Abstract
This article summarizes 4 phase 1 trials that explored interactions between the novel, triazole antifungal isavuconazole and substrates of the drug transporters breast cancer resistance protein (BCRP), multidrug and toxin extrusion protein‐1 (MATE1), organic anion transporters 1/3 (OAT1/OAT3), organic anion‐transporting polypeptide 1B1 (OATP1B1), organic cation transporters 1/2 (OCT1/OCT2), and P‐glycoprotein (P‐gp). Healthy subjects received single doses of atorvastatin (20 mg; OATP1B1 and P‐gp substrate), digoxin (0.5 mg; P‐gp substrate), metformin (850 mg; OCT1, OCT2, and MATE1 substrate), or methotrexate (7.5 mg; BCRP, OAT1, and OAT3 substrate) in the presence and absence of clinical doses of isavuconazole (200 mg 3 times a day for 2 days; 200 mg once daily thereafter). Coadministration with isavuconazole increased mean area under the plasma concentration‐time curves (90% confidence interval) of atorvastatin, digoxin, and metformin to 137% (129, 145), 125% (117, 134), and 152% (138, 168) and increased mean maximum plasma concentrations to 103% (88, 121), 133% (119, 149), and 123% (109, 140), respectively. Methotrexate parameters were unaffected by isavuconazole. There were no serious adverse events. These findings indicate that isavuconazole is a weak inhibitor of P‐gp, as well as OCT1, OCT2, MATE1, or a combination thereof but not of BCRP, OATP1B1, OAT1, or OAT3.
Keywords: atorvastatin, digoxin, isavuconazole, metformin, methotrexate
Invasive fungal infections are a common cause of life‐threatening disease worldwide.1 Patients who are immunocompromised due to malignancy or as a consequence of immunosuppressive therapy are particularly at risk.2, 3, 4 However, despite advances in antifungal pharmacology, currently available medications have a number of limitations, including toxicity, a tendency for drug‐drug interactions, and the emergence of resistance.5, 6 Thus, the development of novel, safe, and effective antifungal drugs is an urgent clinical need.
Isavuconazonium sulfate is a new, broad‐spectrum, water‐soluble, triazole antifungal prodrug that was approved for adults in 2015 by the US Food and Drug Administration for the treatment of invasive aspergillosis and invasive mucormycosis and by the European Medicines Agency for the treatment of invasive aspergillosis and for mucormycosis when amphotericin B is inappropriate, based on the results of phase 3 clinical trials.7, 8 Like most triazole antifungal agents,6 its active moiety, isavuconazole, is metabolized by the cytochrome P450 (CYP) system. Primarily, it is a sensitive substrate and acts as a moderate inhibitor of the CYP3A4 isoenzyme.9 Isavuconazole is also a mild inducer of CYP2B6, but it has no inductive or inhibitory potential for CYP1A2, CYP2C8, CYP2C9, CYP2D6, or CYP2C19 (see accompanying articles9, 10, 11). In addition, isavuconazole is a weak inhibitor of uridine diphosphate glucuronosyltransferase (see also accompanying article11).
Triazole antifungal agents also are known to interact with drug transporters and thereby lead to drug‐drug interactions with medications that are substrates of transporters.12 Preclinical studies have indicated that although isavuconazole is not a substrate of the major drug transporters, it may act as an inhibitor of certain transporters (data on file). For example, the bidirectional transport of [3H]digoxin across monolayers of LLC‐PK1 cells transfected with human P‐glycoprotein (P‐gp) was weakly inhibited by isavuconazole (inhibitory constant [IC50] 25.7 μmol/L), as was bidirectional transport of [3H]prazosin by breast cancer resistance protein (BCRP; IC50 35.3 μmol/L). Varying levels of inhibition by isavuconazole were also observed in human embryonic kidney (HEK293) cells stably transfected to express multidrug and toxin extrusion 1 (MATE1; IC50, 6.31 μmol/L with [14C]metformin substrate), organic anion transporter 1 (OAT1; IC50 >10 μmol/L, with [3H]‐p‐aminohippuric acid substrate), OAT3 (IC50 >10 μmol/L with [3H]estrone sulfate substrate), organic anion‐transporting polypeptide 1B1 (OATP1B1), IC50 11.2 μmol/L with [3H]estradiol 17β‐d‐glucuronide substrate), organic cation transporter 1 (OCT1; IC50 3.74 μmol/L; Ki 1.74 μmol/L, with [14C]tetraethylammonium bromide) and OCT2 (IC50 1.97 μmol/L, and Ki 0.69 μmol/L, with [14C]metformin substrate). Values of IC50 or Ki ≤16 μmol may be suggestive of clinical relevance because, based on recommended clinical dosing (200 mg 3 times daily for 2 days, then 200 mg daily), maximum plasma concentrations of isavuconazole are typically <7 μg/mL (data on file; isavuconazole molecular weight 437.47 g/mol). Because isavuconazole would be used in immunocompromised patients with systemic mycoses who require concomitant medications, phase 1 clinical trials were conducted to examine the potential for drug‐drug interactions between isavuconazole and the transporter substrates atorvastatin (OATP1B1 and P‐gp substrate; also a substrate for CYP3A4),13 digoxin (P‐gp substrate),14 metformin (OCT1, OCT2, and MATE1 substrate),15 and methotrexate (BCRP, OAT1, and OAT3 substrate).16, 17
Methods
Study Design
Signed institutional review board–approved written informed consent was obtained from all subjects at each study site (atorvastatin study, Independent Investigational Review Board, Inc. Plantation, Florida; digoxin, metformin, and methotrexate studies, Aspire IRB, LLC, Santee, California) before any study‐related procedures were carried out. Studies were undertaken in compliance with the good clinical practice guidelines of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use and the principles of the Declaration of Helsinki.
