Abstract
Although synaptic transmission may be unidirectional, the establishment of synaptic connections with specific properties can involve bidirectional signaling. Pyramidal neurons in the hippocampus form functionally distinct synapses onto two types of interneurons. Excitatory synapses onto oriens-lacunosum moleculare (O-LM) interneurons are facilitating and have a low release probability, whereas synapses onto parvalbumin interneurons are depressing and have a high release probability. Here, we show that the extracellular leucine-rich repeat fibronectin containing 1 (Elfn1) protein is selectively expressed by O-LM interneurons and regulates presynaptic release probability to direct the formation of highly facilitating pyramidal-O-LM synapses. Thus, postsynaptic expression of Elfn1 in O-LM interneurons regulates presynaptic release probability, which confers target-specific synaptic properties to pyramidal cell axons.
Introduction
Regulated control of synaptic transmission is essential to the gating of information flow through neuronal circuits. The efficacy of synaptic transmission depends on the probability that an action potential will elicit vesicle release (1). Different synapses on a single axon can have different release properties depending on the identity of the postsynaptic partner, suggesting that cell type-specific signals from the postsynaptic neuron define this property (2, 3). The importance of target-specific synaptic release properties is exemplified by CA1 pyramidal cell axons, which make synapses on different classes of inhibitory interneurons (Fig. 1A). CA1 pyramidal cell synapses onto parvalbumin (PV)- and somatostatin (Sst)-containing interneurons have different release probabilities (4-6). High release probability at pyramidal-PV cell synapses results in initially strong but rapidly depressing excitatory synaptic currents. (4). In contrast, pyramidal-Sst cell synapses have much lower release probability and are strongly facilitating (5, 7). The presynaptic pyramidal neurons must therefore receive target-derived information from the postsynaptic cells to assemble synapses with distinct properties. Although these properties are critical for regulating the dynamics of hippocampal circuits, the transsynaptic signals that assemble pyramidal cell terminals with differing release probability are unknown.
Fig 1.
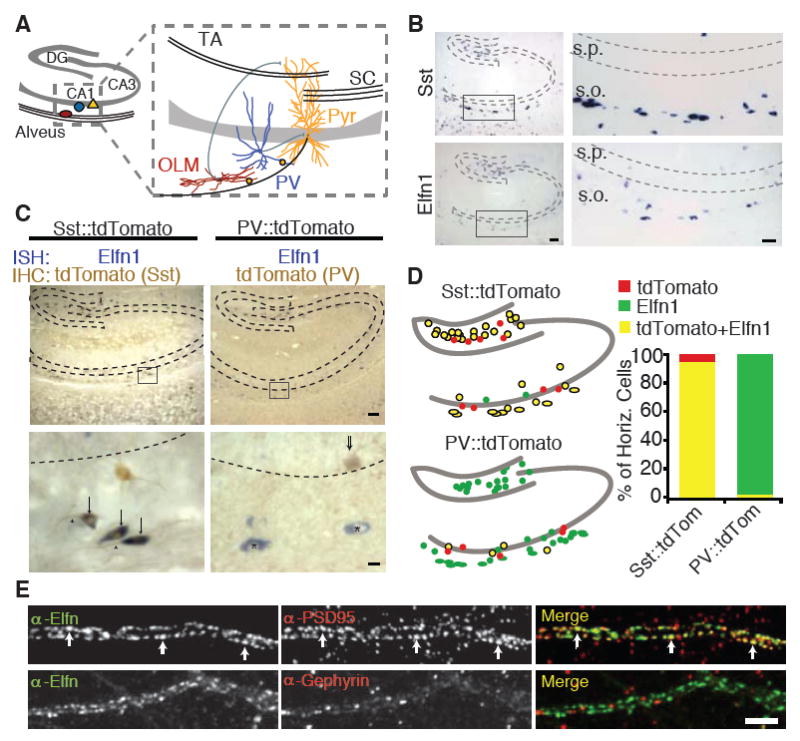
Elfn1 is expressed in hippocampal O-LM interneurons. (A) CA1 axons contact PV cells and O-LM cells, which provide feedback inhibition. DG, dentate gyrus; Pyr, pyramidal cell. (B) ISH for Elfn1 and somatostatin at P14, both showing expression in the stratum oriens and hilus of the hippocampus. s.p., stratum pyramidale; s.o., stratum oriens. Scale bars indicate 25 (left) or 10 (right) mm. (C) ISH (blue) for Elfn1 and immunostaining (brown) for tdTomato in transgenic mouse lines in which tdTomato is expressed in Sst or PV interneurons. Elfn1 expression is highest near the alveus in somata (arrows) and proximal dendrites (arrowheads) of Sst-tdTomato neurons. Elfn1-expressing cells show little colocalization in tdTomato-PV mice (asterisks). Scale bars indicate 25 or 2.5 μm. (D) Quantification of the percent of horizontal cells in each mouse line that contain Elfn1, tdTomato, or both. Sst:tdTomato, n = 145 cells, PV∷tdTomato, n = 157 cells. (E) Dendrites of neurons from dissociated hippocampal cultures stained for Elfn1 and PSD95 or gephyrin. Scale bar, 5 μm.
