Abstract
This study details the nutritional and digestive properties of protein isolates that are extracted from carp (Cyprinus Carpio L.) muscle using pH shifting methods. Alkaline (ALPI) and acid (ACPI) protein isolates exhibit higher protein yields (87.6%, 76.3%, respectively). In addition to the high recovery of myofibrillar protein, a portion of the water‐soluble proteins is also recovered. The moisture contents of ACPI and ALPI are 85.5% and 88.5%, respectively, and the crude protein contents of these two fractions are 83.20% and 83.0%, respectively, both contents of which are higher than those for fresh muscle. Most part of the ash and fat are removed in the separation process. The protein isolation is also found to be lighter and whiter than the fresh muscle and there is no difference between amino acid content of protein isolation and that of fresh muscle. The maximum solubility of water washed surimi is 73.21%, while solubility of ACPI‐2 and ALPI‐2 (pH 7.0) are 66.67% and 62.08%, respectively. The digestibility of ALPI and ACPI is improved after being treated with chymotrypsin, which is about 7–8 times as that of fresh muscle. The results indicate that the protein isolates have better nutritional and digestive properties than the fresh muscle does in food processing.
Practical Applications
Common carp is a lower additional value fish that exists in large amount in China. This study investigates nutritional and digestive properties of protein from carp extracted by pH shifting methods. According to the obtained data in this study, pH shifting method is a good protein recovery method that can effectively remove bone spurs, skin, fat and other impurities. In addition, sarcoplasmic proteins can also be recovered. The nutritional properties of protein isolates of carp were suitable for supplementing as an ingredient for human consumption. The pH‐shift process greatly improves the protein digestibility. Therefore, there are broad application prospects of the protein isolation as protein ingredients in food industry.
Introduction
The pH‐shift method is widely used in the food processing industry for separating proteins. This method is initially applied to the extraction and separation of vegetable proteins, such as the commercialization of soy protein isolates (SPI). Because of its outstanding functional properties, SPI has been used widely in the food industry as a protein additive (Nolsoe and undeland 2009). In recent years, the pH‐shift method has also been applied to the development of aquatic protein resources. According to the China fisheries statistics yearbook, the total output of freshwater fish aquaculture in 2013 of China was 24,800,000 tons (Bureau of Fisheries in Ministry of Agriculture 2014 China Fisheries Statistical Yearbook). The use of raw materials and extensive processing of low value aquatic by‐products is a contemporary great challenge in Chinese aquatic product industry. From the perspective of developing functional protein ingredients, the pH‐shift method can efficiently recover the fish muscle protein, including myofibrillar protein and the water‐soluble proteins. The recovered proteins have a good color and excellent functional properties, and are safe for consumption. As a result, the use of pH‐shift as a new method for recovering fish protein is of a great interest.
Recently, many researchers who are interested in fish protein separation have focused on the gel properties of the protein isolate products from fish protein and protein recovery yield. The study of the recovery yield and functional properties of protein isolates from white fish such as Mullet (Mugil cephalus) (Kristinsson and Demi 2003), Mulloway (Umbrina) (Perez et al. 2004) and Grouper (Epinephelus adscensionis) (Yongsawatdigul and Park 2004) was conducted and compared with water washed surimi, the pH‐shift method contributed to better recovery for acid soluble proteins and better gel forming for alkali soluble proteins. But for Pacific whiting (Thawornchinsombut and Park 2007), the protein isolates exhibited poorer gel forming ability than the water washed surimi. In addition, Kim studied the effect of different pHs on the gel properties of Pacific cod (Morhua Gadus) (Kim et al. 2003). The result showed that the best gel properties of the protein isolates from Pacific cod (Morhua Gadus) was obtained at a pH of 11. All results suggest that the different varieties of fish and pH have an important influence on the gel properties of protein isolate products. Studies on the protein isolates from red meat fish such as Sardina (Pilchardus) (Batista et al. 2007) show that the recovery yield of protein is higher but the gel forming ability is poorer compared with the water washed surimi. In addition, the research on the preparation of silver carp protein isolates by Taskaya et al. (2009a, 2009b, 2009c) suggested that the gel quality of this product was poor without the addition of functional additives. The study of Tilapia (Oreochromis niloticus) by Foh et al. (2012) found that alkali soluble protein had good emulsifying and foaming properties. Many researchers have also reported productions of various physiologically active peptides from exogenous protease hydrolysis of low value fish as a means for improving effective utilization of other worthless products.
In the process of separation, fish proteins will undergo a series of changes on their molecular structure, conformation, physical and chemical properties, because of the influence of pH, temperature, ionic strength and other factors. These changes will have an effect on the nutrition, safety and food functions of the protein products. Therefore, in this research, we first analyzed the changes of the recovery yield as well as composition of protein. Then, we analyzed the changes of the solubility, the composition of amino acids as well as the digestive characters of protein isolates.
Materials and Methods
Raw Materials
Live carp (Cyprinus Carpio L) were obtained from a Dalian local market during the summer seasons (August and September) in 2014. The fish were immediately placed on ice and transported to our laboratory. The fish were beheaded, and the dorsal white muscle was finely chopped. The resulting meat paste was used for this study.
