Abstract
This report describes the phase 1 trials that evaluated the metabolism of the novel triazole antifungal isavuconazole by cytochrome P450 3A4 (CYP3A4) and isavuconazole's effects on CYP3A4‐mediated metabolism in healthy adults. Coadministration of oral isavuconazole (100 mg once daily) with oral rifampin (600 mg once daily; CYP3A4 inducer) decreased isavuconazole area under the concentration‐time curve (AUCτ) during a dosing interval by 90% and maximum concentration (Cmax) by 75%. Conversely, coadministration of isavuconazole (200 mg single dose) with oral ketoconazole (200 mg twice daily; CYP3A4 inhibitor) increased isavuconazole AUC from time 0 to infinity (AUC0‐∞) and Cmax by 422% and 9%, respectively. Isavuconazole was coadministered (200 mg 3 times daily for 2 days, then 200 mg once daily) with single doses of oral midazolam (3 mg; CYP3A4 substrate) or ethinyl estradiol/norethindrone (35 μg/1 mg; CYP3A4 substrate). Following coadministration, AUC0‐∞ increased 103% for midazolam, 8% for ethinyl estradiol, and 16% for norethindrone; Cmax increased by 72%, 14%, and 6%, respectively. Most adverse events were mild to moderate in intensity; there were no deaths, and serious adverse events and adverse events leading to study discontinuation were rare. These results indicate that isavuconazole is a sensitive substrate and moderate inhibitor of CYP3A4.
Keywords: ethinyl estradiol/norethindrone, isavuconazole, ketoconazole, midazolam, rifampin
Invasive fungal diseases are a major cause of morbidity and mortality in immunocompromised patients.1, 2 Individuals with hematological malignancies or undergoing stem cell transplants are particularly at risk.3 Despite advances in antifungal therapy, the emergence of resistant and rare pathogenic fungal species, as well as limits of the efficacy and tolerability of existing treatments, necessitate continued development of new alternatives.
Isavuconazole is a novel broad‐spectrum triazole antifungal agent. It is the active moiety of the water‐soluble prodrug isavuconazonium sulfate, which was approved in 2015 by the US Food and Drug Administration for the primary treatment of adults with invasive aspergillosis and invasive mucormycosis and by the European Medicines Agency for the primary treatment of adults with invasive aspergillosis and for mucormycosis when amphotericin B is inappropriate, based on the results of phase 3 clinical trials.4, 5 Unlike the other second‐generation triazole antifungal agents posaconazole and voriconazole, for which limitations in solubility and bioavailability have presented challenges, the ester prodrug isavuconazonium sulfate has excellent pharmacokinetic properties. It undergoes rapid hydrolysis by esterases in the gut and blood to release the poorly soluble but highly permeable active drug.6, 7 Oral bioavailability was shown to be 98%, and there was no evidence of a clinically relevant food or pH effect.8 Isavuconazole may also have an advantage with respect to tolerability. For example, in the SECURE trial that compared isavuconazole and voriconazole for the treatment of patients with invasive mold disease caused by Aspergillus spp and other filamentous fungi, patients treated with isavuconazole had a lower frequency of hepatobiliary disorders, eye disorders, and skin and subcutaneous tissue disorders.4
As a class, triazole antifungal agents such as isavuconazole can alter the pharmacokinetics (PK) of drugs that are metabolized by the cytochrome P450 system (CYP).9, 11 All triazoles are inhibitors of the CYP isoenzymes, and particularly CYP3A4, although the potency of inhibition and range of other affected CYP isoenzymes vary for the different triazoles.10 Ketoconazole is a particularly potent inhibitor of CYP3A4 that had been used frequently as a positive control in clinical PK drug‐drug interaction studies until safety concerns were raised in a 2013 regulatory guidance that recommended against its use.11 Clinical studies of the inhibitory potential of triazole antifungal agents are especially important because their effects in vivo are generally larger than those predicted by studies in vitro.10
The results of preclinical studies suggest that CYP3A4, and to a lesser extent CYP3A5, are the isoenzymes predominantly responsible for metabolism of isavuconazole (data on file). In studies performed with pooled human liver microsomes, metabolism of isavuconazole was most strongly correlated with CYP3A4/5 activity, as measured by hydroxylation of testosterone and midazolam (P < .001 and r > 0.82 for both), whereas weaker correlations were observed for CYP2B6 activity (S‐mephenytoin demethylation; P < .01, r = 0.6464) and CYP2C8 activity (paclitaxel hydroxylation; P < .05, r = 0.5729). No correlations were observed with activities of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP4A11. In vitro experiments in which isavuconazole was incubated with CYP‐expressing human liver microsomes revealed that the ratio of the remaining isavuconazole was lowest for CYP3A4 (33.8%) or CYP3A5 (68.4%) compared with CYP2B6, CYP2C8, or CYP3A7 (all >98%). Further, isavuconazole had been found to exhibit both inhibitory and inductive effects on CYP3A4 