Results
To identify cues on the postsynaptic cell that might direct the development of cell type-specific synapses, we used mRNA expression data from the Allen Brain Atlas to identify genes expressed in distinct hippocampal interneuron subpopulations (8). Comparison of expression patterns of candidate genes with those of Sst and PV revealed similar expression patterns between the leucine-rich repeat (LRR) protein Elfn1 and a group of Sst-expressing cells, oriens-lacunosum moleculare (O-LM) cells (Fig. 1B). LRR proteins have been shown to function as transsynaptic regulators (9, 10), suggesting that Elfn1 may function as a signal on Sst-positive O-LM interneurons to specify synaptic properties.
To determine whether individual cells coexpress Elfn1 and somatostatin, we combined in situ hybridization (ISH) for Elfn1 mRNA with immunohistochemical (IHC) detection for interneuron cell type. We crossed mice with a Cre-conditional tdTomato reporter with mice expressing Crerecombinase under Sst or PV promoters, Sst∷tdTomato and PV∷tdTomato, respectively (Fig. 1C) (11-13). In CA1 and the hilus, 75% of Sst interneurons contained Elfn1 mRNA. However, horizontally oriented cells adjacent to the alveus, putative O-LM cells, showed 95% colabeling of Sst and Elfn1 (Fig. 1D). In contrast, PV-containing cells comprised only 5% of all Elfn1-expressing cells (fig. S1). These data show that Elfn1 is selectively expressed in perialvear Sst-positive interneurons.
To assess the cellular distribution of Elfn1 protein, we cultured primary hippocampal neurons and immunostained them with cell-type markers and a pan-Elfn antibody (figs. S2 and S3). We found strong dendritic staining in somatostatin interneurons (fig. S3). Because these neurons do not express Elfn2 (fig. S2), we concluded that Elfn1 is localized to the dendrites of Sst+ interneurons. Similar to the in situ expression, 69% of Sst cells in culture expressed Elfn1 protein (Table 1 and fig. S3). O-LM cells are a subset of these Sst-containing cells and can be identified by their strong expression of metabotropic glutamate receptor 1a (mGluR1a). Accordingly, 88% of mGluR1a-expressing cells contained Elfn1 (Table 1). In hippocampal slices, Elfn1 immunoreactivity was enriched in the outer oriens next to the alveus, where O-LM cells lie, consistent with enrichment of Elfn1 in O-LM cell dendrites (fig. S3). Elfn1 was found in discrete punta along these dendrites, and colocalized with glutamatergic, but not g-aminobutyric acid-mediated, synaptic markers (Fig. 1E). Taken together, these data indicate that Elfn1 is localized to excitatory synapses onto Sst-containing O-LM interneurons.
Table 1.
Elfn1 expression by cell type.
| Cell type % of Elfn+ cells with marker | % of each cell type with Elfn | |
|---|---|---|
| GAD | 97 | 18 |
| CamKII | 0 | 0 |
| Sst | 96 | 69 |
| PV | 4 | 3 |
| MGluR1α | 97 | 88 |
To determine whether Elfn1 was necessary for short-term facilitation at synapses between pyramidal and O-LM cells, we examined the functional consequence of knocking down Elfn1 in vivo. We used Sst∷tdTomato mice to identify Sst-containing interneurons for electrophysiological recording (fig. S4). To knock down Elfn1 in vivo, we generated a lentiviral construct containing green fluorescent protein (GFP) and a short hairpin RNA (shRNA) targeting Elfn1 (Fig. 2A). We injected lentivirus into the CA1 region of hippocampus at postnatal day 6 (P6) and recorded from perialvear Sst interneurons at P13 to P16. O-LM interneurons receive synapses from pyramidal cell axon collaterals as they exit CA1 in the alveus, where they can be selectively stimulated (7) (Fig. 2B). Control GFPinfected neurons showed a strong facilitation to alvear stimulation (Fig. 2C). In marked contrast, short-term facilitation was substantially reduced in neurons expressing the Elfn1 shRNA (Fig. 2C). The deficit in short-term facilitation was seen across several different interstimulus intervals, suggesting that Elfn1 regulates shortterm facilitation across a range of physiologically relevant input frequencies (Fig. 2D). When the viral injection was performed earlier (at P1), the effect of Elfn1 knockdown was even stronger, suggesting that the presence of Elfn1 is particularly important when these synapses are established (Fig. 2C). Elfn1 knockdown did not affect the short-term plasticity of disynaptic inhibition onto O-LM cells (Fig. 2E). Thus, Elfn1 selectively regulates the magnitude of synaptic facilitation at pyramidal-O-LM synapses.
