Abstract
Today a cochlear implant (CI) may significantly restore auditory function, even for people with a profound hearing loss. Because the efficacy of a CI is believed to depend mainly on the remaining population of spiral ganglion neurons (SGNs), it is important to understand the timeline of the degenerative process of the auditory neurons following deafness. Guinea pigs were transtympanically deafened with neomycin, verified by recording auditory brainstem responses (ABRs), and then sacrificed at different time points. Loss of SGNs as well as changes in cell body and nuclear volume were estimated. To study the effect of delayed treatment, a group of animals that had been deaf for 12 weeks was implanted with a stimulus electrode mimicking a CI, after which they received a 4‐week treatment with glial cell‐derived neurotrophic factor (GDNF). The electrical responsiveness of the SGNs was measured by recording electrically evoked ABRs. There was a rapid degeneration during the first 7 weeks, shown as a significant reduction of the SGN population. The degenerative process then slowed, and there was no difference in the amount of remaining neurons between weeks 7 and 18. © 2016 The Authors Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Keywords: spiral ganglion, delayed treatment, degeneration, cochlear implant
Impaired auditory function is caused primarily by damage to the sensory cells (hair cells) in the cochlea. Clinically, severe impairment can be treated with a cochlear implant (CI), by which the malfunctioning sensory cells are bypassed directly to stimulate the neurons at the next functional level, the spiral ganglion. However, loss of the sensory cells results in a subsequent degeneration of the spiral ganglion neurons (SGNs; Ylikoski et al., 1974; Webster and Webster, 1981; Zappia and Altschuler, 1989), which drastically reduces the efficacy of the CI. Several animal studies have shown a correlation between electrical responsiveness measured by obtaining electrically evoked auditory brainstem responses (eABRs) and the amount of remaining SGNs in deafened animals (Shinohara et al., 2002; Shepherd et al., 2005; Agterberg et al., 2009; Landry et al., 2011). Although the degeneration is rapid and severe, it is known from previous animal studies that approximately 10% of the SGN will not degenerate even after more than 1 year of deafness (Spoendlin and Suter, 1976). In humans the secondary degeneration of SGNs occurs over several years following hair cell damage, whereas in the guinea pig the process is much more rapid, and reduced electrical responsiveness can be detected within a few weeks of sensory hair cell loss. The guinea pig model is thus useful to mimic the clinical situation in which patients have been deaf for a longer period before receiving a CI.
Because animal models are widely used to study factors influencing CI function, this study was designed to monitor SGN degeneration over a longer period to define the therapeutic window for using a CI. An experimental model was used in which the animals were transtympanically deafened with the ototoxic agent aminoglycoside neomycin sulfate. A neomycin infusion results in rapid degeneration of the hair cells, and, within 1 week, a progressive degeneration of the SGNs starts. It has previously been shown (Shepherd et al., 2008; Agterberg et al., 2009) that the degenerative process following deafness affects not only the number of SGNs but also the size or volume of the cell bodies. In the present study, a stereology technique was applied to estimate not only the degree of cell loss within the spiral ganglion but also changes in the volume of the cell bodies and the nuclei at different time points after deafening. Because it has been demonstrated that an intracochlear infusion of glial cell‐derived neurotrophic factor (GDNF) during the first stages of neuronal degeneration has a beneficial effect on the SGN population (Maruyama et al., 2008; Scheper et al., 2009; Fransson et al., 2010), we tested to determine whether neurotrophic treatment would still have a positive effect at a time point when most of the degenerative changes are manifested.
Our group previously investigated a 6‐week‐delayed neurotrophic treatment (Yamagata et. al, 2004) in which it was clearly seen that a 6‐week‐delayed treatment compared with a 2‐week delay showed significantly higher eABR threshold and lower SGN density. Here we included a small pilot study of three GDNF‐treated animals with a CI to investigate whether there was any electrical response from the SGNs after 12 weeks of deafness.
MATERIALS AND METHODS
Subjects
Normally hearing albino guinea pigs (200–320 g; Harlan Laboratories) of both sexes were used. Animals were kept in an enriched environment and housed in small groups with lights on between 7am and 7pm, with temperature maintained at 21 °C and humidity of 60%.