activity. Inhibition constant (Ki) values for CYP3A4 were 0.62 μmol/L and 1.93 μmol/L using both midazolam and testosterone as probes, respectively. The IC50 or Ki values for the remaining CYPs were CYP1A2, 38.50 μmol/L (phenacetin‐O‐dealkylase activity); CYP2B6, 15.10 μmol/L (bupropion hydroxylase activity); CYP2C8, 2.86 μmol/L (amodiaquine N‐desethylase activity; CYP2C9, 4.78 μmol/L (diclofenac 4ʹ‐hydroxylase activity); CYP2C19, 5.40 μmol/L (S‐[+]‐mephenytoin 4ʹ‐hydroxylase activity); CYP2D6, 4.82 μmol/L ([±]‐bufuralol 1ʹ‐hydroxylase activity); CYP2A6 and CYP2E1, >100 μmol/L (coumarin 7‐hydroxylase and chlorzoxazone 6‐hydroxylase activities, respectively). Because the recommended clinical dosing of isavuconazole (200 mg 3 times daily for 2 days, then 200 mg daily) typically results in maximum plasma concentrations of <7 μg/mL (data on file), IC50 or Ki values of ≤16 μmol/L might indicate particular clinical relevance (isavuconazole molecular weight, 437.47 g/mol). In studies performed to assess induction of CYPs in cultured human hepatocytes, isavuconazole caused up to 3.4‐fold increases in testosterone hydroxylation activity and 6.4‐fold increases in CYP3A4 mRNA. Mixed effects were observed for other CYP isoenzymes. No isavuconazole‐induced increases were observed for the S‐(+)‐mephenytoin 4ʹ‐hydroxylation activity or mRNA levels for CYP2C19. Maximum increases for the activity and mRNA for other CYPs tested were: CYP1A2, 2.8‐fold (phenacetin O‐dealkylase activity) and 5.4‐fold (mRNA); CYP2B6, 13.4‐fold (bupropion hydroxylase activity) and 11.4‐fold (mRNA); CYP2C8, 2.6‐fold (amodiaquine N‐dealkylase activity) and 4.3‐fold (mRNA); and CYP2C9, none (diclofenac 4ʹ‐hydroxylase activity) and 3.1‐fold (mRNA).
Because patients requiring antifungal treatment frequently require concomitant medications related to other underlying conditions, it is important to establish potential drug‐drug interactions between agents whose metabolism is mediated by CYP3A4. This article reports the results of clinical trials that examined CYP3A4‐mediated drug‐drug interactions of isavuconazole in healthy adults using the probes rifampin (strong CYP3A4 inducer), ketoconazole (strong CYP3A4 inhibitor and substrate), midazolam (CYP3A4 substrate), and ethinyl estradiol/norethindrone (CYP3A4 substrate), as per regulatory guidance from the US Food and Drug Administration and European Medicines Agency.
Methods
Study Design
All clinical study protocols were approved by the Institutional Review Board for each participating study site (rifampin study, Stichting Beoordeling Ethiek Bio‐Medisch, Assen, Netherlands; ketoconazole study, Chesapeake IRB, Columbia, Maryland; midazolam and oral contraceptive studies, Independent Investigational Review Board, Inc., Plantation, Florida, USA). The studies were conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation guidelines, and local regulations. Participating subjects provided written, informed consent prior to any study procedures.
These studies were phase 1 single‐center open‐label trials conducted to evaluate potential interactions of isavuconazole (administered as isavuconazonium sulfate; CRESEMBA® oral capsules, Astellas Pharma US, Inc., Northbrook, Illinois) with rifampin (EREMFAT® oral tablets, Riemser Pharma, Berlin, Germany; trial conducted June to October 2005 at Xendo Drug Development B.V., Groningen, Netherlands; not registered), ketoconazole (generic oral tablets; trial conducted May to June 2012 at Spaulding Clinical Research, LLC, West Bend, Wisconsin; ClinicalTrials.gov identifier NCT01657838), midazolam (generic oral syrup; trial conducted May to June 2011 at Covance Clinical Research, Madison, Wisconsin; NCT01406171), and ethinyl estradiol/norethindrone (ORTHO‐NOVUM 1/35® oral tablets, Janssen Pharmaceuticals, Inc., Beerse, Belgium; trial conducted April to May 2012 at Clinical Pharmacology of Miami, Inc., Miami, Florida; NCT01597986).
Dosing and Sampling Schedules
In this article, dosing information is expressed as the isavuconazole equivalent of the prodrug: oral capsules each contained isavuconazonium sulfate 186 mg, equivalent to isavuconazole 100 mg. Only the active metabolite isavuconazole will be referred to hereafter. In the rifampin study, an isavuconazole 100 mg once daily (QD) maintenance dose was used based on data obtained in an early phase 2 trial.12 As more trial data were made available, a maintenance dose of isavuconazole 200 mg QD was selected for the remainder of the phase 1 study program.
Rifampin
Healthy male subjects aged 18 to 65 years and with a body mass index (BMI) of 18 to 30 kg/m2 were enrolled in this study. Subjects were included only if they had normal renal function (creatinine clearance ≥80 mL/min) and values of liver function, cortisol, and adrenocorticotropic hormone tests within normal ranges.
After screening (days –21 to –2), subjects checked in at the study center on day –1. Subjects were permitted to leave the study center each day following study drug administration with the exception of day 44 (first day of coadministration), when subjects remained at the study center until the following morning. A follow‐up assessment was conducted on day 73 (±1 day).
