Abstract
Purpose
To investigate central and peripheral corneal endothelial cell density (ECD) in relation to Baerveldt (BV) glaucoma drainage device (GDD) tube corneal (TC) distance.
Methods
Prospective study of all patients scheduled for glaucoma tube surgery with 36 months follow‐up. A BV GDD was inserted into the anterior chamber (AC). Anterior segment optical coherence tomography (AS‐OCT) scans were made to determine the TC distance. Central and peripheral ECD was measured, preoperatively and at 3, 6, 12, 24 and 36 months postoperatively.
Results
Fifty‐three eyes were included [primary open‐angle glaucoma, (n = 13); secondary glaucoma, (n = 30); and primary angle‐closure glaucoma, (n = 10)]. Central ECD significantly decreased during follow‐up, with a mean decrease of 4.54% per year (p < 0.001), and 6.57% in the peripheral quadrant closest to the BV GDD tube (PQC, p < 0.001). In the PQC, a yearly decrease of 1.57% was shown after transiridial tube placement versus 7.43% after placement ‘free’ into the AC (p = 0.006). Endothelial cell (EC) loss was related to TC distance (mean 1.69 mm), with a central loss of 6.20% and 7.25% in the PQC per year with shorter TC distances, versus a central loss of 4.11% and 5.77% in the PQC per year with longer TC distances (outside mean ± 2SD, p < 0.001). A difference in EC loss by glaucoma subtype was not identified.
Conclusion
The TC distance is of significant influence on corneal ECD, a shorter TC distance causing more severe EC loss, especially in the PQC. Transiridial placement of the BV GDD tube seems safer than placement ‘free’ into the AC.
Keywords: anterior segment OCT, corneal endothelial cell loss, glaucoma, glaucoma drainage implants
Introduction
Aqueous shunts are becoming increasingly popular in the surgical treatment of glaucoma. Although mainly used in cases of previously failed trabeculectomy or in cases with uveitic, neovascular or other forms of refractory glaucoma, they are increasingly used as a primary surgical procedure. Recent studies have demonstrated good short‐term results with early postoperative complication rates even lower than after trabeculectomy (Gedde et al. 2012a,b).
One of the most worrisome long‐term complications after aqueous shunt implantation is the development of corneal decompensation. A few studies have reported on corneal endothelial cell (EC) loss after aqueous shunt implantation (McDermott et al. 1993; Topouzis et al. 1999; Gedde et al. 2007; Kim et al. 2008; Minckler et al. 2008; Stein et al. 2008). The presence of the tube in the anterior chamber (AC) is thought to accelerate the loss of endothelial cells (McDermott et al. 1993; Kim et al. 2008; Mendrinos et al. 2009; Hau & Barton 2009). Endothelial cell loss was reported after Molteno glaucoma drainage device (GDD) insertion (McDermott et al. 1993). Recently, an 8% decrease in central endothelial cell density (ECD) was found after 6 months and 12.6% after 12 months of Ahmed GDD implantation. In that study, the superotemporal area, which was closest to the tube, showed the largest ECD decrease (Kim et al. 2008; Mendrinos et al. 2009; Lee et al. 2009). In cases of corneal decompensation, the central corneal thickness (CCT) was increased.
As far as we are aware of, no study prospectively investigated the corneal ECD after the implantation of the Baerveldt (BV) GDD in the long term. Therefore, the aim of the present study was to investigate the central and peripheral ECD and the CCT up to and including three years postoperatively after the implantation of the BV GDD. In addition, we studied the relation between the BV tube corneal (TC) distance and EC loss as well as the relation between tube position in the AC and EC loss.
Patients and Methods
This study was conducted in accordance with the World Medical Association Declaration of Helsinki. Glaucoma patients were eligible for inclusion if they were scheduled for Baerveldt BG 101‐350 (Abbott Medical Optics®, Chicago, Illinois, USA) glaucoma implantation with placement of the drainage tube into the AC during the period 2009–2011. All surgeries were conducted by a single surgeon (HB) at the University Eye Clinic, Maastricht, The Netherlands. The central and peripheral ECD, the CCT and the TC distance were measured preoperatively and at 3, 6, 12, 24 and 36 months postoperatively. Patients requiring additional intra‐ocular surgery during follow‐up were included until the moment of the additional surgery and thereafter excluded from further analysis.