These studies were phase 1, single‐center, open‐label, sequential‐dosing trials, conducted in healthy subjects to assess drug‐drug interactions between isavuconazole (administered as isavuconazonium sulfate; CRESEMBA® oral capsules; Astellas Pharma US, Inc., Northbrook, Illinois) and the transporter substrates atorvastatin calcium (LIPITOR® oral tablets, Pfizer Inc. New York, New York; trial conducted in August 2012 at Clinical Pharmacology of Miami, Inc. Miami, Florida; ClinicalTrials.gov identifier NCT01635946), digoxin (LANOXIN® oral tablets, Covis Pharmaceuticals, Inc. Cary, North Carolina; trial conducted April to May 2012 at PAREXEL International, Baltimore, Maryland; NCT01582412), metformin (GLUCOPHAGE® oral tablets, Bristol‐Myers Squibb Company, Princeton, New Jersey; trial conducted January to February 2013 at California Clinical Trials Medical Group, Glendale, California; NCT01884558), and methotrexate sodium (generic oral tablets; trial conducted January to March 2013 at California Clinical Trials Medical Group, Glendale, California; NCT01884636). These agents are recommended by the US Food and Drug Administration as validated substrates to examine potential interactions with transporters in vivo.
Healthy, medication‐free, male and female subjects aged 18 to 55 years, weighing ≥45 kg, with a body mass index of 18 to 32 kg/m2, and with no clinically significant disease history were enrolled in these studies. Only male subjects were enrolled in the methotrexate study.
Dosing and Sampling Schedules
Dosing information is expressed in this report as the isavuconazole equivalent of the prodrug, isavuconazonium sulfate. Each oral capsule contained isavuconazonium sulfate 186 mg, equivalent to isavuconazole 100 mg. The clinically targeted dose of isavuconazole is 200 mg 3 times a day loading dose (TID), followed by 200 mg once daily (QD).
Atorvastatin
Subjects were screened between day –21 and day –2. Following screening, subjects checked in at the study center on day –1, where they remained until completion of all study procedures on day 16. Follow‐up was performed by telephone on day 24 ± 2 (Figure 1A).
Figure 1.
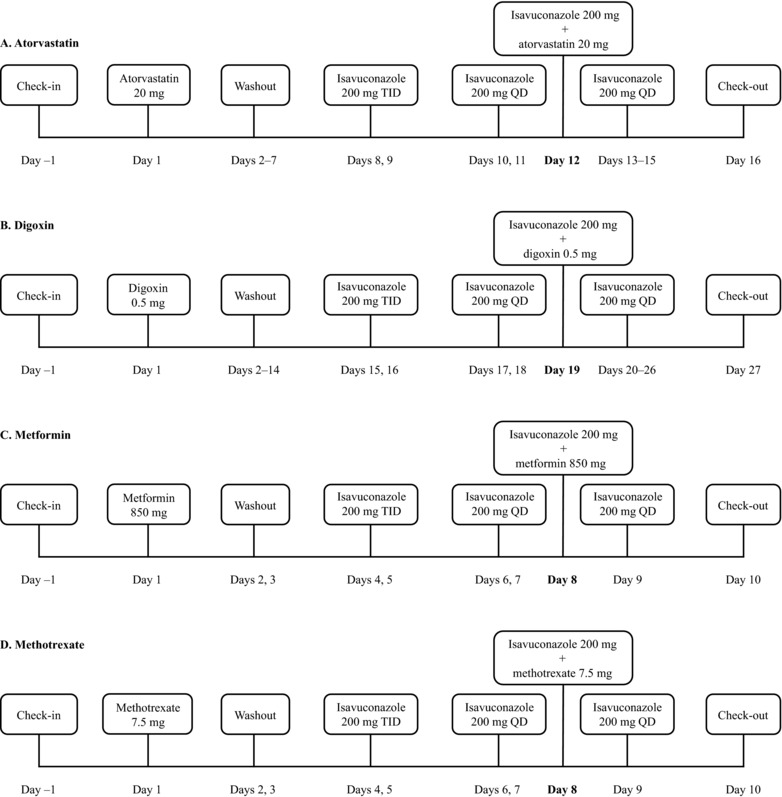
Atorvastatin, digoxin, metformin, and methotrexate study dosing and sampling schedules. QD, once daily; TID, 3 times a day.
