Abstract
Background
In patients with motor fluctuations complicating Parkinson's disease (PD), delays in time‐to‐ON with levodopa are common. This open‐label study aimed to assess the effect of apomorphine on time‐to‐ON in PD patients with morning akinesia.
Methods
The safety population included 127 enrolled patients, and the full analysis set (FAS) included 88 patients. Patients completed a 7‐day levodopa baseline period recording their time‐to‐ON following each morning dose of levodopa. Patients were titrated to an optimal dose of apomorphine (2–6 mg) while taking trimethobenzamide antiemetic therapy. Apomorphine was injected each morning for a 7‐day treatment period and time‐to‐ON was self‐recorded in 5‐minute blocks. The primary efficacy variable was time‐to‐ON in the apomorphine treatment period versus the baseline levodopa period. Secondary assessments included and global impression scales. Safety and tolerability were assessed through adverse events (AEs).
Results
Patients receiving apomorphine achieved mean ± standard deviation (SD) time‐to‐ON 23.72 ± 14.55 minutes, reduced from 60.86 ± 18.11 minutes with levodopa (P < 0.0001). Dose failures (defined as time‐to‐ON >60 minutes) were more commonly reported with levodopa versus apomorphine (46% vs. 7% of diary entries, respectively).
Secondary endpoints supported the primary efficacy findings, with significant improvements from levodopa baseline to apomorphine treatment period (all P < 0.0001). The most common AEs were nausea and dizziness. Most patients who discontinued because of AEs did so in the titration phase.
Conclusions
Apomorphine injections significantly reduced time‐to‐ON in PD patients experiencing delayed onset of their morning levodopa dose, and was well tolerated in most patients. After apomorphine treatment, fluctuating patients with morning akinesia experienced rapid and reliable improvement of time‐to‐ON.
Keywords: apomorphine, Parkinson's disease, morning akinesia, l‐dopa
The development of motor fluctuations is a key limitation to the long‐term management of Parkinson's disease (PD) with levodopa (l‐dopa). After motor complications develop, some patients can experience multiple OFF episodes per day, and with disease progression, the cumulative daily OFF time can account for ≤50% of a patient's waking day.1, 2, 3 The latency from l‐dopa intake to the patient turning ON can often be a major contributor to total daily OFF time.4, 5, 6 Delays in turning ON reflect a delay in the absorption of l‐dopa and a subsequent delay in crossing the blood–brain barrier. This may be a result of delayed gastric emptying (gastroparesis), presence of intestinal protein that competes with l‐dopa absorption, bacterial overgrowth, and/or pharmacodynamic effects.6, 7, 8 Prolonged morning akinesia, during which time patients remain in an OFF state in advance of a therapeutic response from their first morning l‐dopa dose, is a common clinical manifestation in patients with delayed‐ON. Because intestinal protein is not usually present on awakening, it is likely that prolonged morning akinesia reflects delayed gastric emptying or ill‐defined pharmacodynamics factors.
Conventional oral adjunctive therapy to l‐dopa, including oral dopamine agonists, monoamine oxidase (MAO) inhibitors, and catechol‐O‐methyl transferase (COMT) inhibitors, is often used to manage the symptoms of end‐of‐dose wearing‐off, However, although these treatments reduce total daily OFF time, most patients continue to experience residual OFF time, which may reflect delayed time‐to‐ON, dose failures, and suboptimal ON response. None of these oral strategies effectively shorten the time to onset of l‐dopa.4, 9 The potent dopamine agonist, apomorphine, can be administered as an acute subcutaneous injection and thus its onset is not affected by gastroparesis nor by impaired intestinal absorption. Several randomized, controlled studies have demonstrated that in patients with motor fluctuations, subcutaneous injection of apomorphine provides a rapid and reliable l‐dopa‐like ON effect within 8 to 15 minutes in most patients.10, 11, 12
The aim of this study was to assess the effect of a subcutaneous injection of apomorphine in PD patients with prolonged morning akinesia relating to delayed or unreliable onset of benefit after their first morning dose of l‐dopa.
