Abstract
Background and purpose
Dravet syndrome (DS) is a severe, drug‐resistant epilepsy. Fenfluramine has been reported to have a long‐term clinically meaningful anticonvulsive effect in patients with DS.
Methods
This prospective, open‐label study assessed the safety and effectiveness of low‐dose fenfluramine in a new cohort of patients with DS. Following a 3‐month baseline period, fenfluramine was added to each patient's current antiepileptic drug regimen at a dose of 0.25–1.0 mg/kg/day (max. 20 mg/day). The incidence of major motor seizures (tonic, clonic, tonic–clonic, atonic and myoclonic seizures lasting >30 s) in both the baseline and treatment periods was assessed via a seizure diary. Periodic echocardiographic examinations during the treatment period were used to assess cardiovascular safety.
Results
Nine patients (aged 1.2–29.8 years) enrolled in the study and were treated with fenfluramine for a median duration of 1.5 (range, 0.3–5.1) years. Median frequency of major motor seizures was 15.0/month in the baseline period. All patients demonstrated a reduction in seizure frequency during the treatment period with a median reduction of 75% (range, 28–100%). Seven patients (78%) experienced a ≥50% reduction in major motor seizure frequency. The most common adverse events were somnolence (n = 5) and anorexia (n = 4). No evidence of cardiac valvulopathy or pulmonary hypertension was observed.
Conclusions
The effectiveness and safety of low‐dose fenfluramine as an add‐on therapy for DS in this new prospective cohort supports previous findings.
Keywords: clinical trial, Dravet syndrome, epileptic encephalopathy, orphan drug, refractory epilepsy, serotonin, severe myoclonic epilepsy in infancy
Introduction
Dravet syndrome (DS) is a severe and drug‐resistant epilepsy syndrome. The onset of seizures is usually before the age of 12 months in developmentally normal infants. The first seizure type is usually clonic, generalized or unilateral and triggered by fever. Subsequently, other seizure types develop, including tonic–clonic, myoclonic, atypical absence and focal seizures 1. The diagnosis of DS is based on the clinical phenotype; however, mutations in the SCN1A gene can be found in 75–85% of patients with DS 2, 3. The estimated incidence of DS ranges from 1 in 15 700 to 1 in 40 000 live births 2, 4.
The current treatment strategy for patients with DS involves the use of multiple antiepileptic drugs (AEDs), including combinations of valproate, topiramate, clobazam, stiripentol and others 5, 6. In a recent survey of European patients with DS, about 40% of patients were treated with three AEDs and approximately 40% were treated with four AEDs 6. Despite these multidrug regimens, 45% continue to experience ≥4 tonic–clonic seizures/month 6. To reduce frequent and disabling seizures, alternative treatment options are needed.
Fenfluramine (3‐trifluoromethyl‐N‐ethylamphetamine), originally approved as an appetite suppressant 7, has also been reported to exhibit activity in refractory epilepsy 8. Due to the retrospective character of previous studies 9, 10, we wanted to confirm our data in a well‐designed prospective trial in a new group of patients with DS.
Here we describe the effectiveness, tolerability and safety of fenfluramine in a new cohort of patients with DS who began treatment after 2010. This prospective study used a standardized protocol of assessments.
Methods
Patients
Patients of between 6 months and 50 years of age with a DS diagnosis based on the core phenotype 1 with or without SCN1A mutation and whose epilepsy was uncontrolled despite adequate therapy with standard AEDs were eligible. The major exclusion criteria included cardiovascular pathology, hypertension treated with medication, glaucoma, or hypersensitivity to fenfluramine or any other amphetamine‐like drug. All patients who enrolled between January 2011 and December 2015 and who had at least 3 months of observation after starting fenfluramine were included in this analysis.
Study design
This prospective open‐label study began with a 3‐month baseline period during which patients continued treatment with their current AEDs. A daily diary was used to record the date, time, type and duration of seizures. A 24‐h electroencephalographic study was conducted during the baseline period as was an echocardiographic examination to evaluate cardiac valvular structure and function. AED blood levels were checked to confirm compliance with treatment.
