Abstract
The retina is a specialized sensory organ, which is essential for light detection and visual formation in the human eye. Inherited retinal degenerations are a heterogeneous group of eye diseases that can eventually cause permanent vision loss. UPR (unfolded protein response) and ER (endoplasmic reticulum) stress plays an important role in the pathological mechanism of retinal degenerative diseases. mTOR (the mammalian target of rapamycin) kinase, as a signaling hub, controls many cellular processes, covering protein synthesis, RNA translation, ER stress, and apoptosis. Here, the hypothesis that inhibition of mTOR signaling suppresses ER stress-induced cell death in retinal degenerative disorders is discussed. This review surveys knowledge of the influence of mTOR signaling on ER stress arising from misfolded proteins and genetic mutations in retinal degenerative diseases and highlights potential neuroprotective strategies for treatment and therapeutic implications.
Keywords: mTOR, ER stress, retinal degeneration, unfolded protein response, retinal neuroprotection, apoptosis
1. Retinal Degeneration
The retina arises from the neuroectoderm during embryogenesis and is the part of the eye that perceives light stimuli, as well as integrates and transmits electrical impulses through the optic nerve to the visual cortex in the brain. The retina is comprised of six major types of neurons, including retinal ganglion cells, bipolar, horizontal and amacrine interneurons, Müller glia and photoreceptors. These neurons are organized in three cellular layers separated by synaptic layers [1]. In the outer nuclear layer (ONL), the photoreceptor cells are morphologically compartmentalized cells having inner and outer segment regions, connected by a narrow cilium. The outer segment of the photoreceptor consists of a stack of disk membranes surrounded by a plasma membrane [2]. Continual replenishment of disks in the photoreceptor leads to a high rate of protein turnover and ER biogenesis post-translationally modifies and controls the quality of many outer segment (OS) proteins, including opsin. Retinal pigment epithelial (RPE) cells, located at the outer layer of the retina, provide nourishment (e.g., vitamin A metabolites) and clear OS debris of the overlying photoreceptor cells, via daily phagocytosis of OS tips, and participate in regeneration of visual pigment regeneration [3]. The outer plexiform layer (OPL) consists of synaptic interactions between photoreceptors and horizontal cells and bipolar cells [4]. The inner nuclear layer (INL) contains horizontal, bipolar, and amacrine cell bodies, which play different roles in visual formation. The inner plexiform layer (IPL) is composed of synapses between bipolar and retinal ganglion cells (RGCs), and the ganglion cell layer (GCL) consists of RGC nuclei. The RGC axons form the optic nerve from the output of the retina to the brain, transferring visual information to the centers [5].
Inherited retinal degenerations (IRD) are a heterogeneous group of eye diseases, which affect more than 2 million people worldwide, that can eventually cause permanent vision loss [6]. The inheritance patterns, onset age, and severity of visual dysfunction in IRD are different. Syndromic and nonsyndromic forms of retinal dystrophies, which include autosomal, X-linked, and mitochondrial inheritance are classified. Phenotypic categories cover retinitis pigmentosa (RP), macular degeneration, cone or cone-rod dystrophy, congenital stationary night blindness, and Leber congenital amaurosis (LCA) etc. [7]. Dysfunctions of rod and cone photoreceptor cells, can involve the outer segment structure, phototransduction, the cilium structure and transport connection, inner segment protein and vesicle trafficking, lipid metabolism, chaperone function, RNA splicing and transcription, synaptic function, and retinal development. Affected mechanisms in the RPE cover membrane trafficking, ion transport and visual cycle reactions [7]. Certain mutations in secondary retinal neurons such as ganglion cells and Müller cellscan also lead to IRD [8]. However, the exact molecular mechanisms involved in mutant genes causing dysfunctional retinal neurons to undergo apoptosis and lead to the development of IRDs are still unclear. Animal models, which carry mutations in various genes that mimic human IRDs have been used to describe the modes of retinal cell death. These animals include (1) retinal degeneration (rd) mice; (2) retinal degeneration slow (rds) mice; (3) transgenic mice carrying P347S and Q344ter mutations in the rhodopsin gene; (4) knockout mice deficient for the b2-subunit of Na1/K1-ATPase expressed in retinal Müller cells; and (5) Royal College of Surgeons (RCS) rats, in which photoreceptors were detected that diedvia the apoptotic pathway as evidenced by histological morphology, TUNEL (terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labeling) assays, and/or by retinal DNA laddering with gel electrophoresis. Results from these studies indicate that it is likely that photoreceptors in human IRDs are dying similarly via the apoptotic pathway [9].
2. Proteostasis and ER Stress
Protein homeostasis is critical for cellular function andi s tightly controlled by the synthesis and clearance of proteins [10]. The concept of proteostasis is simple. Protein synthesis (including protein folding and protein transport) must match the rate of degradation [11,12]. A healthy proteomeis maintained through a series of complex surveillance systems, which ensure that each protein is functionally folded or assembled [13]. Cells have evolved many mechanisms to cope with misfolded proteins, such as the ubiquitin-proteasome system (UPS) [14], ER-associated protein degradation (ERAD) [15] and the unfolded protein response (UPR) [16]. These proteostasis networks play important roles in maintaining correctly folded proteins and removing misfolded proteins.
The endoplasmic reticulum (ER) is responsible for the quality control of newly synthesized proteins, including protein folding, post-translational modification and transportation [17], thus it is a key component of cellular proteostasis. ER cisternae have been historically classified as ribosome-bound “rough” ER and ribosome-free “smooth” ER. As indicated, smooth ER includes ribosome-free areas, where fusion and vesicle budding take place, whereas the rough ER performs the functions of proper protein folding and modification [18]. The newly synthesized, unfolded proteins are initially generated from the ribosomes and are then transported into the cisternal space of the ER, where these polypeptide chains are properly folded and oligomerized, disulfide bonds are formed, and N-linked oligosaccharides are attached for a glycoprotein. After folding and post-translational modification, mature proteins are disassociated from ER chaperones and transported to the Golgi apparatus [19].