On day 1, subjects received a single dose of oral atorvastatin calcium 20 mg. After a 7‐day washout period, subjects received oral isavuconazole 200 mg TID (approximately 8 hours apart) on days 8 and 9, followed by oral isavuconazole 200 mg QD on days 10 to 15. On day 12, a concomitant dose of oral atorvastatin calcium 20 mg was administered immediately following isavuconazole. Subjects fasted for at least 10 hours prior to administration of both doses of atorvastatin on days 1 and 12 and prior to isavuconazole dosing on day 11; they continued to fast for 4 hours after administration. On day 12, isavuconazole was administered immediately following atorvastatin.
Blood samples were obtained for pharmacokinetic (PK) assessment of atorvastatin on days 1 and 12 prior to dosing, and 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24, 48, 72, and 96 hours postdose; and for PK assessment of isavuconazole on days 11 and 12 prior to dosing and 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, and 20, and 24 hours postdose.
Digoxin
Subjects were screened (days –28 to –2) before checking in at the study center (day –1), where they remained through day 9 and between days 14 and 27. A follow‐up visit was conducted 7 days after final dosing of isavuconazole (day 34 ± 2) (Figure 1B).
On day 1, subjects received a single oral dose of digoxin 0.5 mg. After a 2‐week washout, subjects received oral isavuconazole 200 mg TID (approximately 8 hours apart) on days 15 and 16, followed by oral isavuconazole 200 mg QD on days 17 to 26. On day 19, another single dose of oral digoxin 0.5 mg was administered immediately prior to isavuconazole. Subjects fasted for at least 10 hours prior to digoxin dosing on days 1 and 19, and for 4 hours after administration. Isavuconazole was administered immediately following digoxin on day 19.
Blood samples were obtained for PK assessment of digoxin on days 1 and 19 prior to dosing and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24, 48, 72, 96, 120, 144, 168, and 192 hours postdose; and for PK assessment of isavuconazole on days 18 and 19 prior to dosing and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, and 24 hours postdose.
Metformin
Subjects were screened from day –21 to day –2, prior to check in at the study center on day –1, where they remained through completion of study procedures on day 10, and follow‐up was performed by telephone on day 16 (±2 days) (Figure 1C). On day 1, subjects received a single dose of oral metformin hydrochloride 850 mg. After a 3‐day washout, subjects received oral isavuconazole 200 mg TID (approximately 8 hours apart) on days 4 and 5, followed by oral isavuconazole 200 mg QD on days 6 through 9. On day 8, a further single oral dose of metformin hydrochloride 850 mg was administered immediately after isavuconazole. On days 1, 7, and 8, doses were administered after a 10‐hour fast, and subjects continued to fast for 4 hours after administration of the drugs. Metformin was dosed after isavuconazole on day 8.
Blood samples for PK assessment of metformin were obtained on days 1 and 8 prior to dosing and 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16, 24, 36, and 48 hours postdose and for PK assessment of isavuconazole on days 7 and 8 prior to dosing, and 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16, and 24 hours postdose.
Methotrexate
The screening, dosing, and follow‐up schedule in this study was identical to that for the metformin study (Figure 1D). Subjects received single doses of oral methotrexate 7.5 mg alone and concomitantly with oral isavuconazole. Blood samples for PK analysis of methotrexate and its metabolite 7‐hydroxymethotrexate (also transported by BCRP18) were obtained on days 1 and 8 prior to dosing and 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16, 24, and 36 hours postdose; and for PK analysis of isavuconazole on days 7 and 8 prior to dosing, and 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16, and 24 hours postdose.
Pharmacokinetic Assessments
Measurement of plasma concentrations of all analytes was conducted by liquid chromatography–mass spectrometry/mass spectroscopy (see Supplementary Materials for details). The primary PK parameters evaluated were area under the plasma concentration–time curve (AUC) from time of dosing extrapolated to infinity (AUC∞), AUC from time of dosing to the last measurable concentration (AUClast), and maximum observed plasma concentration (Cmax). Other PK parameters calculated were AUC during the time interval between consecutive dosing (AUCτ), time to Cmax (tmax), apparent volume of distribution (Vz/F), apparent body clearance after extravascular dosing (CL/F), and half‐life (t1/2).