After the 3‐month baseline period, fenfluramine was added at a dose of 0.25–1.0 mg/kg/day. The daily dose could be adjusted during the study based on efficacy or tolerability issues, with a maximum of 20 mg/day. Concomitant AEDs were kept stable during the first 3 months, thereafter adjustments could be made if necessary (Figure S1). Echocardiographic examinations were conducted every 3 months during the first year of treatment, every 6 months during the second year of treatment and annually thereafter. Beginning in 2014 all echocardiograms were evaluated by a pediatric (F.M.) and an adult (B.P.P.) cardiologist at Antwerp University Hospital. All data from inclusion until the last study visit (before April 2016) were analyzed.
Fenfluramine
Fenfluramine was obtained from Zogenix International Limited, Emeryville, CA, USA and its purity was assessed and confirmed by the Belgian Pharmaceutical Association. Fenfluramine was compounded into capsules by the pharmacy of Antwerp University Hospital at doses of 2.5, 5, 10 and 20 mg/capsule. Most patients in this study swallowed the capsules but, for a few, the contents were sprinkled prior to consumption.
Study objectives
The key objective of this study was to assess the overall change in frequency of all major motor seizures during the fenfluramine treatment duration compared with the 3‐month baseline period. In addition, the change in major motor seizure frequency was assessed at specific time points (3, 6, 9 and 12 months post‐initiation of fenfluramine treatment). Major motor seizures were defined as tonic–clonic, tonic, clonic, atonic and myoclonic seizures lasting >30 s. Seizure frequency (seizures/month) during the baseline period was calculated as the total number of seizures divided by the number of days in the baseline period multiplied by 30. During treatment with fenfluramine, seizure frequency was similarly calculated at each study visit. Taking into account the small sample size and the non‐Gaussian distribution, a Wilcoxon signed‐rank test for paired data was used to analyze the treatment effect. Statistical analysis was performed with SPSS software (IBM Corp SPSS Statistics for Windows, version 23.0, Armonk, NY, USA). Adverse events were monitored at each visit during the study. Global impression of change, quality of life (QoL) and sleep quality were assessed at each study visit since June 2015. The QoL and sleep quality of parents and patients were assessed using a visual analog scale (0, extremely bad to 10, very good). Global impression of change was evaluated with a five‐point scale (2, very much improved; 1, much improved; 0, no change; −1, much worse; and −2, very much worse).
Study ethics
Following the withdrawal of fenfluramine in 1997, its use as an AED for the treatment of intractable epilepsy was allowed in Belgium following the issuance of a Royal Decree (KB 2002/22215). The study protocol was reviewed and approved by the Ethics Committee at Antwerp University Hospital prior to the initiation of this study (registration no. B30020108998). The parents or guardians of each patient were fully informed about the study and provided written informed consent prior to participation in the study.
Results
Nine patients with DS between the ages of 1.2 and 29.8 years enrolled in the study between January 2011 and December 2015, and had been treated with fenfluramine for a median duration of 1.5 (range, 0.3–5.06) years at the time of the last assessment. Individual patient demographic and clinical information are presented in Tables 1 and 2. The initial dose of fenfluramine in each patient was 5 or 10 mg/day, divided and administered as two separate doses, with a mean weight‐adjusted daily dose of 0.24 (range, 0.16–0.50) mg/kg/day. Fenfluramine doses were increased in six patients in order to optimize effectiveness during their treatment and, at the most recent study visit, the mean dose was 0.35 (range, 0.16–0.69) mg/kg/day.
Table 1.