Methods
This was a Phase IV, multicenter, open‐label study of subcutaneous apomorphine injections in PD patients with morning akinesia resulting from delayed or unreliable onset of oral l‐dopa. The study was sponsored by US WorldMeds LLC, and was conducted from December 2012 through April 2014 at 12 U.S. study sites. It was conducted in accordance with Good Clinical Practice guidelines; U.S. Code of Federal Regulations (CFRs) dealing with Protection of Human Subjects (U.S. 21 CFR Part 50); the Nuremberg Code; and the Declaration of Helsinki. The study was approved by each of the participating site's Institutional Review Boards, and all patients provided written informed consent to participate. The study is registered with ClinicalTrials.gov (NCT01770145).
Study Population
This study recruited adult PD patients (>18 years old) currently treated with l‐dopa and with morning akinesia. Eligible patients had to have a modified Hoehn and Yahr stage of I to III during the ON state and receive optimized l‐dopa therapy at a steady maintenance dose for at least 4 weeks. Morning akinesia was defined as a minimum subject‐reported time‐to‐ON of 45 minutes or more following their usual first daily l‐dopa dose for a minimum of 3 days during a 1‐week baseline diary period. Eligible patients had to be able to adequately differentiate between and describe variations in ON and OFF states. Key exclusion criteria included: use of 5‐HT3 antagonists, use of medications for gastroparesis (erythromycin, cisapride, metoclopramide), a history of poor compliance and/or follow‐up, and any serious medical or psychiatric conditions that, in the investigators’ judgment, would make study participation unsafe or make treatment compliance difficult.
Study Design
A screening window of ≤5 days was permitted to allow investigators adequate time to determine eligibility criteria (Visit 1). At this visit, Unified Parkinson's Disease Rating Scale (UPDRS) motor scores were assessed while patients were in their reported “best ON” state. After they entered the study, patients completed a 7‐day baseline period during which, after taking their usual first l‐dopa dose, they recorded in a supplied diary their motor state (i.e., OFF or ON) every 5 minutes until ON was achieved or until 60 minutes had passed (practically defined as dose failure). This record of daily time‐to‐ON following their regularly scheduled morning dose of l‐dopa was then reviewed to determine whether the inclusion criteria for morning akinesia were met. If so, at the end of the baseline period all patients started trimethobenzamide antiemetic therapy (3 days) and returned to the clinic for apomorphine titration to identify optimal apomorphine dose (Visit 2; throughout a maximum period of 11 days). During titration, dose increases were permitted at the subject's next observed OFF period, but no sooner than 2 hours after the previous apomorphine dose. A maximum of 3 clinic visits (during the 11 days) were permitted for dose optimization. Optimal doses were defined in the protocol as the apomorphine dose achieving ≥90% of the subject's “best ON” UPDRS motor score (as recorded during the screening visit) within 15 minutes after subcutaneous injection of apomorphine and without intolerable side effects. In the United States, apomorphine is only available at a concentration of 10 mg/mL and is not dilutable. Therefore a 2‐mg dose is always administered in 0.2 mL, 3 mg as 0.3 mL, and so forth.
Before initial injection of apomorphine, UPDRS motor examination was recorded in the OFF state. During the titration period, the dose of apomorphine was gradually escalated in increments ≤2 mg from a starting dose of 2 mg (0.2 mL) until each individual subject achieved an optimal dose, but not exceeding 6 mg (0.6 mL). After the optimal dose was identified, patients entered the 7‐day treatment period during which their morning l‐dopa dose was replaced by a subcutaneous injection of apomorphine at the same usual time, followed by diary recording of motor ON/OFF state at 5‐minute intervals. All other PD medications (agents, doses, and timings) remained unchanged during the study. At the end of the study (within 2 days after the apomorphine treatment period), patients returned to the clinic for final assessments (Visit 3).
