Abstract
Objectives
Transmission of drug‐resistant HIV‐1 has decreased in the UK since the early 2000s. This analysis reports recent trends and characteristics of transmitted drug resistance (TDR) in the UK from 2010 to 2013.
Methods
Resistance tests conducted in antiretroviral treatment (ART)‐naïve individuals between 2010 and 2013 were analysed for the presence of transmitted drug resistance mutations (TDRMs), defined as any mutations from a modified 2009 World Health Organization surveillance list, or a modified 2013 International Antiviral Society‐USA list for integrase tests. Logistic regression was used to examine associations between demographics and the prevalence of TDRMs.
Results
TDRMs were observed in 1223 (7.5%) of 16 425 individuals; prevalence declined from 8.1% in 2010 to 6.6% in 2013 (P = 0.02). The prevalence of TDRMs was higher among men who have sex with men (MSM) compared with heterosexual men and women (8.7% versus 6.4%, respectively) with a trend for decreasing TDRMs among MSM (P = 0.008) driven by a reduction in nucleoside reverse transcriptase inhibitor (NRTI)‐related mutations. The most frequently detected TDRMs were K103N (2.2%), T215 revertants (1.6%), M41L (0.9%) and L90M (0.7%). Predicted phenotypic resistance to first‐line ART was highest to the nonnucleoside reverse transcriptase inhibitors (NNRTIs) rilpivirine and efavirenz (6.2% and 3.4%, respectively) but minimal to NRTIs, including tenofovir, and protease inhibitors (PIs). No major integrase TDRMs were detected among 101 individuals tested while ART‐naïve.
Conclusions
We observed a decrease in TDRMs in recent years. However, this was confined to the MSM population and rates remained stable in those with heterosexually acquired HIV infection. Resistance to currently recommended first‐line ART, including integrase inhibitors, remained reassuringly low.
Keywords: drug resistance, HIV‐1, mutations, transmitted, transmitted drug resistance, transmitted drug resistance mutations
Introduction
The majority of individuals initiating combination antiretroviral treatment (ART) suppress virus replication and are less likely to acquire drug resistance compared with individuals who were exposed to older ART regimens. However, approximately one‐third of individuals in the UK experiencing virological failure still have drug‐resistant strains of HIV 1. Onward transmission of drug‐resistant HIV can have an adverse effect on the success of first‐line treatment and may negatively impact an individual's prognosis 2. National guidelines in the UK 3 and many other European countries 4 recommend that newly diagnosed individuals have a resistance test to detect transmitted drug resistance (TDR) and help selection of a fully active first‐line treatment regimen.
Surveillance of TDR in the UK has monitored the impact of improved ART regimens and resistance testing guidelines over time. Previous work reported a decrease in the prevalence of TDR between 2001 and 2007 5, 6. The prevalence of TDR in individuals with a subtype B infection was higher than in those with non‐B infections between 2001 and 2006, reflecting long‐standing free access to ART in the UK and limited availability of ART in countries where non‐B infections were probably acquired 5. The prevalence of TDR in individuals with a subtype B infection increased slightly between 2007 and 2009, driven by an increase in resistance to the nucleoside reverse transcriptase inhibitor (NRTI) drug class, leading to the hypothesis that onward transmission of persistent thymidine analogue mutations (TAMs) among undiagnosed men who have sex with men (MSM) may keep levels of TDR stable in this population 6.
The European SPREAD. collaborative study reported a similar pattern of a higher prevalence of TDR among MSM compared with individuals who acquired their infection heterosexually between 2002 and 2007 7. Other studies in high‐income countries have reported comparable rates of TDR; the European CHAIN collaboration reported a prevalence of 9.5% between 1998 and 2008 2, while a prevalence of 15% was reported in a US study conducted between 1999 and 2011 8.
Of increasing interest in recent years is the surveillance of TDR to integrase inhibitors, a relatively new drug class approved for use in HIV treatment in Europe in 2008. The integrase inhibitors raltegravir, dolutegravir and elvitegravir are increasingly likely to be used as first‐line ART in combination with two NRTIs. Other than a small number of isolated case reports 9, 10, 11, studies have failed to detect transmitted major integrase mutations [as defined by the International Antiviral Society (IAS)‐USA 2013 list 12] in ART‐naïve individuals 13, 14, 15. Recently, the French PRIMO cohort study detected isolated E157Q mutations in 1.5% of baseline samples 16.
The objective of this report was to examine the recent prevalence, patterns and predictive factors of TDR among ART‐naïve persons living with diagnosed HIV infection in the UK.
Methods
The UK HIV Drug Resistance Database (UK HDRD) collects information from all National Health Service and Public Heath England (PHE) virology laboratories performing resistance tests as part of routine clinical care in the UK. Nucleotide sequences of the protease and reverse transcriptase and integrase genes are collected. Resistance data are linked to demographic and clinical patient data held in the UK Collaborative HIV Cohort study (UK CHIC) database 17 and the national HIV/AIDS Reporting System (HARS) database held at PHE 18. Eighty‐three per cent of resistance tests are successfully linked to one or both sources.
Resistance testing of polymerase (pol) (protease and reverse transcriptase genes) started in the UK in 1998, but testing of ART‐naïve individuals only became widespread from 2005 following the release of British HIV Association (BHIVA) guidelines recommending routine monitoring of transmitted resistance in this population 3. Sequencing of the integrase gene was first performed in the UK in 2007 but to date only a small number of integrase sequences have been reported from ART‐naïve individuals. Findings in the pol and integrase genes are described separately in this report. We selected the first sequence per individual aged 15 years or over who had not yet received ART at the time of sampling. Individuals infected via mother‐to‐child transmission were excluded.