Fig 2.
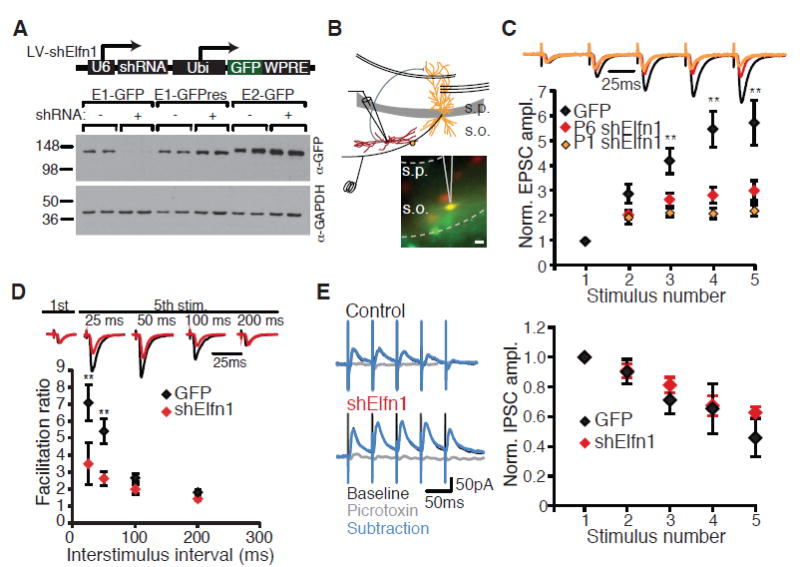
Elfn1 knockdown reduces short-term facilitation at CA1-O-LM synapses. (A) Western blot from human embryonic kidney (HEK) cells expressing Elfn1-GFP cotransfected with Elfn1 shRNA, blotted for GFP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Knockdown is rescued by a point mutation in the target sequence of the Elfn1-GFP cDNA (E1-GFPres). (B) Recording configuration and epifluorescence images of tdTomato and GFP in stratum oriens. Scale bar, 20 mm. (C) (Top) Average postsynaptic response of control and shElfn1-expressing cells to five stimuli to the alveus at 20 Hz, normalized to the amplitude of the first EPSC. Black, GFP; red, shElfn1 at P6; orange, shElfn1 at P1. (Bottom) Population data for EPSC amplitude normalized to first EPSC. GFP, n = 13; P6 shElfn1, n = 20; P1 shElfn1, n = 7. (D) (Top) Example cells comparing first to fifth EPSC at different interstimulus intervals. (Bottom) Population data for facilitation ratio, calculated as the amplitude ratio of the fifth EPSC to the first EPSC. GFP, n = 12; shElfn1, n = 8. ** P < 0.01, analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD). (E) (Left) Example recordings of inhibitory synaptic responses in control and shElfn1-expressing cells to a 20-Hz stimulus before and after picrotoxin application. (Right) Average inhibitory postsynaptic current (IPSC) amplitude, normalized to the first IPSC amplitude. GFP, n = 7; shElfn1, n = 5. Error bars in all figures indicate SEM.
Facilitating synapses have low release probabilities. Their release depends on accumulation of calcium through several action potentials (14-16). If the reduced facilitation mediated by Elfn1 shRNA is due to increased release probability, evoked transmission onto Elfn1 shRNA-expressing neurons should be increased compared with control neurons. To test this prediction, we compared pyramidal cell inputs onto neighboring infected and uninfected neurons (17). We found that cells infected with the control lentivirus did not differ from uninfected neighbors in the strength of their pyramidal cell input, whereas shElfn1- infected neurons showed an increase in the evoked response when compared with simultaneously recorded neighboring cells (Fig. 3, A and B). Although this is consistent with an effect of Elfn1 on release probability, it could also reflect a role of Elfn1 on the postsynaptic response to glutamate. However, we found no effect of Elfn1 knockdown on AMPA/N-methyl-D-aspartate (NMDA) ratio, decay kinetics of the AMPA receptor (AMPAR)- and NMDA receptor (NMDAR)-mediated components, miniature excitatory postsynaptic current (mEPSC) amplitude, or rectification (fig. S5). Together these observations suggest that Elfn1 does not exert a significant effect on postsynaptic properties.