Individual patient demographic and clinical information
| Patient | Sex | Age at start of FFA (years) | Height at start (cm) | Weight at start (kg) | Mutation in SCN1A | Initial epilepsy treatment regimen at study entry |
|---|---|---|---|---|---|---|
| 1 | M | 11.9 | 144 | 35 | De‐novo nonsense mutation (c.4497delT) | VPA, CLB, VNS |
| 2 | F | 1.2 | 78 | 10 | De‐novo mis‐sense mutation (c.296T>A) | VPA, TPM, CLB |
| 3 | M | 5.9 | 107 | 17 | De‐novo nonsense mutation (c.969T>G) | VPA, TPM |
| 4 | M | 11.9 | 149 | 40 | De‐novo duplication (c.3427‐4002 + ?dup) | Bromide, VPA, TPM |
| 5 | F | 13.5 | 164 | 50 | De‐novo nonsense mutation (c.58C>T) | STP, TPM, VPA, ethyl loflazepate |
| 6 | M | 19.8 | 168 | 48 | De‐novo splice site mutation (IVS22 + 1 G>A) | VPA, TPM, ethyl loflazepate, STP |
| 7 | M | 20.3 | 165 | 60 | De‐novo splice site mutation (c.2589 + 3A>T) | VPA, LEV, CLB, TPM, VNS |
| 8 | M | 7.2 | 124 | 24 | De‐novo frameshift mutation (c.657‐658delAG) | VPA, TPM, ethyl loflazepate |
| 9 | F | 29.8 | 165 | 64 | De‐novo mis‐sense mutation (c.2875T>C) | VPA, TPM, ethyl loflazepate, VNS |
CLB, clobazam; FFA, fenfluramine; LEV, levetiracetam; STP, stiripentol; TPM, topiramate; VNS, vagal nerve stimulation; VPA, valproic acid.
Table 2.
Individual patient therapeutic information
| Patient | Initial FFA dose | Most recent FFA dose | Treatment duration (years) | Major motor seizures/montha | ||||
|---|---|---|---|---|---|---|---|---|
| mg/day | mg/kg/day | mg/day | mg/kg/day | 3‐month baseline period | FFA treatmentb | Percent reductionc | ||
| 1 | 10 | 0.29 | 20 | 0.44 | 5.06 | 15.0d | 4.5d | −70% |
| 2 | 5 | 0.50 | 12.5 | 0.69 | 4.70 | 2.5d | 0.4d | −84% |
| 3 | 5 | 0.29 | 10 | 0.62 | 0.78 | 0.4d | 0d | −100% |
| 4 | 10 | 0.25 | 15 | 0.36 | 1.50 | 39.7d | 7.3d | −82% |
| 5 | 5 | 0.10 | 15 | 0.25 | 1.64 | 2.0d | 0.7d | −68% |
| 6 | 10 | 0.21 | 10 | 0.19 | 1.57 | 2.3d | 1.5d | −37% |
| 7 | 10 | 0.17 | 15 | 0.27 | 1.02 | 18.3 | 13.2 | −28% |
| 8 | 5 | 0.21 | 5 | 0.17 | 0.63 | 20.4 | 0.8 | −96% |
| 9 | 10 | 0.16 | 10 | 0.16 | 0.30 | 23.8 | 6.0 | −75% |
| Mean | 7.8 | 0.24 | 12.5 | 0.35 | 1.9 | 13.8 | 3.8 | −71% |
| Median | 10 | 0.23 | 12.5 | 0.29 | 1.5 | 15.0 | 1.5 | −75% |
FFA, fenfluramine. aMajor motor seizures were defined as tonic–clonic, tonic, clonic, atonic and myoclonic seizures lasting >30 s. bMonthly seizure frequency was calculated as the total number of seizures during the treatment period divided by the total number of treatment days multiplied by 30 days/month. cPercent reduction refers to the entire treatment period compared with the seizure frequency per month in the baseline period. dTonic–clonic seizures were the only major motor seizures observed in these patients both before and during treatment with fenfluramine.
During the 3‐month baseline period, the frequency of major motor seizures ranged from 0.4 to 39.7/month (median, 15.0/month). Only tonic–clonic seizures were observed in Patients 1–6, whereas additional types of major motor seizures were observed in Patients 7–9. All patients experienced a decrease in the frequency of major motor seizures after treatment with fenfluramine was initiated (Table 2). Compared with the 3‐month baseline period, a reduction in the median frequency of major motor seizures was observed from baseline (15.0/month) to 3 months (2.0/month), 6 months (1.1/month), 9 months (1.1/month) and 12 months (1.6/month) (Fig. 1). A Wilcoxon signed‐rank test showed a significant difference between the 3‐month baseline period compared with the first 3 months of treatment with fenfluramine (Z = −2.67, P = 0.008). Over the entire treatment period the median percentage reduction in major motor seizures was 75% (range, 28–100%).
Figure 1.