In this report, the term ‘transmitted drug resistance mutations’ (TDRMs) is used to describe (a) for pol, the presence of one or more mutations from the World Health Organisation (WHO) 2009 surveillance list 19 with the addition of E138K, a mutation known to cause resistance to the second‐generation nonnucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine, and all changes at codon T215 as they are likely to be ancestrally related to T215F or Y mutations 20; (b) for integrase, the presence of mutations from the IAS‐USA 2013 list or accessory mutations reported by the Stanford HIVdb algorithm v7.0 (HIV Drug Resistance Database, Stanford University, Stanford, CA). HIV‐1 subtypes were determined using the rega v3.0 genotyping tool (REGA Institute, Katholieke Universiteit Leuven, Belgium). Predicted phenotypic resistance to ART drugs currently recommended (preferred or alternative) for first‐line treatment of HIV infection in the UK 21 was examined using the Stanford HIVdb algorithm v7.0. Scores of low‐level, intermediate or high‐level resistance were used to predict phenotypic resistance.
Univariate and multivariate logistic regression was used to examine the association between demographic and clinical factors and the prevalence of TDRMs. Demographic and clinical factors included in logistic regression models were: year of resistance test, transmission group, ethnicity, age at diagnosis, region of treatment centre and baseline CD4 T‐cell count and HIV‐1 viral load. Recent Infection Testing Algorithm (RITA) avidity score was not included in multivariate analysis given the close relationship to baseline CD4 cell count and the availability of more CD4 data. Viral subtype was not included as a separate variable in either the univariate or multivariate analyses because of the close association with ethnicity and transmission group. Where demographic information was missing, an unknown category was created to allow individuals to be included in multivariate analyses. A priori, an interaction between year of resistance test and transmission group was added to the adjusted model. Wald tests were used to assess the effect of demographic factors on the prevalence of TDRMs in the adjusted model. Individuals were defined as being recently infected when this was confirmed with a RITA avidity score <80% and their first resistance test was within 3 months of HIV diagnosis. The closest CD4 cell count and HIV‐1 viral load measured within the year before or the 6 months after the resistance test and before receiving ART were included in analyses.
Analyses of time trends of TDRMs in pol are presented from 2005 onwards to provide contextual data for recent years (2010‐2013), which are the focus of the report. More detailed analyses of predicted phenotypic resistance and predictors of TDRMs are limited to 2010‐2013 to be most relevant to contemporary clinical practice. Data on TDRMs conferring resistance to integrase inhibitors cover the period 2007 to 2013.
All analyses were carried out using stata statistical software version 13.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 40 549 individuals were tested for TDRMs (pol) while ART‐naïve between 2005 and 2013 (Table 1). Of the 16 425 individuals who were tested in recent years (2010–2013 inclusive), 66.5% were male and 49.7% of white ethnicity. MSM comprised 45.9% of individuals, with 39.4% acquiring their infection heterosexually. Median age at HIV diagnosis in recent years was 35 years [interquartile range (IQR) 28–44 years]. Almost half of all samples (45.6%) came from treatment centres in London, followed by 19.5% from the Midlands and East England region. Median CD4 cell count at the time of the resistance test was 350 cells/mL (IQR 175–530 cells/μL). As expected, the median CD4 cell count was higher in those with a recent infection confirmed by a RITA assay: 546 cells/μL (1098 individuals) compared with 305 cells/μL (4864 individuals) in nonrecent infections. The median viral load at the time of the resistance test was 4.76 log10 HIV‐1 RNA copies/mL (IQR 4.12–5.31 copies/mL). The median time from HIV diagnosis to first resistance test was 9 days. Subtype B viruses were the most commonly detected (49.2%), followed by subtype C (21.7%), subtype CRF02_AG (7.0%) and subtype A (5.8%). Only 16.0% of heterosexual men and women were infected with subtype B virus, while 42.6% had subtype C infection. Conversely, almost 80% of MSM had subtype B virus and only 3.5% subtype C. Demographic characteristics of ART‐naïve individuals tested for drug resistance in recent years were comparable to those of individuals newly diagnosed with HIV infection in the UK between 2010 and 2013 22, suggesting that our cohort is broadly representative of this population in the UK.
Table 1.