Surgical technique
A fornix‐based conjunctival flap was made in the superotemporal quadrant. A 101–350 mm2 BV GDD was placed underneath the lateral and superior rectus muscles, and the plate was sutured to the globe with two nylon 8‐0 sutures (Ethicon–Johnson & Johnson, Somerville, New Jersey, USA). The BV GDD tube was tied off using a Vicryl 7‐0 suture (Ethicon–Johnson & Johnson, Somerville, New Jersey, USA) and fixated to the sclera with one nylon 8‐0 suture. The AC was entered using a 23‐G needle after which the BV GDD tube (with an intra‐ocular tube length of 3 mm) was inserted bevel up into the AC. To prevent conjunctival erosion, the extra ocular part of the tube was patched with donor sclera before closing the conjunctival wound.
A tube positioned parallel to the iris plane, with the tube lying flat on the iris, was preferred. In pseudophakic eyes, especially with a more shallow AC, an additional technique was adopted to keep the tube away from the cornea by placing the tube transiridial through a peripheral iridectomy (PI). In eyes with a previously performed trabeculectomy, the previously created iridectomy was used, and in the other eyes, a new iridectomy was created using the 23‐G needle that was used to enter the AC. There were no differences in AC maintainer between the two subgroups (BV GDD tube ‘free’ in the AC and transiridial placement of the tube). Subanalyses were performed for these two subgroups.
Corneal endothelium
The corneal endothelium and the CCT were analysed by specular microscopy (Konan Noncon ROBO Pachy SP‐9000). A ‘center‐dot’ method was used to measure the ECD. The ECD and CCT measurements were performed preoperatively and at 3, 6, 12, 24 and 36 months postoperatively.
The central ECD was measured in all eyes and in four peripheral locations (at 2, 6, 10 and 12 o’ clock) at 3 mm from the centre of the cornea (Fig. 1). Patients were asked to look at the internal fixation light. When they were unable to see the fixation light peripherally due to visual field loss, a peripheral measurement could not be obtained. Because of the known variability in ECD data, three consecutive endothelial images of the central and each peripheral corneal quadrant were obtained and analysed using the dot method, after which the centres of 50 or more contiguous cells were marked. The mean values of these three measurements were used for further statistical analyses.
Figure 1.
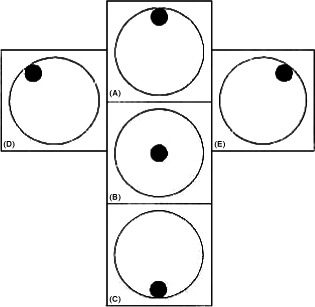
Endothelial cell density (ECD) measurement, (A) Superior corneal ECD, (B) Central corneal ECD, (C) Inferior corneal ECD, (D) Left superior corneal ECD, (E) Right superior corneal ECD.
Tube to cornea distance
All patients underwent anterior segment imaging using Visante™ optical coherence tomography (OCT) (Carl Zeiss Meditec Inc, Dublin, California, USA). At all follow‐up visits, the patients were asked to look at the internal fixation light (through an undilated pupil). The research assistants were instructed not to indent the eyeball during the examination.
Two anterior segment single (ASS) scans were acquired in the angle parallel to the BV GDD tube in the AC, at 3, 6, 12, 24 and 36 months postoperatively, to measure the TC distance. The mean of these two TC distances was used for further statistical analyses.
Scan analysis
All scans were analysed using Zeiss software (version 2.0.1.88) as available on the Visante™ OCT. This software has a claimed accuracy of 0.01 mm in measurements.
The distance between the superior tip of the BV GDD tube and the corneal endothelium was determined using the ‘Safety Centre tool’. The upper end of this tool automatically adheres perpendicular to the corneal endothelium after which the other end can be dragged to the superior tip of the BV GDD tube (Fig. 2).
Figure 2.

Anterior segment optical coherence tomography (AS‐OCT) showing the Baerveldt tube and the tube corneal distance in pink. (A) Tube ‘free’ in the AC, (B) Transiridial placement of the tube.
Extreme (short and long) TC distances were defined as outside mean ± 2SD.