All animals were tested for normal hearing by recording acoustically evoked ABRs prior to the experiment. All animal procedures were performed in accordance with the ethical guidelines of Karolinska Institutet and were consistent with national regulations for the care and use of animals. The ethical permit was approved by Stockholms Norra Djurförsöksetiska nämnd (approval No. 14/10).
Experimental Design
Cochleae from 24 normally hearing albino guinea pigs were transtympanically and bilaterally deafened by the ototoxic drug neomycin. Animals that did not attain a threshold shift of 60 dB SPL or higher were excluded from the study. After deafening, the animals were divided into normal (cochleae = 6), 1‐week (cochleae = 6), 4‐week (cochleae = 6), 7‐week (cochleae = 6), 10‐week (cochleae = 3), 12‐week (cochleae = 6), and 18‐week (cochleae = 6) groups. From the 12‐week group, six animals were divided into two groups. One group (n = 3) received a stimulus electrode mimicking a CI and an osmotic pump for cochlear infusion of the neurotrophic factor GDNF (Amgen, Thousand Oaks, CA) for 4 weeks (Maruyama et al., 2008). The other group (n = 3) served as a control group and received a CI and an osmotic pump containing artificial perilymph (AP; Ringer‐acetate; Fresenius Kabi Sweden). For animals staying in the experiment beyond 1 week, click‐evoked ABRs were measured every third week. At the end of the experiment, frequency‐specific ABRs were recorded at 4, 8, 12.5, and 20 kHz. After 4 weeks of treatment, the pumps were removed, but the animals stayed in the study for an additional 2 weeks (Fig. 1). To reduce the number of animals used in the study, the right ear from the implanted subjects served as the 18‐week group.
Figure 1.
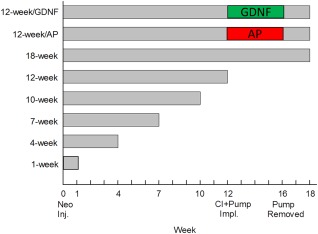
Graphic presentation of the experimental design. ABRs were measured every third week throughout the experiment. For animals implanted with an electrode mimicking a cochlear implant, eABRs were measured every week. [Color figure can be viewed at wileyonlinelibrary.com]
Deafening Procedure
Animals were anesthetized with xylazine (10 mg/kg, i.m.; Bayer) and ketamine (40 mg/kg, i.m.; Pfizer AB). Ophthalmic ointment was applied to the eyes to prevent corneal ulcers resulting from the lack of blink reflex caused by ketamine. The animal was placed on its side, and a small amount of the local anesthetic Marcain (AstraZenica) was administered subcutaneously on the tragus. A small piece of the tragus was removed to provide free access to the transtympanic membrane. A thin needle (30 G) was placed in the upper left corner of the transtympanic membrane, and approximately 0.15 ml of 10% neomycin solution (Sigma‐Aldrich) was infused into the middle ear. The needle was withdrawn, the animal stayed lying on its side for 20 min and was then turned over, and the procedure was repeated for the contralateral ear.
Implantation Surgery
Guinea pigs were deeply anesthetized with xylazine (10 mg/kg, i.m.) and ketamine (40 mg/kg, i.m.). The procedure for implanting a stimulus electrode mimicking a CI and an osmotic pump for intracochlear infusion has previously been described (Brown et al., 1993; Fransson et al., 2009). Briefly, the middle ear was exposed by a postauricular approach to visualize the cochlea. A small hole was drilled in the basal turn of the cochlea to insert the cannula (inner diameter 0.72 mm; Scientific Commodities), which was preloaded with 24 µl AP and connected to an osmotic pump (infusion rate 0.5 µl/hr; model 2002 Alzet; DURECT). The stimulus electrode (stimulus electrode, Ø 0.074 mm; ground electrode Ø 0.125 mm; ADVENT) was inserted into the scala tympani through the round window, and the ground electrode was placed in the middle ear cavity adjacent to the middle ear wall. The percutaneous connector connected to the electrodes was cemented to the skull with dental cement. Finally, a preloaded osmotic pump containing GDNF or AP was connected to the cannula and placed under the skin on the back of the animal. The reservoir was replaced after 2 weeks, and after another 2 weeks the osmotic pump was removed and the cannula was sealed.
