Abstract
Background and Objectives
Cryolipolysis is a safe, effective non‐surgical procedure to reduce fat. For most cryolipolysis treatments, tissue is pulled between parallel cooling plates with a treatment duration of 60 minutes. A novel contoured cup, medium‐sized applicator was developed to increase tissue contact with reduced skin tension and reduced treatment time. This prototype contoured cup was investigated with a standard cryolipolysis applicator to evaluate safety, efficacy, and patient preference.
Study Design/Material and Methods
A prototype CoolCup medium‐sized vacuum applicator (CoolSculpting System, ZELTIQ Aesthetics) was used to treat n = 19 subjects in the flanks. Randomly assigned, one flank received standard treatment with the CoolCore applicator (−10°C for 60 minutes). The contralateral flank received treatment from the CoolCup (−11°C for 35 minutes). The clinical study primary efficacy endpoint was 70% correct identification of baseline photographs by independent physician review. Incidence of adverse device effects was monitored. Fat layer reduction was measured by ultrasound and subject surveys were administered 12 weeks post‐treatment.
Results
Equivalent efficacy was demonstrated between the CoolCore standard treatment and the prototype CoolCup. Independent review from three blinded physicians found 81% correct identification of baseline photographs for the standard treatment and 79% for the CoolCup. Ultrasound measurements indicated mean fat layer reduction of 4.38 mm for the standard treatment and 4.40 mm for the CoolCup; no statistically significant difference was found when comparing treatment efficacy of the two applicators (P = 0.96). Patient questionnaires revealed 85% preferred CoolCup because of shorter treatment duration and greater comfort. Procedural assessments revealed 45% lower pain scores for CoolCup. Immediate post‐treatment clinical assessments revealed 82% less bruising. Typical side effects, such as numbness and erythema, were similar. There were no adverse events.
Conclusion
This clinical study of a prototype medium‐sized vacuum applicator with a cooled contoured surface indicates that the CoolCup produces equivalent safety and efficacy to the standard CoolCore cryolipolysis applicator. With a 42% reduction in treatment time, the procedure was found to be more comfortable because of lower vacuum skin tension and shorter treatment duration. Lasers Surg. Med. 49:63–68, 2017. © 2016 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: cryolipolysis, flanks, non‐invasive body contouring, non‐surgical fat reduction, subcutaneous fat, contoured cooled cup
INTRODUCTION
Cryolipolysis (CoolSculpting System, ZELTIQ Aesthetics, Pleasanton, CA) is a safe, effective non‐surgical procedure to reduce fat. For most cryolipolysis treatments, tissue is pulled by vacuum suction between two parallel cooling plates for a treatment duration of 60 minutes. While the parallel cooling plate applicator has been shown to be safe and effective 1, 2, 3, 4, 5, 6, efforts were made to improve the efficiency of tissue cooling. To improve the uniformity of the targeted tissue cooling, the cryolipolysis applicator was redesigned to create a contoured cup surface to maximize tissue contact with the cooling surface. A contoured, cooled cup had been successfully developed for small volume fat reduction, such as in the submental area 7, but a significantly larger cryolipolysis cooled treatment cup had not been clinically tested. Rather than pulling the tissue deeply into a parallel plate vacuum applicator, the tissue was drawn gently into the cooled cup, allowing it to seat fully against the entire cup surface.
In addition to improving cooling efficiency by increasing direct tissue contact with the cooled surface, the cooling surface was set to a lower temperature in order to reduce the overall procedure time. While current parallel plate vacuum cryolipolysis commercial treatments are delivered at −10°C for 60 minutes, lower temperature treatments have been investigated and shown to be safe and efficacious. A prior study of flank treatments using a commercially‐available parallel plate vacuum cryolipolysis applicator demonstrated safety and efficacy with parameters of −15°C for 45 minutes 8. Those study results were submitted for FDA clearance of lower temperature cryolipolysis treatments. Currently, there are two commercially‐available cryolipolysis applicators that deliver at temperatures lower than the typical −10°C. The non‐vacuum surface applicator (CoolSmooth PRO) delivers treatment to areas such as the lateral thighs at −13°C for 75 minutes while the small volume cup applicator (CoolMini) delivers treatment to sites such as the submental area at −11°C for 45 minutes 9.