Safety Assessments
Treatment‐emergent adverse events (TEAEs) were monitored and assessed throughout the studies. TEAEs were defined as any untoward medical events (not necessarily caused by study drug administration) that occurred from the time of study drug administration (day 1) to the end of the study. Safety was also evaluated using vital‐sign measurements, 12‐lead ECG, clinical laboratory testing (hematology, chemistry, and urinalysis), and physical examinations.
Statistics
Descriptive statistics were used to summarize demographics, baseline characteristics, and TEAEs in all subjects who received ≥1 dose of the study drug. The PK parameters were assessed in all subjects who received ≥1 dose of the study drug and whose PK data were adequate for the calculation of ≥1 primary PK parameter (AUC and Cmax). Levels of analyte below the level of quantification were entered as 0 for calculations. To assess the effect of isavuconazole on the PK of atorvastatin, digoxin, metformin, methotrexate, and 7‐hydroxymethotrexate, log‐transformed parameters (AUC∞, AUClast, and Cmax) were analyzed using a mixed‐effects model with treatment (substrate alone and substrate plus isavuconazole) as a fixed effect and subject as a random effect. For the primary PK parameters, 90% confidence intervals (CIs) were constructed around the geometric least‐squares mean ratios of transporter substrate plus isavuconazole vs transporter substrate alone. Parameters were calculated using noncompartmental analysis with Phoenix® WinNonlin® version 6.2 (Certara USA, Inc. Princeton, New Jersey). All PK and safety data analyses were performed using SAS® version 9.1 or higher (SAS® Institute Inc. Cary, North Carolina).
Results
Pharmacokinetics
Atorvastatin
Twenty‐four subjects enrolled in and completed the atorvastatin study (Table 1). Mean atorvastatin AUC∞, AUClast, and Cmax were 37%, 38%, and 3% higher following coadministration with isavuconazole vs atorvastatin alone, respectively (Tables 2 and 3; see Figure 2 for concentration‐time profile). Isavuconazole PK parameters were comparable in the presence and absence of atorvastatin (Table 4).
Table 1.
Baseline Demographics and Subject Characteristics
| Parameter | Atorvastatin (n = 24) | Digoxin (n = 24) | Metformin (n = 24) | Methotrexate (n = 24) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 13 (54.2) | 18 (75.0) | 18 (75.0) | 24 (100) |
| Female | 11 (45.8) | 6 (25.0) | 6 (25.0) | 0 |
| Race, n (%) | ||||
| White | 18 (75.0) | 7 (29.2) | 14 (58.3) | 14 (58.3) |
| Black or African | 6 (25.0) | 16 (66.7) | 7 (29.2) | 7 (29.2) |
| American | ||||
| Asian | 0 | 1 (4.2) | 3 (12.5) | 2 (8.3) |
| Middle Eastern | 0 | 0 | 0 | 1 (4.2) |
| Ethnicity, n (%) | ||||
| Not Hispanic or Latino | 15 (62.5) | 21 (87.5) | 17 (70.8) | 18 (75.0) |
| Age, years, mean (SD) | 43.1 (9.0) | 37.1 (11.2) | 31.8 (9.6) | 33.5 (10.2) |
| Weight, kg, mean (SD) | 82.2 (13.4) | 82.1 (14.1) | 75.2 (12.2) | 80.1 (12.1) |
| BMI, kg/m2, mean (SD) | 27.8 (3.1) | 26.8 (3.1) | 25.1 (3.2) | 25.9 (3.6) |
BMI, body mass index; SD, standard deviation.
Table 2.
