Abstract
Aims
To investigate the effects of exercise in combination with a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), liraglutide, or placebo for the treatment of type 2 diabetes.
Methods
Thirty‐three overweight, dysregulated and sedentary patients with type 2 diabetes were randomly allocated to 16 weeks of either exercise and liraglutide or exercise and placebo. Both groups had three supervised 60‐minute training sessions per week including spinning and resistance training.
Results
Glycated haemoglobin (HbA1c) levels dropped by a mean ± standard deviation of 2.0% ± 1.2% (from 8.2% ± 1.4%) in the exercise plus liraglutide group vs 0.3% ± 0.9% (from 8.0% ± 1.2%) in the exercise plus placebo group ( P < .001), and body weight was reduced more with liraglutide (−3.4 ± 2.9 kg vs −1.6 ± 2.3 kg; P < .001). Compared with baseline, similar reductions were seen in body fat (exercise plus liraglutide: −2.5% ± 1.4% [ P < .001]; exercise plus placebo: −2.2% ± 1.9% [ P < .001]) and similar increases were observed in maximum oxygen uptake (exercise plus liraglutide: 0.5 ± 0.5 L O2/min [ P < .001]; exercise plus placebo: 0.4 ± 0.4 L O2/min [ P = .002]). Greater reductions in fasting plasma glucose (−3.4 ± 2.3 mM vs −0.3 ± 2.6 mM, P < .001) and systolic blood pressure (−5.4 ± 7.4 mm Hg vs −0.6 ± 11.1 mm Hg, P < .01) were seen with exercise plus liraglutide vs exercise plus placebo. The two groups experienced similar increases in quality of life during the intervention.
Conclusions
In obese patients with type 2 diabetes, exercise combined with GLP‐1RA treatment near‐normalized HbA1c levels and caused a robust weight loss when compared with placebo. These results suggest that a combination of exercise and GLP‐1RA treatment is effective in type 2 diabetes.
Keywords: exercise, GLP‐1, GLP‐1 receptor agonist, glucagon‐like peptide‐1, liraglutide, randomized study, type 2 diabetes
1. INTRODUCTION
Physical activity is one of the three cornerstone recommendations (along with diet and medication) in the treatment of type 2 diabetes.1, 2, 3, 4, 5 Exercise training improves aerobic capacity and muscular strength and is often associated with fat loss and increased muscle mass. Furthermore, exercise training has a beneficial effect on insulin‐mediated glucose uptake and is associated with increased glucose transporter 4 (GLUT4) expression and improved insulin signalling in skeletal muscle.6, 7 Despite several studies reporting significant reductions in glycated haemoglobin (HbA1c) of 0.4% to 0.6% after increasing the level of physical activity, it is often not sufficient to normalize glycaemic control in patients with type 2 diabetes.2, 8, 9 Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone secreted from enteroendocrine L cells located in the distal jejunum and ileum in response to meal ingestion. GLP‐1 is known mainly for its glucose‐lowering and satiety‐promoting effects.10 Since 2006, GLP‐1 receptor agonists (GLP‐1RAs) have been used for the treatment of patients with type 2 diabetes.11 Liraglutide is a once‐daily administered GLP‐1RA with beneficial effects on HbA1c levels, body weight and body composition in obese dysregulated patients with type 2 diabetes.11 Nevertheless, a substantial proportion of GLP‐1RA‐treated patients do not reach glycaemic targets.
Exercise and GLP‐1RAs have each been used for the treatment of patients with type 2 diabetes for several years; however, the combination of the two has, to our knowledge, never been evaluated in prospective trials before. We hypothesized that exercise training combined with GLP‐1RA treatment would result in clinically relevant improvements in HbA1c and a range of other health‐related variables, in obese, sedentary and dysregulated patients with established type 2 diabetes.
2. METHODS
2.1. Study design and endpoints
The study was a 16‐week, randomized, double‐blind, placebo‐controlled study conducted at the Center for Diabetes Research, Gentofte Hospital, University of Copenhagen, Denmark. The study was conducted in accordance with the international Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. The study was designed and conducted by the authors, and the authors vouch for the data and analyses, as well as the fidelity of this report to the study protocol. The study was an investigator‐initiated study, sponsored by Novo Nordisk, but Novo Nordisk had no role in the study design, data analysis or reporting of results. The primary endpoint was change in HbA1c (from baseline until end of study (16 weeks)). Secondary outcomes included changes in body weight, body composition, maximum oxygen uptake (VO2max), fasting plasma glucose (FPG), serum insulin, serum C‐peptide, plasma glucagon, plasma concentrations of incretin hormones, liver enzymes, lipids, blood pressure, heart rate and quality of life (QoL). Further secondary outcomes, insulin resistance and β‐cell function, were evaluated by homeostatic model assessment (HOMA‐IR and HOMA‐β, respectively).