Assessments
Using a diary, patients self‐recorded their time‐to‐ON by marking either “yes” or “no” every 5 minutes until onset of ON for the first 60 minutes after each morning dose of l‐dopa during the 7‐day l‐dopa baseline phase and following apomorphine injection in the 7‐day treatment phase. ON was defined as when the subject first felt that their medication was working; all patients received diary training and their interpretation of motor state ON and OFF correlated with the investigator during the screening period.
Investigators and subjects rated Clinical Global Impressions of Severity (CGI‐S) and Patient Global Impressions of Severity (PGI‐S), respectively, and the EuroQol (EQ‐5D) (with respect to morning akinesia during the previous 7 days) was assessed before the first apomorphine dose at Visit 2 and at Visit 3. Modified Hoehn and Yahr stage and UPDRS version 3.1 motor scores were assessed during Visit 1 in the best‐ON state, at Visit 2 in the OFF state before the first apomorphine injection, and at 15 minutes after each dose of apomorphine (to identify optimal‐dose ON state). Safety and tolerability were assessed through adverse event (AE) reporting, vital signs measurements, neurological exams, and physical exams.
Statistical Analyses
Efficacy analyses were performed on the full analysis set (FAS) defined as all eligible patients who completed ≥5 of 7 days of diary entries in the apomorphine treatment period. Baseline and safety outcomes were assessed using the safety population, which included all patients who took at least 1 dose of study drug.
The primary efficacy endpoint was a change from l‐dopa baseline in average daily time‐to‐ON by subject diary. For the analysis purposes, a “dose failure” was practically defined as a failure to turn ON within 60 minutes on the home diary and these dose failures were imputed to 100 minutes. Key secondary efficacy outcomes were changed from baseline in CGI‐S, PGI‐S, EQ‐5D‐3L index score, and EQ‐5D‐3L visual analogue scale (VAS) score. Changes in modified Hoehn and Yahr stage and UPDRS motor scores from the preapomorphine OFF state to the postapomorphine ON state (for the optimal apomorphine dose only) were also analyzed as secondary variables. All treatment effects were analyzed using the Student t test.
Results
Subject Disposition
The safety population included 127 enrolled patients, and 97 patients completed the study (Fig. 1). The FAS included 88 (69%) patients; 3 patients did not complete the required 5 of 7 diary days per baseline l‐dopa or apomorphine treatment phase, and 6 patients were later discounted from the analyses because they did not meet the original eligibility criteria of having a time‐to‐ON of >45 minutes for 3 of 7 l‐dopa days.
Figure 1.
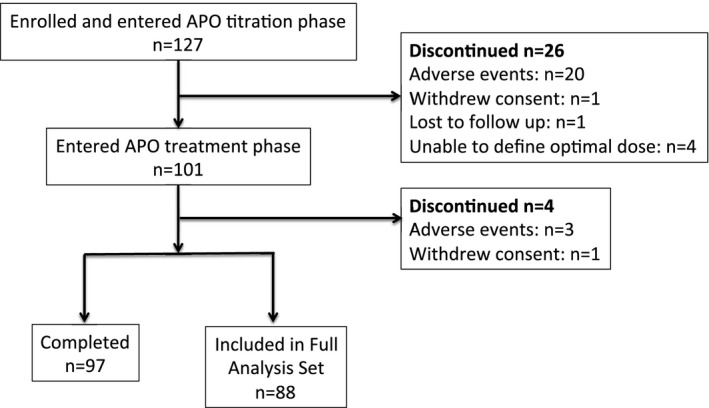
Subject disposition. The FAS included all eligible patients who completed at least 5 of 7 days of diary entries in the apomorphine treatment period.
Baseline Characteristics and Optimal Apomorphine Dose Levels
Subject demographics and baseline PD characteristics of the safety population are presented in Table 1. The population comprised predominantly white, non‐Hispanic individuals with mean ± standard deviation (SD) age 65.20 ± 9.72 years. Almost half (45.6%) of patients had been diagnosed with PD for ≤10 years, the mean ± SD daily l‐dopa dose was 965 ± 990 mg, and most patients received oral adjunctive medication (dopamine agonists, COMT inhibitors, and MAO‐B inhibitors) (Table 1). The optimal apomorphine dose level was identified as 2 mg in 25 patients (28.4% of FAS), 3 mg in 12 patients (13.6%), 4 mg in 35 patients (39.8%), 5 mg in 12 patients (13.6%), and 6 mg in 4 patients (4.5%). The mean dose of apomorphine in the FAS was 3.5 mg.
