braschi E., Xu L., McBride H.M. Translocation of SenP5 from the nucleoli to the mitochondria modulates Drp1-dependent fission during mitosis. J. Biol. Chem. 2009;284:17783–17795. doi: 10.1074/jbc.M901902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo C., Hildick K.L., Luo J., Dearden L., Wilkinson K.A., Henley J.M. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 2013;32:1514–1528. doi: 10.1038/emboj.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gawlowski T., Suarez J., Scott B., Torres-Gonzalez M., Wang H., Schwappacher R., Han X., Yates J.R., 3rd, Hoshijima M., Dillmann W. Modulation of dynamin-related protein 1 (Drp1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J. Biol. Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bossy B., Petrilli A., Klinglmayr E., Chen J., Lutz-Meindl U., Knott A.B., Masliah E., Schwarzenbacher R., Bossy-Wetzel E. S-Nitrosylation of Drp1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J. Alzheimers Dis. 2010;20(Suppl. S2):S513–S526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Udagawa O., Ishihara T., Maeda M., Matsunaga Y., Tsukamoto S., Kawano N., Miyado K., Shitara H., Yokota S., Nomura M., et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr. Biol. 2014;24:2451–2458. doi: 10.1016/j.cub.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 77.Leduc-Gaudet J.P., Picard M., St-Jean Pelletier F., Sgarioto N., Auger M.J., Vallee J., Robitaille R., St-Pierre D.H., Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6:17923–17937. doi: 10.18632/oncotarget.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashrafian H., Docherty L., Leo V., Towlson C., Neilan M., Steeples V., Lygate C.A., Hough T., Townsend S., Williams D., et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikeda Y., Shirakabe A., Maejima Y., Zhai P., Sciarretta S., Toli J., Nomura M., Mihara K., Egashira K., Ohishi M., et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 80.Filichia E., Hoffer B., Qi X., Luo Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci. Rep. 2016;6:32656. doi: 10.1038/srep32656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T.W., Iijima M., Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Touvier T., de Palma C., Rigamonti E., Scagliola A., Incerti E., Mazelin L., Thomas J.L., D’Antonio M., Politi L., Schaeffer L., et al. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death Dis. 2015;6:e1663. doi: 10.1038/cddis.2014.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L., Ye X., Zhao Q., Zhou Z., Dan J., Zhu Y., Chen Q., Liu L. Drp1 is dispensable for mitochondria biogenesis in induction to pluripotency but required for differentiation of embryonic stem cells. Stem Cells Dev. 2014;23:2422–2434. doi: 10.1089/scd.2014.0059. [DOI] [PubMed] [Google Scholar]
- 84.Parker D.J., Iyer A., Shah S., Moran A., Hjelmeland A.B., Basu M.K., Liu R., Mitra K. A new mitochondrial pool of cyclin E, regulated by Drp1, is linked to cell-density-dependent cell proliferation. J. Cell Sci. 2015;128:4171–4182. doi: 10.1242/jcs.172429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Palma C., Falcone S., Pisoni S., Cipolat S., Panzeri C., Pambianco S., Pisconti A., Allevi R., Bassi M.T., Cossu G., et al. Nitric oxide inhibition of Drp1-mediated mitochondrial fission is critical for myogenic differentiation. Cell Death Differ. 2010;17:1684–1696. doi: 10.1038/cdd.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong Z., Kutty S., Toth P.T., Marsboom G., Hammel J.M., Chamberlain C., Ryan J.J., Zhang H.J., Sharp W.W., Morrow E., et al. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ. Res. 2013;112:802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uo T., Dworzak J., Kinoshita C., Inman D.M., Kinoshita Y., Horner P.J., Morrison R.S. Drp1 levels constitutively regulate mitochondrial dynamics and cell survival in cortical neurons. Exp. Neurol. 2009;218:274–285. doi: 10.1016/j.expneurol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Todd L.R., Gomathinayagam R., Sankar U. A novel Gfer-Drp1 link in preserving mitochondrial dynamics and function in pluripotent stem cells. Autophagy. 2010;6:821–822. doi: 10.4161/auto.6.6.12625. [DOI] [PubMed] [Google Scholar]
- 89.Todd L.R., Damin M.N., Gomathinayagam R., Horn S.R., Means A.R., Sankar U. Growth factor erv1-like modulates Drp1 to preserve mitochondrial dynamics and function in mouse embryonic stem cells. Mol. Biol. Cell. 2010;21:1225–1236. doi: 10.1091/mbc.E09-11-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pradeep H., Sharma B., Rajanikant G.K. Drp1 in ischemic neuronal death: An unusual suspect. Curr. Med. Chem. 2014;21:2183–2189. doi: 10.2174/0929867321666131228203513. [DOI] [PubMed] [Google Scholar]