Patients were recruited from the Diabetes Outpatient Clinic at Gentofte Hospital, Denmark, and through advertisement. Oral and written informed consent were obtained before inclusion. Inclusion criteria were: age >18 years; type 2 diabetes treated with diet and/or metformin; HbA1c between 7% and 11% (53‐97 mmol/mol), body mass index (BMI) > 25 kg/m2; and sedentary lifestyle (self‐reported physical activity <150 min/wk). Exclusion criteria were clinically relevant cardiovascular disease, impaired liver function, anaemia and/or impaired renal function. Eligible patients were randomly assigned to either exercise training plus liraglutide (exercise plus liraglutide) or to exercise training plus placebo (exercise plus placebo) for 16 weeks (Figure 1). An employee otherwise not involved in the study carried out the randomization at a 1:1 ratio from a prespecified randomization list. The exercise training consisted of three 60‐minute supervised training sessions per week (in groups). Twice per week the patients participated in spinning sessions (Indoor Body Bike Supreme; Pedan, Køge, Denmark) with an average intensity of 65% to 85% of maximum heart rate (monitored by RS300X; Polar, Kempele, Finland). The programme for spinning sessions was modelled on traditional spinning programs with music and focusing on cardio‐training with incorporated high‐intensity intervals of 30‐second sprints (4‐10 repetitions) followed by 1 minute of active recovery. The third session consisted of 8 different resistance exercises (squat, lunges, biceps, triceps, whole‐body [modulated Olympic lift], bench press, shrugs and plank), and 3 sets of 12 repetitions with 30‐second to 45‐second rests between each set in order to recruit the major muscle groups. Resistance exercise training was performed with barbells (Reebok, Canton, Massachusetts) with individually adjustable weight loads. Exercise training compliance was defined as no less than 85% participation. All exercise sessions were conducted under the supervision of trained exercise physiologists (P.M. and S.N.). The patients were repeatedly instructed not to change their diet or physical activity during the study period except for the study intervention. A questionnaire describing the frequency and composition of the daily food intake before, midway and at the end of the study was used. The study drugs used were the GLP‐1 analogue, liraglutide (Victoza), and placebo, which were supplied by Novo Nordisk in blinded pens for subcutaneous (s.c.) injections. After baseline evaluation, the patients received once‐daily s.c. injections of 0.1 mL of study medication (0.6 mg liraglutide or placebo) in the evening for 1 week, 0.2 mL (1.2 mg liraglutide or placebo) the following week and thereafter 0.3 mL (1.8 mg liraglutide or placebo) for the remaining study period. Patients were examined in the fasting state at baseline and at the end of the study with blood samples, blood pressure, resting heart rate, dual‐energy X‐ray absorptiometry scan and physical fitness test. End of study examinations were performed after the last liraglutide or placebo dose was given, the evening before and 42 to 75 hours after the last bout of exercise.
Figure 1.
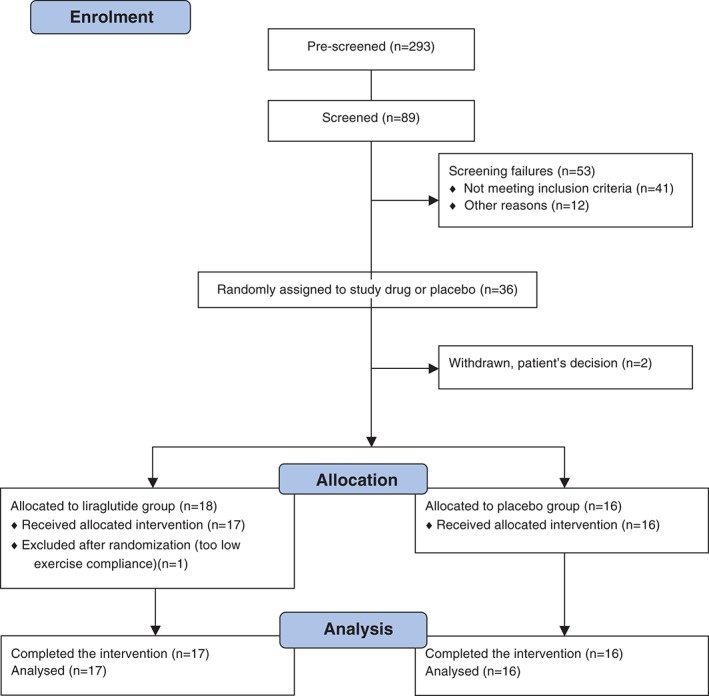
Consort flow diagram showing patient numbers at each stage of the study.
2.2. Experimental procedures
A dual‐energy X‐ray absorptiometry scan (DPX–IQ Lunar, General Electric Lunar Corporation, Madison, Wisconsin) was performed to assess body composition. Fat distribution was evaluated as the relative proportion of fat tissue in the android (abdominal) and the gynoid (hip and thigh) regions. The physical fitness level of the patients (VO2max) was estimated from a two‐step sub‐maximum bicycle test at baseline and at the end of the intervention. Each patient worked at two different workloads on a stationary bicycle ergometer (Monark ergomedic 839 E, Monark, Vansbro, Sweden). The workload was increased until a heart rate of 110 to 120 beats/min was achieved. At steady‐state heart rate, oxygen uptake was measured (MasterScreen CPX system; Intramedic, Gentofte, Denmark). The workload was then increased to achieve a heart rate of 140 to 160 beats/min and oxygen uptake at steady state was measured again. The duration of the test was 10 to 12 minutes. VO2max was estimated from the measurements of VO2 during the two‐point step test and an estimation of maximum heart rate.12 Resting heart rate and blood pressure were measured on the left arm after the patients had rested for 10 minutes. A health‐related questionnaire, the Short‐Form‐36 Health Survey, was used to evaluate changes in QoL.13
2.3. Analytical assessment
A colorimetric assay was used to measure HbA1c (Vitros 5.1 FS; Ortho‐Clinical Diagnostics, Linden, New Jersey). Concentrations of glucose were measured by the glucose oxidase method, using a glucose analyser (Yellow Springs Instrument model 2300 STAT plus analyser; YSI Inc., Yellow Springs, Ohio). Insulin and C‐peptide concentrations were measured using a two‐sided electrochemiluminescence immunoassay (Siemens Healthcare, Ballerup, Denmark).14, 15 Concentrations of total GLP‐1, glucose‐dependent insulinotropic polypeptide (GIP) and glucagon were measured by radioimmunoassays, as previously described.16, 17 Assessment of triglycerides and lipids was measured using reflectance spectrophotometry (Vitros 5.1, Johnson & Johnson, Ortho‐Clinical Diagnostics, New Brunswick, New Jersey).