Protein Solubility at Different pHs
Protein solubility was determined using the procedure of Kristinsson and Hultin (2003). Precooled distilled deionized water (dd H2O) was added to carp meat in the ratio of 9:1 (water : meat, v : w). The mixture was subjected to homogenization using a homogenizer (HG‐200, HSIANGTAI, Japan) at a temperature of 0C. The pH of this homogenate was adjusted to different pHs in the range 2.0 to 13.0 using 1 M HCl or 1 M NaOH within 10 min, followed by continuous stirring for 30 min. The homogenate was then centrifuged (Z326K, HERMLE, Germany) at 12,000 g for 30 min and the supernatant carefully collected. The exception here was one homogenate that before centrifugation, was dissolved in 2 M NaOH followed by gentle shaking overnight to ensure complete solubilization prior to protein content determination. The protein concentration in each step was measured according to the biuret method (Gomall et al. 1949), using bovine serum albumin (BSA) as the standard.
The resulting supernatants were subjected to isoelectric precipitation by adjusting the pH to 5.0, 5.5 and 6.0, respectively, followed by centrifugation at 12,000 g for 20 min. To evaluate the final yield of the protein recovery, we determined the protein content in the supernatant and calculated the relative protein recovery yield according to the following formula:
where [supernatant protein] refers to the protein content and volume of the supernatant after precipitation, respectively. Total protein amount (mg) is the amount of protein in the carp muscle homogenate.
Preparation of Protein Isolates from Carp Muscle
Precooled dd H2O was added to each carp muscle homogenate at a ratio of 9:1 (water : muscle, v : w). Each mixture was then homogenized once again. Based on preliminary experiments, the homogenates were adjusted to pH 2.5 or 12.5, respectively, using 1 M HCl or 1 M NaOH within 10 min, and then were centrifuged at 10,000 g for 10 min at 4C. The supernatants were collected and the pH was adjusted to 5.5 using 1 M NaOH or 1 M HCl, followed by centrifugation at 12,000 g for 10 min. The resulting precipitates were diluted threefold in dd H2O (w/v) and the pH of the homogenates was adjusted to 5.5 and then recentrifuged. The precipitates were labeled according to their treatment; ACPI‐I (recovered by solubilization at pH 2.5 and subsequent precipitation at pH 5.5) and ALPI‐I (recovered by solubilization at pH 12.5 and subsequent precipitation at pH 5.5), the pH of ACPI‐I was adjusted to 7.0 using 1 M NaOH as ACPI‐II and pH of ALPI‐I adjusted to 7.0 using 1 M HCl as ALPI‐II.
Proximate Composition
The proximate composition of fresh muscle, surimi, ACPI and ALPI samples was determined by using the standard method of the Association of Official Analytical Chemists (AOAC) by the Agriculture Experiment Station Chemical Laboratories at the University of Missouri‐Columbia (1995). The moisture content was determined by drying a sample of the isolate in an oven at 103C until the weight became constant. Ash content was analyzed by incinerating a sample in a muffle furnace at 600C for 48 h and expressed as a percentage (dry weight basis). The total crude protein content (N×6.25) was determined by Kjeldahl assay. The lipid content of the carp protein was determined according to the method of Folch et al. (1957). All of the results were expressed as the mean value of three independent determinations.
Color Measurement
The color of the fresh muscle, surimi, ALPI and ACPI samples was measured by using a hand held Minolta Chroma meter (model: CR‐410, Minolta Co., Ltd., China). Those samples were equilibrated to a transparent glass sample cup at room temperature before the color measurement. At least five samples per treatment were tested. The test results were reported in CIE L*, a* and b*. where L* represents lightness, a* represents red/green hue and b* represents blue/yellow hue. The whiteness values of the sample were calculated according to the following formula:
Amino Acid Composition Analysis
The analysis of amino acids was conducted according to AOAC method 982.30 E (a, b, c) (1995). The fresh muscle, surimi, ACPI and ALPI were hydrolyzed by using 6 M HCl for 24 h. Following neutralization by NaOH, a portion of the filtrate was used for amino acid analysis by using an amino acid analysis system (Agilent 1260 LC; Agilent Technologies Corp, China) equipped with a column (Elite‐AAK; length 250 mm, inner diameter 4.5 mm; column temperature 27C; Pump flow rate: 1.2 mL/min; pump pressure: 14 MPa; injection volume: 10 μL,Elite Corp.). Amino acids were detected with a fluorescence detector (G4212A; Elite Corp). The amino acid composition of carp protein, including the starting material, was determined and expressed as g/100 g protein. To estimate if the amino acid content of the carp protein could meet human amino acid nutritional requirements, common carp muscle and ACPI, ALPI were compared to amino acid requirements for human adults and infants as recommended by the World Health Organization/ Food and Agriculture Organization of United Nations/ United Nations University (FAO/WHO/UNO (2007).