A novel contoured cup, medium‐sized applicator was developed to increase tissue contact with reduced skin tension and reduced treatment time. Based upon computational modeling, the treatment parameters for the prototype applicator were developed. A multilayer, three‐dimensional finite element model of transient heat conduction was created to predict the subcutaneous fat temperature profile resulting from cryolipolysis treatment with the parallel plate and contoured cup applicators. Based upon the theoretical model, the cooling surface for a prototype medium volume cup vacuum applicator was set to −11°C for 35 minutes to achieve similar tissue temperature to the current parallel plate vacuum applicators set to −10°C for 60 minutes. This prototype contoured cup was investigated with a standard cryolipolysis applicator to evaluate safety, efficacy, and patient preference.
MATERIALS AND METHODS
This was a prospective, open label, interventional cohort study. The bilateral treatment study compared the prototype applicator to a standard parallel cooling plate applicator. The prototype device was created by modifying a commercially available parallel plate cryolipolysis vacuum applicator. A machined metal insert was installed in the standard applicator to create a cooled, contoured cup surface (Fig. 1).
Figure 1.
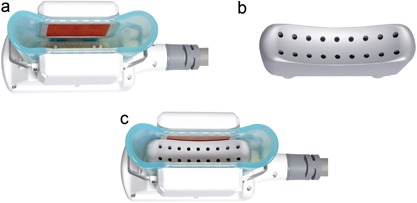
The CoolCup prototype device was created by modifying a commercially‐available CoolCore parallel plate cryolipolysis vacuum applicator (a) with a machined metal insert (b) to provide full cooling surface contact to the sides and top of the tissue drawn into the cooling cup (c).
Each subject received one treatment cycle on each flank consisting of either a −10°C, 60 minute cooling cycle delivered with the standard parallel cooling plate vacuum applicator (CoolCore) or a −11°C, 35 minute cooling cycle delivered using a prototype medium volume contoured cooled cup vacuum applicator (CoolCup). The standard and prototype applicator treatments were randomly assigned to each subject's left or right flank.
Eligible subjects were male or female, between 22 and 65 years of age, and with clearly visible subcutaneous flank fat and Body Mass Index (BMI) up to 30. For the duration of the study, subjects were instructed to avoid implementing major diet or exercise routine changes in order to maintain their weight within 5% of baseline measurement.
The primary safety endpoint was defined by monitoring the incidence of device‐ and/or procedure‐related adverse events. Immediately following treatment and at the 4‐ and 12‐week follow‐up visits, clinical assessments were performed to evaluate the treatment areas. One week post‐treatment, subjects were also contacted by telephone to assess the condition of the treatment areas. Safety was monitored by documentation of adverse events and clinical assessment of the treatment site. Subjects were assessed throughout the study for adverse events. Subject satisfaction data was collected by a written questionnaire at the 12‐week post‐treatment visit.
The efficacy endpoint was defined as 70% or greater correct identification of the pre‐treatment images by three blinded, independent physician reviewers. Photos were taken at pre‐treatment and 12‐week post‐treatment visits. At the baseline and follow‐up visits, photographs were acquired using a standardized photography set‐up (Nikon D810, Nikon 85 mm lens, DynaLite strobes) to ensure consistency. Study subjects were positioned with their feet separated at a fixed distance using a foot positioning guide and instructed to keep their arms raised and crossed at chest level. Clothing was carefully rearranged to reveal the flank treatment area with shirts raised and pinned out of the way and shorts lowered to avoid squeezing the flank area and inadvertently modifying the appearance of subcutaneous fat. At the post‐treatment visit, the photographer referred to baseline photographs while capturing the follow‐up photographs to ensure consistency in subject positioning and exposure. Subsequently, photos taken at the 12‐week post‐treatment visit were compared with those taken at baseline by a blinded independent panel of three physicians board‐certified in either dermatology or plastic surgery. Independent photo review data was generated by randomizing pre‐treatment and post‐treatment photograph pairs of each subject, then asking the reviewers to determine which image was the pre‐treatment image.