Demographic and clinical patient characteristics, 2005–2013
| Patient characteristics | Year of resistance test | Year of HIV diagnosis | |
|---|---|---|---|
| 2005–2009 | 2010–2013 | 2010–2013a | |
| Total n | 24 124 | 16 425 | 24 828 |
| Sex | |||
| Male | 16050 (66.5) | 10917 (66.5) | 17769 (71.6) |
| Female | 7133 (29.6) | 3820 (23.3) | 7059 (28.4) |
| Unknown | 1941 (8) | 1688 (10.3) | 0 |
| Transmission group | |||
| MSM | 10057 (41.7) | 7533 (45.9) | 11678 (47.0) |
| Heterosexual male | 4045 (16.8) | 2833 (17.2) | 4540 (18.3) |
| Heterosexual female | 6833 (28.3) | 3635 (22.1) | 6119 (24.6) |
| IDU | 465 (1.9) | 298 (1.8) | 530 (2.1) |
| Other | 642 (2.7) | 125 (0.8) | 506 (2.0) |
| Unknown | 2082 (8.6) | 2001 (12.2) | 1455 (5.9) |
| Ethnicity | |||
| White | 11361 (47.1) | 8169 (49.7) | 13222 (53.3) |
| Black African | 7792 (32.3) | 3932 (23.9) | 6741 (27.2) |
| Black Caribbean | 830 (3.4) | 565 (3.4) | 708 (2.9) |
| Asian | 621 (2.6) | 720 (4.4) | 1219 (4.9) |
| Other | 1529 (6.3) | 1139 (6.9) | 1772 (7.1) |
| Unknown | 1991 (8.3) | 1900 (11.6) | 1166 (4.7) |
| Age at diagnosis (years) | |||
| [median (IQR)] | 34 (28–41) | 35 (28–44) | 36 (29–45) |
| Region of treatment centre/diagnosis | |||
| London | 11557 (47.9) | 7493 (45.6) | 10823 (43.6) |
| South England | 2811 (11.7) | 2175 (13.2) | 3542 (14.3) |
| North England | 3778 (15.7) | 2237 (13.6) | 3997 (16.1) |
| Midlands and East England | 3638 (15.1) | 3208 (19.5) | 4370 (17.6) |
| Wales | 497 (2.1) | 441 (2.7) | 562 (2.3) |
| Scotland | 1316 (5.5) | 435 (2.6) | 1135 (4.6) |
| Northern Ireland | 198 (0.8) | 252 (1.5) | 358 (1.4) |
| Other/unknown | 329 (1.4) | 184 (1.1) | 41 (0.2) |
| Baseline CD4 count | |||
| <200 cells/mL | 4935 (20.5) | 3249 (19.8) | 5557 (22.4) |
| 200–349 cells/mL | 3909 (16.2) | 2508 (15.3) | 4259 (17.2) |
| 350–499 cells/mL | 3353 (13.9) | 2528 (15.4) | 4541 (18.3) |
| ≥500 cells/mL | 4032 (16.7) | 3342 (20.3) | 6728 (27.1) |
| Unknown | 7895 (32.7) | 4798 (29.2) | 3743 (15.1) |
| CD4 count (cells/mL) [median (IQR)] | 320 (160–497) | 350 (175–530) | 371 (187–558) |
| Baseline VL | |||
| <4.0 log10 copies/mL | 2579 (10.7) | 1262 (7.7) | – |
| 4.0 to <5.0 log10 copies/mL | 3833 (15.9) | 2396 (14.6) | – |
| ≥5.0 log10 copies/mL | 2659 (11) | 2268 (13.8) | – |
| Unknown | 15053 (62.4) | 10499 (63.9) | – |
| VL [median (IQR)] | 4.54 (3.91–5.11) | 4.76 (4.13–5.31) | – |
| Subtype | |||
| A | 1364 (5.7) | 957 (5.8) | – |
| B | 11761 (48.8) | 8085 (49.2) | – |
| C | 6742 (27.9) | 3557 (21.7) | – |
| G | 586 (2.4) | 438 (2.7) | – |
| CRF01_AE | 587 (2.4) | 560 (3.4) | – |
| CRF02_AG | 1324 (5.5) | 1150 (7) | – |
| Other | 1760 (7.3) | 1678 (10.2) | – |
| Time from diagnosis to resistance test (days) [median (IQR)]) | 19 (4–239) | 9 (1–36) | – |
IDU, injecting drug use; IQR, interquartile range; MSM, men who have sex with men; VL, viral load.
Values are n (%), unless otherwise stated.
Data from PHE HIV surveillance data tables 2015 (https://www.gov.uk/government/statistics/hiv-data-tables) 22.
The prevalence of TDRMs has declined over time, from 9.3% in 2005 to 6.6% in 2013 (test for trend P = 0.02) (Fig. 1). As reported previously, the rate stabilized between 2008 and 2010, but has declined in recent years from 8.1% in 2010 to 6.6% in 2013 (test for trend P = 0.02). This recent decline was driven by a decrease in the prevalence of observed TDRMs in MSM (9.8% in 2010 to 7.5% in 2013; test for trend P = 0.008), while the prevalence of TDRMs in heterosexual men and women has remained stable in recent years (6.7% in 2010 and 6.2% in 2013; test for trend P = 0.56). The decrease in observed TDRMs in MSM was driven by a reduction in the prevalence of TDRMs conferring resistance to the NRTI drug class (from 5.2% in 2010 to 3.7% in 2013; test for trend P = 0.01), while the prevalence of TDRMs conferring resistance to NNRTIs and protease inhibitors (PIs) was unchanged over the 4 years (test for trend P = 0.63 for NNRTIs; P = 0.12 for PIs).
Figure 1.
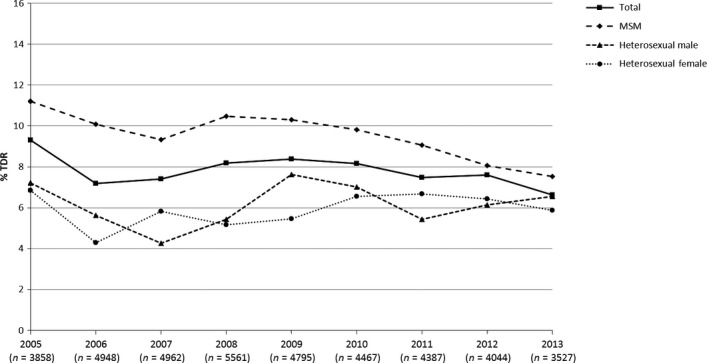
Prevalence of transmitted drug resistance mutations (TDRMs) by transmission group, 2005−2013. MSM, men who have sex with men.