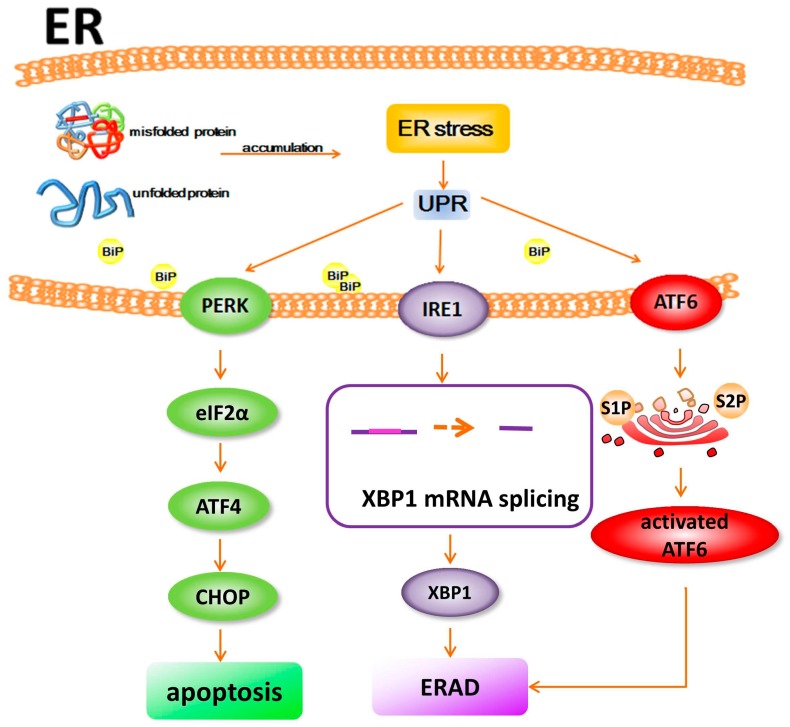
The cellular protein folding capacity is tightly regulated in the ER via activation of intracellular signal pathways [20]. When the accumulation of misfolded or unfolded proteins causes an imbalance in ER homeostasis, it leads to ER stress, which further causes the activation of the UPR (unfolded protein response) [21,22]. The UPR promotes protein folding and suppresses protein translation to reduce the load of proteins within the ER and increases autophagy and ERAD to promote degradation of misfolded proteins [21]. There are three known stress sensors that trigger UPR in ER: inositol-requiring protein 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [23]. PERK phosphorylates initiation factor eIF2α, leading to cap independent translation of ATF4. ATF4 activates C/EBP homologous protein (CHOP), which can stimulate apoptosis. IRE1 is a kinase that leads to activation of RNAse activity. This induces the splicing of XBP1mRNA and further activates ERAD. Following immunoglobulin binding protein (BiP) dissociation, ATF6 is cleaved by the S1P and S2P proteases into an active form in the Golgi. The activated ATF6 then causes activation of ERAD to restore ER homeostasis. The initial transcriptional and translational effects of IRE1, PERK, and ATF6 signaling help cells adapt to ER stress. However, if these actions fail to restore ER homeostasis and ER stress persists, UPR signaling triggers maladaptive proapoptotic programs and cell death. ER stress functions as a critical mechanism relevant to pathogenesis in IRD [19] (Figure 1).
Figure 1.
The unfolded protein response (UPR).There are three known pathways triggering UPR in ER. (i) PERK (protein kinase RNA-like ER kinase) phosphorylates initiation factor eIF2α, leading to cap independent translation of ATF4 (activating transcription factor 4). ATF4 activates CHOP (C/EBP homologous protein), which can stimulate apoptosis; (ii) IRE1 (inositol-requiring protein 1), is a kinase that leads to activation of RNAse activity. This induces the splicing of XBP1 mRNA and further activates ERAD (ER-associated protein degradation); (iii) following BiP (Immunoglobulin binding protein) dissociation, ATF6 is cleaved by the S1P and S2P proteases into an active form in the Golgi. The activated ATF6 will then cause the activation of ERAD to restore ER (endoplasmic reticulum) homeostasis.
3. Disturbance of Proteostasis and ER Stress in Retinal Degeneration
The generation of visual information from retinal cells depends on functional proteins, such as rhodopsin (Rh) [24]. ER is an important intracellular apparatus responsible for the protein quality control. Only properly folded proteins are released from the ER, yet misfolded proteins are degraded to prevent the generation of dysfunctional or potentially toxic proteins [25]. The ER stress and UPR, caused by protein misfolding, have been recently regarded as a contributing factor to IRD [26,27]. Bhootada et al. reported that the expression of T17M Rh in rod photoreceptors induces the activation of ER stress-related UPR signaling, which results in severe retinal degeneration. ATF4 knockdown blocked retinal degeneration and promoted photoreceptor survival in one-month-old T17M mice [28]. Thus far, over 250 different heritable mutations have been identified that cause the production of abnormal proteins by retinal cells, which lead to retinal degeneration and vision loss [29]. The imbalance of proteostasis has been implicated in IRD [30,31]. UPR induction might function as a common pathway, which is activated in cases of retinal degeneration, involving the degenerative process.
As the predominant protein within photoreceptors, mutations in Rh are the most common cause of inherited RP [32]. The IRE1 signaling pathway of UPR was robustly activated in a Drosophila model of retinal degeneration caused by Rh misfolding [33]. In addition, selective activation of UPR signaling pathways were detected in P23H animals, which is expressed at different levels of P23H Rh compared to wild-type siblings [34,35,36,37]. Griciuc et al. studied Drosophila, in which Rh1 (P37H) was expressed in photoreceptors, and genetically increased the levels of misfolded Rh1 (P37H), which further caused the activation of the Ire1/Xbp1 ER stress pathway [38]. In animals expressing misfolded Rh, proapoptotic UPR molecules, such as cleaved ATF6, pEIF2, and CHOP, markedly increased before the loss of photoreceptors, raising the possibility that UPR activation caused by misfolded Rh may directly result in photoreceptor cell death [34,35]. However, overexpressing BiP with adeno-associated virus type 5 (AAV5) vector in transgenic rat retina led to a reduction in CHOP and photoreceptor apoptosis [35].
The mutations in the ELOVL4 (the elongation of very long chain fatty acids) gene resulted in Stargardt macular dystrophy and early childhood blindness [39]. ELOVL4 encodes a membrane protein targeted to the ER, which is an enzyme involved in the generation of long-chain fatty acids [40,41]. Not only do photoreceptors express high levels of ELOVL4, but other types of cells in the eye have been found to express ELOVL4 as well [42]. From a molecular point of view, ELOVL4 mutations cause premature truncations of the protein, leading to loss of an ER retention motif [43,44,45]. In transfected cells, misfolded ELOVL4 aggregates in the ER and induces the UPR, which is closely associated with photoreceptor cell death [35].