Summary of Plasma PK Parameters of Atorvastatin, Digoxin, Metformin, and Methotrexate Alone and During Coadministration With Isavuconazole
| Atorvastatin | Digoxin | Metformin | Methotrexate | 7‐Hydroxymethotrexate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parametera | Atorvastatin Alone (n = 24)b , c | Atorvastatin + Isavuconazole (n = 24) | Digoxin Alone (n = 24) | Digoxin + Isavuconazole (n = 21)d | Metformin Alone (n = 23)e | Metformin + Isavuconazole (n = 20)e , f | Methotrexate Alone (n = 24) | Methotrexate + Isavuconazole (n = 23)g | Methotrexate Alone (n = 24) | Methotrexate + Isavuconazole (n = 23)g |
| AUC∞, h·ng/mL | 44.4 (17.9) | 58.7 (21.1) | 34.4 (10.9)h | 42.1 (10.6) | 9303 (2543) | 14,170 (4308) | 619.8 (132.2) | 600.1 (124.2) | 635.8 (309.3) | 837.3 (345.8) |
| AUClast, h·ng/mL | 40.5 (17.8) | 55.0 (20.8) | 32.9 (10.3)h | 40.1 (9.8) | 9119 (2524) | 13,697 (4162) | 606.5 (131.7) | 586.7 (126.2) | 554.6 (242.6) | 683.2 (272.5) |
| Cmax, ng/mL | 7.5 (3.8) | 8.1 (4.8) | 1.6 (0.6) | 2.2 (0.8) | 1467 (449) | 1792 (533) | 156.3 (32.4) | 139.7 (29.5) | 32.1 (13.4) | 36.7 (14.0) |
| tmax, h | 0.8 (0.5‐2.0) | 0.8 (0.5‐5.0) | 1.0 (1.0‐3.0) | 1.5 (0.5‐3.0) | 2.5 (1.0‐6.0) | 2.0 (1.0‐4.0) | 1.0 (1.0‐2.5) | 1.0 (1.0‐3.0) | 6.0 (5.0‐10.0) | 6.0 (3.5‐10.0) |
| t1/2, h | 10.1 (4.4) | 11.2 (3.6) | 40.9 (4.7) | 44.1 (6.0) | 12.5 (3.8) | 14.3 (6.2) | 3.1 (0.5) | 3.7 (1.0) | 11.9 (2.9) | 13.0 (2.3) |
| CL/F, L/h | 522.9 (206.4) | 386.2 (145.9) | 16.0 (5.1)h | 12.7 (3.7) | 76.6 (20.9) | 50.4 (13.4) | 12.3 (4.0) | 12.5 (2.9) | ND | ND |
AUC∞, area under the plasma concentration‐time curve from time 0 extrapolated to infinity; AUClast, AUC from time 0 to the last measurable concentration; CL/F, apparent body clearance after oral dosing; Cmax, maximum observed concentration; ND, not done; t1/2, apparent terminal elimination half‐life; tmax, time to reach Cmax.
Values are expressed as arithmetic mean (standard deviation) except tmax, which is expressed as median (range).
Day 1: AUC∞, CL/F, and Vz/F, n = 23; values for 1 subject were unreliable due to the percentage of AUC∞ that was extrapolated being >20%.
Days 1 and 12: t1/2, n = 23; concentrations for 1 subject were below the lower limit of quantification in the terminal phase.
Two subjects discontinued the study prior to day 18 due to a treatment‐emergent adverse event (TEAE); 1 subject discontinued the study prior to day 18 due to prohibited use of a concomitant medication.
Concentrations for 1 subject were all below the lower limit of quantification during metformin and isavuconazole coadministration (day 8) due to potential noncompliance, and they were excluded from the PK analysis set.
Two subjects discontinued due to a TEAE, and 1 subject withdrew consent prior to metformin and isavuconazole coadministration (day 8).
One person withdrew consent during isavuconazole administration (day 6).
n = 23: 1 subject discontinued due to prohibited use of a concomitant medication.
Table 3.
Statistical Comparison of Log‐Transformed Atorvastatin, Digoxin, Metformin, and Methotrexate PK Parameters
| Geometric Least‐Squares Mean Ratio, % (90% Confidence Interval)a | |||||
|---|---|---|---|---|---|
| Parameter | Atorvastatin | Digoxin | Metformin | Methotrexate | 7‐Hydroxymethotrexate |
| AUC∞ | 137 (129, 145) | 125 (117, 134) | 152 (138, 168) | 97 (90, 104.6) | 129 (119, 141) |
| AUClast | 138 (129, 149) | 124 (116, 133) | 150 (137, 165) | 97 (90, 104.7) | 124 (114, 135) |
| Cmax | 103 (88, 121) | 133 (119, 149) | 123 (109, 140) | 89 (83, 97) | 115 (104, 127) |
AUC, area under the concentration‐time curve; Cmax, maximum concentration.
Results based on a mixed‐effects model of natural log‐transformed parameters, with treatment as a fixed effect and subject as a random effect.
Figure 2.