On days 1 to 14, subjects received a course of oral isavuconazole (400 mg single loading dose on day 1, then 100 mg QD on days 2 to 14), followed by a washout on days 15 to 35. Subjects then received a course of oral rifampin (600 mg QD) on days 36 to 71, with isavuconazole coadministered on days 44 to 57 (400 mg single dose on day 44, then 100 mg QD on days 45 to 57) (Figure 1A). Isavuconazole and rifampin were administered under fasting conditions (≥8 hours). On days of coadministration, isavuconazole was administered 2 hours after rifampin.
Figure 1.
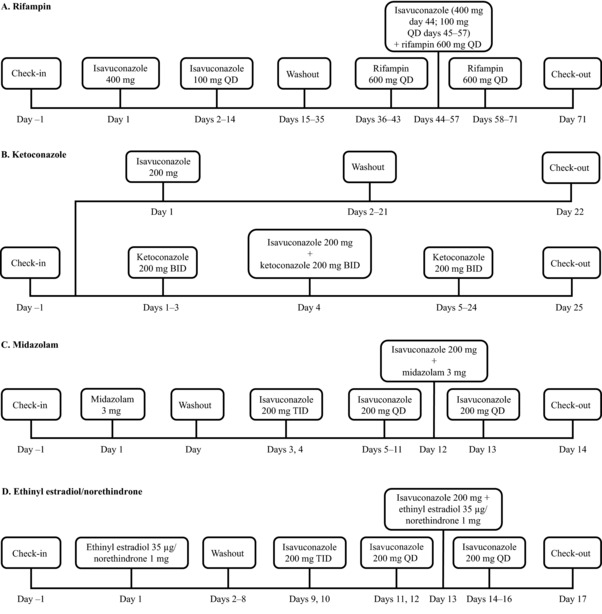
Clinical study designs. Isavuconazole 100 mg was administered as oral isavuconazonium sulfate 186 mg. BID, twice daily; QD, once daily; TID, 3 times daily.
Blood samples were collected for PK analysis of rifampin on day 43 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 24, and 48 hours postdose. Blood samples were collected for PK analysis of isavuconazole on days 14 and 57 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 24, 48, 72, 96, 144, 192, 240, 288, and 336 hours postdose.
Ketoconazole
Healthy male and female subjects aged 18 to 55 years old with a body weight ≥45 kg and a BMI of 18 to 32 kg/m2 were enrolled in this study. After screening (days –28 to –2), subjects checked in at the study center on day –1, where they remained until day 22 or day 25 (arm 1 or arm 2, respectively).
Subjects were randomized 1:1 to 2 treatment arms (Figure 1B). Subjects in arm 1 received a single oral dose of isavuconazole 200 mg on day 1, whereas subjects in arm 2 received oral doses of ketoconazole 200 mg twice daily (BID; approximately 12 hours apart) on days 1 to 24 and a single oral dose of concomitant isavuconazole 200 mg on day 4. Subjects fasted for ≥10 hours prior to isavuconazole administration and continued to fast for 4 hours following administration. Subjects also fasted before and after ketoconazole administration on day 3. On day 4, isavuconazole was administered immediately after ketoconazole.
In arm 1, blood samples were collected for PK analysis of isavuconazole on day 1 at predose and at 0.5, 1, 2, 4, 6, 8, 10, 12, 20, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216, 240, 264, 288, 312, 336, 360, 384, 408, 432, 456, 480, and 504 hours postdose. In arm 2, blood samples were collected for PK analysis of isavuconazole on day 4 at predose and at 0.5, 1, 2, 4, 6, 8, 10, 12, 20, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216, 240, 264, 288, 312, 336, 360, 384, 408, 432, 456, 480, and 504 hours postdose. Samples also were collected from subjects in arm 2 for PK analysis of ketoconazole on days 2, 3, and 4 at predose and on day 4 at 0.5, 1, 2, 4, 6, 12, and 24 hours postdose.
Midazolam
Healthy male and female subjects aged 18 to 55 years with a body weight ≥45 kg and a BMI of 18 to 32 kg/m2 were enrolled in this study. After screening (days –28 to –2), subjects checked in at the study center on day –1, where they remained until day 14 and returned for a follow‐up visit on day 21 (±1 day).
Subjects received a single oral dose of midazolam 3 mg on day 1. Following a 1‐day washout, subjects received an oral loading dose of isavuconazole 200 mg 3 times daily (TID; 8 hours apart) on days 3 and 4, then 200 mg QD on days 5 to 13. A single oral dose of midazolam 3 mg was coadministered with isavuconazole on day 12 (Figure 1C). Isavuconazole and midazolam were administered under fed conditions. On day 12, isavuconazole was administered immediately after midazolam.
Blood samples were collected for PK analysis of midazolam and its metabolite 1‐hydroxy‐midazolam on days 1 and 12 at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, and 48 hours postdose. Samples were collected for PK analysis of isavuconazole on day 11 at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 hours postdose as well as on day 12 at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, and 48 hours postdose.