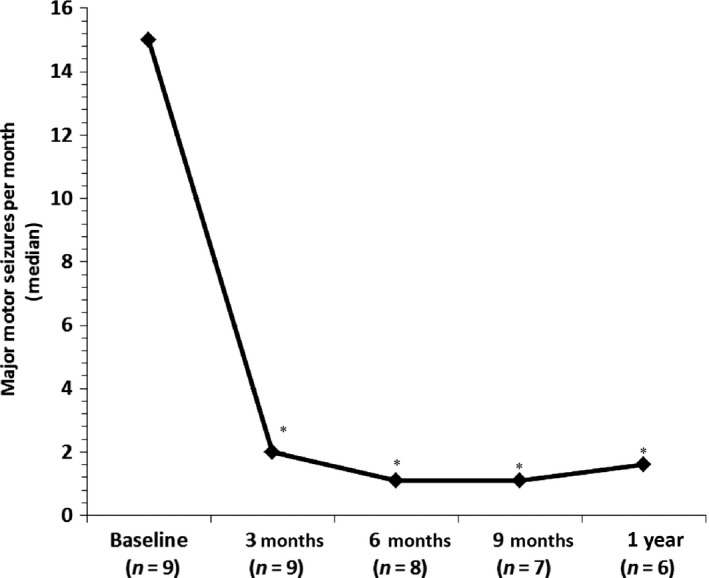
The effect of add‐on fenfluramine on the frequency of major motor seizures in patients with Dravet syndrome. *Significant differences in Wilcoxon non‐parametric test (P < 0.05) compared with baseline period.
Six of the nine (67%) patients demonstrated ≥50% reduction (defined as a ‘responder’) in major motor seizures by Month 3 and remained responders throughout the entire observation period (Table 2, Fig. 1). Of the remaining three patients, one became a responder at Month 6 and remained a responder for the rest of the observation period. Thus, as of the last clinic visit, 78% (seven of nine patients) of patients were 50% responders. The AEDs were kept stable during the first 3 months except in one patient (Patient 8). The parents of this boy suddenly stopped his ethyl loflazepate 1 week after starting fenfluramine; 2 weeks after this he experienced an increase in seizures with development of a non‐convulsive status epilepticus for which he was hospitalized. He recovered after the addition of clobazam. Due to the long follow‐up period and good seizure control in some patients, we tried to optimize the AED treatment regime. In three patients, one of the other AEDs (clobazam, bromide or stiripentol) was successfully discontinued without any increase in seizures.
Several patients experienced clinical benefits in addition to reductions in seizure frequency. At the most recent visit, mean sleep quality for patients and parents was 8.1/10 and 7.9/10, respectively (10, very good), mean QoL scores were 7.4/10 and 7.6/10, respectively, and five parents indicated a much to very much improved global impression. Parents never indicated a global deterioration. Patient 3, a 5.9‐year‐old boy, had a low frequency of major motor seizures during the baseline observation period. Prior to starting fenfluramine he regularly experienced atypical absences with increasing somnolence and the impression of decreasing cognitive function. After starting fenfluramine, all seizure activity ceased, including the absence seizures and his school performance improved. He also had his first febrile episode without seizures. Patient 4, an 11.9‐year‐old boy, typically had motor seizures occurring in clusters and, during treatment with fenfluramine, the duration of the clusters was diminished with a faster recovery between clusters. Patient 7, a 20.3‐year‐old male, demonstrated the smallest decrease in motor seizure frequency in this cohort (−28%); however, his seizures were described as less severe and his level of alertness throughout the day as being increased.
All patients experienced one or more treatment‐emergent adverse events (TEAEs) during treatment with fenfluramine. The most commonly reported TEAEs were somnolence (n = 5) and anorexia (n = 4). In addition, fatigue (n = 3), sleep difficulties (n = 2) and non‐convulsive status epilepticus (n = 3) occurred in two or more patients during treatment with fenfluramine. Except for episodes of non‐convulsive status epilepticus, all TEAEs were deemed to be of mild to moderate severity. One patient (Patient 2), who was hospitalized 11 times for status epilepticus before the addition of fenfluramine, experienced four separate episodes of status epilepticus with fever due to respiratory infection during the 4.7 years of treatment with fenfluramine.
Between two and nine echocardiographic examinations were performed in each patient (including the baseline examination). No evidence of change in cardiac valve structure or function was observed in any patient during the entire treatment observation period and nor were there any echocardiographic findings suggestive of pulmonary arterial hypertension.