Further analysis of individuals tested between 2010 and 2013 revealed that, of the 1233 (7.5%) individuals with one or more resistance mutations, 6.5% had resistance to a single drug class, 0.9% had dual drug class resistance and 0.1% had triple drug class resistance (Table 2). Overall, 3.5% had TDRMs conferring resistance to drugs from the NRTI class, 3.3% to the NNRTI class and 1.7% to the PI drug class. The most frequently detected mutations were T215 revertants (not F/Y) (268; 1.6%) and other TAMs [M41L (141; 0.9%) and K219Q/N (104; 0.6%)] conferring resistance to the NRTI drug class; K103N (354; 2.2%) and Y181C (66; 0.4%) conferring resistance to the NNRTI drug class, and L90M (111; 0.7%) and M46L (48; 0.3%) conferring resistance to the PI drug class. Despite TAMs remaining the most commonly detected NRTI resistance mutations, their prevalence has been decreasing in recent years, from 4.5% in 2010 to 3.1% in 2013 (test for trend P = 0.001). K65R was only detected in 10 (0.06%) individuals tested between 2010 and 2013.
Table 2.
Transmitted drug resistance mutations (TDRMs) by drug class, 2010–2013
| TDRMs by drug class | n | % |
|---|---|---|
| Any | 1233 | 7.5 |
| Single | ||
| NRTI only | 425 | 2.6 |
| NNRTI only | 405 | 2.5 |
| PI only | 241 | 1.5 |
| Dual | ||
| NRTI + NNRTI | 121 | 0.7 |
| NRTI + PI | 17 | 0.1 |
| NNRTI + PI | 11 | 0.1 |
| Triple | ||
| NRTI + NNRTI + PI | 13 | 0.1 |
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Table 3 shows a comparison between the prevalence of TDRMs by drug class and predicted phenotypic resistance to currently recommended first‐line ART in the UK. Very little relevant resistance was observed to currently recommended first‐line NRTIs or PIs: 1.2% and 0.9%, respectively. In particular, the frequently detected TAMs are no longer of direct clinical relevance in light of the replacement of zidovudine by tenofovir in first‐line therapy. A higher incidence of predicted phenotypic resistance was seen to first‐line NNRTIs, with 8.2% of individuals having predicted low‐, intermediate‐ or high‐level resistance to current drugs in this class; 6.2% of individuals had predicted resistance to rilpivirine and 3.4% to efavirenz (Fig. 2). Resistance to rilpivirine was largely low‐level resistance and probably a consequence of cross‐resistance to other NNRTIs, given the limited use of the drug in this period.
Table 3.
Comparison of transmitted drug resistance mutations (TDRMs) and predicted phenotypic resistance (Stanford scores) by drug class, 2010–2013
| ART drug class | Surveillance mutations | Predicted phenotypic resistance to currently recommended first‐line ART | ||
|---|---|---|---|---|
| % any TDRM | % any low level/intermediate/high level | % any intermediate/high level | % any high level | |
| Any class | 7.5 | 9.6 | 3.9 | 2.8 |
| NRTI | 3.5 | 1.2 | 0.8 | 0.6 |
| NNRTI | 3.3 | 8.2 | 3.5 | 2.6 |
| PI | 1.7 | 0.9 | 0.2 | 0.1 |
ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Figure 2.
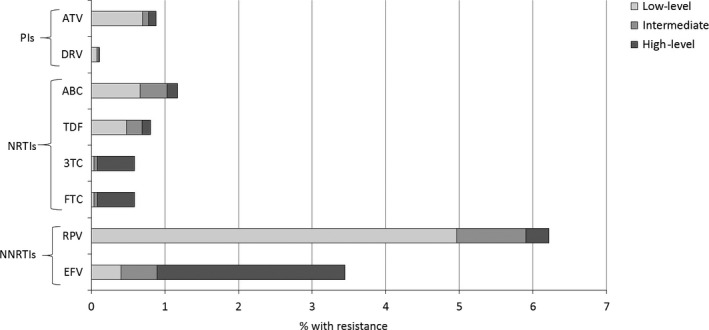
Predicted phenotypic resistance (Stanford scores) for antiretroviral drugs currently recommended for first‐line combination therapy in the UK, 2010−2013. 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; RPV, rilpivirine; TDF, tenofovir.
The results of a multivariate logistic regression model of potential predictive factors for TDRMs are shown in Table 4. Although a test for interaction between year of resistance test and transmission group did not reach conventional statistical significance (P = 0.65), the interaction term was retained because of the strong clinical plausibility of this effect. In an additional model which excluded this interaction term, the overall odds ratios (ORs) for heterosexual men and heterosexual women versus MSM were 0.78 [95% confidence interval (CI) 0.64–0.95] and 0.79 (95% CI 0.65–0.97), respectively. The only significant predictor of TDRMs was geographical region of attending treatment centre in the UK. Compared with individuals attending London clinics, the odds of having detectable TDRMs was lower in individuals attending treatment centres in Northern Ireland, Wales, the Midlands and East of England and the South of England (52%, 49%, 40% and 20% lower, respectively). This association persisted in a sensitivity analysis limited to individuals with subtype B infection (P < 0.001). Neither baseline CD4 cell count nor recency of infection defined by RITA was associated with risk of TDRMs.
Table 4.