Ethinyl Estradiol/Norethindrone
Healthy postmenopausal female subjects (≥2 years since their last regular menstrual cycle and follicle stimulating hormone >30 IU/L) aged 50 to 65 years old with a body weight ≥45 kg and a BMI of 18 to 32 kg/m2 were enrolled in this study. After screening (days –21 to –2), subjects checked in at the study center on day –1, where they remained until day 17, and a follow‐up telephone call was made on day 24 (±2 days).
Subjects received a single oral dose of ethinyl estradiol 35 μg/norethindrone 1 mg on day 1. Following washout (days 2 to 8), subjects received an oral loading dose of isavuconazole 200 mg TID (8 hours apart) on days 9 and 10, then 200 mg QD on days 11 to 16. A single oral dose of ethinyl estradiol 35 μg/norethindrone 1 mg was coadministered with isavuconazole on day 13 (Figure 1D). Subjects fasted for ≥10 hours prior to ethinyl estradiol/norethindrone administration on days 1 and 13 and continued to fast for 4 hours following administration. Subjects also fasted before and after administration of isavuconazole on day 12. On all other days, subjects received isavuconazole 1 hour prior to their morning meal. On day 13, ethinyl estradiol/norethindrone was administered immediately after isavuconazole.
Blood samples were collected for PK analysis of ethinyl estradiol and norethindrone on days 1 and 13 at predose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 20, 24, 48, 72, and 96 hours postdose. Samples were collected for PK analysis of isavuconazole on days 12 and 13 at predose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 20 hours postdose.
Pharmacokinetic Assessments
Plasma concentrations of all analytes were measured by liquid chromatography–mass spectrometry/mass spectrometry (see Supplementary Data S1 for methods). The primary PK parameters analyzed for isavuconazole and each of the coadministered drugs (and their metabolites) included the area under the concentration‐time curve (AUC) from time 0 to infinity (AUC0‐∞), AUC from time of dosing to time of last measurable concentration (AUClast), and maximum concentration (Cmax). In the rifampin study, AUC during a dosing interval (AUCτ) was also a primary PK parameter. Additional PK parameters included time to Cmax (tmax), elimination half‐life (t½), apparent clearance (CL/F), and volume of distribution (Vz/F).
Safety Assessments
Safety and tolerability were assessed by monitoring the incidence, nature, and severity of treatment‐emergent adverse events (TEAEs) as well as by vital‐sign measurements, 12‐lead electrocardiograms, clinical laboratory testing (hematology, chemistry, and urinalysis), and physical examinations.
Statistics
Descriptive statistics were used to summarize demographics, baseline characteristics, and TEAEs in all subjects who received ≥1 dose of study drug. The PK parameters were assessed in all subjects who received ≥1 dose of study drug and whose PK data were adequate for the calculation of ≥1 primary PK parameter. Levels of analyte below the level of quantification were entered as 0 for calculations. To assess the effect of isavuconazole on the PK of rifampin, midazolam, ethinyl estradiol, and norethindrone as well as the effect of each probe on isavuconazole, log‐transformed AUC and Cmax values were analyzed using a mixed‐effects model with treatment as a fixed effect and subject as a random effect. The effect of ketoconazole on isavuconazole PK was assessed using analysis of variance of log‐transformed AUC and Cmax values with treatment as a factor. The 90% confidence intervals (CI) were constructed around the geometric least‐squares mean ratios of the probe plus isavuconazole to isavuconazole alone for the primary PK parameters of isavuconazole. In addition, confidence intervals were constructed around the geometric least‐squares mean ratios of the probe plus isavuconazole to the probe alone for the primary PK parameters of the probes. Parameters were calculated using noncompartmental analysis with Phoenix® WinNonlin® version 5.3 (Certara USA, Inc., Princeton, New Jersey). All data processing, summarization, and analyses were conducted using SAS® version 9.1 (Statistical Analysis Software, Cary, North Carolina).
Results
Pharmacokinetics
Rifampin
Twenty‐six healthy subjects were enrolled, and 24 completed the study that examined the PK effects of the strong CYP3A4 inducer, rifampin, on isavuconazole (Table 1). Mean AUCτ, AUC0–∞, AUClast, and Cmax values for isavuconazole were 90%, 97%, 97%, and 75% lower during coadministration with rifampin, compared with isavuconazole alone, respectively (Table 2; see Figure 2 for concentration–time profile).
Table 1.
Demographics and Baseline Characteristics
| Ketoconazole | |||||
|---|---|---|---|---|---|
| Parameter | Rifampin Total (n = 26) | Isavuconazole Alone (n = 12) | Ketoconazole + Isavuconazole (n = 12) | Midazolam Total (n = 23) | Ethinyl Estradiol/ NorethindroneTotal (n = 24) |
| Sex, n (%) | |||||
| Male | 26 (100) | 6 (50.0) | 8 (66.7) | 16 (69.6) | 0 |
| Female | 0 | 6 (50.0) | 4 (33.3) | 7 (30.4) | 24 (100) |
| Race, n (%) | |||||
| White | 24 (92.3) | 7 (58.3) | 7 (58.3) | 14 (60.9) | 22 (91.7) |
| Black or African American | 0 | 4 (33.3) | 4 (33.3) | 3 (13.0) | 2 (8.3) |
| Other | 2 (7.7) | 1 (8.3) | 1 (8.3) | 6 (26.1) | 0 |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | NS | 10 (83.3) | 9 (75.0) | 21 (91.3) | 7 (29.2) |
| Hispanic or Latino | NS | 2 (16.7) | 3 (25.0) | 2 (8.7) | 17 (70.8) |
| Age [years], mean (SD) | 39.2 (14.4) | 35.3 (12.2) | 30.1 (9.3) | 32.0 (11.2) | 57.8 (4.5) |
| Weight [kg], mean (SD) | 83.4 (10.4) | 73.8 (11.0) | 77.0 (14.2) | 78.4 (10.4) | 68.6 (7.4) |
| BMI [kg/m2], mean (SD) | 25.0 (2.8) | 26.1 (4.0) | 26.5 (3.6) | 26.4 (3.0) | 26.5 (2.7) |
BMI, body mass index; NS, not specified; SD, standard deviation.