The rd1 mutation leads to a remarkable reduction in PDE6-β protein, which is a catalytic subunit of a phosphodiesterase, regulating cGMP levels in photoreceptors [46,47]. Absence of the PDE6-β causes accumulation of cGMP and, in turn, results in significant increases of intracellular calcium and photoreceptor degeneration [48]. In a recent study it was demonstrated that multiple UPR signaling pathways were activated and UPR protein levels of BiP and peIF2α were increased in a time-dependent manner in the retinas of rd1 mice, which suggests that the ER stress contributes to retinal degeneration in rd1 mice [49].
As a post-translational modification, N-linked glycosylation can influence protein folding efficiency. The fibulin-3 gene encodes an N-glycoprotein, which is expressed and secreted by RPE cells. The R345W mutation leads to fibulin-3 misfolding and retention in the ER and causes Malattia Leventinese and Doyne honeycomb retinal dystrophy (ML/DHRD) [50,51,52]. In a cell study, over-expression of mutant R345W fibulin-3 resulted in activation of UPR and increased vascular endothelial growth factor (VEGF) expression when compared with over expression of the wild-type protein [50,53]. These results suggest that misfolded fibulin-3 may trigger RPE dysfunction via activation of UPR pathways, yet further enhance the VEGF level leading to choroidal neovascularization.
Various gene mutations target different retinal neurons and encode misfolded proteins in various types of retinal degenerative diseases. As misfolded or unfolded proteins accumulate in the ER, the UPR is triggered to restore proteostasis. However, an excessive imbalance in proteostasis results in prolonged ER stress, which initiates programmed cell death. The ER stress response has been recently proposed as an important contributing factor to retinal degenerative disease (Table 1).
Table 1.
Summary of inherited retinal degenerations (IRD) models and dysregulated components.
| Animal Model | Mutant Gene | Dysregulated Components | Related Retinal Degeneration | ER Stress Activation | Reference |
|---|---|---|---|---|---|
| Drosophila | RhoP23H | rhodopsin | ADRP | + | [33] |
| Xenopus laevis | RhoP23H | rhodopsin | RP | + | [54] |
| Rats | RhoP23H | rhodopsin | RP | + | [50] |
| Mice | RhoT17M | rhodopsin | ADRP | + | [28] |
| Transfected cell | ELOVL4 | an enzyme involved in the generation of long-chain fatty acids | Stargardt macular dystrophy | + | [39] |
| Rd1 mouse | PDE6-β | a catalytic subunit of a phosphodiesterase, regulating cGMP levels in photoreceptors | RP | + | [49] |
| ARPE-19 cells | R345W | N-Linked glycosylation | Malattia Leventinese and Doyne honeycomb retinal dystrophy | + | [55] |
ADRP, autosomal dominant retinitis pigmentosa; RP, retinitis pigmentosa; ER, endoplasmic reticulum.
4. mTOR and mTOR Signal
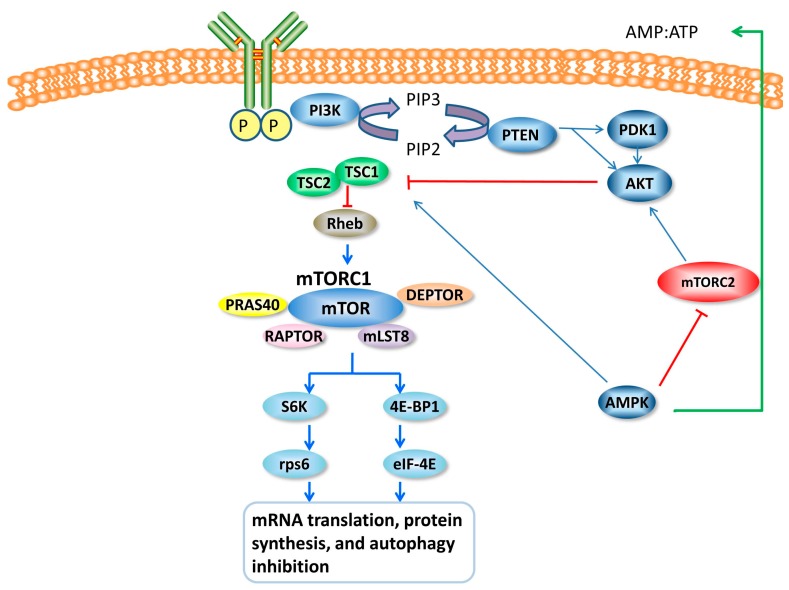
The mTOR (the mammalian target of rapamycin) is an evolutionarily-conserved serine/threonine kinase, which belongs to PIKK (the PI3K-kinase-related kinase) superfamily [19,56]. mTOR functions as two physically and functionally distinct signaling complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2) [57]. mTORC1 is composed of RAPTOR (regulatory associated protein of mTOR), PRAS40 (40 kDa pro-rich AKT1 substrate 1), mLST8 (mammalian lethal with SEC13 protein 8), and DEPTOR (DEP domain-containing mTOR-interacting protein). mTORC2 consists of RICTOR (rapamycin-insensitive companion of mTOR), PRR5 (pro-rich protein 5), mSIN1 (stress-activated MAP kinase-interacting protein 1), mLST8, and DEPTOR [58]. mTORC1 is sensitive to rapamycin, yet mTORC2 is not directly inhibited by rapamycin.
mTORC1 is potently activated by a small GTPase known as Rheb (Ras homolog enriched in brain), yet Rhebcanbe is suppressed by TSC (tuberous sclerosis complex), which consists of TSC1 and TSC2 [59]. In addition, AKT also activates the mTOR signal directly by inhibiting PRAS40 [60]. PTEN (phosphatase and tensin homolog deleted on chromosome 10), which is further upstream, can be suppressed PI3K, thus inactivating the mTOR pathway [61]. In addition, functioning as a key sensor of relative energy status, AMPK (AMP-dependent kinase) is activated by a high AMP/ATP ratio and suppresses mTOR in the cell as an upstream signal [62]. The two most well-known downstream targets of mTORC1 are S6K1 (S6 kinase 1) and 4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1). The main function of mTORC1 activity is the stimulation of mRNA translation, protein synthesis, and autophagy inhibition [62] (Figure 2).
Figure 2.