Fig 3.
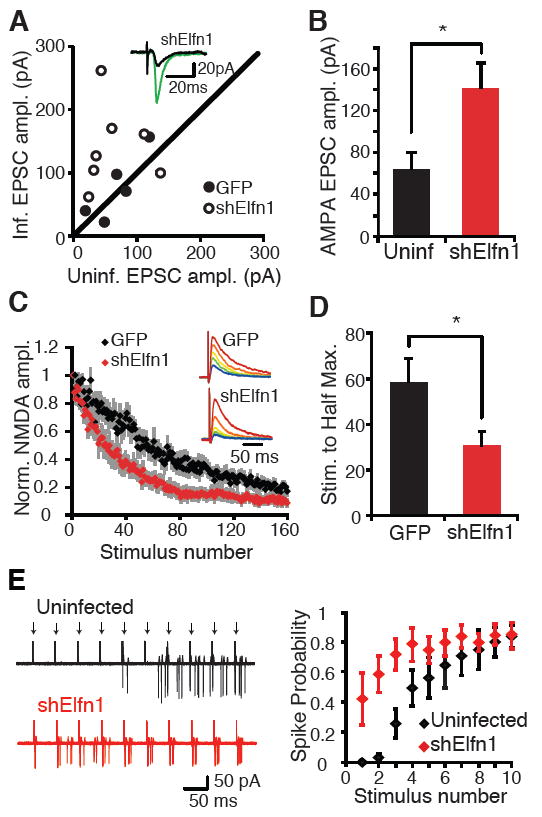
Postsynaptic Elfn1 regulates release prob- ability at CA1-O-LM synapses. (A) Evoked EPSC amplitude recorded from neighboring O-LM interneurons, one uninfected and one infected with a lentivirus expressing GFP or GFP plus shElfn1. Each point represents one pair of simultaneously recorded neurons. Solid circle, GFP control; open circle, shElfn1. (Inset) Example traces of evoked EPSC amplitude from uninfected (black) and in- fected (green) neurons. (B) Average EPSC amplitude of data in (A). n = 7 pairs. P < 0.05, paired t test. (C) Kinetics of NMDA block in control and shElfn1-expressing neurons. NMDA EPSC is recorded at a holding potential of +40 mV in DNQX, and the alveus is stimulated in the presence of MK801 (40 μM) at 0.2 Hz. Peak NMDA-mediated current is normalized to the initial peak NMDA EPSC amplitude. (D) Average time to half maximum NMDA EPSC amplitude. GFP, n = 8; shElfn1, n = 10. P < 0.05, t test. (E) Cell-attached, simultaneous recording from neighboring uninfected and shElfn1-infected O-LM cells. Ten stimuli are delivered to the alveus (arrows) at 20 Hz. (Left) Overlayed traces of subsequent sweeps to show spiking distribution. (Right) Spiking probability is plotted as a function of stimulus number. n = 5 pairs.
We also examined whether Elfn1 has synaptogenic properties, because other LRR proteins have been shown to regulate synaptic density or synaptic differentiation (18-20). We found that Elfn1 does not regulate synapse number in vitro (fig. S6). In addition, Elfn1 expressed in heterologous cells did not induce hemisynapse formation in contacting axons, unlike other LRRcontaining proteins (LRRTMs, NGLs, and TrkC) (18, 20, 21) (fig. S7). These observations suggest that Elfn1 exerts a selective effect synaptic transmission. The robust effect on short-term facilitation at CA1-O-LM synapses, while leaving other synaptic parameters unaffected, strongly suggests that Elfn1 acts in a target-selective manner to control presynaptic release at pyramidalO-LM synapses.
To further verify that Elfn1 knockdown regulates release probability at pyramidal-O-LM synapses, we recorded from control and Elfn1 shRNA- expressing O-LM interneurons while repeatedly stimulating the alveus in the presence of the irreversible, use-dependent NMDAR antagonist MK801. Because MK801 blocks open NMDARs, synapses that have a higher probability of release will release glutamate more often, and NMDARs at those synapses will be blocked more quickly (22). Neurons expressing Elfn1 shRNA showed a much faster block of NMDA EPSCs compared with control infected neurons (Fig. 3, C and D), indicating that Elfn1 knockdown increases release probability, producing a larger evoked response and reduced short-term facilitation.