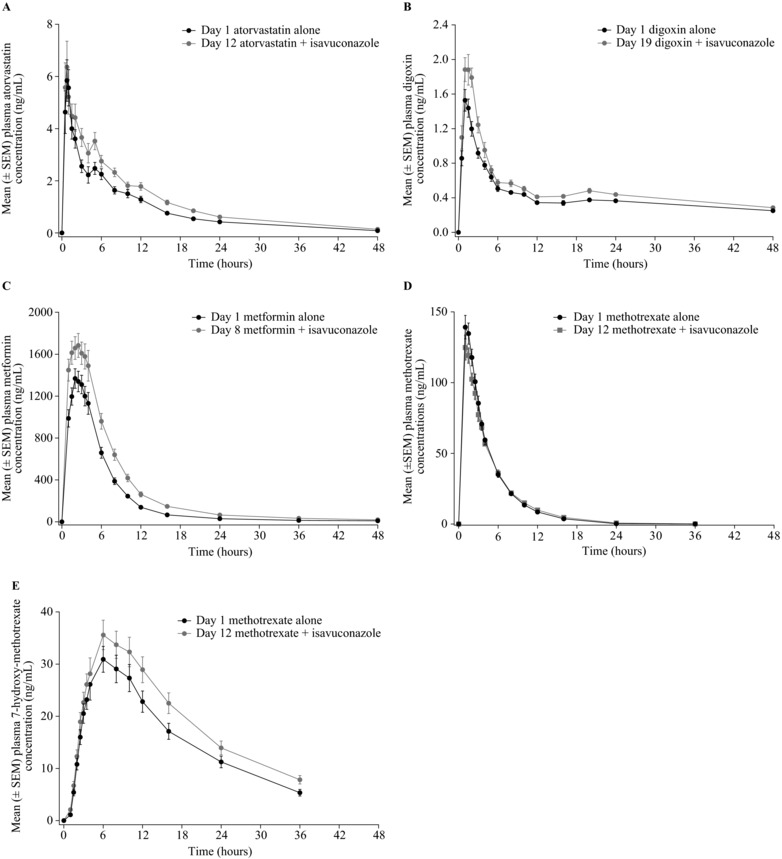
Mean plasma concentration‐time profiles of atorvastatin (A), digoxin (B), metformin (C), methotrexate (D), and 7‐hydroxymethotrexate (E) in the presence and absence of isavuconazole. SEM, standard error of the mean.
Table 4.
Summary of Plasma PK Parameters of Isavuconazole
| Atorvastatin | Digoxin | Metformin | Methotrexate | |||||
|---|---|---|---|---|---|---|---|---|
| Parametera | Isavuconazole Alone (n = 24) | Isavuconazole + Atorvastatin (n = 24) | Isavuconazole Alone (n = 21)b | Isavuconazole + Digoxin (n = 21)b | Isavuconazole Alone (n = 20)c | Isavuconazole + Metformin (n = 20)c | Isavuconazole Alone (n = 23)d | Isavuconazole + Methotrexate (n = 23)d |
| AUCτ, h·μg/mL | 77.3 (22.0) | 81.2 (22.3) | 101.8 (30.2) | 101.1 (29.5) | 105.7 (31.0) | 111.7 (33.7) | 99.4 (30.8) | 102.7 (29.5) |
| Cmax, μg/mL | 5.3 (1.2) | 5.5 (1.3) | 6.1 (1.7) | 6.2 (1.7) | 7.1 (2.2) | 7.2 (2.0) | 6.2 (1.5) | 6.4 (1.6) |
| tmax, hours | 3.0 (1.5‐4.0) | 2.5 (1.5‐4.0) | 3.0 (2.0‐4.1) | 3.0 (2.0‐5.0) | 3.0 (1.5‐4.0) | 2.8 (2.0‐4.0) | 3.0 (2.0‐4.0) | 3.0 (2.0‐4.0) |
AUC, area under the plasma concentration‐time curve; Cmax, maximum observed concentration; TEAE, treatment‐emergent adverse event; tmax, time to reach Cmax.
Values are expressed as arithmetic mean (standard deviation), except tmax, which is expressed as median (range).
Two subjects discontinued the study prior to day 18 due to TEAEs; 1 subject discontinued the study prior to day 18 due to prohibited use of a concomitant medication.
Two subjects discontinued due to a TEAE, and 1 subject withdrew consent prior to metformin and isavuconazole coadministration (day 8).
One person withdrew consent during isavuconazole administration (day 6).
Digoxin
Twenty‐four subjects enrolled and 21 completed the digoxin study (Table 1). Mean AUC∞, AUClast, and Cmax of digoxin were 25%, 24%, and 33% higher in the presence vs absence of isavuconazole, respectively (Tables 2 and 3; see Figure 2 for concentration‐time profile). Isavuconazole PK were similar in the presence and absence of digoxin (Table 4).
Metformin
Twenty‐four subjects enrolled and 21 completed the metformin study (Table 1). One subject was excluded from the PK analysis set because his concentrations on day 8 were all below the lower limit of quantification (2 ng/mL for metformin; 100 ng/mL for isavuconazole). Mean AUC∞, AUClast, and Cmax of metformin were 52%, 50%, and 23% higher in the presence vs absence of isavuconazole, respectively (Tables 2 and 3; see Figure 2 for concentration‐time profile). Isavuconazole PK was similar in the presence and absence of digoxin (Table 4).
Methotrexate
Twenty‐four subjects enrolled, and 23 completed the methotrexate study (Table 1). Mean AUC∞, AUClast, and Cmax of methotrexate were 3%, 3%, and 11% lower when coadministered with isavuconazole than during administration of methotrexate alone, respectively. By contrast, mean 7‐hydroxymethotrexate AUC∞, AUClast, and Cmax were 29%, 24%, and 15% higher during concomitant administration with isavuconazole, respectively (Tables 2 and 3; see Figure 2 for concentration‐time profile). Isavuconazole PK parameters were unchanged in the presence and absence of methotrexate (Table 4).