2.4. Calculations and statistical methods
Steady‐state insulin resistance (HOMA‐IR) and β‐cell function (HOMA‐β) were estimated using the HOMA2 calculator.18 The statistical analyses were performed using STATA 12.1 (STATA Corp, LP, College Station, Texas). Data are reported as mean ± standard deviation; in case of non‐normal distribution, data were log‐transformed and back‐transformed. Estimates are reported as geometric means with 95% confidence interval (CI). Differences between mean values in the two groups were analysed using Student's t‐test. Analyses of between‐group differences were performed by analysis of covariance (ANCOVA) with the end‐of‐study value as the dependent variable and the baseline value as the independent variable. P values of <.05 were considered to indicate statistical significance. The statistical analyses were performed by P.M. and M.T.J.
2.5. Study registration
The study was approved by the Danish Medicines Agency (EudraCT number: 2011‐002739‐24) and the Scientific‐Ethical Committee of the Capital Region of Denmark (registration number: H‐4‐2011‐073), notified to the Danish Data Protection Agency (reference number 2007‐58‐0015) and registered at ClinicalTrials.gov (ID: NCTO1455441). The study conformed to the latest revision of the Declaration of Helsinki. The study was monitored by the Good Clinical Practice unit of University of Copenhagen and was conducted in compliance with the protocol and national guidelines including Clinical Trials Directive 2001/20/EC.
3. RESULTS
From December 2011 to March 2013, a total of 34 patients were enrolled in the study, of whom 33 completed the full study. One patient was excluded because of lack of exercise compliance. Patient characteristics were similar in the two groups (Table 1). Medical treatment was similar in the groups and remained unchanged during the study. Besides metformin, patients received no medication known to interfere with glucose metabolism.
Table 1.
Baseline characteristics of the patients
| Exercise plus liraglutide | Exercise plus placebo | P | |
|---|---|---|---|
| Baseline (n = 17) | Baseline (n = 16) | ||
| Age, years | 56.5 ± 9 | 55.6 ± 12 | .79 |
| Sex: female, n (%) | 4 (24) | 6 (38) | .38 |
| Duration of diabetes, years | 6.0 ± 5.2 | 3.7 ± 3.3 | .20 |
| Diet/metformin, n | 0/17 | 3/13 | |
| Daily dose metformin, mg | 1374 ± 607 | 1628 ± 936 | |
| FPG, mM | 9.9 ± 2.7 | 10.2 ± 3.1 | .91 |
| HbA1c, % | 8.2 ± 1.4 | 8.0 ± 1.2 | .58 |
| HbA1c, mmol/mol | 66.0 ± 16.0 | 64.0 ± 13.0 | |
| Body weight, kg | 101.0 ± 14.5 | 96.8 ± 17.4 | .45 |
| BMI, kg/m2 | 32.5 ± 3.7 | 32.4 ± 5.2 | .95 |
| VO2max estimated, L O2/min | 2.9 ± 0.9 | 2.5 ± 0.7 | .11 |
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; VO 2max, maximum oxygen uptake.
Data are presented as mean ± standard deviation.
3.1. Glycated haemoglobin
Patients receiving exercise plus liraglutide experienced pronounced reductions in HbA1c, whereas no significant change was observed in the exercise plus placebo group (Table 2, Figure 2A). In the exercise plus liraglutide group, 77% of patients obtained an HbA1c <7% (53 mmol/mol) and 53% normalized their HbA1c concentration (<6.0% [42 mmol/mol]). In the exercise plus placebo group, the corresponding values were 25% and 6%, respectively (Figure 2F).
Table 2.