ABR
To monitor auditory function and to verify the deafening procedure, click‐evoked ABRs were recorded. Animals were anesthetized as previously described and placed in a sound‐proof box. The stimulus signal was generated through a signal analyzer (Tucker‐Davis Technologies) controlled by a personal computer and delivered by an earphone positioned within the ear canal. Neural responses were collected with a subdermal electrode placed at the vertex (active), one subdermal needle electrode placed subcutaneously above the bulla on the deafened ear (reference), and a ground electrode (subcutaneous in the thigh). Threshold was defined as the lowest stimulus intensity that produced a visually consistent waveform and was reproducible.
Click ABRs showed that, after deafening, all animals were profoundly deaf (>60 dB threshold shift). Prior to sacrificing the animals, we tested them with both click ABR and frequency‐specific ABR (4, 8, 12.5, and 20 kHz). At this time, it was not possible to elicit any thresholds with the present equipment.
eABR
Animals were anesthetized and placed in a sound‐proof box. The eABRs were recorded with a SigGen System 2 signal analyzer (Tucker‐Davies Technologies). This method has been described previously (Hall, 1990). Responses are summed to alternate polarity current pulses, in which each pair provides charge balancing. In all, 2,048 responses to 50‐µsec computer‐generated monophasic current pulses presented at 50 pps with an alternating polarity on each presentation were collected from an epidural electrode placed at vertex (active) and one subdermal needle electrode placed subcutaneous above the bulla on the deafened ear (reference) and one in the hind leg (ground).
Histology
After the last measurement, animals were deeply anesthetized with pentobarbital sodium (25 mg/kg, i.p.) and were transcardially perfused with saline (37 °C), followed by cold glutaraldehyde 2.5% in 0.1 M phosphate buffer. The temporal bones were removed, and the bulla was opened to expose the cochlea. Small fenestrations were made in the apex and the round window, and fixative was gently flushed through the cochlea. The temporal bones were decalcified for 2 weeks in 0.1 M EDTA in 0.1 M phosphate buffer, rinsed, dehydrated, and embedded in a 2‐hyroxyethyl methacrylate‐based resin (Technovit 7100; Heraeus Kulzer GmbH). For stereological analysis, 24‐µm‐thick sections were prepared, sectioned consecutively throughout the whole cochlea, mounted on slides, and stained with hematoxylin and eosin.
SGN Counting
The total number of SGNs in the cochlea was estimated with the optical fractionator technique (Gundersen et al., 1988; Tandrup, 1993; Watanabe et al., 2010). The number of sections counted varied from six to 15, the higher number for the group with the longest time of deafness to collect enough cells. The technique requires that 100 cells or more be counted in each animal. A Zeiss Axioplan microscope with a motorized stage (Prior ProScan II) and an electronic microcator (Haidenhain MT2) with a digital readout for measuring movements in the z‐direction were interfaced with a digital camera (Pixel link) and a personal computer running newCAST software (Visiopharm Denmark). Tissue from the inner ear was divided into sampling fractions with the optical fractionator. The fractions were determined in three levels: 1) the section of the sampling fraction (the ratio of counted sections per cochlea), 2) the area of the sampling fraction (percentage size of counting frame out of the area of interest), and 3) the height sampling of the fraction, defined by dividing the height of the counting frame with the average section thickness. The SGNs were outlined at ×10, and counting was performed with a ×100 oil immersion objective (NA = 1.3). Counting frames with an area of 1,600 µm2 and height of 12 µm were digitally placed over sections. SGNs were counted in all predetermined sections. The total number of cells was estimated by multiplying the counted number with the inverted number of each of the fractions.