Statistical analysis
To analyse the corneal ECD during the follow‐up period, linear mixed model (LMM) analyses were performed. This model was chosen because it uses all available ECD data of each eye to fit the best linear model. The LMM was fitted with ECD as a dependent variable with time as covariate and assuming a random intercept per eye. To test for possible differences in EC loss, the TC distance was also included in the model as well as an interaction term ‘time’ x ‘TC distance’. Our approach was to fit a linear mixed model using the following equation: y i (t,d) = α + α i + β 1 * t + β 2 *d + β 3 * t * d + ε i, where y i (t,d) is the ECD count of an eye i after a follow‐up of t months with TC distance d; α represents the intercept; α i represents the random intercept per eye; β 1 is the effect of time after a follow‐up of t months; β 2 is the effect distance; β 3 is the interaction effect of time and TC distance with a follow‐up of t months and TC distance d; ε i is the residual error. All data were analysed using the statistical software package spss ® version 18.0 (SPSS Inc., Chicago, IL, USA). Firstly, the central EC loss was determined for the total study group, after which the peripheral EC loss, for the quadrant closest to the BV tube (PQC) and the other quadrants, was assessed. Secondly, central and peripheral EC losses were compared between glaucoma subtypes [primary open‐angle glaucoma (POAG), secondary glaucoma and primary angle‐closure glaucoma (PACG)]. The TC distance was included in the model to analyse the influence of TC distance on central and peripheral EC loss. Finally, central and peripheral EC losses were compared between patients with the tube positioned ‘free’ in the AC or with transiridial fixation.
The central corneal thickness (CCT) was evaluated preoperatively and at all time‐points during follow‐up.
Results
Baseline data
Fifty‐three eyes of 35 patients (mean age 61 ± 14 years, 54% female) were included. Fifty‐one per cent were right eyes. A total of 45 eyes were pseudophakic at the time of surgery, and there were no aphakic eyes. Fifty‐six per cent of subjects had secondary glaucoma, 24.5% POAG and 18% PACG. Sixty‐seven per cent of eyes underwent trabeculectomy in the past. Baseline characteristics are listed in Table 1. All included patients gave their informed consent.
Table 1.
Baseline characteristics
| No of eyes (n) | 53 |
| Mean age in years (mean ± SD) | 61 ± 14 |
| Gender (% men) | 46 |
| Eye (% right eye) | 51 |
| Lens status (% pseudophakic) | 84.9 |
| Glaucoma type | |
| Primary open‐angle glaucoma (%) | 24.5 (n = 13) |
| Secondary glaucoma (%) | 56.6 (n = 30) |
| Primary angle‐closure glaucoma (%) | 18.9 (n = 10) |
| Previous trabeculectomy (%) | 67.8 |
| Endothelial cell density preoperatively (cells/mm2) | |
| Tube ‘free’ in the anterior chamber (mean ± SD) | 2091 ± 344 |
| Transiridial placement of the tube (mean ± SD) | 2017 ± 547 |
Preoperatively, the mean central ECD was 2052 ± 572 cells/mm2, with no statistically significant difference between the peripheral quadrants and the central ECD. There were no statistically significant differences in baseline ECD in phakic versus pseudophakic eyes. The mean central ECD was 2176 ± 105 in phakic eyes and 1887 ± 181 in pseudophakic eyes (p = 0.12). Preoperatively, the mean ECD was 2091 ± 344 in the group where the BV GDD was placed ‘free’ into the AC and the mean ECD was 2017 ± 547 preoperatively in the transiridial group.
A statistically significant difference in EC loss by glaucoma subtype could not be identified in baseline ECD.
The BV GDD tube was placed ‘free’ into the AC in 31 eyes; in 22 eyes, the BV GDD tube was placed transiridial (11 through pre‐existent PI; 11 through a newly created PI). Preoperatively, the AC depth was 3.6 ± 0.6 mm in eyes where the BV GDD was placed ‘free’ into the AC and 3.3 ± 0.7 mm in eyes with transiridial placement.
In the total study population, two eyes underwent a reoperation with repositioning of the BV tube because of a very short tube corneal distance (one eye had a tube corneal touch). One eye developed cornea decompensation after prolonged hypotony, which persisted after tying off the BV drainage tube. These eyes were excluded from further analyses.
Central and peripheral ECD
Table 2 shows the absolute central ECD during follow‐up. The central ECD significantly decreased during follow‐up, with a mean decrease of 4.54% per year (p < 0.001). In the PQC, a yearly decrease of 6.57% was found (p < 0.001), versus 4.53% in the other peripheral quadrants. The decrease in the PQC was significantly larger compared to the central (p = 0.005) and the other peripheral quadrants (p = 0.003). The β‐coefficients of the LMM analysis and their 95% confidence interval (CI) are shown in Table 3.
Table 2.
Central endothelial cell density (ECD) at different time points
| Period | Mean ECD (cells/mm2) ± SD |
|---|---|
| Preoperatively | 2052 ± 572 |
| 3 months postop | 2016 ± 592 |
| 6 months postop | 2012 ± 607 |
| 12 months postop | 1911 ± 640 |
| 24 months postop | 1898 ± 657 |
| 36 months postop | 1771 ± 662 |
Table 3.