Changes between baseline and end of study
| Exercise plus liraglutide | Exercise plus liraglutide | Difference | Exercise plus placebo | Exercise plus placebo | Difference | P value between groups | |
|---|---|---|---|---|---|---|---|
| Baseline (n = 17) | End of study (n = 17) | Baseline (n = 16) | End of study (n = 16) | ||||
| Glucose | |||||||
| HbA1c, % | 8.2 ± 1.4 | 6.2 ± 0.7*** | −2.0 ± 1.2 | 8.0 ± 1.2 | 7.7 ± 1.5 | −0.3 ± 0.9 | <.001 |
| HbA1c, mmol/mol | 66.0 ± 16.0 | 44.0 ± 8.0*** | −22.0 ± 14.0 | 64.0 ± 13.0 | 61.0 ± 17.0 | −3.0 ± 10.0 | .001 |
| FPG, mM | 10.4 ± 3.4 | 7.0 ± 1.6*** | −3.4 ± 2.3 | 10.0 ± 3.1 | 9.8 ± 3.7 | −0.3 ± 2.6 | .010 |
| Incretin hormones and glucagon (fasting levels) | |||||||
| Glucagon, pM | 9.6 ± 2.9 | 9.4 ± 3.2 | −0.3 ± 3.2 | 9.4 ± 3.7 | 8.2 ± 2.1 | −1.2 ± 3.2 | .23 |
| GLP‐1, pM | 18.7 ± 3.9 | 19.3 ± 5.6 | +0.6 ± 6.3 | 20.1 ± 8.2 | 18.1 ± 5.7 | −2.0 ± 5.4 | .30 |
| GIP, pM | 13.9 ± 4.0 | 16.3 ± 5.2 | +2.4 ± 7.1 | 13.1 ± 4.0 | 17.0 ± 8.5 | +4.0 ± 9.3 | .82 |
| Body composition | |||||||
| Body weight, kg | 101.0 ± 14.5 | 97.6 ± 14.9*** | −3.4 ± 2.9 | 96.8 ± 17.4 | 95.2 ± 17.7 | −1.6 ± 3.2 | .10 |
| Body mass index, kg/m2 | 32.5 ± 3.7 | 31.3 ± 3.4*** | −1.1 ± 1.1 | 32.4 ± 5.2 | 31.8 ± 5.1* | −0.5 ± 1.0 | .11 |
| Fat percent, % | 34.3 ± 6.3 | 31.8 ± 6.8*** | −2.5 ± 1.4 | 37.0 ± 6.5 | 34.8 ± 7.0*** | −2.2 ± 1.9 | .77 |
| Gynoid fat, % | 34.4 ± 9.1 | 32.5 ± 9.6*** | −2.0 ± 1.6 | 38.8 ± 9.2 | 36.3 ± 8.6*** | −2.4 ± 1.9 | .53 |
| Android fat, % | 44.6 ± 4.5 | 41.4 ± 5.2*** | −3.2 ± 2.3 | 45.9 ± 6.5 | 42.8 ± 7.8*** | −3.1 ± 3.0 | .97 |
| Lean body mass, kg | 63.3 ± 12.2 | 63.4 ± 12.8 | 0.1 ± 2.2 | 58.0 ± 12 | 58.7 ± 12.1 | 0.7 ± 1.5 | .42 |
| Blood pressure and heart rate | |||||||
| SBP, mm Hg | 136.2 ± 8.9 | 130.8 ± 8.8** | −5.4 ± 7.4 | 136.4 ± 11.0 | 135.8 ± 11.6 | −0.6 ± 11.1 | .11 |
| DBP, mm Hg | 82.1 ± 7.0 | 81.5 ± 7.2 | −0.5 ± 4.5 | 84.1 ± 7.0 | 81.8 ± 8.0 | −2.3 ± 5.5 | .41 |
| Resting heart rate ‐ bpm | 69.9 ± 8.9 | 71.3 ± 9.4 | +1.4 ± 7.6 | 70.1 ± 11.1 | 68.1 ± 12.7 | −2.0 ± 7.7 | 0.22 |
| VO2max, L/O2/min | 2.9 ± 0.9 | 3.4 ± 1.1*** | +0.5 ± 0.5 | 2.5 ± 0.7 | 2.9 ± 0.8** | +0.4 ± 0.4 | .92 |
| Lipid profile | |||||||
| Total cholesterol, mM | 4.7 ± 1.1 | 4.4 ± 1.3 | −0.3 ± 0.8 | 4.2 ± 0.9 | 4.3 ± 0.9 | +0.1 ± 0.9 | .44 |
| HDL cholesterol, mM | 1.2 ± 0.4 | 1.2 ± 0.3 | −0.02 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 0.4 | +0.1 ± 0.2 | .50 |
| LDL cholesterol, mM | 2.6 ± 1.0 | 2.4 ± 1.2 | −0.2 ± 0.8 | 2.2 ± 0.9 | 2.3 ± 0.8 | +0.1 ± 0.8 | .35 |
| VLDL cholesterol, mM | 0.8 ± 0.5 | 0.7 ± 0.4 | −0.1 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.3 | −0.07 ± 0.2 | .65 |
Abbreviations: DBP, diastolic blood pressure; FPG, fasting plasma glucose; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; SBP, systolic blood pressure; VO 2max, maximum oxygen uptake.
Data are mean ± standard deviation
P < .05,
P < .01 and
P < .001 compared with baseline value within the group.
Figure 2.