Safety
Among all of the studies, there were no serious TEAEs and few discontinuations. Most TEAEs experienced by subjects were mild in intensity.
In the atorvastatin study, the most common TEAEs were vertigo (n = 3) and myositis (n = 2) (Supplementary Table S1). No subjects discontinued this study due to TEAEs.
The most common TEAEs experienced in the digoxin study were nervous system disorders including headache (n = 4) and dizziness (n = 2) (Supplementary Table S2). One TEAE of muscle spasm was moderate in intensity and considered to be possibly related to isavuconazole treatment by the study investigator. One subject discontinued this study on day 1 due to use of a prohibited concomitant medication, and 1 subject discontinued from the study due to a mild TEAE of increased aspartate aminotransferase, which was not considered to be related to study‐drug administration. One additional subject was lost to follow‐up.
The most common TEAEs experienced in the metformin study were gastrointestinal disorders including diarrhea (n = 6) and nausea (n = 4) as well as nervous system disorders including headache (n = 6) and dizziness (n = 5) (Supplementary Table S3). One TEAE of diarrhea was moderate in intensity. Two subjects discontinued the study due to TEAEs: 1 on day 5 due to a mild TEAE of intermittent vomiting, which was considered to be probably related to isavuconazole administration, and 1 on day 3 due to a mild TEAE of muscle spasms, which was considered to be possibly related to metformin administration. In addition, 1 subject withdrew from the study on day 4 for personal reasons.
In the methotrexate study the most common TEAEs were nervous system disorders including headache (n = 2) and gastrointestinal disorders including diarrhea (n = 2) (Supplementary Table S4). One TEAE of a viral upper respiratory tract infection was moderate in intensity. One subject withdrew from the study on day 6 for personal reasons.
Discussion
This report describes the clinical studies conducted to evaluate potential interactions between isavuconazole and the transporter substrates atorvastatin (OATP1B1 and P‐gp substrate),13 digoxin (P‐gp substrate),14 metformin (OCT1, OCT2, and MATE1 substrate),15 and methotrexate (BCRP, OAT1, and OAT3 substrate)16, 17 in healthy human subjects. Methotrexate exposure was largely unaffected, but coadministration with isavuconazole resulted in approximately 37%, 25%, and 52% increases in the exposure of atorvastatin, digoxin, and metformin, respectively. These findings indicate that isavuconazole is a weak inhibitor of P‐gp as well as OCT1, OCT2, MATE1, or a combination thereof.
Given that atorvastatin is known to be a sensitive substrate of OATP1B1,19, 20 it was notable that coadministration with isavuconazole only weakly inhibited atorvastatin exposure. In addition, the PK of the OATP1B1 substrate repaglinide is unchanged in the presence and absence of isavuconazole.10 Together, these observations suggest that OATP1B1 is unaffected by isavuconazole in vivo and that the small change in atorvastatin exposure was likely due instead to inhibition of CYP3A4 by isavuconazole. Atorvastatin is metabolized by CYP3A4,21 and isavuconazole is known to be a moderate inhibitor of this isoenzyme.9 Moreover, coadministration of itraconazole (strong CYP3A4 inhibitor) and atorvastatin also results in increased (approximately 47%) exposure of atorvastatin, and this effect is attributed to CYP3A4 inhibition by itraconazole.22 Due to the increased exposure of atorvastatin observed in this study, monitoring of patients for atorvastatin‐related adverse reactions is recommended during coadministration with isavuconazole.
The P‐gp‐mediated increase in plasma digoxin concentrations during coadministration with isavuconazole was markedly lower than that reported for itraconazole (approximately 80%).23 Posaconazole has also been reported to increase plasma concentrations of digoxin (see NOXAFIL® package insert), although voriconazole does not appear to affect levels of digoxin when these agents are given together.24 Potential interactions between fluconazole and digoxin have not been examined in vivo; however, in vitro studies indicate that fluconazole is not an inhibitor of P‐gp‐mediated active transport.25 As digoxin has a narrow therapeutic profile, monitoring of digoxin levels during coadministration with isavuconazole is advised.7
Isavuconazole displayed weak inhibitory effects on the OCT1, OCT2, and MATE1 substrate, metformin; however, the relative contributions of each transporter to this finding were not examined further. Nonetheless, this interaction is not expected to cause any major safety concerns or to disrupt the therapeutic efficacy of metformin. Last, the results obtained in the methotrexate study indicate that isavuconazole is not an inhibitor of OAT1 and OAT3 in vivo. The results of this study also indicate that isavuconazole is not an inhibitor of BCRP in vivo, based on guidance by the US Food and Drug Administration. However, it should be noted that methotrexate is not listed as a sensitive substrate of BCRP by the European Medicines Agency, and further testing of a potential interaction between isavuconazole and a specific substrate of BCRP would be beneficial. There are no published trials of metformin or methotrexate coadministration with other currently approved triazole antifungals.