Digestion of Fresh Muscle and Protein Isolates
The digestion of fresh muscle and protein isolates was studied in a medium containing 0.1 M HCl (pH 2.0), by using pepsin to proteins ratios of 1/1,000 (w/w) at 37C, and in another medium containing 0.5 M NaCl, 20 mM Tris‐HCl (pH 7.5), 1 mM CaCl2, by using α‐chymotrypsin to proteins ratios of 1/1,000 (w/w) at 20C, then the digested products were analyzed by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). We focused on the degradation of myosin for its high content and susceptibility. The content of logarithmic remaining of myosin heavy chain (MHC) is in proportion to digestion time, therefore, we can regard the slope of the straight line as digestive rate of proteins for comparative study. The staining intensity of the MHC band on SDS‐PAGE was measured by using the Fuji Film Multi Gauge 2.0 system (Fujifilm Co. Tokyo, Japan) after scanning of the gel on CanoScan 8000F (Canon Co., Tokyo, Japan).
Polyacrylamide Gel Electrophoresis
SDS‐PAGE electrophoresis was performed according to the method of Laemmli (1970), by using 7.5% polyacrylamide gels containing 0.1% SDS. Proteins of 10 μg /well were applied to the gel. Prestained protein marker (TaKaRa Co., LTD., JP) including nine molecular weight markers (Ma): Myosin (200 kDa); β‐galactosidase (116 kDa); Phosphorylase B (97.2 kDa); Serum Albumin (66.4 kDa); Ovalbumin (44.2 kDa); Carbonic angydrase (29 kDa); Trypsin inhibitor (20.1 kDa); lysozyme (14.3 kDa); and aprotinin (6.5 kDa). Gels were stained with 0.1% Coomassie brilliant blue R‐250 and destained with a solution containing 45% methanol and 9% acetic acid.
All experiments were repeated at least three times to confirm the results and the data were expressed as means ± standard deviations (SD). The differences between variables were evaluated by Student–Newman–Keuls statistical method using SPSS 17.0 multiple range tests. The results with P < 0.05 were considered to be statistically significant.
Results and Discussion
Solubilization of Carp Muscle Protein at Different pHs
Figure 1 shows the dissolution variation of carp muscle protein in the pH range of 1.5–13.0 and displays an obvious pH effect on protein solubility. The fish protein has its lowest solubility at pH 5.5 and the protein solubility increases when the pH reaches to extreme acidity (pH 2.0–3.0) or alkalinity (pH 12.0–13.0), which are 82.3% and 94.6%, respectively. These results are similar to the findings reported for Tilapia (Chomnawang and Yongsawatdigul 2013), headed gutted silver carp (Paker et al. 2013) and North Pacific Krill (Sun et al. 2014). In extremely acidic or alkaline pH conditions, the protein, with the same type of positive or negative charges, easily combines with water molecules. In addition, there exists a strong mutual electrostatic repulsion between the proteins, which makes it difficult for precipitation and polymerization to occur. That is why the solubility is the highest in extremely acidic or alkaline pH conditions (Lone et al. 2015). When the pH (pH 5.0–6.0) is at the isoelectric point, the protein net charge becomes zero, causing a lack of electrostatic repulsion between the protein molecules. This promotes hydrophobic interactions and protein aggregation, thus it results in the lowest protein solubility (Nolsoe and Undeland 2009).
Figure 1.
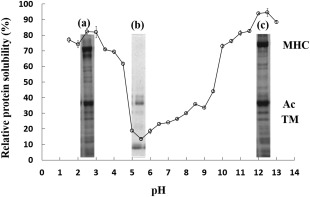
Protein Solubility at Different pHs. pH 2.5‐Soluble Fraction (a), pH 5.5‐ Soluble Fraction (b) and pH 12.5‐Soluble Fraction (c) were Studied on SDS‐Page
MHC is myosin heavy chain component.
In the acidic condition of pH 2.5, the SDS‐PAGE of dissolved protein is shown in Fig. 1a. The dissolved protein consists of a 200 kDa myosin heavy chain (MHC), 42 kDa actin, 34 kDa tropomyosin and 17–20 kDa myosin light chains etc. The MHC shows obvious degradation (160 kDa) under acidic pH conditions. In the alkaline condition of pH 12.5, just as shown in Fig. 1c with the SDS‐PAGE of the dissolved protein, the protein composition is similar to that shown in Fig. 1a except for the MHC degraded components (160 kDa). From pH 5.5 to pH 9.0, little MHC dissolves and the dissolved protein content remains around 42 kDa at pH 5.5.
It has been reported that the acidic protein isolates from Herring muscle also undergoes apparent myofibrillar protein degradation with two new bands appearing at 130–150 kDa and 35–37 kDa (Marmon and Undeland 2010). In addition, Kristinsson also found that the myofibrillar protein of Atlantic Cod experienced an obvious degradation (Kristinsson 2001) during the extraction process. Kristinsson and Hultin (2003) found that protein molecules of Cod became completely dissociated in extremely acidic conditions (pH 2.5), but remained undegraded in extremely alkaline conditions (pH 11.0).