Table 1.
Baseline Characteristics
| Baseline Characteristic | FAS (N = 88) | Safety (N = 127) |
|---|---|---|
| Male; n (%) | 56 (63.6%) | 84 (66.1%) |
| Age (yr); mean ± SD | 65.63 ± 10.14 | 65.20 ± 9.72 |
| Duration of PD (yr); mean ± SD | 11.63 ± 5.95 | 10.38 ± 5.74 |
| Duration of morning akinesia (yr); mean ± SD | 4.53 ± 3.27 | 4.23 ± 2.71 |
| UPDRS Motor Score (ON); mean ± SD | 20.36 ± 9.71 | 20.05 ± 0.97 |
| Duration of l‐dopa treatment (mo); mean ± SD | 57.49 ± 85.49 | 52.09 ± 85.66 |
| Daily l‐dopa dose (mg); mean ± SD | 841 ± 512 | 965 ± 990 |
| Use of adjunct medications; n (%) | ||
| Dopamine agonists | 67 (76.1%) | 83 (65.4%) |
| MAO‐B inhibitors | 45 (51.1%) | 59 (46.5%) |
| COMT inhibitors | 42 (47.7%) | 51 (40.2%) |
| Amantadine | 22 (25.0%) | 28 (22.0%) |
SD, standard deviation; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale; MAO‐B, monoamine oxidase; COMT, catechol‐O‐methyl transferase.
Efficacy Assessments
Patients with morning akinesia related to delayed onset of oral l‐dopa had a rapid turning‐ON after apomorphine injection. The mean ± SD time‐to‐ON reduced from 60.86 ± 18.11 minutes at baseline with l‐dopa therapy to 23.72 ± 14.55 minutes at the end of the treatment period (reduction of 37.14 ± 20.51 minutes; P < 0.0001 vs. baseline) (Fig. 2).
Figure 2.
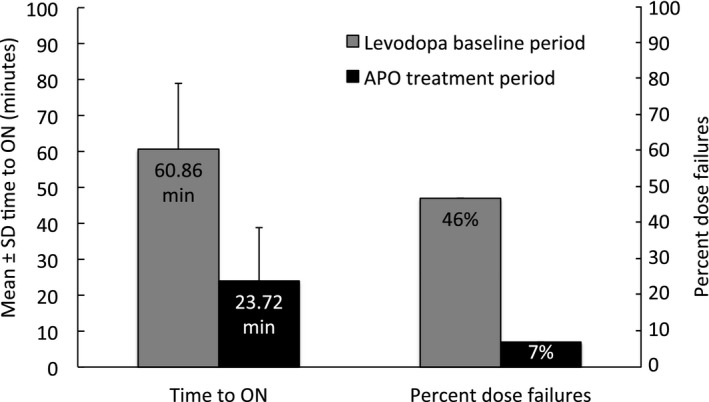
Time to ON and percent dose failures during the l‐dopa baseline period and apomorphine treatment period (FAS; n = 88). Patients recorded their time to ON after their l‐dopa or apomorphine dose in a diary every 5 minutes by marking either “yes” or “no” until onset of ON ≤60 minutes. A value of 100 was imputed for patients that did not report turning ON within 60 minutes.
Time‐to‐ON was highly reliable in the apomorphine injection phase. Almost all subjects (84 of 88, 95.5%) had improvement in time‐to‐ON. Although dose failures (pragmatically defined as time‐to‐ON >60 minutes) were reported for 144 of 310 (46%) of completed diary entries during the l‐dopa baseline week, they were much less frequent with apomorphine injections (20 of 307 [7%] of diary entries during the apomorphine treatment week).