Table 2.
Plasma Pharmacokinetics of Isavuconazole in the Presence and Absence of Rifampin and Ketoconazole
| Isavuconazole PK (± Rifampin) | Isavuconazole PK (± Ketoconazole) | |||
|---|---|---|---|---|
| Parametera | Isavuconazole Alone (n = 25)b | Isavuconazole + Rifampin (n = 24)c | Isavuconazole Alone (n = 12) | Isavuconazole + Ketoconazole (n = 12) |
| AUC0‐∞, h·μg/mL | 233.1 (122.9) | 5.8 (1.4) | 84.8 (22.2) | 466.4 (199.4) |
| AUClast, h·μg/mL | 206.0 (89.3) | 5.6 (1.4) | 64.9 (17.5) | 238.5 (45.4) |
| AUCτ, h·μg/mL | 38.0 (8.2) | 3.9 (0.8) | ND | ND |
| Cmax, μg/mL | 2.4 (0.5) | 0.6 (0.2) | 2.2 (0.5) | 2.4 (0.6) |
| tmax, hours | 2.0 (1.0−8.0) | 2.0 (1.0−4.0) | 2.0 (2.0−4.0) | 3.0 (2.0−4.0) |
| t½, hours | 83.6 (38.9) | 19.7 (4.9) | 117.0 (65.6) | 430.1 (198.0) |
| CL/F, L/h | 2.8 (0.6) | 27 (6.2) | 2.5 (0.6) | 0.5 (0.2) |
| Geometric Least‐Squares Mean Ratio, % (90% CI) | ||||
| AUC0‐∞ | 3 (2, 3) | 522 (409, 666) | ||
| AUClast | 3 (3, 3) | 373 (319, 435) | ||
| AUCτ | 10 (9, 11) | ND | ||
| Cmax | 25 (23, 27) | 109 (93, 127) | ||
AUC, area under the concentration–time curve; Cmax, maximum concentration; CL/F, clearance; CI, confidence interval; ND, not done; PK, pharmacokinetics; t½, half‐life; tmax, time to Cmax.
Values are expressed as arithmetic mean (standard deviation) except tmax, for which median (range) is provided.
One subject discontinued the study during isavuconazole‐alone administration (day 9).
Two subjects discontinued the study during isavuconazole‐alone administration (days 9 and 14).
Figure 2.
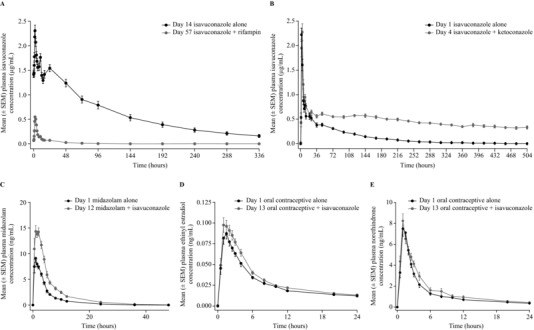
Mean plasma concentration‐time profiles of isavuconazole (A, ± rifampin; B, ± ketoconazole), midazolam (C), ethinyl estradiol (D), and norethindrone (E). SEM, standard error of the mean.
Ketoconazole
Twenty‐four healthy subjects (12 in each study arm) were enrolled and completed the study that evaluated the effects of the strong CYP3A4 inhibitor, ketoconazole, on the PK of isavuconazole (Table 1). Mean isavuconazole AUC0‐∞, AUClast, and Cmax values were 422%, 273%, and 9% higher in the presence vs absence of ketoconazole, respectively (Table 2; see Figure 2 for concentration‐time profile). Plasma exposure of ketoconazole was increased by coadministration with isavuconazole compared with ketoconazole alone (geometric least‐squares mean ratios [90%CI]: AUCτ, 127% [112, 144]; Cmax, 118% [105, 134]; Supplementary Table S1).
Midazolam
Twenty‐three healthy subjects were enrolled and 22 completed the study that assessed the PK effects of isavuconazole on a single dose of the CYP3A4 substrate, midazolam (Table 1). Mean midazolam AUC0‐∞, AUClast, and Cmax values were 103%, 106%, and 72% higher in the presence vs absence of isavuconazole, respectively (Table 3; see Figure 2 for concentration‐time profile). Mean AUC0‐∞ and AUClast values of 1‐hydroxy‐midazolam were also higher, whereas Cmax was similar, during coadministration with isavuconazole (Figure S1 and Table S1). Mean AUCτ and Cmax values of isavuconazole were comparable in the presence and absence of midazolam (Table 4).