Schematic representation of the mTOR signaling pathway. mTORC1 is activated by Rheb (Ras homolog enriched in brain); the two most well-known downstream targets of mTORC1 are S6K1 (S6 kinase 1) and 4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1). As an upstream signal, TSC (tuberous sclerosis complex) can suppress Rheb to negatively regulate mTORC1. Furthermore, PTEN (phosphatase and tensin homolog deleted on chromosome 10) can be suppressed by PI3K (phosphoinositide 3-kinase), thus inactivating the mTOR pathway. In addition, AMPK (AMP-dependent kinase) is activated by a high AMP/ATP ratio and suppresses mTOR. The main function of mTORC1 activity is the stimulation of mRNA translation, protein synthesis, and autophagy inhibition. PIP2/PIP3, Phosphatidylinositol 4,5-bisphosphate/Phosphatidylinositol 3,4,5-triphosphate; PDK1, 3-Phosphoinositide-dependent protein kinase 1; AKT1, AKT serine/threonine kinase 1; PRAS40, 40 kDa pro-rich AKT1 substrate 1; RAPTOR, regulatory associated protein of mTOR; mLST8, mammalian lethal with SEC13 protein 8; DEPTOR, DEP domain-containing mTOR-interacting protein.
5. Inhibition of mTOR Suppresses ER Stress and Attenuates Retinal Degeneration
Rapamycin (a.k.a. Rapa Nui), as an mTOR inhibitor, was discovered in 1970 on Easter Island. In all eukaryotes, the intracellular rapamycin receptor is a 12-kDa protein, the FK506-binding protein (FKBP12) [63]. When rapamycin conjugates to FKBP12, it forms a ternary complex with a conserved mTOR domain, shutting down downstream signals [64,65].
Rapamycin inhibits the function of mTORC1, whereas newly-developed mTOR kinase inhibitors interfere with the actions of both mTORC1 and mTORC2. mTOR inhibition suppresses cellular protein synthesis by regulating the initiation and elongation phases of translation and ribosome biosynthesis [66]. Two main downstream signals of mTOR are p70S6K and eIF4E (4E-BP1) [67]. Blocking mTOR affects the activity of p70S6K and the function of 4E-BP1, leading to inhibition of protein synthesis [68]. Through inhibition of p70S6K, rapamycin also blocks the translation of 5′-TOP (5′-terminal oligopyrimidine tract) mRNAs to suppress mRNA translation and protein synthesis [69]. In addition to affecting p70S6K, 4E-BP1 is a translation inhibitor, which is phosphorylated and inactivated in response to a growth signal [70]. In fact, rapamycin treatment causes the dephosphorylated 4E-BP1 to bind and inhibit the translation initiation factor eIF4E, which then dissociates the cap structure at the 5′ termini of mRNAs, thereby suppressing cap-dependent translation [71]. Thus, inhibiting the mTOR signal strongly reduces intracellular protein synthesis and alleviates the protein load in the ER which, in turn, remarkably suppresses ER stress. In addition, the mTOR signal can directly influence ER stress through a specific Ire1α–ASK1–JNK signal. Inhibiting mTORC1 blocks the Ire1α–ASK1–JNK pathway and suppresses the activation of JNK, which mitigates ER-stress-induced apoptosis [72].
Inhibition of the mTOR signal augments the autophagy process to remove damaged macromolecules and misfolded proteins. Inhibition of mTORC1 leads to increased ULK1/2 (UNC-5 like autophagy activating kinase 1/2) activity, which further phosphorylates ATG13 (autophagy related gene 13) to activate the autophagy process [73,74]. At the transcriptional level, inhibition of mTORC1 also modulates autophagy through regulating the localization of TFEB (transcription factor EB), an important autophagy gene regulator. Many studies have demonstrated that mTOR inhibition strongly induces autophagy in various model systems, even in the presence of nutrients [75].
Inhibition of mTOR signal by rapamycin and its analogs leads to a reduction in the synthesis of misfolded proteins and an increase in the degradation of damaged proteins, which further suppresses the ER stress caused by gene mutations in cases of retinal degeneration. Many previous studies have demonstrated that inhibition of mTOR has neuroprotective effects, including rescue of photoreceptors and or RPE from mutant gene-induced apoptosis, which slow down the process of retinal degeneration [76].
P23H, one of the Rh mutations, misfolds and accumulates in the ER. If degradation fails, the protein can aggregate to further trigger photoreceptor death. Moreover, mutantP23H negatively competes with the function of the wild-type (WT) protein. However, the toxic and negative properties of mutantP23Hcan beremarkablyattenuated by mTOR inhibition, since P23H aggregation, caspase activation, and apoptosis have been found to be significantly reduced by mTOR inhibition [77]. In an animal model, the activation of UPR and a consistent increase in BiP and CHOP gene expression was detected in P23H Rh retinas with autosomal dominant retinitis pigmentosa (ADRP), which might stimulate apoptotic signaling in these animals. Injections of rapamycin protected the P23H Rh rod photoreceptors from experiencing physiological decline and slowed the rate of retinal degeneration [78].
In P37H mutant retina, chronic suppression of TOR signaling, using the inhibitor rapamycin, was found to strongly mitigate photoreceptor degeneration and chronic P37H proteotoxic stress [79]. Genetic inhibition of the ER stress-induced JNK/TRAF1 pathway and the APAF-1/caspase-9 pathway dramatically suppressed P37H-induced photoreceptor degeneration. These findings suggest that chronic P37H proteotoxic stress disrupts cellular proteostasis, further leading to metabolic imbalance and mitochondrial failure. Inhibiting the mTOR signal can normalize metabolic function and alleviate ER stress-induced retinal degeneration.
RPE dysfunction has been implicated in various retinal degenerative diseases. Impairment of RPE mitochondria in mice induces gradual epithelium dedifferentiation, loss of RPE features, and cellular hypertrophy through activation of the AKT/mTOR pathway. Treatment with rapamycin has been shown to mitigate key features of dedifferentiation and maintain photoreceptor function [77]. However, Rajala et al. reported that light damage caused the activation of the phosphoinositide 3-kinase and the AKT pathway in rod photoreceptor cells. AKT activation, especially the AKT2 signal, plays a neuroprotective role in light-induced retinal degeneration. AKT2 knock-out mice exhibited a significantly greater sensitivity to stress-induced cell death than rods in heterozygous or wild-type mice. These findings suggest that AKT, as a regulator of the mTOR signal, can also function as a therapeutic target when treating retinal degenerative diseases [80,81].
In a rat model of NMDA (N-methyl-d-aspartate)-induced retinal neurotoxicity, intravitreal injection of NMDA caused a marked increase of leukocytes and microglia and significant capillary degeneration. However, the NMDA-induced changes were significantly reduced by the simultaneous injection of rapamycin. These findings indicate that mTOR inhibition prevents inflammation and capillary degeneration during retinal injury [82].