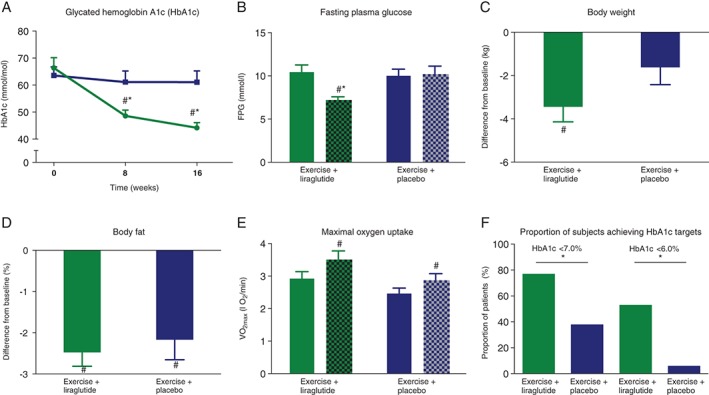
Glycated haemoglobin ( HbA1c), fasting plasma glucose (FPG), body composition and maximum oxygen uptake (VO2max). A, HbA1c in exercise plus liraglutide (green at baseline and green pattern at end of study), exercise plus placebo (blue at baseline and blue pattern at end of study). B, FPG in exercise plus liraglutide (green at baseline and green pattern), exercise plus placebo (blue at baseline and blue pattern at end of study). C, Differences in body weight in exercise plus liraglutide (green blue at baseline and green pattern at end of study), exercise plus placebo (blue at baseline and blue pattern at end of study). D, Differences in fat mass in exercise plus liraglutide (green at baseline and blue pattern at end of study), exercise plus placebo (blue at baseline and blue pattern at end of study). E, Estimated VO2max in exercise plus liraglutide (green at baseline and green pattern at end of study), exercise plus placebo (blue at baseline and blue pattern at end of study). F, Proportions of patients archiving HbA1c targets after the intervention. Exercise plus liraglutide (green) exercise plus placebo (blue). Data are mean ± standard error of the mean. Significant differences ( P < .05) between the groups are indicated by asterisks (*) and significant differences ( P < .05) within the groups are indicated by hatch signs (#).
3.2. Body weight and body composition
The mean (range) body weight was reduced by 3.4 (−8.5 kg to +2.3) kg in patients treated with exercise plus liraglutide, whereas it remained unchanged with exercise alone (−1.6 [−9.3 to +2.8] kg; Figure 2C). A significant reduction in body fat (%) was observed in both groups (Table 2, Figure 2D). Furthermore, gynoid and android fat (%) decreased significantly in both groups, with no difference between the groups. Lean body mass was unchanged from baseline in both groups (Table 2). Data from the simple questionnaire inquiring about the frequency and composition of the daily food intake before, midway and at the end of the study showed little or no change in food intake between the groups over 16 weeks.
3.3. Exercise compliance and physical fitness
Overall exercise compliance was 89% ± 4%, equal to ~2 hours and 41 min/wk during the 16‐week intervention period. During spinning sessions, the mean intensity was 77% ± 2% of estimated maximum heart rate, with no difference between the groups. VO2max increased significantly, by ~17% in both groups, after 16 weeks (Figure 2E).
3.4. FPG, insulin, C‐peptide and HOMA‐IR/β
A significant effect on FPG was seen with exercise plus liraglutide, whereas exercise plus placebo had no effect on FPG level (Figure 2B). No changes were observed in fasting levels of insulin and C‐peptide, respectively, in any of the groups. The estimated HOMA‐IR and estimated steady‐state β‐cell function, HOMA‐β, were both significantly improved with exercise plus liraglutide, whereas no changes were observed with exercise plus placebo (Table 3).
Table 3.
Changes in insulin resistance, β‐cell function and liver enzymes
| Exercise plus liraglutide | Exercise plus liraglutide | Difference | Exercise plus placebo | Exercise plus placebo | Difference | P value between groups | |
|---|---|---|---|---|---|---|---|
| Baseline (n = 17) | End of study (n = 17) | Baseline (n = 16) | End of study (n = 16) | ||||
| Insulin, C‐peptide and HOMA2 | |||||||
| Insulin, pM | 118 (98–141) | 111 (91–135) | −7 | 102 (71–147) | 93 (63–138) | −9 | .66 |
| C‐peptide, pM | 901 (781–1040) | 913 (814–1023) | +12 | 873 (700–1087) | 799 (634–1008) | −74 | .21 |
| HOMA2‐IR, insulin | 2.6 (2.2–3.1) | 2.2 (1.8–2.7)** | −0.4 | 2.2 (1.5–3.2) | 2.0 (1.4–3.1) | −0.2 | .63 |
| HOMA2‐IR, C‐peptide | 2.6 (2.3–3.0) | 2.2 (2.0–2.5)** | −0.4 | 2.4 (1.9–3.1) | 2.4 (1.9–3.1) | +0.02 | .023 |
| HOMA2‐β, insulin | 45.4 (32.7–63.1) | 86.5 (70.1–106.7)*** | +41.1 | 44.6 (30.1–66.3) | 45.7 (30.3–69.1) | +1.0 | <.0001 |
| HOMA2‐β, C‐peptide | 45.1 (33.4–60.9) | 86.7 (70.7–106.3)*** | +41.6 | 47.9 (35.5–64.6) | 51.6 (36.8–72.3) | +3.8 | <.0001 |
| Triglycerides and liver enzymes | |||||||
| Triglycerides, mM | 1.7 (1.2–2.6) | 1.5 (1.0–2.1) | −0.3 | 1.6 (1.2–2.3) | 1.4 (1.1–1.8) | −0.2 | 0.95 |
| ALT, U/L | 47.0 (35.5–62.3) | 32.9 (23.2–46.5)** | −14.1 | 38.0 (29.2–49.6)** | 28.4 (22.7–35.4) | −9.6 | 0.84 |
| AST, U/L | 35.7 (28.6–44.5) | 33.2 (27.5–40.1) | −2.5 | 31.2 (25.4–38.4) | 27.6 (23.7–32.1) | −3.6 | 0.21 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA, homeostatic model assessment; HOMA‐β, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance.