Table 3.
Plasma Pharmacokinetics of Midazolam, Ethinyl Estradiol, and Norethindrone in the Presence and Absence of Isavuconazole
| Midazolam PK | Ethinyl Estradiol PK | Norethindrone PK | ||||
|---|---|---|---|---|---|---|
| Parametera | Midazolam Alone (n = 23) | Midazolam + Isavuconazole (n = 22)b | Ethinyl Estradiol/Norethindrone Alone (n = 23)c | Ethinyl Estradiol/Norethindrone + Isavuconazole (n = 23)d | Ethinyl Estradiol/Norethindrone Alone (n = 24) | Ethinyl Estradiol/Norethindrone + Isavuconazole (n = 23)d |
| AUC0‐∞, h·ng/mL | 46.0 (14.7) | 93.4 (33.0) | 1.1 (0.3) | 1.2 (0.4) | 40.9 (20.8) | 46.9 (25.4) |
| AUClast, h·ng/mL | 44.5 (14.8) | 91.2 (32.6) | 1.0 (0.3) | 1.1 (0.4) | 38.1 (20.8) | 44.8 (25.2) |
| Cmax, ng/mL | 10.7 (4.6) | 18.1 (6.9) | 0.09 (0.03) | 0.1 (0.04) | 8.3 (3.2) | 8.6 (2.8) |
| tmax, h | 1.0 (0.5−3.0) | 1.6 (0.5−3.0) | 1.5 (1.0−2.5) | 1.5 (0.5−4.0) | 1.0 (1.0−2.0) | 1.0 (0.6−2.5) |
| t½, h | 5.4 (1.9) | 6.4 (1.8) | 20.5 (3.0) | 20.0 (4.7) | 13.1 (2.5) | 11.6 (3.1) |
| CL/F, L/h | 72.1 (24.6) | 35.2 (9.8) | 35.5 (9.7) | 32.6 (8.2) | 28.9 (11.6) | 26.1 (11.6) |
| Geometric Least‐Squares Mean Ratio, % (90%CI) | ||||||
| AUC0‐∞ | 203 (173, 238) | 108 (103, 113) | 116 (109, 123) | |||
| AUClast | 206 (175, 243) | 110 (104, 116) | 116 (109, 123) | |||
| Cmax | 172 (144, 205) | 114 (103, 126) | 106 (93, 120) | |||
AUC, area under the concentration‐time curve; Cmax, maximum concentration; CL/F, clearance; CI, confidence interval; ND, not done; PK, pharmacokinetics; t½, half‐life; tmax, time to Cmax.
Values are expressed as arithmetic mean (standard deviation), except tmax, for which median (range) is provided.
One subject discontinued the study during isavuconazole administration (day 8).
One subject was excluded due to outlier concentrations at all time points, including detectable levels at baseline (predose).
One subject discontinued the study following oral contraceptive administration (day 7).
Table 4.
Plasma Pharmacokinetics of Isavuconazole in the Presence and Absence of Midazolam, Ethinyl Estradiol, and Norethindrone
| Isavuconazole PK (± Midazolam) | Isavuconazole PK (± Ethinyl Estradiol/Norethindrone) | |||
|---|---|---|---|---|
| Parametera | Isavuconazole Alone (n = 22)b | Isavuconazole+ Midazolam (n = 22)b | Isavuconazole Alone (n = 23)c | Isavuconazole + Ethinyl Estradiol/Norethindrone(n = 23)c |
| AUCτ, h·μg/mL | 89.8 (22.9) | 88.2 (22.0) | 80.8 (18.7) | 81.4 (19.0) |
| Cmax, μg/mL | 5.2 (1.4) | 5.0 (1.1) | 5.8 (1.3) | 6.1 (1.5) |
| tmax, hours | 4.0 (3.0−23.9) | 4.6 (2.0−23.9) | 2.5 (2.0−3.0) | 2.0 (1.0−4.0) |
AUC, area under the concentration‐time curve; Cmax, maximum concentration; PK, pharmacokinetics; tmax, time to Cmax.
Values are expressed as arithmetic mean (standard deviation) except tmax, for which median (range) is provided.
One subject discontinued the study during isavuconazole administration (day 8).
One subject discontinued the study following oral contraceptive administration (day 7).
Ethinyl Estradiol/Norethindrone
Twenty‐four healthy subjects were enrolled and 23 completed the study that assessed the PK effects of isavuconazole on single doses of the CYP3A4 substrates, ethinyl estradiol and norethindrone (Table 1). Mean ethinyl estradiol AUC0‐∞, AUClast, and Cmax values were 8%, 10%, and 14% higher in the presence vs absence of isavuconazole, respectively (Table 3; see Figure 2 for concentration–time profile). Mean norethindrone AUC0‐∞ and AUClast values were 16% higher, and Cmax was 6% higher, during coadministration with isavuconazole (Table 3; see Figure 2 for concentration‐time profile). Mean AUCτ and Cmax values for isavuconazole were comparable in the presence and absence of ethinyl estradiol/norethindrone (Table 4).
Safety
Across all studies, most TEAEs were mild or moderate in intensity, and both serious TEAEs and TEAEs leading to study discontinuation were rare.