Volume of SGN Cell Bodies and Nuclei
Volumes of the cell bodies and the nuclei were estimated in the experimental normal, 4‐week, 10‐week, and 18‐week groups and the two groups with cochlear implant receiving GDNF or AP treatment. The microscope was set up as previously described for the counting of the SGNs. The average volume of the type I SGN cell body and nucleus was estimated with the nucleator technique (Gundersen et al., 1988; Tandrup, 1993). The average length of four randomly placed test lines, from the most centrally located nucleolus to the cell membrane for SGN cell body and from the most centrally located nucleolus to the nucleus membrane, was calculated. The number of measured cell bodies varied from 93 to 245 per animal.
Statistical Analysis
For statistical analyses in the calculation of the remaining SGNs and for the investigation of the cell body and nucleus volume, one‐way ANOVA was used, followed by multiple comparisons vs. control group, Holm‐Sidak method. The results are presented as mean ± SEM.
RESULTS
SGN Population After Deafening
After only 1 week of deafness (1‐week group), the number of remaining SGNs was significantly reduced (P < 0.05) compared with the normal group (Fig. 2, Table 1). When the normal group and the groups that had been been deaf for 4 weeks or longer were compared, the effect was even more evident (P < 0.001). There was a significant difference (P < 0.001) between the 1‐week group and the 4‐week group. In the 1‐week group, 83% of the SGNs remained, whereas only 41% of the neurons remained at 4 weeks after deafening. For the groups that had been deaf for longer periods (7, 10, 12, and 18 weeks), approximately 20% of the SGNs remained compared with the group with normal animals. Thus, there was no significant difference among the groups that had been deaf for 7, 10, 12, or 18 weeks, suggesting that the major degenerative changes in SGNs occur within the early weeks after ototoxic trauma. Table 1 shows the percentage of remaining SGNs for all groups compared with the normal group of animals.
Figure 2.
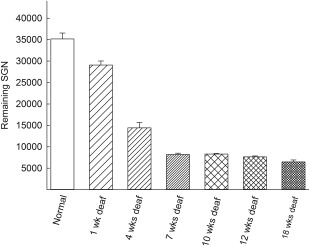
Total number of remaining spiral ganglion neurons after neomycin deafening. After 1 week of deafness, there were significant reductions in the number of SGNs (P < 0.05), which increased over time (normal vs. 4 weeks of deafness, P < 0.001; normal vs. 7 weeks of deafness, P < 0.001). There were also significant differences among the deafened groups (1 week vs. 4 weeks, P < 0.001; 4 weeks vs. 7 weeks, P < 0.001).
Table 1.
Percentage of Remaining SGNs After Deafening Compared With Normal (Not‐Deafened) Animals
| Group | Remaining SGNs compared with normal (%) |
|---|---|
| 1‐week | ∼83 |
| 4‐week | ∼41 |
| 7‐week | ∼23 |
| 10‐week | ∼23 |
| 12‐week | ∼22 |
| 18‐week | ∼19 |
| 12‐week + GDNF | ∼21 |
| 12‐week + AP | ∼15 |
Changes in Cell Body and Nucleus Volumes
The volumes of the cell bodies and the nuclei were estimated in a representative selection of the groups (normal, 4‐week, 10‐week, and 18‐week groups; see Fig. 4). In all deafened groups, the volume of the cell bodies was smaller compared with the control group (4‐week and 10‐week groups, P < 0.001; 18‐week group, P < 0.05). In the 18‐week group, it was particularly difficult to measure the cell body size because of the fact that, in many neurons, the myelin sheaths were absent or broken, and the edges of the cell bodies were much frayed. The volume of the cell body was measured only in cells that had an intact nucleus. In the group that had been deaf for 18 weeks, there was also a substantial amount of extensive intracellular cavities and vesicles in the cytoplasm, which was not observed to a similar extent in the other groups.
Figure 4.