Protein Recovery Yield
Based on the results that carp muscle protein solubility was comparably high under both acidic (pH 1.5–2.5) and alkaline (pH 12.0–13.0) conditions and that there was a minimum solubility in the pH range of 5.0–6.6, various pH combinations were evaluated to find the optimum pH for the best protein recovery from carp muscle. As shown in Fig. 2a, the protein recovery yield in alkaline conditions is slightly better than that in acidic conditions. The maximum recovery yield can attain 87.6% in alkaline conditions at pH 12.5 compared with 76.3% in acidic conditions at pH 2.5. Although the recovery yield of the precipitated protein is slightly higher at pH 5.5, there is no obvious difference. This finding is consistent with Fennema (1996). From the perspective of protein recovery, the pH shift method results in a significantly higher protein recovery yield than the conventional technology does (50%), and it is similar to the separated protein recovery (60% to 80%) from other fish (Nolsoe and undeland 2009).
Figure 2.
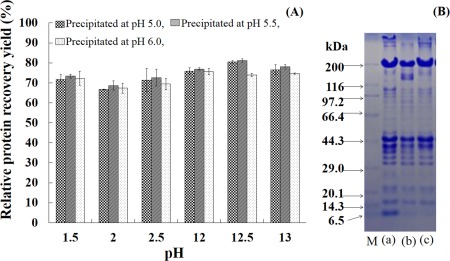
Recovery Yields and Patterns of Protein
(A). Protein recovery yields at different pHs during isoelectric solubilization/ precipitation. (B). Patterns of fresh muscle and protein isolates. (a), fresh muscle; (b), ACPI (acid‐aided protein isolates obtained by solubilization at pH 2.5 and precipitation at pH 5.5); (c), ALPI (alkali‐aided protein isolates obtained by solubilization at pH 12.5 and precipitation at pH 5.5).
These experiment, were conducted with various pHs (at 2.5, 12.5) by using SDS‐PAGE for analysis of the carp protein isolates from the precipitation at pH 5.5. As shown in Fig. 2b, in addition to the full recovery of the composition of myofibrillar protein, a portion of water‐soluble proteins can also be recovered. Moreover, during the acid solution process the degradation products of MHC can be also recycled.
Elementary Chemical Composition Analysis of Protein
To assess the quality of protein recovered from carp muscle protein, the moisture, total fat, crude protein and ash content of carp protein were determined, as reported in Table 1. The carp muscle has a moisture content of 77.8%, protein content of 78.2% (dry basis), ash content of 12.6% (dry basis) and lipid content of 5.3% (dry basis). Surimi, ACPI and ALPI have moisture contents of 89.50%, 85.53% and 88.45%, all of which are higher than those of fresh muscle. The crude protein contents resulting from above three processes are 91.88%, 83.20% and 82.96%, all of which are higher than that from fresh muscle. Most of the ash and fat are removed in the separation process. Compared with the 12.56% in fresh muscle, the fat content from ACPI and ALPI was much lower at 2.02% and 4.88%, while the low fat content is more conducive to storage; the ash content declined to 8.88% and 8.83%, which surpassed the 5.76% in traditional surimi processing. These results indicate that the pH change can lead to an increase in metal ions during the process of protein separation, thus to increase the ash content. Overall, ACPI and ALPI prepared by pH‐shift method are a superior quality protein source with a high content of protein and low content of fat. Although the fat and ash content of ACPI and ALPI are slightly higher than that of surimi, the pH‐shift method is more convenient from the perspective of operational processes. Surimi was treated four times in this study, if protein isolates processes were conducted the same number of times (e.g., washed three times with the solution of pH 5.5), the fat and ash contents from protein isolates process may have achieved equally to the level that of the Surimi process. In addition, it is reported that the heavy metal content in fish protein isolates can be greatly reduced by using the pH‐shift method (Taskaya et al. 2009a, 2009b, 2009c), which is an advantage in protein isolated process.
Table 1.
Proximate Compositions of Fresh Carp Muscle and Protein Isolates (n = 3)
| Methods | Moisture (%) | Protein dry basis (%) | Ash dry basis (%) | Lipid dry basis (%) |
|---|---|---|---|---|
| fresh muscle | 77.79 ± 0.35a | 78.18 ± 0.72a | 12.56 ± 0.09c | 5.26 ± 0.17b |
| Surimi(washed) | 89.50 ± 0.31d | 91.88 ± 1.64c | 5.76 ± 0.55a | 1.69 ± 0.32a |
| ACPI(2.5/5.5) | 85.53 ± 0.10b | 83.20 ± 2.87b | 8.88 ± 0.36b | 2.02 ± 0.41a |
| ALPI(12.5/5.5) | 88.45 ± 0.07c | 82.96 ± 1.80b | 8.83 ± 0.21b | 4.88 ± 0.21b |
Surimi obtained by washed; ACPI, carp fresh muscle obtained by solubilization at pH 2.5 and precipitation at pH 5.5; ALPI, carp fresh muscle obtained by solubilization at pH 12.5 and precipitation at pH 5.5. Different letters (a, b, c or d) within a column indicate significant differences (P < 0.05).