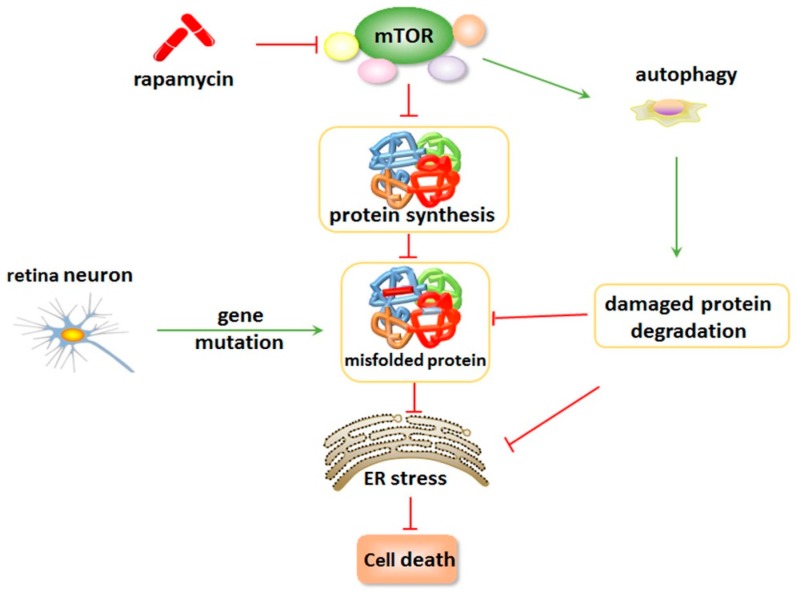
Inhibition of mTOR maintains cellular proteostasis and attenuates ER stress by reducing misfolded protein synthesis and augmenting autophagy to remove misfolded proteins with gene mutations. The mTOR pathway plays an exquisitely complex role in the regulation of retina protein biosynthesis and degradation, as well as in ER stress-induced apoptosis (Figure 3).
Figure 3.
Inhibition of the mTOR signal suppresses ER stress-induced cell death. Inhibition of mTOR maintains cellular proteostasis and attenuates ER stress by reducing misfolded protein synthesis. In addition, inhibition of mTOR can augment autophagy to remove misfolded proteins generated from mutant genes. mTOR inhibition can suppress ER stress-induced apoptosis by regulating retina protein biosynthesis and degradation.
6. Conclusions
In summary, UPR and ER stress are critical factors that contribute to retinal degeneration, yet inhibition of mTOR maintains cellular proteostasis and attenuates ER stress by reducing misfolded protein synthesis and augmenting autophagy to remove misfolded proteins caused by gene mutations [83]. Further studies are needed to investigate the detailed regulatory network, such as the sensitive surveillance mechanisms of mTORC1 and ER stress, and ER-related apoptosis in retinal neurons. Although the exact mechanism(s) that lead to rapamycin’s neuroprotective effects are unclear, it is tempting to speculate that reduction of misfolded protein synthesis and induction of autophagy help prevent the accumulation of abnormal proteins seen in retinal degenerative disorders, and aids in cell survival in the setting of gene mutation injury. Considering that the field of combined mTOR/UPR research is new, significant progress is likely still ahead.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81570864), the Norman Bethune Program of Jilin University (No. 2012208) and the Natural Science Foundation of Jilin Province (No. 20160101004JC; No.20160414045GH).
Abbreviations
| ATF4 | Activating transcription factor 4 |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| ATF6 | Activating transcription factor 6 |
| ATG13 | Autophagy related gene 13 |
| AMP | Adenosine monophosphate |
| AMPK | AMP-dependent kinase |
| AMD | Age-related macular degeneration |
| AAV5 | Adeno-Associated Virus Type 5 |
| AKT1 | AKT serine/threonine kinase 1 |
| adRP | Autosomal dominant RP |
| BiP | Immunoglobulin binding protein |
| cGMP | Cyclic guanosine-mono-phosphate |
| CHOP | C/EBP homologousprotein |
| cKO | Conditional knockout |
| DHRD | Doyne honeycomb retinal dystrophy |
| DEPTOR | Disheveled, Egl-10, and pleckstrin domain-containing mTOR-interacting protein |
| ERAD | ER-associated protein degradation |
| ER | Endoplasmic reticulum |
| ELOVL4 | Elongation of very long chain fatty acids |
| ERK | Extracellular signal related kinase |
| F3 | Fibulin-3 |
| FKBP12 | 12 kDa Protein FK506-binding protein |
| GCL | Ganglion cell layer |
| GRP78 | Glucose-regulated protein 78 |
| GAP | GTPase activating protein |
| HSR | Heat shock response |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| IRD | Inherited retinal degeneration |
| IRE1 | Inositol-requiring protein 1 |
| Ire1α | Inositol-requiring kinase 1 |
| JNK | c-Jun N-terminal kinase |
| LCA | Leber congenital amaurosis |
| ML | Malattia Leventinese |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | mTOR complex 1 |
| mTORC2 | mTOR complex 2 |
| mLST8 | Mammalian lethal with SEC13 protein 8 |
| mSIN1 | Stress-activated MAP kinase-interacting protein 1 |
| ONL | Outer nuclear layer |
| OS | Outer segment |
| OPL | Outer plexiform layer |
| PERK | Protein kinase RNA-like ER kinase |
| PDE6 | Phosphodiesterase |
| PDK1 | 3-Phosphoinositide-dependent protein kinase 1 |
| PIKK | PI3K-kinase-related kinase |
| PI3K | Phosphoinositide 3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-triphosphate |
| PRAS40 | 40 kDa Pro-rich AKT1 substrate 1 |
| PRR5 | Pro-rich protein 5 |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| PN | Photoreceptor neuron |
| p90-RSK | Ribosomal S6 kinase |
| p70S6K | 40S ribosomal protein S6 kinase |
| Rheb | Ras homolog enriched in brain |
| RICTOR | Rapamycin-insensitive companion of mTOR |
| Rh | Rhodopsin |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| RAPTOR | Regulatory associated protein of mTOR |
| S6K1 | S6 kinase 1 |
| TSC | Tuberous sclerosis complex |
| TFEB | Transcription factor immunoglobulin E box-binding proteins |
| TOR | Target of rapamycin |
| T17M | Threonine-to-methionine mutation at the 17th residue of rhodopsin |
| UPS | The ubiquitin-proteasome system |
| UPR | The unfolded protein response |
| ULK | UNC-5 like autophagy activating kinase |
| VEGF | Vascular endothelial growth factor |
| WT | Wild-type |
| 4E-BP1 | 4E-binding protein 1 |
| 5′-TOP | 5′-Terminal oligopyrimidine tract |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Brzezinski J.A., Reh T.A. Photoreceptor cell fate specification in vertebrates. Development. 2015;142:3263–3273. doi: 10.1242/dev.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman M.S., Hughes S.E., Valentino T.L., Liu Y. Photoreceptor transplantation: Anatomic, electrophysiologic, and behavioral evidence for the functional reconstruction of retinas lacking photoreceptors. Exp. Neurol. 1992;115:87–94. doi: 10.1016/0014-4886(92)90227-H. [DOI] [PubMed] [Google Scholar]