Discussion
In this new cohort we prospectively evaluated nine patients with DS treated with fenfluramine for a median duration of 1.5 (range, 0.3–5.06) years. All patients demonstrated a reduction in major motor seizure frequency during treatment, with a median reduction of 75%. Seven patients (78%) experienced a ≥50% reduction in major motor seizure frequency. In addition, a tendency towards a positive effect on the sleep quality and QoL of both patients and parents was seen in combination with an improved global impression. Importantly, no echocardiographic evidence of cardiac valvulopathy or pulmonary hypertension was observed with repeated echocardiography. Thus, this study confirms the previously published original cohort of patients with DS who were successfully treated with fenfluramine 10, 11.
Ceulemans et al. 10 have followed patients with DS treated with fenfluramine for periods of up to 27 years. In their study of the original cohort of 12 patients with DS, seven of the 10 patients with DS who were still being treated as of the end of 2010 had been seizure free for ≥1 year at their last study visit. A recent 5‐year follow‐up report on the 10 patients from this original cohort demonstrated continued long‐term efficacy and good tolerability 11. Three patients were seizure free for the entire 5 years and four patients experienced seizure‐free intervals of at least 2 years during the new study period. Two patients had mild (stable) valve thickening on the last echocardiography, which was deemed clinically insignificant. No patient had any clinical or echocardiographic signs of pulmonary hypertension.
The lack of effective medical treatment has led to investigation of new agents for treatment of DS 12, 13. Devinsky and colleagues have recently reported the results of an open‐label trial of cannabidiol add‐on therapy in 214 patients with childhood‐onset intractable epilepsy, including 32 patients with DS 14. The patients with DS had a 49.8% median reduction in monthly motor seizures and 16 patients (50%) had a reduction of ≥50%. Multiple logistic regression analysis revealed that only treatment with clobazam independently predicted being a responder (defined by a ≥50% decrease in seizure frequency). Somnolence was the most common adverse event, reported by 25% of patients, followed by decreased appetite (19%), diarrhea (19%) and fatigue (13%). In the present study, acknowledging the small sample size, we did not observe any association between the degree of seizure frequency reduction with fenfluramine in patients who were and were not being treated with clobazam or any other anticonvulsant (Tables 1 and 2).
It remains to be fully assessed whether the association of fenfluramine with cardiac valvulopathy 7, 15 that was seen at higher doses (>60 mg/day), often in combination with phentermine, in the treatment of adult obesity translates to its use in low doses in children with DS. It was removed from the market in 1997 following a report of 113 cases of valvulopathy described by the United States Centers for Disease Control and Prevention. In those cases, only 2% involved fenfluramine monotherapy and 79% occurred with both fenfluramine and phentermine 16. Although no echocardiographic evidence of changes in cardiac valve structure or function during fenfluramine treatment was observed in the present study, the relatively short period of exposure in this new cohort of patients may be insufficient for valvulopathy to become evident. In the original cohort of patients with DS treated with fenfluramine, eight of 10 patients had normal echocardiograms at their most recent study visit (average treatment duration of 16.6 years), whereas two patients had slight, but stable, cardiac valve thickening without any clinical manifestations 11. It is important to note that cardiac valvulopathy has been reported to be dose related. Li et al. showed that, in adult obese patients, severe valvulopathy was more common with ≥60 mg/day compared with patients treated with <40 mg/day 17. In the present study, lower doses of fenfluramine were employed; the maximum dose in these patients was 20 mg/day and the median dose was 10 mg/day.
Major limitations of the current study include the small sample size, use of a study diary, open‐label design, absence of systematic electroencephalographic monitoring and lack of a control group. Sleep quality, QoL and global impression were not assessed at baseline for the majority of the patients. Therefore, the findings need to be viewed in this context. It is, however, encouraging that the findings in this group of patients are consistent with those reported in the original cohort 10. The ongoing Phase 3 randomized, placebo‐controlled studies on the use of fenfluramine in DS will be required to confirm these initial observations and aid in further defining the benefit–risk profile in patients with DS.
Conclusions
These results on the use of low‐dose fenfluramine in this new cohort of patients with DS further support the observations reported in the original cohort. Patients with DS experienced sustained periods of clinically meaningful improved seizure control during fenfluramine treatment with a favorable tolerability and no echocardiographic or clinical evidence of cardiac valvulopathy or pulmonary hypertension.