Since the color of protein isolates affects its application in food processing, the chroma becomes a very important parameter. As the results in Table 2 show, ACPI and ALPI prepared by pH‐shift method, significantly increase the L* and b*s value of the product, but decrease the a* value resulting in the product was much whiter than that of fresh muscle, but not as white as Surimi. This color improvement is probably due to the removal of pigments (Marmon and Undeland 2010) during the pH‐shift process. The decrease of the a* values also shows that, during the protein isolated process (at extreme pH), the protein hemoglobin is separated from proteins because of the changes in protein structure. After being adjusted with acid and base on the isoelectric point, the heme proteins unfold, oxidize, denature and become brown associating with the increased yellow of the product (Kristinsson et al. 2005). This is probably because the b* values in ACPI or ALPI are higher than that in the surimi.
Table 2.
Colour Characteristics for Fresh Muscle and Protein Isolates (n = 3)
| Methods | L* | a* | b* | Whiteness |
|---|---|---|---|---|
| fresh muscle | 49.45 ± 0.16a | 9.53 ± 0.26c | 6.68 ± 0.20c | 48.13 ± 0.14a |
| Surimi(washed) | 70.78 ± 1.50b | −2.44 ± 0.06b | 2.68 ± 1.01a | 70.54 ± 1.41b |
| ACPI(2.5/5.5) | 71.31 ± 0.09b | −1.7 ± 0.10a | 7.65 ± 0.11b | 70.26 ± 0.07b |
| ALPI(12.5/5.5) | 72.17 ± 0.09b | −1.65 ± 0.06b | 10.03 ± 0.1b | 70.37 ± 0.11b |
Different letters (a b or c) within a column indicate significant differences (P < 0.05).
Amino Acid Composition
According to the results of amino acid composition of the samples shown in Table 3, fresh muscle, surimi, ACPI and ALPI have a total amount of amino acids of 91.21 g/100 g, 98.99 g/100 g, 93.97 g/100 g and 92.68 g/100g, respectively. The amino acid content in ACPI and ALPI is higher than that of fresh muscle. Surimi has the highest total amount of amino acid, where the content of glutamic acid, arginine, proline and glycine are higher than that in the other three kinds of proteins. Most of the water‐soluble proteins are removed during the acidic/alkaline solubilization process, while the myofibrillar protein and matrix proteins are retained. Glycine is the major amino acid in collagen, reaching its highest level in the connective tissue and membrane proteins (Kittiphattanabawon et al. 2005). The glycine content in ACPI and ALPI is lower than that in the fresh muscle. This shows that the collagen was not sufficiently recovered during the preparation of the protein isolates, since only a small part of ACPI can be recovered.
Table 3.
Amino Acid Compositions of Fresh Muscle and Protein Isolates(Dry Basis)
| Amino acid | Fresh muscle (g/100g) | Surimi | ACPI | ALPI | FAO/WHO/UNU Adult (infant) |
|---|---|---|---|---|---|
| Essential amino acids | |||||
| Histidine | 6.22 ± 0.05a | 6.39 ± 0.75a | 5.53 ± 0.09a | 5.56 ± 0.21a | 1.5 (1.6) |
| Isoleucine | 3.33 ± 0.06b | 1.86 ± 1.67a | 3.71 ± 0.02b | 3.66 ± 0.01b | 3.0 (3.1) |
| leucine | 7.02 ± 0.10b | 5.49 ± 1.74a | 7.63 ± 0.03b | 7.55 ± 0.01b | 5.9 (6.1) |
| Lysine | 9.20 ± 0.04a | 9.44 ± 0.08b | 9.47 ± 0.10b | 9.47 ± 0.02b | 4.5 (4.8) |
| Methionine | 2.77 ± 0.01a | 3.09 ± 0.09b | 3.01 ± 0.04b | 3.00 ± 0.03b | 2.2 (2.4) |
| Threonine | 4.01 ± 0.07a | 4.26 ± 0.06b | 4.27 ± 0.01b | 4.25 ± 0.01b | 2.3 (2.5) |
| Valine | 4.27 ± 0.03a | 4.37 ± 0.04b | 4.56 ± 0.01c | 4.58 ± 0.02c | 3.9 (4.0) |
| Tryptophan | 5.087 ± 0.04b | 8.77 ± 3.89c | 4.80 ± 0.20a | 4.50 ± 0.10a | 0.6 (0.7) |
| Phenylalanine | 3.57 ± 0.03c | 2.81 ± 0.61b | 2.26 ± 0.21ab | 2.06 ± 0.06a | 3.8 (4.1) |
| Toto EAAs | 45.48 ± 0.29a | 46.50 ± 2.12a | 45.25 ± 0.31a | 44.64 ± 0.03a | |
| Non‐essential amino acids | |||||
| Cysteine | 0.58 ± 0.01a | 0.66 ± 0.01b | 0.70 ± 0.02c | 0.73 ± 0.01d | |
| Tyrosine | 2.12 ± 0.02a | 2.26 ± 0.03b | 2.42 ± 0.08c | 2.61 ± 0.03d | |
| Alanine | 5.39 ± 0.04a | 5.86 ± 0.18b | 5.41 ± 0.01a | 5.22 ± 0.01a | |
| Aspartate | 9.18 ± 0.08a | 9.08 ± 0.35a | 9.71 ± 0.07b | 9.73 ± 0.01b | |
| Glutamate | 12.74 ± 0.16a | 15.22 ± 0.21c | 14.08 ± 0.01b | 14.00 ± 0.01b | |
| Glycine | 4.06 ± 0.02c | 5.25 ± 0.25d | 3.55 ± 0.04b | 3.30 ± 0.04a | |
| Proline | 2.79 ± 0.01a | 3.99 ± 0.12c | 3.09 ± 0.01b | 3.04 ± 0.01b | |
| Arginine | 5.04 ± 0.05a | 6.03 ± 0.29c | 5.66 ± 0.08b | 5.47 ± 0.16b | |
| Serine | 3.82 ± 0.05a | 4.14 ± 0.06b | 4.11 ± 0.05b | 3.93 ± 0.14a | |
| Total Acids | 91.21 ± 0.68a | 98.99 ± 3.54b | 93.97 ± 0.65a | 92.68 ± 0.33a | |
Different letters (a b c or d) within a row indicate significant differences (P < 0.05).