- 91.Iwasawa R., Mahul-Mellier A.L., Datler C., Pazarentzos E., Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556–568. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martorell-Riera A., Segarra-Mondejar M., Munoz J.P., Ginet V., Olloquequi J., Perez-Clausell J., Palacin M., Reina M., Puyal J., Zorzano A., et al. Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J. 2014;33:2388–2407. doi: 10.15252/embj.201488327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ban-Ishihara R., Ishihara T., Sasaki N., Mihara K., Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome C. Proc. Natl. Acad. Sci. USA. 2013;110:11863–11868. doi: 10.1073/pnas.1301951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Germain M., Mathai J.P., McBride H.M., Shore G.C. Endoplasmic reticulum BIK initiates Drp1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cribbs J.T., Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian W., Wang J., Roginskaya V., McDermott L.A., Edwards R.P., Stolz D.B., Llambi F., Green D.R., van Houten B. Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells. Oncotarget. 2014;5:4180–4194. doi: 10.18632/oncotarget.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim B., Kim J.S., Yoon Y., Santiago M.C., Brown M.D., Park J.Y. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R927–R938. doi: 10.1152/ajpregu.00502.2012. [DOI] [PubMed] [Google Scholar]
- 98.Wu S., Zhou F., Zhang Z., Xing D. Bax is essential for Drp1-mediated mitochondrial fission but not for mitochondrial outer membrane permeabilization caused by photodynamic therapy. J. Cell. Physiol. 2011;226:530–541. doi: 10.1002/jcp.22362. [DOI] [PubMed] [Google Scholar]
- 99.Brooks C., Cho S.G., Wang C.Y., Yang T., Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am. J. Physiol. Cell Physiol. 2011;300:C447–C455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clerc P., Ge S.X., Hwang H., Waddell J., Roelofs B.A., Karbowski M., Sesaki H., Polster B.M. Drp1 is dispensable for apoptotic cytochrome c release in primed MCF10A and fibroblast cells but affects Bcl-2 antagonist-induced respiratory changes. Br. J. Pharmacol. 2014;171:1988–1999. doi: 10.1111/bph.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J., Xu P., Wang Y., Wang M., Li H., Lin S., Mao C., Wang B., Song X., Lv C. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission. J. Cell. Mol. Med. 2015;19:2215–2231. doi: 10.1111/jcmm.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin H.Y., Lai R.H., Lin S.T., Lin R.C., Wang M.J., Lin C.C., Lee H.C., Wang F.F., Chen J.Y. Suppressor of cytokine signaling 6 (SOCS6) promotes mitochondrial fission via regulating Drp1 translocation. Cell Death Differ. 2013;20:139–153. doi: 10.1038/cdd.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clavier A., Ruby V., Rincheval-Arnold A., Mignotte B., Guenal I. The Drosophila retinoblastoma protein, Rbf1, induces a Debcl- and Drp1-dependent mitochondrial apoptosis. J. Cell Sci. 2015;128:3239–3249. doi: 10.1242/jcs.169896. [DOI] [PubMed] [Google Scholar]
- 104.Su Y.C., Chiu H.W., Hung J.C., Hong J.R. B-nodavirus B2 protein induces hydrogen peroxide production, leading to Drp1-recruited mitochondrial fragmentation and cell death via mitochondrial targeting. Apoptosis. 2014;19:1457–1470. doi: 10.1007/s10495-014-1016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryu S.W., Choi K., Yoon J., Kim S., Choi C. Endoplasmic reticulum-specific BH3-only protein BNIP1 induces mitochondrial fragmentation in a Bcl-2- and Drp1-dependent manner. J. Cell. Physiol. 2012;227:3027–3035. doi: 10.1002/jcp.23044. [DOI] [PubMed] [Google Scholar]
- 106.Zuo W., Zhang S., Xia C.Y., Guo X.F., He W.B., Chen N.H. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: The role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology. 2014;86:103–115. doi: 10.1016/j.neuropharm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Zepeda R., Kuzmicic J., Parra V., Troncoso R., Pennanen C., Riquelme J.A., Pedrozo Z., Chiong M., Sanchez G., Lavandero S. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. 2014;63:477–487. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 108.Sharp W.W., Fang Y.H., Han M., Zhang H.J., Hong Z., Banathy A., Morrow E., Ryan J.J., Archer S.L. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Din S., Mason M., Volkers M., Johnson B., Cottage C.T., Wang Z., Joyo A.Y., Quijada P., Erhardt P., Magnuson N.S., et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc. Natl. Acad. Sci. USA. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Wang P., Wei J., Fan R., Zuo Y., Shi M., Wu H., Zhou M., Lin J., Wu M., et al. Inhibition of Drp1 by Mdivi-1 attenuates cerebral ischemic injury via inhibition of the mitochondria-dependent apoptotic pathway after cardiac arrest. Neuroscience. 2015;311:67–74. doi: 10.1016/j.neuroscience.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 111.Park S.W., Kim K.Y., Lindsey J.D., Dai Y., Heo H., Nguyen D.H., Ellisman M.H., Weinreb R.N., Ju W.K. A selective inhibitor of Drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest. Ophthalmol. Vis. Sci. 2011;52:2837–2843. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L., Jiang F., Chen Y., Luo J., Liu S., Zhang B., Ye Z., Wang W., Liang X., Shi W. Necrostatin-1 attenuates ischemia injury induced cell death in rat tubular cell line NRK-52E through decreased Drp1 expression. Int. J. Mol. Sci. 2013;14:24742–24754. doi: 10.3390/ijms141224742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chuang Y.C., Lin T.K., Yang D.I., Yang J.L., Liou C.W., Chen S.D. Peroxisome proliferator-activated receptor-γ dependent pathway reduces the phosphorylation of dynamin-related protein 1 and ameliorates hippocampal injury induced by global ischemia in rats. J. Biomed. Sci. 2016;23:44. doi: 10.1186/s12929-016-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rappold P.M., Cui M., Grima J.C., Fan R.Z., de Mesy-Bentley K.L., Chen L., Zhuang X., Bowers W.J., Tieu K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat. Commun. 2014;5:5244. doi: 10.1038/ncomms6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ju W.K., Liu Q., Kim K.Y., Crowston J.G., Lindsey J.D., Agarwal N., Ellisman M.H., Perkins G.A., Weinreb R.N. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest. Ophthalmol. Vis. Sci. 2007;48:2145–2151. doi: 10.1167/iovs.06-0573. [DOI] [PubMed] [Google Scholar]
- 116.Kim K.Y., Perkins G.A., Shim M.S., Bushong E., Alcasid N., Ju S., Ellisman M.H., Weinreb R.N., Ju W.K. Drp1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6:e1839. doi: 10.1038/cddis.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Arriba G., Calvino M., Benito S., Parra T. Cyclosporine A-induced apoptosis in renal tubular cells is related to oxidative damage and mitochondrial fission. Toxicol. Lett. 2013;218:30–38. doi: 10.1016/j.toxlet.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 118.Tang W.X., Wu W.H., Qiu H.Y., Bo H., Huang S.M. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J. Nephrol. 2013;26:1073–1082. doi: 10.5301/jn.5000268. [DOI] [PubMed] [Google Scholar]
- 119.Zhang B., Davidson M.M., Zhou H., Wang C., Walker W.F., Hei T.K. Cytoplasmic irradiation results in mitochondrial dysfunction and Drp1-dependent mitochondrial fission. Cancer Res. 2013;73:6700–6710. doi: 10.1158/0008-5472.CAN-13-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramachandran A., McGill M.R., Xie Y., Ni H.M., Ding W.X., Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao Y., Chu S., Zhang Z., Zuo W., Xia C., Ai Q., Luo P., Cao P., Chen N. Early stage functions of mitochondrial autophagy and oxidative stress in acetaminophen-induced liver injury. J. Cell. Biochem. 2016 doi: 10.1002/jcb.25788. [DOI] [PubMed] [Google Scholar]
- 122.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid β with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: Implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L., Trushin S., Christensen T.A., Bachmeier B.V., Gateno B., Schroeder A., Yao J., Itoh K., Sesaki H., Poon W.W., et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci. Rep. 2016;6:18725. doi: 10.1038/srep18725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su Y.C., Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 2013;22:4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]
- 125.Wang D.B., Garden G.A., Kinoshita C., Wyles C., Babazadeh N., Sopher B., Kinoshita Y., Morrison R.S. Declines in Drp1 and parkin expression underlie DNA damage-induced changes in mitochondrial length and neuronal death. J. Neurosci. 2013;33:1357–1365. doi: 10.1523/JNEUROSCI.3365-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roy M., Itoh K., Iijima M., Sesaki H. Parkin suppresses Drp1-independent mitochondrial division. Biochem. Biophys. Res. Commun. 2016;475:283–288. doi: 10.1016/j.bbrc.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merrill R.A., Slupe A.M., Strack S. N-terminal phosphorylation of protein phosphatase 2A/Bβ2 regulates translocation to mitochondria, dynamin-related protein 1 dephosphorylation, and neuronal survival. FEBS J. 2013;280:662–673. doi: 10.1111/j.1742-4658.2012.08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li C., Zhao W., Liu B., Xu G., Liu L., Lv H., Shang D., Yang D., Damirin A., Zhang J. Cytotoxicity of ultrafine monodispersed nanoceria on human gastric cancer cells. J. Biomed. Nanotechnol. 2014;10:1231–1241. doi: 10.1166/jbn.2014.1863. [DOI] [PubMed] [Google Scholar]