Secondary endpoints evaluating quality of life scores and global impression scales (CGI‐S, PGI‐S, EQ‐5D‐3L index scores, and EQ‐5D VAS scores) supported the primary efficacy findings, with consistent and statistically significant changes from l‐dopa baseline to apomorphine treatment period (Table 2). Objective assessments of motor function confirmed that subcutaneous apomorphine injections significantly improved motor function as assessed by improvements in Hoehn and Yahr staging and UPDRS motor scores.
Table 2.
Secondary Efficacy Variables (FAS)
| Efficacy variable | FAS (n = 88) |
|---|---|
| CGI‐S | |
| l‐dopa baseline score | 4.26 ± 0.92 |
| APO treatment score | 3.73 ± 0.93 |
| Treatment effect | 0.53 ± 0.92 |
| P value | P < 0.0001 |
| PGI‐S | |
| l‐dopa baseline score | 4.34 ± 0.99 |
| APO treatment score | 3.37 ± 1.30 |
| Treatment effect | 0.98 ± 1.53 |
| P value | P < 0.0001 |
| EQ‐5D visual analogue scale (VAS) | |
| l‐dopa baseline score | 50.38 ± 21.28 |
| APO treatment score | 65.67 ± 20.86 |
| Treatment effect | 15.28 ± 22.11 |
| P value | P < 0.0001 |
| EQ‐5D‐3L index score | |
| l‐dopa baseline score | 3.430 ± 1.51 |
| APO treatment score | 2.18 ± 1.60 |
| Treatment effect | 1.11 ± 1.72 |
| P value | P < 0.0001 |
| Hoehn and Yahr stage | |
| Pre‐APO score (during OFF) | 2.79 ± 0.66 |
| 15 minutes post‐APO (during ON) | 2.31 ± 0.54 |
| Treatment effect | 0.48 ± 0.58 |
| P value | P < 0.0001 |
| UPDRS motor score | |
| Pre‐APO score (during OFF) | 35.53 ± 9.79 |
| 15 minutes post‐APO (during ON) | 17.32 ± 8.81 |
| Treatment effect | 18.22 ± 8.80 |
| P value | P < 0.0001 |
APO, apomorphine; FAS, full analysis set; CGI‐S, Clinical Global Impressions of Severity; PGI‐S, Patient Global Impressions of Severity; UPDRS, Unified Parkinson's Disease Rating Scale.
Safety and Tolerability
No new safety issues with apomorphine were observed during the study. AEs occurring at a level of ≥5% in the safety population were nausea (26.8%), dizziness (16.5%), yawning (10.2%), somnolence (7.9%), hypotension (7.9%), and vomiting (7.1%). Nausea, vomiting, and hypotension were also the most common AEs leading to discontinuation (Table 3), and most (20 of 23) patients who discontinued because of an AE did so in the titration phase. Most AEs were of mild or moderate intensity. Eleven AEs of severe intensity were reported in 6 subjects: vomiting (n = 2), hypotension (n = 2), dizziness, cold sweat, ear pain, jaw pain, reduced mobility, loss of consciousness, and syncope (all n = 1). Of these, just one subject accounted for the reports of “loss of consciousness” and “syncope”; this same subject also had vomiting, dizziness, and hypotension listed as severe (but not serious) AEs. These AEs were reported to emerge 15 to 20 minutes following injection of the starting dose of 2 mg apomorphine in the titration phase, and this subject did not continue in the study.
Table 3.