Essential amino acids in the recovered protein, account for 45.25 g/100 g (ACPI) and 44.64 g/100 g (ALPI) and are concentrated by isoelectric solubilization/precipitation, compared to the initial essential amino acid content (45.48 g/100 g) in the fresh muscle. The contents of all essential amino acids in ACPI and ALPI, meet the amino acid requirements for adults and infants according to FAO/WHO/UNU recommendations.
Solubility of Protein Isolates
The protein solubility at various ionic strengths is shown in Fig. 3. As shown, the higher ionic strength of solution contributes to the increase of solubility for all the four samples of the fresh muscle, surimi, as well as ACPI‐II and ALPI‐II. However, the solubility of each sample reaches a maximum when the ionic strength of KCl in solution is close to 0.4 M. The protein solubility maximum of the fresh muscle is 88.78%; surimi 73.21%, ACPI‐II (pH 7.0) 66.67% and ALPI‐II (pH 7.0) 62.08%. There appears to be no difference in solubility between surimi and protein isolates. Protein solubility, as the basis of all the functions and features, reflects the change in the molecular structure of proteins from its own change. In general, protein denaturation will affect protein solubility, so protein denaturation is an important process parameter and should be measured compared to fresh muscle. The solubility of muscle protein decreases after being washed and isolated using acid and alkali, as well as a post‐separation step. This is due to fish muscle proteins including salt‐soluble myofibrillar protein, water‐soluble sarcoplasmic proteins and muscle matrix proteins. However, some of the water‐soluble protein is lost during the recovery process of washing and separating by using acid or alkali. At low ionic strength (0.1–0.2 M), the water‐soluble proteins mainly dissolve, but as the ionic strength increasing the major dissolved component is the myofibrillar protein. Under low ionic strength (0.1 M KCl) condition, the solubility of ACPI‐II and ALPI‐II is higher than that of surimi. According to the above results, it indicated that a portion of the water‐soluble proteins are recovered from protein isolates. Generally, the functional properties of protein isolates are weaken at the protein isoelectric point, hence, it is very necessary to adjust pH to 7.0 for the purpose of achieving the superior functional properties.
Figure 3.
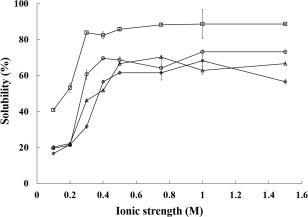
Effects Of Different Ionic Strength on the Solubility of Fresh Muscle and Protein Isolates
□, fresh muscle; ○, surimi; △, ACPI‐II (acid‐aided protein isolates obtained by solubilization at pH 2.5 and precipitation at pH 5.5, then adjusted to pH 7.0); ⋄, ALPI‐II (alkali‐aided protein isolates obtained by solubilization at pH 12.5 and precipitation at pH 5.5, then adjusted to pH 7.0).
Digestion of Fresh Muscle and Protein Isolates
Fig. 4 shows the pepsin digestion patterns of fresh muscle (a), ACPI (b) and ALPI (c). There is almost no change on digestion pattern of the MHC of three samples. The optimum digestion pH of pepsin is 2.0. In this digestive situation, the Mf as a substrate will be influenced by the acid condition. It is hard to determine whether the digestive properties of recovered protein results from the changes of the protein structure during the pH adjustment process. Therefore, we use α‐chymotrypsin which has highly specificity and clear restriction sites. Figure 5 shows the chymotrypsin digestion patterns of fresh muscle (a), ACPI (b) and ALPI (c). The cleavage sites of chymotrypsin have a little difference between them. In Fig. 5a, there are several fragments increased near the 180 kDa, 165kDa and 97 kDa, while the amount of MHC decreased over a longer digestion period. As shown in Fig. 5b,c, in the vicinity of 165 kDa and 66.4 kDa, two obvious bands are present with little difference between the digestion patterns of ACPI and ALPI. It indicated that the site becomes exposed and its 180 kDa band is degraded into smaller molecular fragments, probably due to the unfolding and refolding process of protein structure after the acid‐alkali treatment. With high specificity, the cleavage sites of chymotrypsin only cut the peptide bond at the carboxyl group of tyrosine, tryptophan and phenylalanine (they are all aromatic amino acids). In the condition of 0.5 M KCl and 20 mM Tris‐HCl (pH 7.5), the myosin is cleaved into heavy meromyosin (HMM, 165 kDa) and light meromyosin (LMM, 60 kDa) (Kato and Konno 1993).