- 129.Niu J., Yu M., Wang C., Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J. Neurochem. 2012;122:650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- 130.Yan J., Liu X.H., Han M.Z., Wang Y.M., Sun X.L., Yu N., Li T., Su B., Chen Z.Y. Blockage of GSK3β-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer’s disease. Neurobiol. Aging. 2015;36:211–227. doi: 10.1016/j.neurobiolaging.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Aravamudan B., Kiel A., Freeman M., Delmotte P., Thompson M., Vassallo R., Sieck G.C., Pabelick C.M., Prakash Y.S. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L840–L854. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wikstrom J.D., Israeli T., Bachar-Wikstrom E., Swisa A., Ariav Y., Waiss M., Kaganovich D., Dor Y., Cerasi E., Leibowitz G. AMPK regulates ER morphology and function in stressed pancreatic β-cells via phosphorylation of Drp1. Mol. Endocrinol. 2013;27:1706–1723. doi: 10.1210/me.2013-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J., Chen Z., Zhang Y., Zhang M., Zhu X., Fan Y., Shi S., Zen K., Liu Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62:3927–3935. doi: 10.2337/db13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varadarajan S., Butterworth M., Wei J., Pellecchia M., Dinsdale D., Cohen G.M. Sabutoclax (BI97C1) and BI112D1, putative inhibitors of MCL-1, induce mitochondrial fragmentation either upstream of or independent of apoptosis. Neoplasia. 2013;15:568–578. doi: 10.1593/neo.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie Q., Wu Q., Horbinski C.M., Flavahan W.A., Yang K., Zhou W., Dombrowski S.M., Huang Z., Fang X., Shi Y., et al. Mitochondrial control by Drp1 in brain tumor initiating cells. Nat. Neurosci. 2015;18:501–510. doi: 10.1038/nn.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferreira-da-Silva A., Valacca C., Rios E., Populo H., Soares P., Sobrinho-Simoes M., Scorrano L., Maximo V., Campello S. Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PLoS ONE. 2015;10:e0122308. doi: 10.1371/journal.pone.0122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhan L., Cao H., Wang G., Lyu Y., Sun X., An J., Wu Z., Huang Q., Liu B., Xing J. Drp1-mediated mitochondrial fission promotes cell proliferation through crosstalk of p53 and NF-κB pathways in hepatocellular carcinoma. Oncotarget. 2016;7:65001–65011. doi: 10.18632/oncotarget.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Akita M., Suzuki-Karasaki M., Fujiwara K., Nakagawa C., Soma M., Yoshida Y., Ochiai T., Tokuhashi Y., Suzuki-Karasaki Y. Mitochondrial division inhibitor-1 induces mitochondrial hyperfusion and sensitizes human cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2014;45:1901–1912. doi: 10.3892/ijo.2014.2608. [DOI] [PubMed] [Google Scholar]
- 139.Li G., Zhou J., Budhraja A., Hu X., Chen Y., Cheng Q., Liu L., Zhou T., Li P., Liu E., et al. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget. 2015;6:1834–1849. doi: 10.18632/oncotarget.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Inoue-Yamauchi A., Oda H. Depletion of mitochondrial fission factor Drp1 causes increased apoptosis in human colon cancer cells. Biochem. Biophys. Res. Commun. 2012;421:81–85. doi: 10.1016/j.bbrc.2012.03.118. [DOI] [PubMed] [Google Scholar]
- 141.Tailor D., Hahm E.R., Kale R.K., Singh S.V., Singh R.P. Sodium butyrate induces Drp1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion. 2014;16:55–64. doi: 10.1016/j.mito.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takamura H., Koyama Y., Matsuzaki S., Yamada K., Hattori T., Miyata S., Takemoto K., Tohyama M., Katayama T. TRAP1 controls mitochondrial fusion/fission balance through Drp1 and Mff expression. PLoS ONE. 2012;7:e51912. doi: 10.1371/journal.pone.0051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang P., Liu B., Zhao J., Pang Q., Agrawal S.G., Jia L., Liu F.T. Dynamin-related protein Drp1 is required for Bax translocation to mitochondria in response to irradiation-induced apoptosis. Oncotarget. 2015;6:22598–22612. doi: 10.18632/oncotarget.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]