Logistic regression model of risk factors for transmitted drug resistance mutations (TDRMs), 2010–2013
| Patient characteristics | Total | TDRMs [n (%)] | Univariate OR | Multivariate OR | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| MSM | ||||||
| 2010 | 1977 | 194 (9.8) | Ref | Ref | – | 0.06 |
| 2011 | 2022 | 183 (9.1) | 0.91 | 0.90 | 0.73–1.11 | |
| 2012 | 2057 | 166 (8.1) | 0.81 | 0.79 | 0.63–0.98 | |
| 2013 | 1477 | 111 (7.5) | 0.75 | 0.73 | 0.56–0.94 | |
| Heterosexual male | ||||||
| 2010 | 826 | 58 (7.0) | Ref | Ref | – | 0.51 |
| 2011 | 809 | 44 (5.4) | 0.76 | 0.73 | 0.49–1.10 | |
| 2012 | 618 | 38 (6.1) | 0.87 | 0.83 | 0.54–1.27 | |
| 2013 | 580 | 38 (6.6) | 0.93 | 0.89 | 0.57–1.36 | |
| Heterosexual female | ||||||
| 2010 | 1145 | 75 (6.6) | Ref | Ref | – | 0.86 |
| 2011 | 1018 | 68 (6.7) | 1.02 | 1.00 | 0.71–1.41 | |
| 2012 | 825 | 53 (6.4) | 0.98 | 0.95 | 0.66–1.38 | |
| 2013 | 647 | 38 (5.9) | 0.89 | 0.85 | 0.56–1.28 | |
| Ethnicity | ||||||
| White | 8169 | 682 (8.3) | Ref | Ref | 0.16 | |
| Black African | 3932 | 250 (6.4) | 0.75 | 0.86 | 0.71–1.06 | |
| Black Caribbean | 565 | 35 (6.2) | 0.72 | 0.78 | 0.54–1.12 | |
| Asian | 720 | 50 (6.9) | 0.82 | 0.83 | 0.61–1.12 | |
| Other | 1139 | 107 (9.4) | 1.14 | 1.12 | 0.90–1.40 | |
| Age at diagnosis | ||||||
| 15–24 years | 1907 | 137 (7.2) | 0.85 | 0.82 | 0.68–0.99 | 0.07 |
| 25–39 years | 7232 | 603 (8.3) | Ref | Ref | – | |
| 40–49 years | 3307 | 234 (7.1) | 0.84 | 0.86 | 0.73–1.00 | |
| ≥50 years | 1757 | 119 (6.8) | 0.80 | 0.85 | 0.70–1.05 | |
| Region | ||||||
| London | 7493 | 640 (8.5) | Ref | Ref | – | <0.01a |
| South England | 2175 | 150 (6.9) | 0.79 | 0.80 | 0.66–0.98 | |
| North England | 2237 | 196 (8.8) | 1.03 | 1.02 | 0.84–1.22 | |
| Midlands and East England | 3208 | 165 (5.1) | 0.58 | 0.60 | 0.49–0.73 | |
| Wales | 441 | 20 (4.5) | 0.51 | 0.51 | 0.32–0.81 | |
| Scotland | 435 | 34 (7.8) | 0.91 | 0.86 | 0.59–1.25 | |
| Northern Ireland | 252 | 11 (4.4) | 0.49 | 0.48 | 0.26–0.89 | |
| Baseline CD4 count | ||||||
| <200 cells/mL | 3249 | 235 (7.2) | 0.88 | 1.00 | 0.82–1.23 | 0.8 |
| 200–349 cells/mL | 2508 | 178 (7.1) | 0.86 | 0.91 | 0.74–1.12 | |
| 350–499 cells/mL | 2528 | 206 (8.1) | Ref | Ref | – | |
| ≥500 cells/mL | 3342 | 278 (8.3) | 1.02 | 0.98 | 0.81–1.26 | |
| Baseline VL | ||||||
| <4.0 log10 copies/mL | 1262 | 111 (8.8) | 1.09 | 1.13 | 0.88–1.45 | 0.6 |
| 4.0 to <5.0 log10 copies/mL | 2396 | 195 (8.1) | Ref | Ref | – | |
| ≥5.0 log10 copies/mL | 2268 | 185 (8.2) | 1.00 | 1.02 | 0.82–1.26 | |
| RITA | ||||||
| Nonrecent | 4864 | 358 (7.4) | Ref | – | – | |
| Recent | 1098 | 94 (8.6) | 1.18 | – | – | |
CI, confidence interval; MSM, men who have sex with men; OR, odds ratio; RITA, Recent Infection Testing Algorithm; VL, viral load.
Injecting drug users and other transmission groups are included in the multivariate model but not presented here.
Of the integrase tests reported to the UKHDRD, 101 were performed in ART‐naïve individuals between January 2007 and December 2013. No major mutations were identified in these 101 individuals. Six individuals had minor or accessory mutations: one had L74M, two had V151I and three had E157Q. In isolation, these mutations are not reported to affect susceptibility to integrase inhibitors with the exception of E157Q, which confers low‐level resistance to raltegravir and elvitegravir.
Discussion
Analysis of TDR in the ART‐naïve UK population has revealed a trend for decreasing prevalence of TDRMs from 2005 to 2013, with 7.5% of individuals tested in recent years (2010–2013) having detectable TDRM. Other European cohort studies have also recently reported temporal trends for decreasing TDR and clinically relevant resistance to first‐line treatments, including Spanish cohorts 23, 24 and the PRIMO cohort in France 16.