Ultrasound images were acquired at baseline and 12‐week post‐treatment visits. Flank ultrasounds were obtained with subjects standing with crossed arms and straight posture. Ultrasound areas were marked starting at the midpoint of the treatment area with one additional ultrasound area marked 2.5 cm on either side of the central site, for a total of three ultrasound measurements per flank. A transparent film was applied to each flank to mark the measurement areas and any landmarks (e.g., moles and scars) to facilitate locating the same ultrasound sites in the follow‐up visit. Measurements were conducted by placing the transducer (model L38, bandwidth 10–5 MHz, SonoSite Inc., Bothell, WA) over the flank measurement site and capturing the image on an ultrasound device (SonoSite Titan) to a depth of 3.9 cm. Care was taken to avoid adding pressure or negative pressure during measurement. During the follow‐up visit, subjects were positioned in the same manner as at baseline. The transparent film was applied and matched to the landmarks on the skin; ultrasound sites were marked on the skin and post‐treatment images were captured. Ultrasound images were post‐processed to measure anatomical features in the pre‐treatment and post‐treatment images and the fat layer reduction in the treatment area was calculated.
The cryolipolysis flank treatment was similar for both applicators. A protective gelpad was applied to the skin, either the standard CoolCore or prototype CoolCup applicator was positioned over the flank, and vacuum suction was initiated. The vacuum adhered the applicator to the treatment area and the subject was seated on the treatment table throughout the cryolipolysis treatment. At the conclusion of the treatment cycle, the applicator was removed, revealing firm, frozen tissue. Immediately following removal of the applicator, infrared (IR) thermography images (FLIR Systems, Wilsonville, OR) were obtained to evaluate the thermal profile across the treatment areas. The contralateral flank was then treated using the other applicator.
RESULTS
Nineteen patients were enrolled and completed treatment. The subject ages ranged from 33 to 59, with mean 46.7 years. Weight ranged from 128.2 to 215.0 lbs, with mean 157.0 lbs. Body Mass Index (BMI) ranged from 21.0 to 30.0, with mean BMI 25.2. All subjects remained within the allowed 5% weight change limit; therefore, no subjects were excluded from treatment efficacy analysis due to weight change. The mean weight change between baseline and the 12 week follow‐up visit was −0.5%. Seventeen of the subjects enrolled in the study were female and two were male. The Fitzpatrick Skin Type of the subjects ranged from II to IV, with 58% Type II, 26% Type III, and 16% Type IV. Ethnicity of the study subjects was 84% Caucasian and 16% Hispanic.
Immediate post‐treatment photos are shown in Figures 2 and 3 with the subjects still seated following removal of the applicators. As shown by the IR images obtained immediately following applicator removal, the CoolCup applicator produced a uniform cooling profile since the entire surface area of the treated flank was in contact with the contoured cooling cup. For the standard CoolCore treatment, discrete localized cooling was observed on the sides of the treated flank which were in direct contact with the cooling plates. The post‐treatment images in Figure 3 show that both applicators produced firm, solidified “butter stick” tissue, but there was less bruising produced by the prototype CoolCup.
Figure 2.
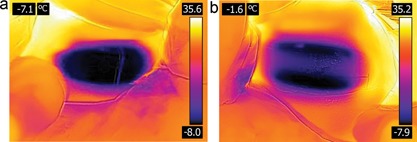
Comparison of immediate post‐treatment IR thermography images show uniform cooling from the CoolCup (a) and localized cooling around the parallel plates for the CoolCore (b). Subject KIL‐016.