Data were log‐transformed due to non‐normal distribution; back‐transformed data are therefore presented as geometric mean (95% CI).
P < .01 and
P < .001 compared with baseline value within the group.
3.5. Lipids, liver enzyme, glucagon, GLP‐1 and GIP concentrations
Lipids were unchanged after the interventions in both groups (Tables 2 and 3). Alanine transaminase decreased in both groups, whereas aspartate transaminase remained unchanged. No changes in fasting glucagon, GLP‐1 or GIP were observed (Table 3).
3.6. Blood pressure and heart rate
A significant reduction in systolic blood pressure was found in the exercise plus liraglutide group. No changes were seen in diastolic blood pressure or heart rate (Table 2).
3.7. Health‐related QoL
The health‐related QoL summary score was significantly improved in the general health component and the physical function component in both groups without differences between the groups in any of the components (data not shown).
3.8. Adverse events
Gastrointestinal side effects (mild/moderate and transient nausea, vomiting and diarrhoea) were observed in 24% of the patients in the exercise plus liraglutide group vs 6% in the exercise plus placebo group. No episodes of hypoglycaemia were reported. No serious adverse events (including pancreatitis) were seen in the study.
4. DISCUSSION
We show, for the first time, that overweight dysregulated and sedentary patients with type 2 diabetes receiving supervised exercise training in combination with the GLP‐1RA liraglutide for 16 weeks achieved: (1) improved glycaemic control; (2) reduction in body weight; and (3) decreased systolic blood pressure compared with patients receiving supervised exercise training combined with placebo. In both groups, improvements in physical fitness, body fat and QoL were observed. Lean body mass was unchanged in both groups, despite weight loss. The overall safety profile of liraglutide was consistent with findings in previous reports.19, 20 Interestingly, no changes in resting heart rate were detected in any of the groups, which is in contrast to previous studies with continuously acting GLP‐1RAs, which reported a sustained increase in heart rate of approximately 2 to 4 beats/min.19, 20, 21 Thus, further studies are warranted to elucidate whether exercise training is able to neutralize the well‐known increase in heart rate seen with this class of glucose‐lowering drugs.
An important limitation of the present study is the relatively small sample size of patients, reducing the power to detect subtle changes and increasing the risk of type II statistical errors. Despite this, we were able to demonstrate clinically relevant differences between the two groups. Another limitation of the study is the absence of diet records, which could have shown possible changes in diet after GLP‐1RA treatment. Strengths of the study include the validated methods used; the study was performed as a single‐centre, randomized, double‐blind, placebo‐controlled study, with only few physicians and experienced exercise physiologists involved (all the exercise sessions were performed under the supervision of the same two exercise physiologists). Supervised training was chosen because previous studies have shown improved results with supervision22 and to support adherence to exercise training and exercise intensity. The period of 16 weeks was selected to allow sufficient time for uptitration of study drug (liraglutide and placebo) so that the maximum recommended dose could be maintained for a full 3‐month period necessary for optimum evaluation of the primary endpoint (HbA1c) and, furthermore to ensure slow adaptation to exercise training to avoid injuries. A GLP‐1RA as a glucose‐lowering drug was selected because of the well‐documented effect on glycaemic control and body weight in type 2 diabetes combined with a low risk of hypoglycaemia.11 Only a limited number of previous trials with liraglutide have observed reductions in HbA1c up to 1.5% (in the majority of trials reductions ranged from 0.9% to 1.1%).11 Pratley et al.23 found HbA1c reduction of 1.5% from baseline HbA1c levels of 8.4% after 26 weeks of treatment. The Liraglutide Effects and Action in Diabetes (LEAD) 4 and 5 studies reported HbA1c decreases of 1.5% and 1.3%, respectively, after 26 weeks of treatment with liraglutide, both in combination with other glucose‐lowering agents (metformin plus rosiglitazone or glimepiride).24, 25 Several possible explanations for the near‐normalization of HbA1c after treatment with exercise plus liraglutide in the present study should be considered. At least part of the improved glycaemic control in patients treated with liraglutide relies on the well‐established glucose‐lowering effects of GLP‐1: the glucose‐dependent stimulation of insulin secretion and the inhibition of glucagon secretion10 and in the brain, respectively.26 Exercise training is known to increase insulin sensitivity of skeletal muscle in both healthy individuals and in patients with type 2 diabetes27, 28 and improves β‐cell function in patients with type 2 diabetes with well‐preserved residual β‐cell function.10 In individuals with impaired glucose tolerance and in patients with type 2 diabetes, β‐cell function has been shown to be a better predictor of improvement in glycaemic control than insulin sensitivity.29 Therefore, the beneficial effect of liraglutide on glycaemic control in the present exercise training study seems at least in part to be attributable to the insulinotropic effect of liraglutide, in line with the marked improvement in HOMA‐β (Table 3). Nevertheless, the substantial improvement in glycaemic control in the exercise plus liraglutide group, which markedly surpasses what previously has been observed with liraglutide in sedentary patients with type 2 diabetes, is probably attributable to the combination of improved β‐cell function, as well as increased peripheral insulin sensitivity. In the exercise plus placebo group we assume that the lack of improvement in glycaemic control despite an expected increase in muscle insulin sensitivity27, 29 may be attributable to inability of the residual β‐cell reserve to benefit from increased insulin sensitivity.