AEs Leading to Discontinuation (Safety Population)
| Preferred Terma | Frequency; n (%) (N = 127) |
|---|---|
| ALL | 23 (18.1) |
| Hypotension | 8 (6.3) |
| Nausea | 7 (5.5) |
| Vomiting/retching | 7 (5.5) |
| Dizziness | 7 (5.5) |
| Yawning | 3 (2.4) |
| Orthostatic hypotension | 3 (2.4) |
| Syncopeb | 2 (1.6%) |
| Hyperhidrosis | 2 (1.6%) |
| Hot flush | 1 (0.8%) |
| Confusional state | 1 (0.8%) |
| Somnolence | 1 (0.8%) |
| Loss of consciousnessb | 1 (0.8%) |
| Dyskinesia | 1 (0.8%) |
| Fall | 1 (0.8%) |
| Fatigue | 1 (0.8%) |
| Vision blurred | 1 (0.8%) |
More than one reason for discontinuation could be given for patients who could not tolerate the 2‐mg dose during titration.
One subject who had received a single 2‐mg dose of apomorphine reported both “loss of consciousness” and “syncope”; the same subject also experienced vomiting, dizziness, and hypotension listed as severe AEs leading to discontinuation.
AEs, adverse events.
Discussion
The results of this open‐label study demonstrate that apomorphine injection significantly reduced time‐to‐ON in advanced PD patients experiencing morning akinesia resulting from a delayed onset of effect or no effect with their morning l‐dopa dose, and the injection was well tolerated in most patients. These patients experienced a prolonged time‐to‐ON with their morning l‐dopa dose—averaging an hour. The reduction in time‐to‐ON (mean reduction of 37.14 minutes) was clinically relevant, as evidenced by the significant improvements in patient‐driven scales of quality of life and global impression.
Early morning akinesia is often cited as one of the first l‐dopa complications that the patient recognizes when awakening.13 An observational study conducted in Europe showed that, when consecutive PD patients are specifically questioned about their morning function, ≤60% report having OFF periods in the early morning.14 However, despite this high prevalence, the results of the present study suggest that it often remains poorly recognized and undertreated despite the frequent use of oral adjunctive medications. Patients had experienced morning akinesia for an average of 4 years, even though movement disorder specialists were treating all patients and the majority was receiving adjunct therapy with dopamine agonists, COMT, and/or MAO‐B inhibitors.
After taking their first morning l‐dopa dose during the 7‐day baseline period, >40% of patients had at least one “dose failure” pragmatically defined as failure to turn ON within 60 minutes of dosing. This probably reflects delayed gastric emptying of l‐dopa into the proximal intestine where it is absorbed (protein was unlikely to be present in the intestine on awakening). It has also been postulated that with slower gastric emptying, more l‐dopa may be metabolized by amino acid decarboxylase in the gastric mucosa7 into dopamine.
The prevalence of dose failures was unexpectedly high, and further study of the frequency of l‐dopa dose failures in fluctuating patients is warranted. Dose failures were much less common with apomorphine treatment, and the mean time‐to‐ON with apomorphine subcutaneous injection was around 24 minutes, suggesting gastrointestinal dysfunction may underlie dose failures. Almost all subjects (95.5%) showed improvement in time‐to‐ON, which mirrors the 95% response rate in previous studies.10 The motor effect of apomorphine was also shown as reliable, with significant improvements in Hoehn and Yahr and UPDRS motor scores paralleling the effect of l‐dopa. Although not directly assessed, improvement in morning akinesia seems likely to be clinically relevant to patients, and to allow them, for example, to get more safely out of bed, to perform their usual morning routine, and to get on with their day. Indeed, it is of interest that patients consistently rated both their baseline disease severity as worse and their degree of improvement with treatment as greater as compared to investigator‐rated GI‐S. This suggests that patients consider morning akinesia a more significant problem than might be currently recognized in clinical practice.