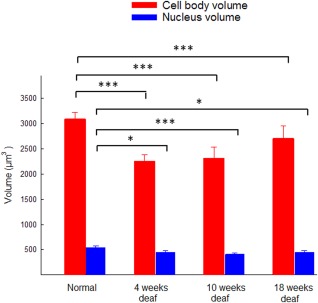
Estimated volume of the spiral ganglion cell bodies and nuclei in normal animals and in animals deafened for 4, 10, or 18 weeks. In comparison with the normal group, there were significant reductions in the volume of the cell bodies in the groups that had been deaf for 4, 10 (*P < 0.001), and 18 weeks (*P < 0.05). The volume of the nuclei was also reduced (4 and 18 weeks, *P < 0.05; 10 weeks, *P < 0.001) compared with the normal group. [Color figure can be viewed at wileyonlinelibrary.com]
All deafened groups displayed a statistical difference in the nucleus volume compared with the normal group (normal vs. 4‐week group, P < 0.05; normal vs. 10‐week group, P < 0.001; normal vs. 18‐week groups, P < 0.05). All nuclei appeared normal but with a smaller volume compared with the nuclei in the normal group.
Effects of GDNF
Twelve weeks after deafness, two groups of animals were implanted with an electrode to elicit electrical responses in the auditory nerve, thus mimicking a CI. By recording these responses, SGN function (i.e., electrical responsiveness) could be monitored following intracochlear infusion of GDNF or AP (control). In these animals, eABR was measured weekly during 4 weeks of treatment and for 2 additional weeks without treatment, i.e., a total of six measurements/animal. However, during these 6 weeks, it was not possible to elicit eABRs from any animal with the present equipment. There were significantly (P > 0.05) more remaining SGNs in the GDNF‐treated group than in the group that received only AP (Fig. 3). The volume of the nuclei was significantly (P < 0.05) smaller in the AP‐treated group compared with the normal group (Fig. 5).
Figure 3.
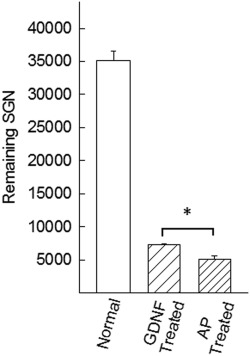
The effect of GDNF treatment showed that the number of remaining SGNs in the GDNF‐treated group was significantly (P < 0.05) higher compared with the AP‐treated (control) group.
Figure 5.
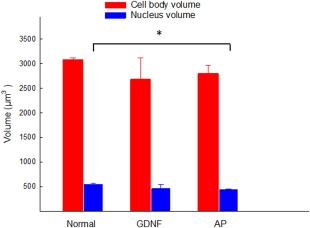
Estimated volumes of the spiral ganglion cell bodies and nuclei in normal animals and in deafened animals implanted with a stimulus electrode mimicking a CI and treated with an intracochlear infusion of GDNF or AP (control). A significant difference (*P < 0.05) was found only for the AP treated group with regard to the volume of the nucleus. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Previous studies in the guinea pig have shown that SGN degeneration is already evident 1 week after aminoglycoside exposure (Dodson and Mohuiddin, 2000; van Loon et al., 2013). Our model seeks to mimic a deaf patient lacking or with only few remaining hair cells that will be followed by a secondary degeneration of a SGNs. It cannot be excluded that this deafening technique with an infusion of aminoglycoside has a direct effect on the SGNs. However, it has been shown that, compared with other ototoxic agents in animal studies (Webster and Webster, 1981; Leake and Hradek, 1988; Zappia and Altschuler, 1989), the density of SGNs in human temporal bones has remained high in the inner ears of some patients who were deafened by aminoglycoside during life (Nadol, 1997).
The rapid progression of degeneration obviously limits the therapeutic window. It has been shown that, when treatment (e.g., infusion of neurotrophic factors) is delayed, the positive effects on CI function are lower compared with when treatment starts shortly after the trauma (Yamagata et al., 2004; Agterberg et al., 2009; Fransson et al., 2010; Ramekers et al., 2015). The present study further demonstrates that the progression of structural degeneration is rapid and complete within 7 weeks. Four weeks after deafening, about 40% of the SGNs remained structurally present, and, at 7 weeks, only 23% of the SGNs remained structurally present. There were no statistical differences among the groups that had been deaf for 7, 10, 12, and 18 weeks. Thus, after the early rapid loss of SGNs, the remaining neural population appears to be resilient. It should be noted that there may be functional differences among the SGNs at the different time points that are not reflected in the number of remaining neurons. It is, however, generally believed that a significant factor deciding the functional outcome of a CI is the number of remaining SGNs. The present study suggests that, at least in the guinea pig, the time window for implantation as well as other therapeutic measures is limited to about 4 weeks after deafening (Fig. 2). This would constitute a time frame during which the efficacy of a CI is expected to be good because of a reasonably large and functional SGN population. This is in line with previous experimental studies that have demonstrated significantly reduced electrical responsiveness at week 6 after deafness (Yamagata et al., 2004). Consequently, during this period, it would also be feasible to initiate pharmacological intervention to rescue SGNs from further degeneration and, thus, maintain the size of the SGN population (Agterberg et al., 2009; Fransson et al., 2010).