Figure 4.
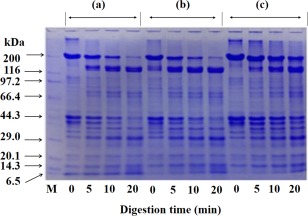
Digestion Pattern of Fresh Muscle and Protein Isolates by Pepsin
Proteins (2.5 mg/ml) in 0.1 N HCl were incubated with pepsin at 37C and using pepsin of 1/1,000 (w/w). (a), fresh muscle; (b), ACPI; (c), ALPI.
Figure 5.
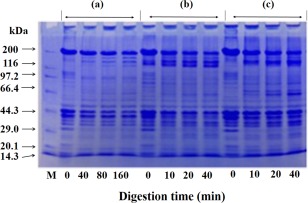
Digestion Patterns of Fresh Muscle and Protein Isolates by α‐Chymotrypsin
Proteins (2.5 mg/ml) in 0.5 M NaCl, 20 mM Tris‐HCl (pH 7.5), 1 mM CaCl2 were incubated at 20C and using α‐chymotrypsin of 1/1,000 (w/w). (a), fresh muscle; (b), ACPI‐II; (c), ALPI‐II.
In addition, as the rates of disappearance of the three MHC shows in Fig. 6, there is no significant difference between ACPI and ALPI, but which exhibited a rate of 7 or 8 times greater than that of the fresh muscle. The effect of the acid‐alkali treatment has also been reported in Tian's (Tian et al. 2010). The protein isolates, either acid or alkaline treatment, some degeneration occurs and there is an increase in the sensitivity to proteases, thus the pH‐shift process greatly improves the protein digestibility. Therefore, use the pH‐shift method for preparing protein isolates and further developing them into fish protein peptides, as well as other fermented products, may have a greater advantage compared with direct extraction of fresh muscle.
Figure 6.
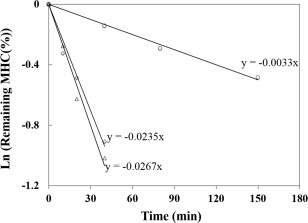
Decrease Rate of MHC Content in Fig. 5 when Incubated with Α‐Chymotrypsin was Analyzed
○, fresh muscle; △, ACPI‐II; ⋄, ALPI‐II.
Conclusions
In an acidic or alkaline range, solubility of muscle proteins increases as much as to 80–90%. Most of the protein can be recovered by adjusting the pH to 5.5, with fish muscle protein recovery yield up to 80%. In addition to myofibrillar proteins, the recovery constituents also include a portion of the water‐soluble proteins. In the recovery process, the fish fat and part of the ash can be effectively removed and the protein isolates have whiteness as good as surimi. Amino acids contents of the protein isolates have a full range and completely conform to the standard FAO recommendations. Moreover, isolated proteins have the similar solubility as surimi, but exhibit better digestibility after being digested by chymotrypsin. Using the pH‐shift method to isolate protein from freshwater fish such as carp and then using enzymes to hydrolyze it, it may have greater advantages to produce functional polypeptides. Some changes should be studied further including the gel properties of protein isolates and the structure of myosin, which are treated with acid and alkali.
Acknowledgment
The present study was part of Project 31271980 funded by National Natural Science Foundation of China.
References
- Association of Official Analytical Chemists . 1995. Official Methods of Analysis, 16th Ed., Assoc. of Official Analytical Chemists, Washington, DC. [Google Scholar]
- Batista, I. , Pires, C. and Nelhas, R. 2007. Extraction of sardine proteins by acidic and alkaline solubilisation. Food Sci. Technol. Int. 13(3), 189–194. [Google Scholar]
- Bureau of Fisheries in Ministry of Agriculture . 2014. 2014 China Fisheries Statistical Yearbook, China Agriculture Press, Beijing. [Google Scholar]
- Chomnawang, C. and Yongsawatdigul, J. 2013. Protein recovery of tilapia frame by‐products by pH‐shift method. J. Aquatic Food Prod. Technol, 22(2), 112–120. [Google Scholar]
- Fennema, O.R. 1996. Food Chemistry, pp. 348–349, 3rd Ed., Marcel Dekker Inc, New York, NY. [Google Scholar]
- Foh, M.B.K. , Xia, W.S. , Amadou, I . and Jing, Q.X. 2012. Influence of pH shift on functional properties of protein isolated of tilapia (Oreochromis niloticus) muscles and of Soy protein isolate. Food Bioproc. Tech. 5(6), 2192–2200. [Google Scholar]
- Folch, J. , Lees, M. and Stanley, G.H.S. 1957. A simple method for theisolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- Gomall, A.G. , Bardawill, C.J. and David, M.M. 1949. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177, 751–766. [PubMed] [Google Scholar]
- Kato, S. and Konno, K. 1993. Isolation of carp myosin rod and its structural stability. Nippon Suisan Gakkaishi 59(3), 539–544. [Google Scholar]
- Kim, Y.S. , Park, J.W. and Choi, Y.J. 2003. New approaches for the effective recovery of fish proteins and their physicochemical characteristics. Fish. Sci. 69(6), 1231–1239. [Google Scholar]