Both O-LM and PV cells provide feedback inhibition to CA1 pyramidal cells. O-LM cells target distal pyramidal cell dendrites, whereas PV cells target pyramidal cell somata. Strongly facilitating inputs engage O-LM cells most effectively after repetitive pyramidal cell activity, producing delayed but persistent inhibition to distal pyramidal cell dendrites. Strong but rapidly depressing inputs to PV cells result in fast but transient inhibition to the soma (7). This arrangement channels inhibition to different somatodendritic compartments at distinct times relative to pyramidal cell spiking. To determine whether Elfn1 regulates the recruitment of O-LM neurons in response to pyramidal cell spiking, we performed simultaneous cell-attached recordings from neighboring uninfected and shElfn1-infected O-LM neurons. As previously demonstrated (7), uninfected O-LM neurons require repetitive stimulation of pyramidal cell axons to reach spike threshold (Fig. 3E). In contrast, shElfn1-infected neurons showed a significant increase in spike probability at early stimuli in the train (Fig. 3E), as predicted by larger underlying EPSC amplitudes. Thus, by regulating short-term facilitation, Elfn1 influences the temporal dynamics of O-LM recruitment in response to pyramidal cell activity.
Next, we investigated whether Elfn1 expression in target cells was sufficient to alter functional properties of CA1 outputs. Pyramidal cells form depressing synapses onto PV interneurons. To determine whether we could convert depressing pyramidal-PV synapses into facilitating synapses, we overexpressed Elfn1-GFP in CA1 by using lentiviral-mediated infection in PV∷tdTomato mice and recorded at pyramidal-PV synapses. Elfn1 overexpression converted pyramidal-PV synapses into moderately facilitating synapses (Fig. 4A), indicating that postsynaptic Elfn1 can instruct the output properties of CA1 pyramidal cell axons.
Fig 4.
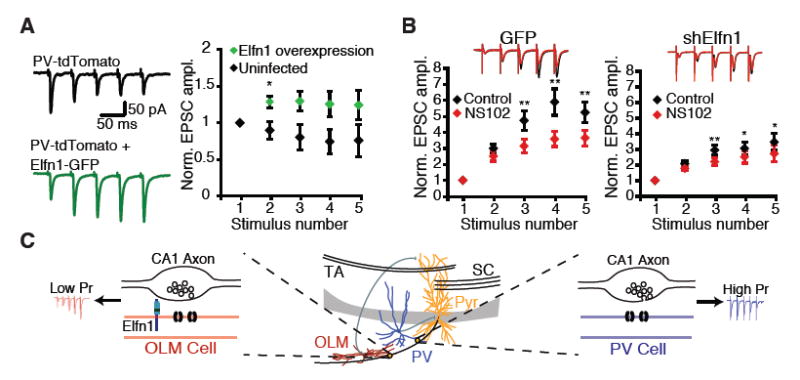
Elfn1 is sufficient to modulate CA1 outputs. (A) Lentivirus overexpressing Elfn1-GFP is injected into P5 PV∷tdTomato mouse pups. Stratum pyramidale or stratum oriens PV neurons in the infected area are targeted for recording. (Left) Response of control and shElfn1-expressing PV neurons to five stimuli delivered to the alveus at 20 Hz. (Right) Quantification of short-term plasticity in Elfn1 overexpressing PV cells. *P < 0.05 by Mann-Whitney U test. (B) (Right) Example recording and quantification of evoked EPSC in GFP-infected Sst neurons before and after application of the GluR6-selective kainate receptor antagonist, NS102 (20 mM). (Left) Average postsynaptic response of GFP-infected Sst interneurons before and after NS102, n = 8. (Right) Average postsynaptic response of shElfn1-infected Sst interneurons before and after NS102, n = 14. * P < 0.05; ** P < 0.01 by ANOVA with Tukey's post-hoc test. (C) Model of Elfn1 function at the synapse. In CA1, Elfn1 is selectively localized to excitatory synapses onto O-LM interneurons. Elfn1 signals transsynaptically to contacting CA1 axons to reduce probability of release and create a facilitating synapse.
Last, we examined whether Elfn1 regulates short-term facilitation by engaging one of the pathways that has been implicated in controlling release probability. Short-term facilitation at synapses onto other types of Sst interneurons has been shown to arise from the activation of presynaptic glutamate receptors, which produces direct depolarization and calcium influx into the presynaptic terminal. At Schaffercollateral synapses, presynaptic GluR5- and GluR6-containing kainate receptors underlie target-specific facilitation at terminals on another group of Sst interneurons in the stratum radiatum (23, 24). In cortex, presynaptic NMDARs have recently been shown to control release probability at pyramidal-Sst interneuron synapses (25). To investigate whether similar mechanisms regulate release at pyramidal-O-LM synapses, we used a pharmacological approach to examine the contribution of these presynaptic receptors. We found that blocking presynaptic GluR5- containing kainate receptors and presynaptic NMDARs had no effect on facilitation (fig. S8). However, the GluR-6-specific kainate receptor antagonist NS102 significantly reduced facilitation, suggesting that these kainate receptors contribute to setting release probability at pyramidalO-LM synapses (Fig. 4B).