The volume of the SGN cell bodies and nuclei was measured in animals deafened for 4, 10, and 18 weeks. In all groups, the cell body and nucleus volume decreased compared with the normal group. At 18 weeks there were distinct structural changes, with extensive extracellular cavities and vesicles in the cytoplasm and disruptions of the myelin sheaths surrounding the cells (Fig. 6). The SGN cell body has been measured in previous studies, and the results have varied (Dodson and Mohuiddin, 2000; Fransson et al., 2010; van Loon et al., 2013). The reason is probably the different measuring techniques that have been applied, different ways of calculating the results, and different deafening procedures.
Figure 6.
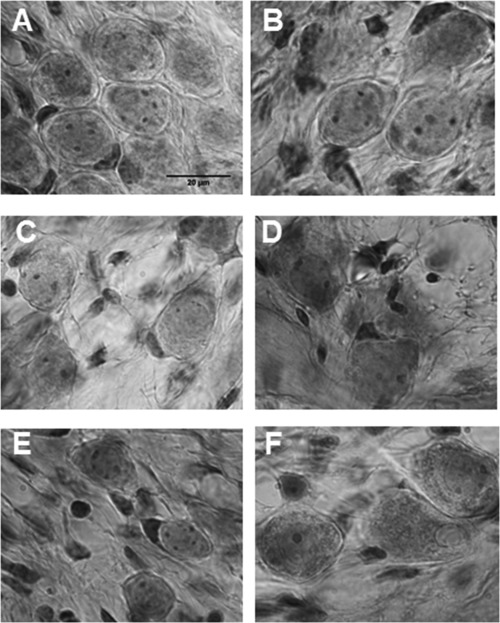
Photomicrographs illustrating the spiral ganglion region in a normal (control) animal (A); animals deafened for 4 weeks (B), 10 weeks (C), and 18 weeks (D); and the spiral ganglion region in 12‐week‐deafened animals followed by GDNF treatment (E) and 12‐week‐deafened animals followed by AP (control) treatment (F). The degenerative changes over time are clearly seen when comparing the different groups. In the 18‐week‐deaf group (D) and the group treated with AP (F), there are multiple vesicles in the cytoplasm, and the myelin sheath is broken. In contrast, the cells from the group treated with GDNF appear healthier and contain fewer vesicles in the cytoplasm, and in most cells there is an intact myelin sheath. Scale bar = 20 µm.
To investigate whether the therapeutic window could be extended beyond 7 weeks, the effect of intracochlear infusion of GDNF was tested in a small pilot group of animals (3) at 12 weeks after deafening. GDNF was infused for 4 weeks, followed by an additional 2‐week period without treatment. However, it was not possible during these 6 weeks to elicit any eABRs from the SGNs with the present equipment. It is possible that, with only about 20% of the SGN population remaining (Fig. 2), the neural activity generated in the auditory pathways was not strong enough to elicit an eABR. However, even though it was not possible to elicit any electrically evoked responses, the group that received GDNF treatment displayed a more normal‐appearing morphology of the SGNs compared with the control AP‐treated group. This is illustrated in Figure 6, showing the structural appearance of the SGNs over the 18‐week experimental period. It should be noted that the SGNs in the animals that received GDNF treatment had a healthier appearance, with fewer vesicles in the cytoplasm and a more intact myelin sheath. Moreover, there was a significant difference (P > 0.05) in the amount of remaining SGNs in the GDNF‐treated group compared with the group that received only AP. Although the findings presented here reflect the situation in an animal model in which degenerative changes occur quite rapidly, our results suggest that inner ear treatment must be initiated soon after trauma.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests.