Twenty‐four (92.3%) subjects experienced TEAEs in the rifampin study: during isavuconazole alone (n = 19), rifampin alone (n = 19), and isavuconazole plus rifampin coadministration (n = 15). The most common TEAEs were headache (n = 13), diarrhea (n = 12), and nausea (n = 9) (Supplementary Table S2). Drug‐related TEAEs were experienced by 22 (84.6%) subjects: during isavuconazole administration (n = 17), rifampin administration (n = 7), and coadministration (n = 13). One subject experienced a serious TEAE of elevation in liver function enzymes on day 36 before the first dose of rifampin, which was considered to be severe in intensity. No treatment was administered for this TEAE, which resolved on day 183. Two subjects withdrew consent on days 9 and 14 and did not complete the study, but no subjects discontinued due to a TEAE.
In the ketoconazole study, TEAEs were reported by 2 (16.7%) subjects during isavuconazole‐alone administration, no subjects during ketoconazole‐alone administration, and 5 (41.7%) subjects during isavuconazole plus ketoconazole coadministration. No TEAE was experienced by >1 subject, and no subjects experienced a serious or drug‐related TEAE (Supplementary Table S3). There were no discontinuations in this study.
In the midazolam study, 20 (87.0%) subjects experienced TEAEs during midazolam alone (n = 9), isavuconazole alone (n = 10), and midazolam plus isavuconazole coadministration (n = 18). The most common TEAE overall was somnolence (n = 15) (Supplementary Table S4). TEAEs were considered by the study investigator to be drug‐related in 16 (69.6%) subjects: during midazolam alone (n = 6), isavuconazole alone (n = 5), and coadministration (n = 15). One subject discontinued on day 8 due to an elevated creatinine level that was already evident on day –1 (115 μmol/L) and that continued to rise during the study before resolving on day 18. There were no serious TEAEs.
Overall, 9 (37.5%) subjects experienced TEAEs in the ethinyl estradiol/norethindrone study during oral contraceptive alone (n = 2), isavuconazole alone (n = 6), and oral contraceptives plus isavuconazole (n = 3). Common TEAEs included gastroesophageal reflux disease (n = 3), flatulence (n = 2), and headache (n = 3) (Supplementary Table S5). In total, 3 subjects experienced drug‐related TEAEs during isavuconazole administration, and 1 subject each experienced drug‐related TEAEs during administration with the oral contraceptives alone and during coadministration. One subject experienced 2 serious TEAEs of continuous gastritis and severe gastroesophageal reflux disease during follow‐up on day 29, which were both severe in intensity. Another subject discontinued the study on day 7 during administration of ethinyl estradiol/norethindrone due to elevated liver enzymes, which resolved on day 20.
Discussion
In this series of studies potential interactions between isavuconazole and an inducer, an inhibitor, and substrates of CYP3A4 were explored in healthy human subjects. Isavuconazole was confirmed to be a sensitive substrate of CYP3A4 based on the profound decrease in isavuconazole exposure when coadministered with the strong CYP3A4 inducer rifampin and the marked increase in isavuconazole exposure when coadministered with the strong CYP3A4 inhibitor ketoconazole. Moreover, isavuconazole significantly increased the exposure of the CYP3A4‐sensitive substrate midazolam, confirming that isavuconazole is a moderate inhibitor of CYP3A4 in vivo. Exposure of the other CYP3A4 substrates—ketoconazole, ethinyl estradiol, and norethindrone—was increased only slightly with coadministration of isavuconazole.
In addition to CYP3A4, rifampin is known to induce UDP‐glucuronosyltransferases (UGT), the transporter P‐glycoprotein, and a number of different CYP isoenzymes including CYP2B6 and CYP2C9.13 Isavuconazole undergoes secondary metabolism by UGT but only after metabolism by CYP3A4 and CYP3A5 (unpublished data). In vitro studies have also shown that isavuconazole is not a substrate of CYP2B6 or CYP2C9 or of P‐glycoprotein (unpublished data). Thus, the decrease in isavuconazole levels described in the current report may be attributed primarily to CYP3A4 induction.
It is well established that coadministration of rifampin with triazole antifungal agents can markedly reduce their plasma concentrations, which in turn may diminish their therapeutic efficacy.9, 13 Accordingly, treatment recommendations generally indicate avoiding coadministration of these agents or increasing the dose of the triazole.9, 13 Isavuconazole is no exception, and coadministration of rifampin and isavuconazole is contraindicated.
Isavuconazole levels increased during coadministration with ketoconazole. Isavuconazole exposure has also been found to increase approximately 2‐fold during coadministration with the strong CYP3A4 inhibitor lopinavir/ritonavir.14 Owing to the strength of the interaction between isavuconazole and ketoconazole and the potential for toxicity, coadministration of isavuconazole and ketoconazole is contraindicated.