Figure 3.
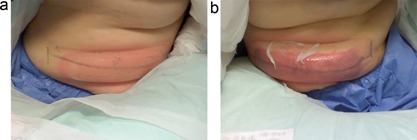
Comparison of immediate post‐treatment photographs showing firm “butter stick” treatment areas from both applicators. No bruising is evident from the CoolCup (a), whereas bruising was observed for the CoolCore (b). Subject KIL‐010.
Figures 4, 5, 6 show representative subjects at baseline and 12 weeks post‐treatment. Visible reduction in flank volume is demonstrated from the pre‐ and post‐treatment photographs. The standard CoolCore and prototype CoolCup applicators produced similar fat reduction, as shown by clinical photographs. From the independent photo review, three blinded, independent physicians reviewed the 19 subjects’ photographs in randomized pairs. For the standard CoolCore flank treatments, the reviewers correctly identified 81% (46/57), whereas for the prototype CoolCup flank treatments, the reviewers correctly identified 79% (45/57). The primary efficacy endpoint of at least 70% correct identification of the pre‐treatment images was met for both applicators.
Figure 4.
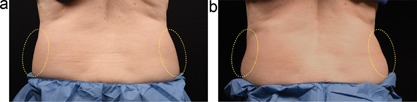
Baseline (a) and 12 week post‐treatment (b) photos for a 59‐year‐old female. The left flank was treated with the CoolCup for 35 minutes and the right flank was treated with the standard CoolCore for 60 minutes. Weight change −1.6 lbs. (−1.2%) from baseline. Subject KIL‐013.
Figure 5.
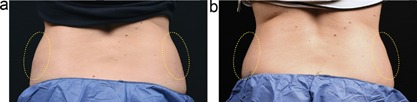
Baseline (a) and 12 week post‐treatment (b) photos for a 41‐year‐old female. The left flank was treated with the CoolCup for 35 minutes and the right flank was treated with the standard CoolCore for 60 minutes. Weight change −2.8 lbs. (−1.7%) from baseline. Subject KIL‐015.
Figure 6.
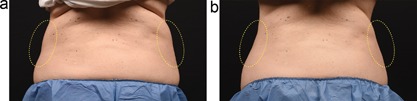
Baseline (a) and 12 week post‐treatment (b) photos for a 49‐year‐old female. The left flank was treated with the CoolCup for 35 minutes and the right flank was treated with the standard CoolCore for 60 minutes. Weight change −2.6 lbs. (−1.8%) from baseline. Subject KIL‐009.
Ultrasound images were analyzed to calculate fat layer reduction. The ultrasound measurement of the CoolCore treatment areas showed a mean fat layer reduction of 4.38 mm, with a standard deviation of 2.65 mm. The ultrasound measurement of the CoolCup treatment areas showed a mean fat layer reduction of 4.40 mm, with a standard deviation of 1.93 mm. Reduction in fat layer thickness was statistically significant (P < 1E‐06) for each applicator. A t‐test comparison demonstrated that there was not a statistically significant difference between the standard CoolCore and prototype CoolCup applicators (P = 0.96). The standard CoolCore and prototype CoolCup applicators demonstrated equivalent treatment efficacy.
While the treatment efficacy was similar between the two applicators, patient survey data provided some differentiation. Patient questionnaires showed three subjects had no preference between the applicators and three did not complete the survey question. Thus of the subjects with preference, 85% (11/13) preferred CoolCup because of shorter duration and greater comfort.
Differentiation between the applicators was also found by analyzing clinical assessment data. Clinical assessments evaluated procedural pain scores from 0 to 10, where 10 is described as the worst pain imaginable. The procedural assessments revealed 45% lower mean pain scores for the CoolCup applicator (0.33) compared to the CoolCore (0.61); it should be noted, however, that both are very low procedural pain scores.