In summary, the results of the current series of studies indicate that isavuconazole is a weak inhibitor of P‐gp and possibly a mild inhibitor of OCT1, OCT2, MATE1, or a combination thereof. By contrast, isavuconazole has no inhibitory potential for BCRP‐, OATP1B1‐, OAT1‐, or OAT3‐mediated transport in vivo. The PK of isavuconazole was unaffected by coadministration with any of the transporters substrates studied.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Acknowledgments
Isavuconazonium sulfate was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. The studies were funded by Astellas Pharma Global Development, Inc. Editorial support was provided by Neil M. Thomas, PhD, CMPP and Tara N. Miller, PhD, CMPP, medical writers at Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. The authors are grateful for the contributions of the investigators and staff who conducted the clinical trials and to the subjects who volunteered for these studies.
Declaration of Conflicting Interests
Isavuconazole was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. T.Y., A.D., C.H., S.A., D.K., C.L., and R.T. are employees of Astellas Pharma Global Development, Inc. R.G. and D.H. are employees of Parexel, who were contracted by Astellas Pharma Global Development, Inc. to perform work related to the digoxin, metformin, and methotrexate studies. K.L. is an employee of Clinical Pharmacology of Miami, Inc., who was contracted by Astellas Pharma Global Development, Inc. to perform work related to the atorvastatin study. D.R. is an employee of Randstad Pharma, who was contracted by Astellas Pharma Global Development, Inc. to perform work related to these studies.
References
- 1. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. [DOI] [PubMed] [Google Scholar]
- 2. Bitar D, Lortholary O, Le Strat Y, et al. Population‐based analysis of invasive fungal infections, France, 2001‐2010. Emerg Infect Dis. 2014;20:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant‐Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis. 2010;50:1091–1100. [DOI] [PubMed] [Google Scholar]
- 4. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 5. Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med. 2015;5:a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nivoix Y, Ubeaud‐Sequier G, Engel P, Leveque D, Herbrecht R. Drug‐drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab. 2009;10:395–409. [DOI] [PubMed] [Google Scholar]
- 7. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised‐controlled, non‐inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 8. Marty FM, Ostrosky‐Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single‐arm open‐label trial and case‐control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 9. Townsend R, Dietz AJ, Hale C, et al. Pharmacokinetic evaluation of CYP3A4‐mediated drug‐drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai A, Yamazaki T, Goldwater R, et al. Pharmacokinetic effects of isavuconazole co‐administration with the cytochrome P450 enzyme substrates bupropion, repaglinide, caffeine, dextromethorphan, and methadone in healthy subjects. Clin Pharmacol Drug Dev. 2017;6:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groll AH, Desai A, Han D, et al. Pharmacokinetic assessment of drug‐drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev. 2017;6:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol. 2014;25:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. [DOI] [PubMed] [Google Scholar]
- 14. de Lannoy IA, Silverman M. The MDR1 gene product, P‐glycoprotein, mediates the transport of the cardiac glycoside, digoxin. Biochem Biophys Res Commun. 1992;189:551–557. [DOI] [PubMed] [Google Scholar]
- 15. Konig J, Zolk O, Singer K, Hoffmann C, Fromm MF. Double‐transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: determinants of uptake and transcellular translocation of organic cations. Br J Pharmacol. 2011;163:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volk EL, Schneider E. Wild‐type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63:5538–5543. [PubMed] [Google Scholar]
- 17. El‐Sheikh AA, Greupink R, Wortelboer HM, et al. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance‐associated protein (MRP) 2 and MRP4. Transl Res. 2013;162:398–409. [DOI] [PubMed] [Google Scholar]
- 18. Breedveld P, Pluim D, Cipriani G, et al. The effect of low pH on breast cancer resistance protein (ABCG2)‐mediated transport of methotrexate, 7‐hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007;71:240–249. [DOI] [PubMed] [Google Scholar]
- 19. Karlgren M, Vildhede A, Norinder U, et al. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug‐drug interactions. J Med Chem. 2012;55:4740–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kantola T, Kivisto KT, Neuvonen PJ. Effect of itraconazole on the pharmacokinetics of atorvastatin. Clin Pharmacol Ther. 1998;64:58–65. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–1146. [DOI] [PubMed] [Google Scholar]
- 23. Partanen J, Jalava KM, Neuvonen PJ. Itraconazole increases serum digoxin concentration. Pharmacol Toxicol. 1996;79:274–276. [DOI] [PubMed] [Google Scholar]
- 24. Purkins L, Wood N, Kleinermans D, Nichols D. Voriconazole does not affect the steady‐state pharmacokinetics of digoxin. Br J Clin Pharmacol. 2003;56(Suppl 1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother. 2002;46:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.