- 3.Wert K.J., Lin J.H., Tsang S.H. General pathophysiology in retinal degeneration. Dev. Ophthalmol. 2014;53:33–43. doi: 10.1159/000357294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb H., Nelson R. The Organization of Photoreceptor to Bipolar Synapses in the Outer Plexiform Layer. Springer; Houten, The Netherlands: 1995. pp. 273–296. [Google Scholar]
- 5.Schiller P.H. Parallel information processing channels created in the retina. Proc. Natl. Acad. Sci. USA. 2010;107:17087–17094. doi: 10.1073/pnas.1011782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahel J.A., Marazova K., Audo I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014;5:a017111. doi: 10.1101/cshperspect.a017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A.J., Bainbridge J.W., Ali R.R. Prospects for retinal gene replacement therapy. Trends Genet. 2009;25:156–165. doi: 10.1016/j.tig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Thompson D.A., Ali R.R., Banin E., Branham K.E., Flannery J.G., Gamm D.M., Hauswirth W.W., Heckenlively J.R., Iannaccone A., Jayasundera K.T., et al. Advancing therapeutic strategies for inherited retinal degeneration: Recommendations from the Monaciano Symposium. Investig. Ophthalmol. Vis. Sci. 2015;56:918–931. doi: 10.1167/iovs.14-16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis G.H. Mechanisms of cell death in the inherited retinal degenerations. Am. J. Hum. Genet. 1998;62:503–508. doi: 10.1086/301772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sin O., Nollen E.A. Regulation of protein homeostasis in neurodegenerative diseases: The role of coding and non-coding genes. Cell. Mol. Life Sci. 2015;72:4027–4047. doi: 10.1007/s00018-015-1985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K., Matsuda N. Proteostasis and neurodegeneration: The roles of proteasomal degradation and autophagy. Biochim. Biophys. Acta. 2014;1843:197–204. doi: 10.1016/j.bbamcr.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Castelao B., Ruiz-Rivas C., Castano J.G. A critical appraisal of quantitative studies of protein degradation in the framework of cellular proteostasis. Biochem. Res. Int. 2012;2012:823597. doi: 10.1155/2012/823597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing G., Wang J.J., Zhang S.X. ER stress and apoptosis: A new mechanism for retinal cell death. Exp. Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crippa V., D'Agostino V.G., Cristofani R., Rusmini P., Cicardi M.E., Messi E., Loffredo R., Pancher M., Piccolella M., Galbiati M., et al. Transcriptional induction of the heat shock protein B8 mediates the clearance of misfolded proteins responsible for motor neuron diseases. Sci. Rep. 2016;6:22827. doi: 10.1038/srep22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuldt A. Protein metabolism: A channel for ERAD. Nat. Rev. Mol. Cell Biol. 2014;15:2. doi: 10.1038/nrm3725. [DOI] [PubMed] [Google Scholar]
- 16.Appenzeller-Herzog C., Hall M.N. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Deldicque L. Endoplasmic reticulum stress in human skeletal muscle: Any contribution to sarcopenia? Front. Physiol. 2013;4:236. doi: 10.3389/fphys.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata Y., Voeltz G.K., Rapoport T.A. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Hiramatsu N., Chiang W.C., Kurt T.D., Sigurdson C.J., Lin J.H. Multiple Mechanisms of unfolded protein response-induced cell death. Am. J. Pathol. 2015;185:1800–1808. doi: 10.1016/j.ajpath.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muoio D.M., Newgard C.B. Biomedicine. Insulin resistance takes a trip through the ER. Science. 2004;306:425–426. doi: 10.1126/science.1104680. [DOI] [PubMed] [Google Scholar]
- 21.Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 22.Perri E.R., Thomas C.J., Parakh S., Spencer D.M., Atkin J.D. The Unfolded protein response and the role of protein disulfide isomerase in neurodegeneration. Front. Cell Dev. Biol. 2015;3:80. doi: 10.3389/fcell.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 24.Chabre M., le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44:9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 25.Powers E.T., Morimoto R.I., Dillin A., Kelly J.W., Balch W.E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 26.Mendes C.S., Levet C., Chatelain G., Dourlen P., Fouillet A., Dichtel-Danjoy M.L., Gambis A., Ryoo H.D., Steller H., Mollereau B. ER stress protects from retinal degeneration. EMBO J. 2009;28:1296–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Athanasiou D., Aguila M., Bevilacqua D., Novoselov S.S., Parfitt D.A., Cheetham M.E. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–2017. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhootada Y., Kotla P., Zolotukhin S., Gorbatyuk O., Bebok Z., Athar M., Gorbatyuk M. Limited ATF4 expression in degenerating retinas with ongoing ER stress promotes photoreceptor survival in a mouse model of autosomal dominant retinitis pigmentosa. PLoS ONE. 2016;11:e0154779. doi: 10.1371/journal.pone.0154779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RetNet: Retinal Information Network. [(accessed on 18 January 2017)]. Available online: https://sph.uth.edu/Retnet.