Clinical assessment of the treatment sites was performed immediately post‐treatment and at the 1‐week, 4‐week, and 12‐week follow‐up visits. All subjects were evaluated for side effects at the treatment sites and assessed for any adverse events. Bruising, erythema/purpura, edema/swelling, numbness, and tingling at the treatment site were evaluated from 0 to 3, where 0 = Absent, 1 = Minor, 2 = Moderate, and 3 = Marked. In addition, any other side effects were also assessed and recorded.
Tables 1 and 2 show the clinical assessment data. The mean scores showed that neither applicator produced significant side effects. Immediately post‐treatment, the most common effects within the treatment area were erythema, edema, and numbness. The incidence of paresthesia was similar for both applicators and there were no reports of neuralgia from the study treatments. The immediate post‐treatment assessments found lower mean bruising for CoolCup (0.16) compared to CoolCore (0.90), thus 82% less bruising for the CoolCup. By the 12‐week post‐treatment visit, all side effects had resolved without intervention for both applicator treatments.
Table 1.
Clinical Assessment of Standard CoolCore Treatments at −10°C for 60 Minutes
| Immediate post‐treatment | 1‐week follow‐up | 4‐week follow‐pp | 12‐week follow‐up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoolCore −10°C/60 minutes assessment parameter | 0 | 1 | 2 | 3 | No | Yes | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Bruising | 5 | 11 | 3 | 0 | 13 | 6 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Erythema/purpura | 1 | 8 | 10 | 0 | 17 | 2 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Edema/swelling | 18 | 0 | 1 | 0 | 14 | 5 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Numbness | 7 | 3 | 6 | 3 | 3 | 16 | 16 | 3 | 0 | 0 | 19 | 0 | 0 | 0 |
| Tingling | 15 | 2 | 2 | 0 | 15 | 4 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Other | 17 | 2 | 0 | 0 | 15 | 4 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
Scale: 0 = absent, 1 = minor, 2 = moderate, and 3 = marked.
Table 2.
Clinical Assessment of Prototype CoolCup Treatments at −11°C for 35 Minutes
| Immediate post‐treatment | 1‐week follow‐up | 4‐week follow‐up | 12‐week follow‐up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoolCup −11°C/35 minutes assessment parameter | 0 | 1 | 2 | 3 | No | Yes | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Bruising | 16 | 3 | 0 | 0 | 17 | 2 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Erythema/purpura | 0 | 14 | 5 | 0 | 17 | 2 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Edema/swelling | 19 | 0 | 0 | 0 | 18 | 1 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Numbness | 10 | 5 | 3 | 1 | 6 | 13 | 16 | 3 | 0 | 0 | 19 | 0 | 0 | 0 |
| Tingling | 15 | 1 | 3 | 0 | 16 | 3 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| Other | 14 | 4 | 1 | 0 | 17 | 2 | 19 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
Scale: 0 = absent, 1 = minor, 2 = moderate and 3 = marked.
The primary safety endpoint for the study was satisfied; there were no device‐ or procedure‐related adverse events and no unanticipated adverse device effects occurred during the study.
DISCUSSION
Previously, a colder temperature flank study was carried out to investigate whether cryolipolysis treatments could be performed in a shorter duration 8. The aforementioned study reduced cryolipolysis treatment time by 25% while maintaining the safety and efficacy of standard treatments 8. This current study is an evolution of the earlier colder temperature, shorter duration flank study because in addition to seeking to reduce treatment times, it also tested a medium cryolipolysis cup designed to maximize tissue contact with the cooling surface. The cup allowed the tissue to seat fully against the entire surface, thus reducing the significant vacuum tension on the skin. The reduced skin tension resulted in an enhanced treatment experience with significantly lower procedural pain scores and lower incidence of post‐treatment bruising and numbness. Coupled with the reduced treatment duration, the prototype CoolCup was preferred by 85% of the study subjects when asked to select between the prototype and the standard applicators.