ROLE OF AUTHORS
AF and MU designed the study. AF collected all data, conducted the analyses, and wrote the first draft of the manuscript. MU contributed to the analyses and the final article.
SIGNIFICANCE For people with profound hearing loss, a cochlear implant is the only therapy available today. The outcome from a cochlear implant depends mainly on the number of the remaining auditory neurons and their electrical responsiveness. Using an animal model, we show that, after experimentally induced deafness, there is rapid neural degeneration within the first 7 weeks, after which the neural population appears resilient. Neurotrophic treatment, known to preserve the neural population and to have beneficial effects on electrical responsiveness, had no detectable effects on functional responses when initiated 12 weeks after deafening. The results suggest a limited therapeutic window for inner ear treatment.
REFERENCES
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. 2009. Enhanced survival of spiral ganglion cells after cessation of treatment with brain‐derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol 10:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. 1993. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res 70:167–172. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. 2000. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol 29:525–537. [DOI] [PubMed] [Google Scholar]
- Fransson A, Jarlebark LE, Ulfendahl M. 2009. In vivo infusion of UTP and uridine to the deafened guinea pig inner ear: effects on response thresholds and neural survival. J Neurosci Res 87:1712–1717. [DOI] [PubMed] [Google Scholar]
- Fransson A, Maruyama J, Miller JM, Ulfendahl M. 2010. Posttreatment effects of local GDNF administration to the inner ears of deafened guinea pigs. J Neurotrauma 27:1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B. 1988. The new stereological tools: disector, fractionator, nucleator, and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881. [DOI] [PubMed] [Google Scholar]
- Hall RD. 1990. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res 49:155–168. [DOI] [PubMed] [Google Scholar]
- Landry TG, Wise AK, Fallon JB, Shepherd RK. 2011. Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment. Hear Res 282:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. 1988. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res 33:11–33. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Miller JM, Ulfendahl M. 2008. Glial cell line‐derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol Dis 29:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB. 1997. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg 117:220–228. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Klis SF, Grolman W. 2015. Temporary neurotrophin treatment prevents deafness‐induced auditory nerve degeneration and preserves function. J Neurosci 35:12331–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, Stover T. 2009. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J Neurosci Res 87:1389–1399. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. 2005. Chronic depolarization enhances the trophic effects of brain‐derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 486:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. 2008. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res 242:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. 2002. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A 99:1657–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H, Suter R. 1976. Regeneration in the VIII nerve. Acta Otolaryngol 81:228–236. [DOI] [PubMed] [Google Scholar]
- Tandrup T. 1993. A method for unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion. J Comp Neurol 329:269–276. [DOI] [PubMed] [Google Scholar]
- van Loon MC, Ramekers D, Agterberg MJ, de Groot JC, Grolman W, Klis SF, Versnel H. 2013. Spiral ganglion cell morphology in guinea pigs after deafening and neurotrophic treatment. Hear Res 298:17–26. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Kirkegaard M, Matsumoto S, Gont C, Mannström P, Ulfendahl M, Fridberger A. 2010. Signaling through erbB receptors is a critical functional regulator in the mature cochlea. Eur J Neurosci 32:717–724. [DOI] [PubMed] [Google Scholar]
- Webster M, Webster DB. 1981. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res 212:17–30. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, Bredberg G. 2004. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin‐deafened guinea pigs. J Neurosci Res 78:75–86. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Wersäll J, Björkroth B. 1974. Degeneration of neural elements in the cochlea of the guinea‐pig after damage to the organ of corti by ototoxic antibiotics. Acta Otolaryngol Suppl 326:23–41. [DOI] [PubMed] [Google Scholar]
- Zappia JJ, Altschuler RA. 1989. Evaluation of the effect of ototopical neomycin on spiral ganglion cell density in the guinea pig. Hear Res 40:29–37. [DOI] [PubMed] [Google Scholar]