Type 2 diabetes develops as a consequence of insulin resistance and impaired β‐cell function, but it is a very heterogeneous disease with a multifactorial background (eg, genes, lifestyle, obesity, dyslipidaemia and inflammation).30 This might partially explain why patients with type 2 diabetes, including the patients in the present study, have heterogeneous responses to glucose‐lowering therapies, such as GLP‐1RAs. Exercise benefits most, but not all, individuals31 and it cannot be ruled out that some of the patients in the present study might have been non‐responders and therefore failed to demonstrate exercise‐induced metabolic improvements. Inflammation in the adipose tissue and dysregulation of adipokines is closely associated with obesity and type 2 diabetes. Also, inflammation has been shown to induce peripheral insulin resistance32 as well as β‐cell dysfunction.33, 34 Several studies have indicated that both GLP‐1 receptor agonists35 and exercise36 have anti‐inflammatory effects. The pronounced effect on glycaemic control during the exercise plus liraglutide group, may to some extent, be explained by the well‐known anti‐inflammatory effects of both treatment methods. In larger and longer‐lasting intervention studies, the effects on HbA1c might be less pronounced, as previously found in large‐scale studies; for example, the LEAD 4 and 5 studies.24, 25, 37
In the present study QoL was not further improved with liraglutide plus exercise despite improved glycaemic control compared with exercise alone. However, QoL was not inferior despite some side effects resulting from liraglutide treatment. These findings could indicate that exercise training is an essential factor for improvements in health‐related QoL. Because adequate lifestyle changes are often difficult to implement and maintain in patients with type 2 diabetes, exercise in combination with liraglutide treatment may be a way to amplify motivation for change when the patients experience noticeable results from the effects of both exercise and liraglutide.
In conclusion, the combination of supervised exercise training and liraglutide induced major improvements in glycaemic control and may be an attractive treatment option for obese, dysregulated patients with type 2 diabetes on diet and/or metformin who are able to perform moderate‐ to high‐intensity exercise training.
ACKNOWLEDGMENTS
The authors thank all participants for spending time on this project, and they are grateful for expert technical assistance from Jytte Purtoft, Nina Kjeldsen, Centre for Diabetes Research, Gentofte Hospital, Hellerup, Denmark and from Lene Albæk, Department of Biomedical Sciences, University of Copenhagen. Finally, the authors are grateful for the cooperation with monitor Stine Hovgaard, The Good Clinical Practice Unit of Copenhagen University, Denmark.
Conflict of interest
P. M, S. N, P. G. J, H. S, M. T. J. and J. S have no conflicts of interest. J. J. H has served on scientific advisory panels for Glaxo, Smith, Kline, Novo Nordisk, Zealand Pharmaceuticals, AstraZeneca, Sanofi, MSD, Intarcia, Hamni, has served as a consultant to Novo Nordisk, and has received research support from Novartis and Merck. B. K. has no conflicts of interest. E.A.R is a shareholder in Novo Nordisk. F. K. K. has served on scientific advisory panels for AstraZeneca, Eli Lilly, Novo Nordisk, Sanofi, Zealand Pharma, has served as a consultant to AstraZeneca, Boehringer Ingelheim, Fractyl, Novo Nordisk, Merck Sharp & Dohme, Zealand Pharma, has received research support from Gubra, Novo Nordisk, Sanofi, Zealand Pharma, and served on the Speakers’ Bureau for AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol‐Myers Squibb, Eli Lilly, Gilead Sciences, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Zealand Pharma. T. V. has served on scientific advisory panels for Amgen, AstraZeneca, Boehringer, Bristol‐Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda, has served as a consultant to AstraZeneca, Boehringer, Bristol‐Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda, has received research support from Novo Nordisk and served on the Speakers Bureau for AstraZeneca, Boehringer, Bristol‐Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Novartis, Sanofi and Takeda.
Author contributions
P. M. contributed to the study design and recruitment of participants, including instruction in study medication, performed screening visits, testing, exercise training and statistical analyses, researched the data and wrote the manuscript. S. N. contributed to the study design and recruitment of participants, performed screening, tests and exercise training and reviewed the manuscript. P. G. J. reviewed/edited the manuscript. H. S. contributed to recruitment of participants and reviewed/edited the manuscript. M. T. J performed the statistical analyses and reviewed/edited the manuscript. J. S. performed the screening visits, and reviewed and edited the manuscript. J. J. H. analysed plasma for incretin hormones and glucagon and reviewed/edited the manuscript. B. K. reviewed/edited the manuscript. E. A. R. contributed to the study design and reviewed/edited the manuscript. F. K. K. contributed to the study design and reviewed/edited the manuscript. T. V. contributed to the study design, contributed to the interpretation of the results, data management and reviewed/edited the manuscript. P. M. and T. V. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentation: Parts of the study were presented at the 74th Scientific Meeting of the American Diabetes Association, San Francisco, California, June 13‐17, 2014 and at the 50th European Association for the Study of Diabetes Annual Meeting, Vienna, Austria, September 15‐19, 2014.