- Kittiphattanabawon, P. , Benjakul, S. , Visessanguan, W. , Nagai, T. and Tanaka, M . 2005. Characterisation of acid‐soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 89(3), 363–372. [Google Scholar]
- Kristinsson, H. and Demir, N. 2003. Functional fish proteín ingredients from fish species of warm and temperate waters: Comparison of acid‐and alkali‐aided processing vs conventional surimi processing In Advances in Seafood Byproducts 2002 Conference Proceedíngs (Bechtel P.J., ed.) pp. 277–295, Alaska Sea Grant College Program University of Alaska, Anchorage, AK. [Google Scholar]
- Kristinsson, H.G. 2001. Evaluation of different methods to isolate Cod (Gadus morhua) muscle myosin. J. Food Biochem. 25(3), 249–256. [Google Scholar]
- Kristinsson, H.G. and Hultin, H.O. 2003. Changes in conformation and subunit assembly of Cod myosin at low and high pH and after subsequent refolding. J.Agric. Food Chem. 51(24), 7187–7196. [DOI] [PubMed] [Google Scholar]
- Kristinsson, H.G. , Theodore, A.E. , Demir, N. , and Ingadottir, B . 2005. A comparative study between acid‐and alkali‐aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J. Food Sci. 70(4), C298–C306. [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during assembly head of bacteriophage T4. Nature 277, 680–685. [DOI] [PubMed] [Google Scholar]
- Lone, D.A. , Wani, N.A. , Wani, I.A. and Masoodi, F.A . 2015. Physico‐chemical and functional properties of Rainbow trout fish protein isolate. Int. Food Res. J. 22(3), 1112–1116. [Google Scholar]
- Marmon, S.K. and Undeland, I. 2010. Protein isolation from Gutted herring (Clupea harengus) using pH‐shift processes. J.Agric. Food Chem. 58(19), 10480–10486. [DOI] [PubMed] [Google Scholar]
- Nolsoe, H. and Undeland, I. 2009. The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food Bioproc. Tech. 2(1), 1–27. [Google Scholar]
- Paker, I. , Beamer, S. , Jaczynski, J. , and MATAK, K.E. 2013. Compositional characteristics of materials recovered from headed gutted silver Carp (Hypophthalmichthys molitrix) by isoelectric solubilization and precipitation using organic acids. J.Food Sci. 78(3), E445–E451. [DOI] [PubMed] [Google Scholar]
- Perez‐Mateos, M. , Amato, P.M. and Lanier, T.C. 2004. Gelling properties of Atlantic croaker surimi processed by acid or alkaline solubilization. J. Food Sci. 69(4), C328–C333. [Google Scholar]
- Sun, L.C. , Chen, Y.L. , Zhong, C. , Okazaki, E. , Cao, M.J. , Weng, W.Y. and Osako, K . 2014. Autolysis of krill protein from North Pacific krill Euphausia pacifica during protein recovery via isoelectric solubilization/precipitation. Fish. Sci. 80(4), 839–847. [Google Scholar]
- Taskaya, L. , Chen, Y.C. and Jaczynski, J. 2009a. Functional properties of proteins recovered from silver Carp (Hypophhalmichthys moli1rix) by isoeIectric solubilization/precipítation. LWT – Food Sci. Technol. 42(6), 1082–1089. [Google Scholar]
- Taskaya, L. , Chen, Y.C. , Beamer, S. , Tou, J.C. , and Jaczynski, J . 2009b. Compositional characteristics of materials recovered from whole gutted silver carp (Hypophthalmichthys molitrix) using isoelectric solubilization/precipitation. J. Agric. Food Chem. 57(10), 4259–4266. [DOI] [PubMed] [Google Scholar]
- Taskaya, L. , Chen, Y.C. , Beamer, S. and Jaczynski, J. 2009c. Texture and colour properties of proteins recovered from whole gutted silver Carp (Hypophthalmichthys molitrix) using isoelectric solubilisation /precipitation. J. Sci. Food Agric. 89(2), 349–358. [Google Scholar]
- Thawornchinsombut, S. and Park, J.W. 2007. Effect of NaCl on gelation characteristics of acid‐ and alkali‐treated pacific whiting fish protein isolates. J. Food Biochem. 31(4), 427–455. [Google Scholar]
- Tian, Y.Y. , Umezawa, E. , Duan, R. and Konno, K. 2010. Three types proteinases in japanese common squid todarodes pacificus hepatopancreas as studied by using carp myofibrils as substrate. Fish. Sci. 76, 365–373. [Google Scholar]
- WHO/FAO/UNU . 2007. Protein and Amino Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, WHO Technical Report Series 935, World Health Organization, Geneva. [Google Scholar]
- Yongsawatdigul, J. and Park, J.W. 2004. Effects of alkali and acid solubilization on gelation characteristics of rockfish muscle proteins. J. Food Sci. 69(7), 499–505. [Google Scholar]