In the UK, the prevalence of TDRMs remains higher in MSM than in heterosexuals, consistent with other studies in high‐income countries 16, 24, 25. This difference has been consistently reported in the UK 5 and can be attributed to historical patterns of HIV acquisition. While most MSM diagnosed in the UK have acquired their infection in the UK, where access to treatment has been free for decades, most heterosexuals historically acquired HIV abroad, where resistant HIV was essentially absent as a consequence of poor availability of ART 5. However, our analyses reveal that the difference in the prevalence of TDRMs between these groups of individuals is narrowing as different temporal patterns continue to be seen in the two populations. As the proportion of heterosexual infections acquired in the UK has increased from 36% in 2005 to 59% in 2013 22, the prevalence of TDRMs in heterosexual men and women has increased since 2006 and now appears to have stabilized between 2010 and 2013. In contrast, there has been a significant decrease in detectable TDRMs among MSM, driven by declining resistance to the NRTI drug class. Resistance to NRTIs is higher in this group particularly as a consequence of historical exposure to the thymidine analogues stavudine and zidovudine, with mutations selected by these drugs known to persist for long periods of time 26 and to be onwardly transmitted from ART‐naïve individuals 27. This report found a decline in these mutations among MSM in recent years, which suggests that they confer a slight fitness disadvantage, and a continued decline in their frequency is anticipated.
The prevalence of TDRMs in recent years, 7.5%, is a little lower than has been reported by other European cohort studies for the years since 2010 16, 23, 24, 28, 29. The incidence of dual or triple class resistance in ART‐naïve individuals is reassuringly very low. The TDRMs detected have a minimal effect at a population level on currently recommended first‐line treatment options in the UK, with predicted phenotypic resistance to individual PIs and NRTIs generally being <1%. In contrast, the prevalence of predicted NNRTI resistance was higher; 6.2% and 3.4% of individuals had resistance to rilpivirine and efavirenz, respectively. Resistance testing prior to ART initiation is therefore particularly useful if patients are to be started on an NNRTI‐containing first‐line regimen to ensure that the virus is fully susceptible to the chosen third agent. The K65R mutation, which confers resistance to tenofovir, was rarely detected as a transmitted mutation, supporting previous work by our group suggesting that this should not affect the success of tenofovir‐containing pre‐exposure prophylaxis (PreP) if its use in the UK becomes more widespread 30.
Our analysis found that region of treatment centre was a significant predictor for the presence of TDRMs in the UK population. Individuals referred for resistance tests from treatment centres in Northern Ireland, Wales, the Midlands and East England and the South of England were less likely to have detectable TDRMs than those referred from London centres. The cause of this is difficult to determine and will be influenced by the extent to which transmission networks are regional or national. Recent work has shown that a high proportion of drug‐resistant HIV transmission in the UK is from ART‐naïve individuals 27, 31. It is possible, therefore, that current geographical variation reflects historical differences in the original emergence of resistant strains, for example, as a result of earlier access to ART drugs or the use of different ART in London. A related explanation is that the average interval between HIV infection and onward transmission could be shorter in London than in other parts of the UK, thereby increasing the chance of onward transmission of resistant virus (rather than the outgrowth of wild‐type virus).
The interpretation of time trends in TDRM prevalence could be affected by temporal changes in the timing of HIV diagnoses as there is evidence for earlier detection of infections in more recent calendar years. We did not observe an association between markers of recent infection, either high CD4 count or a low avidity index, and prevalence of TDRMs. This might appear to be a surprising finding as resistant viruses in patients with long‐standing infection tend to be outcompeted by wild‐type viruses and cease to become detectable 26. However, this effect could be masked by changes in TDRM prevalence by year of infection. It is a limitation of our analysis that baseline CD4 count was missing for 29% of individuals. Baseline HIV‐1 viral load measurements were not available for a large proportion of individuals (64%), making meaningful interpretation of these data difficult.
As integrase resistance testing is not currently recommended in ART‐naïve individuals in the UK, the number of tests available to detect TDRMs conferring resistance to integrase inhibitors was small and possibly selective. No major resistance‐conferring mutations were identified in the 101 individuals analysed. However, with increasing use of integrase inhibitors in the UK, and a move to use them in first‐line regimens, it seems important to monitor TDR to this drug class 32. Some form of sentinel surveillance may be more cost‐effective than universal testing while the prevalence of TDRMs remains low.
In conclusion, our data demonstrate an overall decrease in the prevalence of TDRMs in the UK, although differences were evident between MSM and those living with heterosexually acquired HIV infection. There remains some risk of first‐line therapy with efavirenz or rilpivirine being compromised by the detected TDRMs, but clinically relevant resistance to NRTIs, PIs and integrase inhibitors is rarely seen. We recommend implementing some sentinel surveillance, at selected treatment centres, for TDRMs conferring resistance to integrase inhibitors, given their increasing use as a first‐line treatment in the UK.
Funding
This work was supported by the UK Medical Research Council (Award Number 164587).