The prototype CoolCup was created by modifying an existing parallel plate applicator with a contoured metal insert. In a commercial production version of the medium cup applicator, the cooled contoured cup is integrated into an applicator with multiple body contours (CoolAdvantage). Interchangeable contours allow the same contoured cup applicator to be used on various treatment sites, such as the abdomen, flanks, and inner thighs (Fig. 7). The new contoured cup applicator has a surface area of 122 cm2, whereas the CoolCore standard parallel plate applicator has a typical treatment area of 110 cm2; thus, treatment area is increased by approximately 10% while treatment time is decreased by over 40% with the cup applicator.
Figure 7.
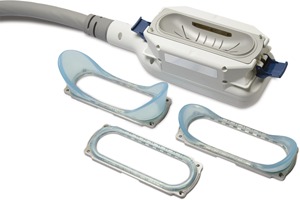
A commercial version of the prototype cooled cryolipolysis cup applicator will have interchangeable contours for flat and curved treatment sites.
As demonstrated in this bilateral flank study, the evolution of the vacuum cryolipolysis applicator from a parallel plate configuration to a contoured cup resulted in increased tissue contact with the cooling surface, more uniform cooling, shorter treatment duration, and an improved patient experience.
CONCLUSION
This clinical study of a prototype medium‐sized vacuum applicator with a cooled contoured surface indicates that the CoolCup produces equivalent safety and efficacy to the standard CoolCore cryolipolysis applicator. With a 42% reduction in treatment time, the procedure was found to be more comfortable because of reduced skin tension and shorter duration.
ACKNOWLEDGMENT
This clinical study was sponsored by ZELTIQ Aesthetics, manufacturer of the CoolSculpting System.
Conflict of Interest Disclosures: Dr. Kilmer is on the medical advisory board for Candela/Syneron, Living Proof/Strateris, Lumenis, Lutronics, and Zeltiq and receives research support from Candela/Syneron, Living Proof/Strateris, Lumenis, Lutronics, Zeltiq, Allergan, Cutera, Cynosure/Palomar, and Valeant.
Dr. Kilmer is the founding director of the Laser and Skin Surgery Center of Northern California and is a Clinical Professor of Dermatology at the University of California, Davis.
REFERENCES
- 1. Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non‐invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two‐cycle treatments. Lasers Surg Med 2014; 46(10):731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med 2015; 47(2):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boey GE, Wasilenchuk JL. Fat reduction in the inner thigh using a prototype cryolipolysis applicator. Dermatol Surg 2014; 40(9):1004–1009. [DOI] [PubMed] [Google Scholar]
- 4. Munavalli GS, Panchaprateep R. Cryolipolysis for targeted fat reduction and improved appearance of the enlarged male breast. Dermatol Surg 2015; 41(9):1043–1051. [DOI] [PubMed] [Google Scholar]
- 5. Lee SJ, Jang HW, Kim H, Suh DH, Ryu HJ. Non‐invasive cryolipolysis to reduce subcutaneous fat in the arms. J Cosmet Laser Ther 2016; 18(3):126–129. [DOI] [PubMed] [Google Scholar]
- 6. Wanitphakdeedecha R, Sathaworawong A, Manuskiatti W. The efficacy of cryolipolysis treatment on arms and inner thighs. Lasers Med Sci 2015; 30(8):2165–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non‐invasive reduction of submental fat. Lasers Surg Med 2016; 48(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilmer S, Boey G, Burns AJ. Safety and efficacy of colder temperature, shorter duration cryolipolysis treatments. Lasers Surg Med 2015; 47(S26):28. [Google Scholar]
- 9.Treatment parameters for CoolSmooth PRO and CoolMini applicators provided by ZELTIQ Aesthetics, Pleasanton, CA.