- 30.Parfitt D.A., Cheetham M.E. Targeting the proteostasis network in rhodopsin retinitis pigmentosa. Adv. Exp. Med. Biol. 2016;854:479–484. doi: 10.1007/978-3-319-17121-0_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrington D.A., Sinha D., Kaarniranta K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 2016;51:69–89. doi: 10.1016/j.preteyeres.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daiger S.P., Bowne S.J., Sullivan L.S. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galy A., Roux M.J., Sahel J.A., Leveillard T., Giangrande A. Rhodopsin maturation defects induce photoreceptor death by apoptosis: A fly model for RhodopsinPro23His human retinitis pigmentosa. Hum. Mol. Genet. 2005;14:2547–2557. doi: 10.1093/hmg/ddi258. [DOI] [PubMed] [Google Scholar]
- 34.Lin J.H., Li H., Yasumura D., Cohen H.R., Zhang C., Panning B., Shokat K.M., Lavail M.M., Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorbatyuk M.S., Knox T., LaVail M.M., Gorbatyuk O.S., Noorwez S.M., Hauswirth W.W., Lin J.H., Muzyczka N., Lewin A.S. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. USA. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhury S., Bhootada Y., Gorbatyuk O., Gorbatyuk M. Caspase-7 ablation modulates UPR, reprograms TRAF2-JNK apoptosis and protects T17M rhodopsin mice from severe retinal degeneration. Cell Death Dis. 2013;4:e528. doi: 10.1038/cddis.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang W.C., Messah C., Lin J.H. IRE1 directs proteasomal and lysosomal degradation of misfolded rhodopsin. Mol. Biol. Cell. 2012;23:758–770. doi: 10.1091/mbc.E11-08-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang W.C., Kroeger H., Sakami S., Messah C., Yasumura D., Matthes M.T., Coppinger J.A., Palczewski K., LaVail M.M., Lin J.H. Robust endoplasmic reticulum-associated degradation of rhodopsin precedes retinal degeneration. Mol. Neurobiol. 2015;52:679–695. doi: 10.1007/s12035-014-8881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karan G., Yang Z., Howes K., Zhao Y., Chen Y., Cameron D.J., Lin Y., Pearson E., Zhang K. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. Mol. Vis. 2005;11:657–664. [PubMed] [Google Scholar]
- 40.Tran H.V., Moret E., Vaclavik V., Marcelli F., Abitbol M.M., Munier F.L., Schorderet D.F. Swiss family with dominant Stargardt Disease caused by a recurrent mutation in the ELOVL4 gene. Klin. Monbl. Augenheilkd. 2016;233:475–477. doi: 10.1055/s-0042-102585. [DOI] [PubMed] [Google Scholar]
- 41.Mandal N.A., Tran J.T., Zheng L., Wilkerson J.L., Brush R.S., McRae J., Agbaga M.P., Zhang K., Petrukhin K., Ayyagari R., et al. In vivo effect of mutant ELOVL4 on the expression and function of wild-type ELOVL4. Investig. Ophthalmol. Vis. Sci. 2014;55:2705–2713. doi: 10.1167/iovs.13-13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karan G., Lillo C., Yang Z., Cameron D.J., Locke K.G., Zhao Y., Thirumalaichary S., Li C., Birch D.G., Vollmer-Snarr H.R., et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: A model for macular degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maugeri A., Meire F., Hoyng C.B., Vink C., van Regemorter N., Karan G., Yang Z., Cremers F.P., Zhang K. A novel mutation in the ELOVL4 gene causes autosomal dominant Stargardt-like macular dystrophy. Investig. Ophthalmol. Vis. Sci. 2004;45:4263–4267. doi: 10.1167/iovs.04-0078. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K., Kniazeva M., Han M., Li W., Yu Z., Yang Z., Li Y., Metzker M.L., Allikmets R., Zack D.J., et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet. 2001;27:89–93. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 45.Logan S., Agbaga M.P., Chan M.D., Kabir N., Mandal N.A., Brush R.S., Anderson R.E. Deciphering mutant ELOVL4 activity in autosomal-dominant Stargardt macular dystrophy. Proc. Natl. Acad. Sci. USA. 2013;110:5446–5451. doi: 10.1073/pnas.1217251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowes C., Li T., Danciger M., Baxter L.C., Applebury M.L., Farber D.B. Retinal degeneration in the rd mouse is caused by a defect in the β subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 47.Pittler S.J., Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase β-subunit gene of the rd mouse. Proc. Natl. Acad. Sci. USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma E.Y., Lewis A., Barabas P., Stearns G., Suzuki S., Krizaj D., Brockerhoff S.E. Loss of Pde6 reduces cell body Ca2+ transients within photoreceptors. Cell Death Dis. 2013;4:e797. doi: 10.1038/cddis.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L.P., Wu L.M., Guo X.J., Tso M.O. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Investig. Ophthalmol. Vis. Sci. 2007;48:5191–5198. doi: 10.1167/iovs.07-0512. [DOI] [PubMed] [Google Scholar]
- 50.Griciuc A., Aron L., Roux M.J., Klein R., Giangrande A., Ueffing M. Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet. 2010;6:e1001075. doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trapani I., Banfi S., Simonelli F., Surace E.M., Auricchio A. Gene therapy of inherited retinal degenerations: Prospects and challenges. Hum. Gene Ther. 2015;26:193–200. doi: 10.1089/hum.2015.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hulleman J.D. Malattia Leventinese/Doyne honeycomb retinal dystrophy: Similarities to age-related macular degeneration and potential therapies. Adv. Exp. Med. Biol. 2016;854:153–158. doi: 10.1007/978-3-319-17121-0_21. [DOI] [PubMed] [Google Scholar]
- 53.Griciuc A., Aron L., Piccoli G., Ueffing M. Clearance of Rhodopsin(P23H) aggregates requires the ERAD effector VCP. Biochim. Biophys. Acta. 2010;1803:424–434. doi: 10.1016/j.bbamcr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Tam B.M., Moritz O.L. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2006;47:3234–3241. doi: 10.1167/iovs.06-0213. [DOI] [PubMed] [Google Scholar]
- 55.Hulleman J.D., Kelly J.W. Genetic ablation of N-linked glycosylation reveals two key folding pathways for R345W fibulin-3, a secreted protein associated with retinal degeneration. FASEB J. 2015;29:565–575. doi: 10.1096/fj.14-255414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroder M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez E., Berna-Erro A., Lopez J.J., Granados M.P., Bermejo N., Brull J.M., Salido G.M., Rosado J.A., Redondo P.C. Role of mTOR1 and mTOR2 complexes in MEG-01 cell physiology. Thromb. Haemost. 2015;114:969–981. doi: 10.1160/TH14-09-0727. [DOI] [PubMed] [Google Scholar]