Mensberg P, Nyby S, Jørgensen PG, Storgaard H, Jensen MT, Sivertsen J, Holst JJ, Kiens B, Richter EA, Knop FK and Vilsbøll T. Near‐normalization of glycaemic control with glucagon‐like peptide‐1 receptor agonist treatment combined with exercise in patients with type 2 diabetes, Diabetes Obes Metab 2017;19(2):172–180.
Funding information The study was an investigator‐initiated study sponsored by Novo Nordisk, who also sponsored the liraglutide and the placebo pens. Novo Nordisk had no role in the study design, data analysis or reporting of results.
REFERENCES
- 1. American Diabetes Association . 4. Prevention or delay of type 2 diabetes. Diabetes Care. 2016;39:36‐38. [Google Scholar]
- 2. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes The American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692‐2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Jung, Zhang W, Guo Q, et al. Duration of exercise as a key determinant of improvement in insulin sensitivity in type 2 diabetes patients. Tohoku J Exp Med. 2012;227:289‐296. [DOI] [PubMed] [Google Scholar]
- 5. George CM, Brujin LL, Will K, Howard‐Thompson A. Management of blood glucose with noninsulin therapies in type 2 diabetes. Am Fam Physician. 2015;92:27‐34. [PubMed] [Google Scholar]
- 6. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JFP, Dela F. Strength training increases insulin‐mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294‐305. [DOI] [PubMed] [Google Scholar]
- 7. Dela F, Ploug T, Handberg A, et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862‐865. [DOI] [PubMed] [Google Scholar]
- 8. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2011;305:1790‐1799. [DOI] [PubMed] [Google Scholar]
- 9. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA. 2001;286:1218‐1227. [DOI] [PubMed] [Google Scholar]
- 10. Holst JJ, Deacon CF, Vilsbøll T, Krarup T, Madsbad S. Glucagon‐like peptide‐1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161‐168. [DOI] [PubMed] [Google Scholar]
- 11. Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon‐like peptide‐1 receptor agonists on weight loss: systematic review and meta‐analyses of randomised controlled trials. BMJ. 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Åstrand P‐O, Ryhming I. A nomogram for calculation of aerobic capacity (Physical Fitness) from pulse rate during submaximal work. J Appl Physiol. 1954;7:218‐221. [DOI] [PubMed] [Google Scholar]
- 13. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Med Care. 1992;30:473‐483. [PubMed] [Google Scholar]
- 14. Bablok W, Passing H, Bender R, Schneider B. A general regression procedure for method transformation. Application of linear regression procedures for method comparison studies in clinical chemistry, Part III. J Clin Chem Clin Biochem Z Für Klin Chem Klin Biochem. 1988;26:783‐790. [DOI] [PubMed] [Google Scholar]
- 15. Hare KJ, Knop FK, Asmar M, et al. Preserved inhibitory potency of GLP‐1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:4679‐4687. [DOI] [PubMed] [Google Scholar]
- 16. Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine‐extended glucagon‐like peptide I in humans. Diabetes. 1994;43:535‐539. [DOI] [PubMed] [Google Scholar]
- 17. Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept. 1984;9:35‐46. [DOI] [PubMed] [Google Scholar]
- 18. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191‐2192. [DOI] [PubMed] [Google Scholar]
- 19. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta‐analysis. BMJ Open. 2013;3(1):pii: e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 21. Pyke C, Heller RS, Kirk RK, et al. GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280‐1290. [DOI] [PubMed] [Google Scholar]
- 22. Umpierre D, Ribeiro PAB, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta‐regression analysis. Diabetologia. 2012;56:242‐251. [DOI] [PubMed] [Google Scholar]
- 23. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet. 2010;375:1447‐1456. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met + TZD). Diabetes Care. 2009;32:1224‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holst JJ. Incretin hormones and the satiation signal. Int J Obes. 2005. 2013;37:1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin‐stimulated muscle glucose clearance in patients with NIDDM: effects of one‐legged physical training. Diabetes. 1995;44:1010‐1020. [DOI] [PubMed] [Google Scholar]
- 28. Wojtaszewski JFP, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 2006;42:31‐46. [DOI] [PubMed] [Google Scholar]
- 29. Solomon TPJ, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic β‐cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab. 2013;98:4176‐4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pessin JE, Kwon H. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dula SB, Jecmenica M, Wu R, et al. Evidence that low‐grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium. 2010;48:133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicol LE, Grant WF, Grant WR, et al. Pancreatic inflammation and increased islet macrophages in insulin‐resistant juvenile primates. J Endocrinol. 2013;217:207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee Y‐S, Jun H‐S, Lee Y‐S, Jun H‐S. Anti‐inflammatory effects of GLP‐1‐based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thyfault JP, Wright DC. “Weighing” the effects of exercise and intrinsic aerobic capacity: are there beneficial effects independent of changes in weight? Appl Physiol Nutr Metab. 2016;41:911‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]