β‐coefficients and their 95% confidence intervals showing difference in endothelial cell density (ECD) loss between the quadrant closest to the Baerveldt (BV) and the other quadrants
| Parameter | Estimate | Sig. | 95% Confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Intercept | 1939 | <0.001 | 1776 | 2101 |
| β 1, the effect of time after a follow‐up of t months | −10.6 | <0.001 | −12.6 | −8.7 |
| β 2, other quadrants | 14.3 | 0.408 | −19.6 | 48.2 |
| β 2, PQC | 0 | |||
| β 3, the interaction effect time – other quadrants | 3.23 | 0.003 | 1.10 | 5.36 |
| β 3,the interaction effect time – PQC | 0 | |||
PQC = peripheral quadrant closest to the BV GDD tube.
Tube position and ECD
The central ECD showed a yearly decrease of 3.54% after transiridial placement and of 5.55% when the BV GDD tube was placed ‘free’ into the AC. However, this difference was not statistically significant (p = 0.37).
In the PQC, we found a yearly decrease of 1.57% after transiridial placement and of 7.43% when the BV GDD tube was placed ‘free’ into the AC. This was statistically different (p = 0.006).
Tube corneal distance and ECD
The mean TC distance was 1.7 ± 0.6 mm for the whole study group at all follow‐up time‐points. The mean TC distance at all follow‐up moments was 1.7 ± 0.5 mm when the tube was placed ‘free’ into the AC, and 1.6 ± 0.7 mm after transiridial placement. Linear mixed model (LMM) analysis revealed that central and peripheral EC loss was significantly influenced by the TC distance (Table 4): the shorter the distance, the higher the loss. A central loss of 6.20% and a loss of 7.25% in the PQC per year was found for a TC distance of 1.1 mm, versus a central loss of 4.11% and a loss of 5.77% in the PQC of per year for a TC distance of 2.0 mm (outside mean ± 2SD, p < 0.001).
Table 4.
β‐coefficients and their 95% confidence intervals showing the difference in central endothelial cell density (ECD) (cells/mm2) loss for different tube corneal (TC) distances
| Parameter | Estimate | Sig. | 95% Confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Intercept (cells/mm2) | 2229 | <0.001 | 1988 | 2470 |
| β 1, the effect of time after a follow‐up of t months | −15.7 | <0.001 | −20.8 | −10.5 |
| β 2, the effect of TC distance | −142.5 | 0.008 | −246.8 | −38.2 |
| β 3, the interaction effect | 4.51 | 0.004 | 1.46 | 7.55 |
Central corneal thickness
The CCT did not statistically change over time. The mean preop CCT was 562.7 μm (549–576), 563.9 μm (547.7–580.1) after 1 year, 565.2 μm (546.2–584.1) after 2 years and 566.4 μm (544.7–588.1) after 3 years of follow‐up; p = 0.38.
Discussion
This three year follow‐up study shows a significant decrease in corneal ECD in eyes with a BV GDD tube placed into the AC. Endothelial cell (EC) loss occurred most extensively in the PQC. Additionally, a tube position closer to the endothelium was found to accelerate EC loss; the shorter the distance, the higher the loss. The CCT did not statistically change over time; however, it may be that with further follow‐up, several eyes may eventually develop corneal decompensation. In normal adult corneas, the central human corneal ECD gradually declines at an average of approximately 0.6% per year (Bourne et al. 1997). Previous studies report a lower ECD in glaucoma patients compared to healthy subjects (Gagnon et al. 1997) (Kocabeyoglu et al. 2016). The secondary glaucoma group of our study consists mainly of uveitic eyes, traumatic eyes and eyes after previous vitrectomy for retinal detachment. Our statistical analyses did not find a statistically significant difference in baseline ECD between secondary glaucoma, POAG or PACG.
Less EC loss was found after transiridial placement of the BV GDD when compared with placement of the BV tube ‘free’ into the AC. In a previous study by the same authors, it was demonstrated that the BV GDD tube remains in a stable position after transiridial placement, whereas the tube moves closer to the endothelium after placement ‘free’ into the AC (Tan et al. 2014). The more stable position of the tube after transiridial placement may explain the lower EC loss in this subgroup. No excessive EC loss seems to occur in the early postoperative stage, implying that there is no additional EC loss according to the surgically induced trauma. An explanation for this might be the recovery capability of the corneal endothelium after intra‐ocular surgery, when lost endothelium might be renewed by stem cells from a niche at the posterior limbus (Whikehart et al. 2005). This phenomenon is also observed in a study of Storr‐Paulsen et al., who studied the central ECD loss after mitomycin C‐augmented trabeculectomy. An ECD loss of 9.5% was found 3 months after mitomycin C‐augmented trabeculectomy, and 10% after 12 months (Storr‐Paulsen et al. 2008).