To determine whether Elfn1 acts by engaging a kainate receptor-mediated mechanism, we examined the effect of NS102 on synaptic transmission onto cells expressing shElfn1. The effect of NS102 was attenuated when recording from Elfn1 knockdown neurons, suggesting that the kainate-mediated component of facilitation is sensitive to postsynaptic Elfn1 levels (Fig. 4B). The mechanism by which Elfn1 signals to the presynaptic terminal is unclear and could include a direct presynaptic interaction, an additional postsynaptic cofactor that bridges the synapse, or a diffusible signal. Our data and previous studies (26) have shown that initial release probability is low at pyramidal-O-LM synapses, which cannot be explained by glutamate-induced activation of kainate receptors (Fig. 3, A and B). Although additional mechanisms may keep initial release probability lower at pyramidal-O-LM synapses (14, 23), our data suggest that kainate receptors contribute to the large increase in release probability that occurs during repetitive activation of pyramidal cells and that Elfn1 acts by engaging a GluR6-dependent mechanism to regulate facilitation.
Discussion
Our observations indicate that postsynaptic Elfn1 regulates target-specific presynaptic properties, namely the differential release probability at CA1-O-LM versus CA1-PV synapses (Fig. 4C). This influences the timing of recruitment of inhibitory neuron subtypes. O-LM neurons target distal CA1 pyramidal cell dendrites, whereas PV neurons target pyramidal cell somata, so the differential recruitment of these interneuron types dictates when inhibition is channeled to distinct somatodendritic compartments to regulate hippocampal output. The activity of O-LM and PV interneurons is synchronized to theta rhythms (27), which are associated with distinct behavioral states, including active exploration and memory formation or retrieval (28-30). Thus, by regulating the temporal dynamics of interneuron recruitment, Elfn1 could exert substantial effect on hippocampus-dependent behavior. Although we have emphasized Elfn1 function in the hippocampus, Elfn1 is expressed in other interneuron populations that receive facilitating synapses, such as somatostatin cells of the neocortex and hilar perforant path-associated (HIPP) cells of the dentate gyrus. Elfn1 may therefore broadly regulate circuit dynamics in the central nervous system and consequently exert considerable influence on cortical output and cognitive function.
Materials and Methods
Plasmids
The full length mouse clone for Elfn1 (Accession# BC059029) was obtained from Open Biosystems (Thermo Fisher Scientific, Huntsville, AL) and subcloned into the expression vector pEGFPN1 (Accession# U55762) to yield a C-terminal GFP fusion construct. For knockdown experiments, an shRNA was obtained from Open Biosystems containing nucleotides 2440-2460 of Elfn1 (gacatcctagactactggaaa), which is 100% conserved between mouse and rat. The U6 promoter and shRNA was subcloned into the lentiviral plasmid FUGW (Addgene), which contains a ubiquitin promoter driving eGFP expression. The Elfn1 rescue construct had a single point mutation in the shRNA target sequence (gacatccttgactactggaaa).
In Situ Hybridization
In situ hybridizations were performed as described in (31), using P7, P14, or P21 mouse or rat brain cryosections. Digoxigenin-labeled cRNA probes were generated from linearized cDNA templates. Full length mouse Elfn1 was subcloned in the reverse orientation into pcDNA3.1(-) to generate anti-sense riboprobes from the coding region using T7 RNA polymerase. For experiments combining in situ hybridization with immunohistochemistry, tissue was prepared using an immunoperoxidase detection kit prior to dehydration (Vectastain Elite ABC; Vector Labs, CA).
Hippocampal Culture
Hippocampal neurons were cultured from P0 Long-Evans rats (Charles River, Wilmington, MA) and plated on poly-D-lysine (Millipore, Temecula, CA), and laminin (Invitrogen, Carlsbad, CA) coated chamber slides (NalgeNunc International, Rochester, NY). Neurons were maintained in Neurobasal-A medium (Invitrogen) supplemented with B27, glucose, glutamax, and penicillin/streptomycin (Invitrogen).
Mixed Culture Assay
Mixed-culture assays were performed as described in (32). HEK 293T cells were grown in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen) and penicillin/streptomycin. Cells were transfected with eGFP or Elfn1-GFP using Fugene6 (Roche). After 24 hours, cells were mechanically triturated and replated on hippocampal neurons (7 DIV) for 2 days and immunostained for synapsin and GFP.