The UK HIV Drug Resistance Database
Steering Committee: Celia Aitken (Gartnavel General Hospital, Glasgow); David Asboe, Anton Pozniak (Chelsea & Westminster Hospital, London); Patricia Cane (Public Health England, Porton Down); David Chadwick (South Tees Hospitals NHS Trust, Middlesbrough); Duncan Churchill (Brighton and Sussex University Hospitals NHS Trust); Duncan Clark (St Bartholomew's and The London NHS Trust); Simon Collins (HIV i‐Base, London); Valerie Delpech (Centre for Infections, Public Health England); Samuel Douthwaite (Guy's and St Thomas’ NHS Foundation Trust, London); David Dunn, Esther Fearnhill, Kholoud Porter, Anna Tostevin, Ellen White (MRC Clinical Trials Unit at UCL, London); Christophe Fraser (Imperial College London); Anna Maria Geretti (Institute of Infection and Global Health, University of Liverpool); Antony Hale (Leeds Teaching Hospitals NHS Trust); Stéphane Hué (London School of Hygiene and Tropical Medicine, London); Steve Kaye (Imperial College, London); Paul Kellam (Wellcome Trust Sanger Institute & University College London Medical School); Linda Lazarus (Expert Advisory Group on AIDS Secretariat, Public Health England); Andrew Leigh‐Brown (University of Edinburgh); Tamyo Mbisa (Virus Reference Department, Public Health England); Nicola Mackie (Imperial NHS Trust, London); Samuel Moses (King's College Hospital, London); Chloe Orkin (St. Bartholomew's Hospital, London); Eleni Nastouli, Deenan Pillay, Andrew Phillips, Caroline Sabin (University College London Medical School, London); Erasmus Smit (Public Health England, Birmingham Heartlands Hospital); Kate Templeton (Royal Infirmary of Edinburgh); Peter Tilston (Manchester Royal Infirmary); Ian Williams (Mortimer Market Centre, London); Hongyi Zhang (Addenbrooke's Hospital, Cambridge).
Coordinating Centre: MRC Clinical Trials Unit at UCL (David Dunn, Keith Fairbrother, Esther Fearnhill, Kholoud Porter, Anna Tostevin and Ellen White).
Centres contributing data: Clinical Microbiology and Public Health Laboratory, Addenbrooke's Hospital, Cambridge (Jane Greatorex); Guy's and St Thomas’ NHS Foundation Trust, London (Siobhan O'Shea, Jane Mullen); PHE – Public Health Laboratory, Birmingham Heartlands Hospital, Birmingham (Erasmus Smit); PHE – Virus Reference Department, London (Tamyo Mbisa); Imperial College Health NHS Trust, London (Alison Cox); King's College Hospital, London (Richard Tandy); Medical Microbiology Laboratory, Leeds Teaching Hospitals NHS Trust (Tracy Fawcett); Specialist Virology Centre, Liverpool (Mark Hopkins, Lynne Ashton); Department of Clinical Virology, Manchester Royal Infirmary, Manchester (Peter Tilston); Department of Virology, Royal Free Hospital, London (Clare Booth, Ana Garcia‐Diaz); Edinburgh Specialist Virology Centre, Royal Infirmary of Edinburgh (Jill Shepherd); Department of Infection & Tropical Medicine, Royal Victoria Infirmary, Newcastle (Matthias L. Schmid, Brendan Payne); South Tees Hospitals NHS Trust, Middlesbrough (David Chadwick); Department of Virology, St Bartholomew's and The London NHS Trust (Spiro Pereira, Jonathan Hubb); Molecular Diagnostic Unit, Imperial College, London (Steve Kaye); University College London Hospitals (Stuart Kirk); West of Scotland Specialist Virology Laboratory, Gartnavel, Glasgow (Rory Gunson, Amanda Bradley‐Stewart, Celia Aitken).
References
- 1. Dolling D NM, Schwenk A, Churchill D, Pillay D, Dunn D, UK HIV Drug Resistance Database , UK Collaborative HIV Cohort Study . Rapid increase in the frequency of wild‐type HIV‐1 drug resistance reports among ART‐experienced patients in the UK. Conference on Retroviruses and Opportunistic Infections (CROI). 2013; Abstract 594.
- 2. Wittkop L, Gunthard HF, de Wolf F et al Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord‐CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11: 363–371. [DOI] [PubMed] [Google Scholar]
- 3. Asboe D, Aitken C, Boffito M et al British HIV Association guidelines for the routine investigation and monitoring of adult HIV‐1‐infected individuals 2011. HIV Med 2012; 13: 1–44. [DOI] [PubMed] [Google Scholar]
- 4. Vandamme AM, Camacho RJ, Ceccherini‐Silberstein F et al European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev 2011; 13: 77–108. [PubMed] [Google Scholar]
- 5. Chilton DN, Castro H, Lattimore S et al HIV type‐1 drug resistance in antiretroviral treatment‐naive adults infected with non‐B subtype virus in the United Kingdom. Antivir Ther 2010; 15: 985–991. [DOI] [PubMed] [Google Scholar]
- 6. Dolling D, Sabin C, Delpech V et al Time trends in drug resistant HIV‐1 infections in the United Kingdom up to 2009: multicentre observational study. BMJ 2012; 345: e5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frentz D, van de Vijver D, Abecasis A et al Patterns of transmitted HIV drug resistance in Europe vary by risk group. PLoS One 2014; 9: e94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchacz K, Young B, Palella FJ Jr, Armon C, Brooks JT. Trends in use of genotypic resistance testing and frequency of major drug resistance among antiretroviral‐naive persons in the HIV Outpatient Study, 1999‐2011. J Antimicrob Chemother 2015; 70: 2337–2346. [DOI] [PubMed] [Google Scholar]