Disclosure of conflicts of interest
A.S.S. is a PhD student at the University of Antwerp and received an educational grant from Zogenix. B.C., L.L. and B.P.P. are members of an advisory board of Zogenix. A.G. and B.S.G. are employees of Zogenix. F.M. and B.G. declare no financial or other conflicts of interest.
Supporting information
Figure S1. The seizure frequency is indicated by a red line (——, left Y‐axis).
Acknowledgements
The authors received professional medical writing and editing assistance that was provided by Edward Weselcouch, PhD of PharmaWrite LLC (Princeton, NJ, USA) and was paid for by Zogenix, Inc. (San Diego, CA, USA). In addition, Zogenix provided financial support to the investigators for the collection of data for this study and for data entry and maintenance of a database specific to this study.
References
- 1. Dravet C. The core Dravet syndrome phenotype. Epilepsia 2011; 52(Suppl. 2): 3–9. [DOI] [PubMed] [Google Scholar]
- 2. Bayat A, Hjalgrim H, Moller RS. The incidence of SCN1A‐related Dravet syndrome in Denmark is 1:22,000: a population‐based study from 2004 to 2009. Epilepsia 2015; 56: e36–e39. [DOI] [PubMed] [Google Scholar]
- 3. Bender AC, Morse RP, Scott RC, Holmes GL, Lenck‐Santini PP. SCN1A mutations in Dravet syndrome: impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy Behav 2012; 23: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunklaus A, Ellis R, Reavey E, Forbes GH, Zuberi SM. Prognostic, clinical and demographic features in SCN1A mutation‐positive Dravet syndrome. Brain 2012; 135(Pt 8): 2329–2336. [DOI] [PubMed] [Google Scholar]
- 5. Shi XY, Tomonoh Y, Wang WZ, et al Efficacy of antiepileptic drugs for the treatment of Dravet syndrome with different genotypes. Brain Dev 2016; 38: 40–46. [DOI] [PubMed] [Google Scholar]
- 6. Aras LM, Isla J, Mingorance‐Le Meur A. The European patient with Dravet syndrome: results from a parent‐reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav 2015; 44: 104–109. [DOI] [PubMed] [Google Scholar]
- 7. Connolly HM, Crary JL, McGoon MD, et al Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med 1997; 337: 581–588. [DOI] [PubMed] [Google Scholar]
- 8. Schoonjans AS, Lagae L, Ceulemans B. Low‐dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv Neurol Disord 2015; 8: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boel M, Casaer P. Add‐on therapy of fenfluramine in intractable self‐induced epilepsy. Neuropediatrics 1996; 27: 171–173. [DOI] [PubMed] [Google Scholar]
- 10. Ceulemans B, Boel M, Leyssens K, et al Successful use of fenfluramine as an add‐on treatment for Dravet syndrome. Epilepsia 2012; 53: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 11. Ceulemans B, Schoonjans A‐N, Marchau F, Paelinck B, Lagae L. Five‐year extended follow‐up of 10 Dravet patients treated with fenfluramine. Epilepsia 2016; 57: e129–e134. [DOI] [PubMed] [Google Scholar]
- 12. Porter BE, Jacobson C. Report of a parent survey of cannabidiol‐enriched cannabis use in pediatric treatment‐resistant epilepsy. Epilepsy Behav 2013; 29: 574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 2015; 45: 49–52. [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Marsh E, Friedman D, et al Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol 2016; 15: 270–278. [DOI] [PubMed] [Google Scholar]
- 15. Abenhaim L, Moride Y, Brenot F, et al Appetite‐suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 335: 609–616. [DOI] [PubMed] [Google Scholar]
- 16. Center for Disease Control and Prevention (CDC). Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb Mortal Wkly Rep 1997; 46: 1061–1066. [PubMed] [Google Scholar]
- 17. Li R, Serdula MK, Williamson DF, Bowman BA, Graham DJ, Green L. Dose‐effect of fenfluramine use on the severity of valvular heart disease among fen‐phen patients with valvulopathy. Int J Obes Relat Metab Disord 1999; 23: 926–928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The seizure frequency is indicated by a red line (——, left Y‐axis).