Immunocytochemistry
Neurons were fixed at P14 in 4% paraformaldehyde in phosphate buffered saline (PBS) and processed for immunofluorescence according to standard procedures. For synapse density experiments, primary antibodies were: rabbit anti-VGlut1, rabbit anti-VGAT, mouse anti- gephyrin, (Synaptic Systems, Goettingen, Germany); mouse anti-PSD-95 (Affinity BioReagents, Rockford, IL); goat anti-GFP, chicken anti-Map2 (Abcam, Cambridge, MA). For mixed culture assay, primary antibodies were: rabbit anti-synapsin (Millipore) goat anti-GFP, (Abcam, Cambridge, MA). For cell type determination, primary antibodies were: mouse anti-GAD6 (Developmental Studies Hybridoma Bank, Iowa City); mouse anti-CamKII (Chemicon, Ramona, CA); rabbit anti-Elfn (anti-lrrc62, Prestige Antibodies-Sigma, St. Louis, MO), rat anti- somatostatin (Millipore), goat anti-mGluR1a (Frontier Institute), sheep anti-PV (R&D Systems). Fluorophore-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA) or Invitrogen.
Image Acquisition and Analysis
Images were captured on a Leica SP5 confocal microscope (Leica Microsystems, Bannockburn, IL). Z-stacks were collapsed in a maximum projection and analyzed using NIH ImageJ software. Images were thresholded using constant settings per experiment and the density of colocalizing pre- and postsynaptic puncta was measured per length of GFP-positive dendrite of transfected neurons. For quantification of mixed-culture assays, images were thresholded and the total area of synapsin puncta was measured and normalized to the total GFP-positive area per cell. For experiments to determine cell-type, z-stack images were collapsed and images were thresholded to determine colocalization of cell type markers. Fields of view at 20x were then counted for each cell type.
Mice
Somatostatin-IRES-Cre mice were created by the Huang lab (17) and Parvalbumin-IRES-GFP mice were generated by the Arber lab (15). Both are available through Jackson Labs. LSL-CAG- tdTomato mice were created by the Allen Institute for Brain Science and acquired from Jackson Labs (16).
Lentivirus Production
For lentivirus production, 293T cells were transfected with control or shElfn1 containing FUGW vector plasmids and helper plasmids MDL, RSV-REV and VSVG using polyethylenimine (PEI). Supernatant was collected 48 hrs after transfection, spun at 2000 rpm to remove debris and filtered through a 0.22 μm filter (Millipore). Viral particles were pelleted using two centrifugation steps at 19500 rpm for 2 hrs each. The final pellet was resuspended in 100 μl PBS and stored at -80°C in 10 μl aliquots.
Electrophysiology
Postnatal day 5-6 mouse pups were anaesthetized with isoflurane and received a subcutaneous injection of bupivacaine prior to craniotomy. Lentivirus injections were targeted to the CA1 region of the hippocampus using stereotaxic coordinates. At postnatal day 13-16, 300 μm slices were maintained in a sucrose substituted solution: 83 NaCl, 2.5 KCl, 1 NaH2PO4, 26 NaHCO3, 22 glucose, 72 sucrose, 0.5 CaCl2, 3.3 MgCl2. Slices were moved to the recording chamber and perfused with an ACSF that consisted of (in mM): 119 NaCl, 2.5 KCl, 26 NaHCO3, 1 NaH2PO4, 2 MgCl2, 2 CaCl2, 11 glucose, 0.1 picrotoxin and bubbled constantly with 95% O2/5% CO2. Somatostatin positive oriens interneurons were identified by tdTomato and eGFP epifluorescence microscopy (Zeiss Axioskop 2). Whole-cell voltage clamp recordings were made under visual guidance aided by infrared differential interference. Neurons lying near the alveus that expressed both eGFP and tdTomato were recorded from using ~3MΩ pipettes pulled on a horizontal micropipette puller (Sutter P-97) and filled with an internal solution that contained (in mM): 130 Cs-methanosulfonate, 5 NaCl, 10 EGTA, 10 HEPES, 10 phosphocreatine, and 2 Mg-ATP, pH 7.3 with CsOH, 280-290 mOsm. Synaptic responses were evoked every 15 s with a bipolar cluster electrode (FHC) placed in the alveus near the subiculum. The signals were low-pass filtered at 2 kHz, digitized at 10 kHz (Molecular Devices Multiclamp 700B) and analyzed with pClamp 9 (Molecular Devices). Series resistance and input resistance were monitored throughout the experiment by a test pulse.