No novel AEs were reported during the apomorphine titration phase or in the rest of the study. Of note, 7 of the 20 patients who discontinued because of an AE in the titration phase were not able to tolerate the 2‐mg dose. Moreover, half of the patients (n = 10) who discontinued because of an AE came from just one site, indicating a relevant site effect in terms of AEs. Site effects were not apparent in the efficacy reporting; results for the safety population were similar to those seen with the FAS (all outcomes were significant at the 5% level). As might be expected, the most common reasons for patients discontinuing were nausea, vomiting, and hypotension. A study of trimethobenzamide to control nausea and vomiting during initiation and continued treatment with subcutaneous apomorphine injection showed that 16% of patients pretreated with trimethobenzamide experienced nausea and/or vomiting when titrating apomorphine.15
Limitations of this study include its open‐label design and the pragmatic definition of “dose failure” in which all patients who did not turn ON within 60 minutes had their time‐to‐ON imputed to 100 minutes. This was an arbitrary threshold and it is possible that this imputation strategy might have led to overestimation of the mean time‐to‐ON in l‐dopa (if patients turned ON within 60–100 minutes) or, conversely, an underestimation (if patients turned ON after >100 minutes). The study was designed to evaluate the time to onset of apomorphine response in patients with morning akinesia and as such does not fully reflect clinical practice in which intermittent subcutaneous apomorphine injections can be added on to oral PD treatments throughout the day.16 Indeed, patients with morning akinesia may well benefit from a combination of subcutaneous apomorphine injection (for rapid relief) with oral l‐dopa (for longer duration of effect). Finally, the study was conducted under U.S. labeling conditions and might therefore not fully represent the clinical situation in other countries. For example, higher doses ≤10 mg apomorphine are licensed in Europe, and the antiemetic domperidone is considered the preferred prophylactic treatment for use concomitantly while introducing apomorphine, but it is not available in the United States.16 Alternative delivery systems of l‐dopa or apomorphine might also improve time to ON during off episodes such as morning akinesia, but these are not registered for use in the United States.
In summary, subcutaneous apomorphine injections were found to provide a rapid and reliable ON state for patients with morning akinesia, presumably as a result of avoiding gastrointestinal delivery and absorption. In addition to reversing OFF episodes, apomorphine injections have clinical utility in improving early morning akinesia by providing rapid and reliable turning‐ON when used during awakening.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
S.I.: 1A, 1B, 1C, 2C, 3A, 3B
M.L.: 1C, 2C, 3B
W.O.: 1C, 2C, 3B
J.H.: 1A, 1B, 2A, 2C, 3B
T.C.: 2A, 2B, 2C, 3B
F.P.: 1C, 2C, 3B
Disclosures
Funding Sources and Conflict of Interest: US WorldMeds LLC funded this study. Stuart Isaacson, Mark Lew, William Ondo, and Fernando Pagan report fees for consultancy and speaker fees from US WorldMeds LLC, and they were investigators in the AM‐IMPAKT trial. US WorldMeds LLC employs Jean Hubble and Thomas Clinch.
Financial Disclosures for the previous 12 months: Stuart Isaacson reports consultancy and/or promotional speaker fees on behalf of: Acadia, Acorda, Adamas, Allergan, Amarantus, Biotie, Brittania, Cynapsus, GE, Impax, Ipsen, Kyowa, Lundbeck, Teva, UCB, and US WorldMeds. In addition, he reports research funding from Abbvie, Acadia, Acorda, Adamas, Addex, Allergan, Biotie, Civitas, Eisai, Ipsen, Kyowa, Lilly, Merck, Michael J Fox Foundation, Parkinson Study Group, Pharma2B, Roche, Serono, Teva, UCB, and US WorldMeds. Mark Lew reports advisor/consultant fees on behalf of Teva, US WorldMeds, Merz, UCB, Acadia, Auspex, Lundbeck, and Abbvie. He reports promotional speaker fees on behalf of Teva, US WorldMeds, UCB, Lundbeck, and Acadia. In addition, he reports research from NIH, Abbott/AbbVie, Parkinson's Study Group, US WorldMeds, Michael J. Fox Foundation, Synosia Pharmaceuticals, Merz, Ipsen, Pharma two B, Civitas, Biotie, Cynapsus, Amarantus, and Pfizer. William Ondo reports speaker compensation from TEVA, Ipsen, UCB Pharma, US WorldMeds, Merz, IMPAX, Avanir, and Lundbeck, and he was a consultant with the companies as well. Fernando Pagan reports speaker and consultancy fees from Teva, Solstice Neurosciences, Novartis, GlaxoSmithKline, Boehringer Ingelheim, Medtronic, Merz, US WorldMeds, Avanir, and Medtronic, and reports research grants from Medtronic and Teva Neuroscience. Jean Hubble and Thomas Clinch are employed by US WorldMeds.