- 58.Yoon M.S., Choi C.S. The role of amino acid-induced mammalian target of rapamycin complex 1 (mTORC1) signaling in insulin resistance. Exp. Mol. Med. 2016;48:e201. doi: 10.1038/emm.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie J., Wang X., Proud C.G. mTOR inhibitors in cancer therapy. F1000Research. 2016 doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin T.D., Chen X.W., Kaplan R.E., Saltiel A.R., Walker C.L., Reiner D.J., Der C.J. Ral and Rheb GTPase activating proteins integrate mTOR and GTPase signaling in aging, autophagy, and tumor cell invasion. Mol. Cell. 2014;53:209–220. doi: 10.1016/j.molcel.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W., Lei C., Fan J., Wang J. miR-18a promotes cell proliferation of esophageal squamous cell carcinoma cells by increasing cylin D1 via regulating PTEN-PI3K-AKT-mTOR signaling axis. Biochem. Biophys. Res. Commun. 2016;477:144–149. doi: 10.1016/j.bbrc.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 62.Li C.M., Narayanan R., Lu Y., Hurh E., Coss C.C., Barrett C.M., Miller D.D., Dalton J.T. 2-Arylthiazolidine-4-carboxylic acid amides (ATCAA) target dual pathways in cancer cells: 5′-AMP-activated protein kinase (AMPK)/mTOR and PI3K/Akt/mTOR pathways. Int. J. Oncol. 2010;37:1023–1030. [PubMed] [Google Scholar]
- 63.Santos R.X., Correia S.C., Cardoso S., Carvalho C., Santos M.S., Moreira P.I. Effects of rapamycin and TOR on aging and memory: Implications for Alzheimer's disease. J. Neurochem. 2011;117:927–936. doi: 10.1111/j.1471-4159.2011.07262.x. [DOI] [PubMed] [Google Scholar]
- 64.Banaszynski L.A., Liu C.W., Wandless T.J. Characterization of the FKBP.rapamycin.FRB ternary complex. J. Am. Chem. Soc. 2005;127:4715–4721. doi: 10.1021/ja043277y. [DOI] [PubMed] [Google Scholar]
- 65.Rogina B., Helfand S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Von Walden F., Liu C., Aurigemma N., Nader G.A. mTOR signaling regulates myotube hypertrophy by modulating protein synthesis, rDNA transcription and chromatin remodeling. Am. J. Physiol. Cell Physiol. 2016;311:C663–C672. doi: 10.1152/ajpcell.00144.2016. [DOI] [PubMed] [Google Scholar]
- 67.Zhuang F., Li M., Gao X., Wang Y., Wang D., Ma X., Ma T., Gu S. The antidepressant-like effect of alarin is related to TrkB-mTOR signaling and synaptic plasticity. Behav. Brain Res. 2016;313:158–171. doi: 10.1016/j.bbr.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 68.Rivera Rivera A., Castillo-Pichardo L., Gerena Y., Dharmawardhane S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE. 2016;11:e0157251. doi: 10.1371/journal.pone.0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdel-Kahaar E., Kabakchiev M., Hartmann B., Wieland E., Shipkova M. Performance of a phosphoflow assay to determine phosphorylation of S6 ribosomal protein as a pharmacodynamic read out for mTOR inhibition. Clin. Biochem. 2016;49:1181–1187. doi: 10.1016/j.clinbiochem.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Bahrami B.F., Ataie-Kachoie P., Pourgholami M.H., Morris D.L. p70 Ribosomal protein S6 kinase (Rps6kb1): An update. J. Clin. Pathol. 2014;67:1019–1025. doi: 10.1136/jclinpath-2014-202560. [DOI] [PubMed] [Google Scholar]
- 71.Wang S., Patsis C., Koromilas A.E. Stat1 stimulates cap-independent mRNA translation to inhibit cell proliferation and promote survival in response to antitumor drugs. Proc. Natl. Acad. Sci. USA. 2015;112:E2149–E2155. doi: 10.1073/pnas.1420671112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonseca B.D., Zakaria C., Jia J.J., Graber T.E., Svitkin Y., Tahmasebi S., Healy D., Hoang H.D., Jensen J.M., Diao I.T., et al. La-related protein 1 (LARP1) represses terminal oligopyrimidine (TOP) mRNA translation downstream of mTOR complex 1 (mTORC1) J. Biol. Chem. 2015;290:15996–16020. doi: 10.1074/jbc.M114.621730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alers S., Loffler A.S., Paasch F., Dieterle A.M., Keppeler H., Lauber K., Campbell D.G., Fehrenbacher B., Schaller M., Wesselborg S., et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–1433. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loffler A.S., Alers S., Dieterle A.M., Keppeler H., Franz-Wachtel M., Kundu M., Campbell D.G., Wesselborg S., Alessi D.R., Stork B. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy. 2011;7:696–706. doi: 10.4161/auto.7.7.15451. [DOI] [PubMed] [Google Scholar]
- 75.He L., Weber K.J., Diwan A., Schilling J.D. Inhibition of mTOR reduces lipotoxic cell death in primary macrophages through an autophagy-independent mechanism. J. Leukoc. Biol. 2016;100:1113–1124. doi: 10.1189/jlb.3A1015-463R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh A.K., Kashyap M.P., Tripathi V.K., Singh S., Garg G., Rizvi S.I. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0129-3. [DOI] [PubMed] [Google Scholar]
- 77.Mendes H.F., Cheetham M.E. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum. Mol. Genet. 2008;17:3043–3054. doi: 10.1093/hmg/ddn202. [DOI] [PubMed] [Google Scholar]
- 78.Sizova O.S., Shinde V.M., Lenox A.R., Gorbatyuk M.S. Modulation of cellular signaling pathways in P23H rhodopsin photoreceptors. Cell. Signal. 2014;26:665–672. doi: 10.1016/j.cellsig.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griciuc A., Roux M.J., Merl J., Giangrande A., Hauck S.M., Aron L., Ueffing M. Proteomic survey reveals altered energetic patterns and metabolic failure prior to retinal degeneration. J. Neurosci. 2014;34:2797–2812. doi: 10.1523/JNEUROSCI.2982-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajala A., Tanito M., Le Y.Z., Kahn C.R., Rajala R.V. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G., Anderson R.E., Tomita H., Adler R., Liu X., Zack D.J., Rajala R.V. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aoki Y., Nakahara T., Asano D., Ushikubo H., Mori A., Sakamoto K., Ishii K. Preventive effects of rapamycin on inflammation and capillary degeneration in a rat model of NMDA-induced retinal injury. Biol. Pharm. Bull. 2015;38:321–324. doi: 10.1248/bpb.b14-00631. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., Yang X., Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell. Signal. 2016;28:1099–1104. doi: 10.1016/j.cellsig.2016.05.007. [DOI] [PubMed] [Google Scholar]