Short-term facilitation was measured at a holding potential of -70mV by delivering a train of 5 stimuli at 5, 10, 20, and 40 Hz. At least 10 sweeps were averaged at each frequency and the amplitude was calculated as the average 1 ms around the peak. The amplitudes were normalized to the amplitude response to the first stimulus in a train to determine the facilitation ratio. To determine rectification of synaptic currents, D-APV (50μM) was added to the perfusion solution. The alveus was stimulated once as described above, then the holding potential was increased from -70mv to +70 mV by 20 mV increments, and repeated 10 times to calculate an average. For MK801 experiments, DNQX (20μM) was used to block non-NMDA currents. The cell was held at +40mV and after a steady baseline was attained, MK801 (40μM) was washed in and the alveus was repetitively stimulated at 5 s intervals. The decrease in NMDA-mediated current was plotted over time and the number of stimuli needed to reach half the initial amplitude was calculated.
For IPSC recordings, cells were held at 0mV and the alveus was stimulated at 20Hz. Picrotoxin (0.1mM) was washed in to block GABA-mediated currents. The resulting trace was subtracted from the baseline to calculate the GABA mediated current. For presynaptic kainate and NMDA experiments, a stable baseline recording was performed and a pre-drug average was calculated from at least 10 sweeps. The antagonist (20μM NS102, 15μM UBP302, or 50μM APV) was washed in and then a second average was calculated. The two averages were each normalized to the amplitude of their first EPSC and a facilitation ratio was calculated for each subsequent EPSC.
For mEPSC recordings, Gabazine (SR 95531 hydrobromide), D-APV, and TTX were added at the time of recording. mEPSCs were identified and isolated using ClampFit software. Using a control culture, a template was created from a composite average of manually identified mEPSCs and was used with a broad threshold for deviation to ensure all events in subsequent recordings were captured. These events were then manually inspected to discard any non-mEPSC traces. Cumulative fractions were calculated by randomly selecting 125 events and plotting the distributions.
Supplementary Material
Acknowledgments
We thank M. Scanziani, P. Scheiffele, and members of the Ghosh lab for suggestions and comments on the manuscript; J. de Wit for generating the Elfn1-GFP construct; and M. Macias, C. Sanchez, and E. Kang for help with virus and protein production. This work was supported by NIH grant R01NS067216 and the Gatsby Charitable Foundation.
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/science.1222482/DC1
Figs. S1 to S8
Supplementary Figure Legends
References and Notes
- 1.Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497. doi: 10.1038/nrn876. [DOI] [PubMed] [Google Scholar]
- 2.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- 3.Reyes A, et al. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 4.Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol. 1998;507:201. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol. 1998;507:185. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacaille J-C, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- 8.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 9.Dolan J, et al. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8:320. doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit J, Hong W, Luo L, Ghosh A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu Rev Cell Dev Biol. 2011;27:697. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- 11.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biró AA, Holderith NB, Nusser Z. Quantal size is independent of the release probability at hippocampal excitatory synapses. J Neurosci. 2005;25:223. doi: 10.1523/JNEUROSCI.3688-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 16.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- 19.Ko J, et al. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50:233. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Linhoff MW, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, et al. Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 23.Sun HY, Dobrunz LE. Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci. 2006;26:10796. doi: 10.1523/JNEUROSCI.2746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun HY, Bartley AF, Dobrunz LE. Calcium-permeable presynaptic kainate receptors involved in excitatory short-term facilitation onto somatostatin interneurons during natural stimulus patterns. J Neurophysiol. 2009;101:1043. doi: 10.1152/jn.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan KA, et al. Target-Specific Expression of Presynaptic NMDA Receptors in Neocortical Microcircuits. Neuron. 2012;75:451. doi: 10.1016/j.neuron.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun HY, Lyons SA, Dobrunz LE. Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats. J Physiol. 2005;568:815. doi: 10.1113/jphysiol.2005.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klausberger T, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 28.Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551. doi: 10.1016/0306-45228990423-5. [DOI] [PubMed] [Google Scholar]
- 29.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347. doi: 10.1016/0006-89938690579-2. [DOI] [PubMed] [Google Scholar]
- 30.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 31.Pasterkamp RJ, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 32.Biederer T, Scheiffele P. Mixed-culture assays for analyzing neuronal synapse formation. Nat Protoc. 2007;2:670. doi: 10.1038/nprot.2007.92. [DOI] [PubMed] [Google Scholar]
- 33.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA. 1995;92:9298. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croce A, Pelletier JG, Tartas M, Lacaille J-C. Afferent-specific properties of interneuron synapses underlie selective long-term regulation of feedback inhibitory circuits in CA1 hippocampus. J Physiol. 2010;588:2091. doi: 10.1113/jphysiol.2010.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