Acknowledgments
We thank all investigators, patients, and their care partners who participated in this project, and Anita Chadha‐Patel (ACP Clinical Communications Ltd. funded by US WorldMeds LLC) for medical writing support (literature searching, referencing, and editing) in the development of this report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 2. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458. [DOI] [PubMed] [Google Scholar]
- 3. Pietz K, Hagell P, Odin P. Subcutaneous apomorphine in late stage Parkinson's disease: a long term follow up. J Neurol Neurosurg Psychiatry 1998;65:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merims D, Djaldetti R, Melamed E. Waiting for ON: a major problem in patients with Parkinson disease and ON/OFF motor fluctuations. Clin Neuropharmacol 2003;26:196–198. [DOI] [PubMed] [Google Scholar]
- 5. Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord 2005;20(Suppl 11):S11–S16. [DOI] [PubMed] [Google Scholar]
- 6. Stocchi F. The levodopa wearing‐off phenomenon in Parkinson's disease: pharmacokinetic considerations. Expert Opin Pharmacother 2006;7:1399–1407. [DOI] [PubMed] [Google Scholar]
- 7. Nyholm D, Lennernas H. Irregular gastrointestinal drug absorption in Parkinson's disease. Expert Opin Drug Metab Toxicol 2008;4:193–203. [DOI] [PubMed] [Google Scholar]
- 8. Heetun ZS, Quigley EM. Gastroparesis and Parkinson's disease: a systematic review. Parkinsonism Relat Disord 2012;18:433–440. [DOI] [PubMed] [Google Scholar]
- 9. Stowe R, Ives N, Clarke CE, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson's disease patients with motor complications. Cochrane Database Syst Rev 2010;7:CD007166. [DOI] [PubMed] [Google Scholar]
- 10. Dewey RB Jr, Hutton JT, LeWitt PA, Factor SA. A randomized, double‐blind, placebo‐controlled trial of subcutaneously injected apomorphine for parkinsonian off‐state events. Arch Neurol 2001;58:1385–1392. [DOI] [PubMed] [Google Scholar]
- 11. Pahwa R, Koller WC, Trosch RM, Sherry JH. Subcutaneous apomorphine in patients with advanced Parkinson's disease: a dose‐escalation study with randomized, double‐blind, placebo‐controlled crossover evaluation of a single dose. J Neurol Sci 2007;258:137–143. [DOI] [PubMed] [Google Scholar]
- 12. Pfeiffer RF, Gutmann L, Hull KL Jr, Bottini PB, Sherry JH, Investigators APOS. Continued efficacy and safety of subcutaneous apomorphine in patients with advanced Parkinson's disease. Parkinsonism Relat Disord 2007;13:93–100. [DOI] [PubMed] [Google Scholar]
- 13. Pahwa R, Lyons KE, eds. Handbook of Parkinson's Disease, 5th ed Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- 14. Rizos A, Martinez‐Martin P, Odin P, et al. Characterizing motor and non‐motor aspects of early‐morning off periods in Parkinson's disease: an international multicenter study. Parkinsonism Relat Disord 2014;20:1231–1235. [DOI] [PubMed] [Google Scholar]
- 15. Hauser RA, Isaacson S, Clinch T. The Tigan/Apokyn Study: randomized, placebo‐controlled trial of trimethobenzamide to control nausea and vomiting during initiation and continued treatment with subcutaneous apomorphine injection. Parkinsonism Relat Disord 2014;20:1171–1176. [DOI] [PubMed] [Google Scholar]
- 16. Trenkwalder C, Chaudhuri KR, Garcia Ruiz PJ, et al. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson's disease: clinical practice recommendations. Parkinsonism Relat Disord 2015;21:1023–1030. [DOI] [PubMed] [Google Scholar]