Isavuconazole impacted CYP3A4 metabolism to a lesser degree than most existing triazole antifungals. Coadministration of midazolam with the strong CYP3A4 inhibitors posaconazole and itraconazole is associated with up to 5‐fold and up to 10‐fold increases in exposure of midazolam, respectively.6 Coadministration with voriconazole is also associated with a large (approximately 10‐fold) increase in midazolam AUC6; however, increases in ethinyl estradiol and norethindrone are relatively modest (less than 2‐fold).15 By comparison, fluconazole is a known moderate inhibitor of the CYP3A4 enzyme and increases the exposure of ethinyl estradiol,16 norethindrone,16 and midazolam17, 18 to a similar degree as isavuconazole. Although the interaction between isavuconazole and the oral contraceptives is not thought to be clinically relevant, caution should be exercised during concomitant use of isavuconazole and midazolam, and possible dose adjustments of midazolam should be considered.
In summary, these studies support that isavuconazole is a sensitive substrate of the CYP3A4 isoenzyme, and together with an additional study in which its effects on sirolimus PK were demonstrated,19 they support that isavuconazole is also a moderate inhibitor of CYP3A4. This is an important factor to consider for any clinical use in multimorbid patients, and appropriate precautions will need to be taken with concomitant medications.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information
Acknowledgments
Isavuconazonium sulfate was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. The rifampin study was funded by Basilea Pharmaceutica International Ltd; the remaining studies were funded by Astellas Pharma Global Development, Inc. Editorial support was provided by Neil M Thomas, PhD, CMPP and Ed Parr, PhD, CMPP, medical writers at Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. The authors are grateful for the contributions of the investigators and staff who conducted the clinical trials and to the subjects who volunteered for these studies.
Declaration of Conflicting Interests
Isavuconazole was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. R.T., S.A., D.K., C.L., H.P., T.Y., A.D. are employees of Astellas Pharma Global Development, Inc. A.S.‐H. is an employee of Basilea Pharmaceutica International Ltd. A.J.D. is an employee of Spaulding Clinical Research, who were contracted by Astellas Pharma Global Development, Inc. to perform work related to the study. C.H. is an employee of Covance Clinical Research, who were contracted by Astellas Pharma Global Development, Inc. to perform work related to the study. K.L. is an employee of Clinical Pharmacology of Miami, who were contracted by Astellas Pharma Global Development, Inc. to perform work related to the study. D.R. is an in‐house contractor employed by Astellas Pharma Global Development, Inc.
References
- 1. Azie N, Neofytos D, Pfaller M, Meier‐Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73:293–300. [DOI] [PubMed] [Google Scholar]
- 2. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. [DOI] [PubMed] [Google Scholar]
- 3. Dignani MC. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000 Prime Rep. 2014;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maertens JA, Raad, II , Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised‐controlled, non‐inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 5. Marty FM, Ostrosky‐Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single‐arm open‐label trial and case‐control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 6. Rybak JM, Marx KR, Nishimoto AT, Rogers PD. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy. 2015;35:1037–1051. [DOI] [PubMed] [Google Scholar]
- 7. Miceli MH, Kauffman CA. Isavuconazole: a new broad‐spectrum triazole antifungal agent. Clin Infect Dis. 2015;61:1558–1565. [DOI] [PubMed] [Google Scholar]
- 8. Schmitt‐Hoffman A, Desai A, Kowalski D, Pearlman H, Yamazaki T, Townsend R. Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH. Int J Clin Pharmacol Ther. 2016; DOI:10.5414/CP202434. [DOI] [PubMed] [Google Scholar]
- 9. Nivoix Y, Ubeaud‐Sequier G, Engel P, Leveque D, Herbrecht R. Drug‐drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab. 2009;10:395–409. [DOI] [PubMed] [Google Scholar]
- 10. Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–180. [DOI] [PubMed] [Google Scholar]
- 11. Greenblatt DJ, Harmatz JS. Ritonavir is the best alternative to ketoconazole as an index inhibitor of cytochrome P450‐3A in drug‐drug interaction studies. Br J Clin Pharmacol. 2015;80:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viljoen J, Azie N, Schmitt‐Hoffmann AH, Ghannoum M. A phase 2, randomized, double‐blind, multicenter trial to evaluate the safety and efficacy of three dosing regimens of isavuconazole compared with fluconazole in patients with uncomplicated esophageal candidiasis. Antimicrob Agents Chemother. 2015;59:1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baciewicz AM, Chrisman CR, Finch CK, Self TH. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin. 2013;29:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Yamazaki T, Desai A, Han D, et al. Pharmacokinetic interaction between isavuconazole and a fixed‐dose combination of lopinavir 400 mg/ritonavir 100 mg in healthy subjects. Clin Pharm Drug Dev. 2017;6:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co‐administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilbert J, Messig M, Kuye O, Friedman H. Evaluation of interaction between fluconazole and an oral contraceptive in healthy women. Obstet Gynecol. 2001;98:218–223. [DOI] [PubMed] [Google Scholar]
- 17. Ahonen J, Olkkola KT, Neuvonen PJ. Effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. Eur J Clin Pharmacol. 1997;51:415–419. [DOI] [PubMed] [Google Scholar]
- 18. Olkkola KT, Ahonen J, Neuvonen PJ. The effects of the systemic antimycotics, itraconazole and fluconazole, on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Anesth Analg. 1996;82:511–516. [DOI] [PubMed] [Google Scholar]
- 19. Groll AH, Desai A, Han D, et al. Pharmacokinetic assessment of drug‐drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information