- 9. Young B, Fransen S, Greenberg KS et al Transmission of integrase strand‐transfer inhibitor multidrug‐resistant HIV‐1: case report and response to raltegravir‐containing antiretroviral therapy. Antivir Ther 2011; 16: 253–256. [DOI] [PubMed] [Google Scholar]
- 10. Boyd SD, Maldarelli F, Sereti I et al Transmitted raltegravir resistance in an HIV‐1 CRF_AG‐infected patient. Antivir Ther 2011; 16: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volpe JM, Ward DJ, Napolitano L et al Five antiretroviral drug class‐resistant HIV‐1 in a treatment‐naive patient successfully suppressed with optimized antiretroviral drug selection. J Int Assoc Provid AIDS Care 2015; 14: 398–401. [DOI] [PubMed] [Google Scholar]
- 12. Johnson VA, Calvez V, Gunthard HF et al Update of the drug resistance mutations in HIV‐1: March 2013. Top Antivir Med 2013; 21: 6–14. [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia‐Diaz A, McCormick A, Booth C et al Analysis of transmitted HIV‐1 drug resistance using 454 ultra‐deep‐sequencing and the DeepChek((R))‐HIV system. J Int AIDS Soc 2014; 17 (4 Suppl 3): 19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stekler JD, McKernan J, Milne R et al Lack of resistance to integrase inhibitors among antiretroviral‐naive subjects with primary HIV‐1 infection, 2007‐2013. Antivir Ther 2015; 20: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volpe JMYO, Petropoulos CJ, Walworth CM. Five antiretroviral drug class‐resistant HIV‐1 in a treatment‐naive patient successfully suppressed with optimized antiretroviral drug selection. ICAAC 2015, September 17‐21, 2015, San Diego. 2015. [DOI] [PubMed]
- 16. Frange P, Assoumou L, Descamps D et al HIV‐1 subtype B‐infected MSM may have driven the spread of transmitted resistant strains in France in 2007‐12: impact on susceptibility to first‐line strategies. J Antimicrob Chemother 2015; 70: 2084–2089. [DOI] [PubMed] [Google Scholar]
- 17. UK Collaborative HIV Cohort Study Steering Committee . The creation of a large UK‐based multicentre cohort of HIV‐infected individuals: the UK Collaborative HIV Cohort (UK CHIC) Study. HIV Med 2004; 5: 115–124. [DOI] [PubMed] [Google Scholar]
- 18. https://www.gov.uk/guidance/hiv-surveillance-systems#hiv-and-aids-reporting-system-hars (accesses 15 March 2016).
- 19. Bennett DE, Camacho RJ, Otelea D et al Drug resistance mutations for surveillance of transmitted HIV‐1 drug‐resistance: 2009 update. PLoS One 2009; 4: e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitsuya Y, Varghese V, Wang C et al Minority human immunodeficiency virus type 1 variants in antiretroviral‐naive persons with reverse transcriptase codon 215 revertant mutations. J Virol 2008; 82: 10747–10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Churchill D, Waters L, Ahmed N et al BHIVA guidelines for the treatment of HIV‐1‐positive adults with antiretroviral therapy 2015. http://www.bhiva.org/HIV-1-treatment-guidelines.aspx (accessed 15 March 2016). [DOI] [PubMed]
- 22. https://www.gov.uk/government/statistics/hiv-data-tables (accessed 15 March 2016).
- 23. Monge S, Guillot V, Alvarez M et al Clinically relevant transmitted drug resistance to first line antiretroviral drugs and implications for recommendations. PLoS One 2014; 9: e90710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vega Y, Delgado E, Fernandez‐Garcia A et al Epidemiological Surveillance of HIV‐1 transmitted drug resistance in Spain in 2004–2012: relevance of transmission clusters in the propagation of resistance mutations. PLoS One 2015; 10: e0125699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pineda‐Pena AC, Schrooten Y, Vinken L et al Trends and predictors of transmitted drug resistance (TDR) and clusters with TDR in a local Belgian HIV‐1 epidemic. PLoS One 2014; 9: e101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castro H, Pillay D, Cane P et al Persistence of HIV‐1 transmitted drug resistance mutations. J Infect Dis 2013; 208: 1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mbisa JL, Fearnhill E, Dunn DT, Pillay D, Asboe D, Cane PA. Evidence of self‐sustaining drug resistant HIV‐1 lineages among untreated patients in the United Kingdom. Clin Infect Dis 2015; 61: 829–836. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt D, Kollan C, Fatkenheuer G et al Estimating trends in the proportion of transmitted and acquired HIV drug resistance in a long term observational cohort in Germany. PLoS One 2014; 9: e104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang WL, Kouyos R, Scherrer AU et al Assessing the paradox between transmitted and acquired HIV type 1 drug resistance mutations in the swiss HIV cohort study from 1998 to 2012. J Infect Dis 2015; 212: 28–38. [DOI] [PubMed] [Google Scholar]
- 30. Dolling D, Phillips AN, Delpech V et al Evaluating the extent of potential resistance to pre‐exposure prophylaxis within the UK HIV‐1‐infectious population of men who have sex with men. HIV Med 2012; 13: 309–314. [DOI] [PubMed] [Google Scholar]
- 31. Mourad R, Chevennet F, Dunn DT et al A phylotype‐based analysis highlights the role of drug‐naive HIV‐positive individuals in the transmission of antiretroviral resistance in the UK. AIDS 2015; 29: 1917–1925. [DOI] [PubMed] [Google Scholar]
- 32. Hurt CB. Transmitted resistance to HIV integrase strand‐transfer inhibitors: right on schedule. Antivir Ther 2011; 16: 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