To determine the course of postoperative EC loss, a linear mixed model analysis was chosen, as this provides the possibility to use all available data to fit a best linear model. A transiridial placement of the BV tube was only chosen for pseudophakic eyes, to prevent cataract formation in phakic eyes.
The present results show a lower ECD decrease in comparison with a previous published paper by Lee et at (Lee et al. 2009), in which EC loss in eyes with an Ahmed glaucoma tube in the anterior chamber was studied. In their paper, a mean central EC loss of 15.4% was found 24 months after the implantation of an Ahmed glaucoma valve S2. In our study, the mean central EC loss after 24 months was 9.08% (4.54% per year). Lee et al. (2009) found an ECD decrease of 22.6% in the superotemporal quadrant (closest to the Ahmed tube) at 24 months. The peripheral EC loss after 24 months was 13.14% in our study. However, we found the TC distance to be of crucial importance in the decline of the number of endothelial cells. A short TC distance of 1.1 mm led to a central EC loss of 6.20% per year and a peripheral EC loss of 7.25% per year.
There are several reasons why our results differ from those of Lee et al. (2009) Their mean follow‐up time was 19 months, whereas our subjects were followed for 36 months. Furthermore, the TC distance was not taken into account in their study. The different designs of the implants could also play a role. The Ahmed tube is valved and might induce more fibrosis compared to the non‐valved BV glaucoma implant (Choritz et al. 2010). Another possible explanation for the difference in EC loss might be the different material of the glaucoma drainage devices. Both the BV GDD and the Ahmed valve have a silicone drainage tube. The Ahmed‐valved plate body and casing are made of polypropylene whereas the BV GDD plate is made of silicone. Despite the plate not being in contact with the corneal endothelium and being situated outside the anterior chamber, it is possible that due to backflow of aqueous humour through the drainage tube immunological inflammation occurs, which might contribute to the difference in EC loss as reported in our study as compared to the study of Lee (Freedman & Iserovich 2013).
Another important observation of our study is the influence of the TC distance on corneal EC loss. This finding underlines the results published by Doors et al. (2010) demonstrating increased EC loss in the event of a shorter distance between a phakic intra‐ocular lens and the corneal endothelium. A recent retrospective study published by Koo et al. (2015) showed that tubes situated close to the cornea seem to lead to an increased EC loss. In our study, a shorter TC distance led to more EC loss, most severe in the PQC. After transiridial placement of the BV GDD tube, outcomes were better, which may be explained by the observation that the distance of the tube to the peripheral corneal endothelium from the entry site in the AC is in general larger than after direct insertion of the tube into the AC through the iridocorneal angle. A difference in EC loss by glaucoma subtype was not identified in our study. Even in uveitic eyes, where transiridial placement of the tube might probably elicit an inflammatory response, the ECD did not show a significant faster decrease as compared to POAG or PACG. But, as we have relatively small numbers, some caution must be taken into account by interpreting these results. However, our findings support that tube placement far away from the corneal endothelium should be preferred to limit EC loss. To reach this goal, a transiridial approach (as an addition to sulcus placement or a pars plana approach) seems a valuable and safe option.
References
- Bourne WM, Nelson LR & Hodge DO (1997): Central corneal endothelial cell changes over a ten‐year period. Invest Ophthalmol Vis Sci 38: 779–782. [PubMed] [Google Scholar]
- Choritz L, Koynov K, Renieri G, Barton K, Pfeiffer N & Thieme H (2010): Surface topographies of glaucoma drainage devices and their influence on human tenon fibroblast adhesion. Invest Ophthalmol Vis Sci 51: 4047–4053. [DOI] [PubMed] [Google Scholar]
- Doors M, Berendschot TT, Webers CA & Nuijts RM (2010): Model to predict endothelial cell loss after iris‐fixated phakic intraocular lens implantation. Invest Ophthalmol Vis Sci 51: 811–815. [DOI] [PubMed] [Google Scholar]
- Freedman J & Iserovich P (2013): Pro‐inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant blebs and their relationship to pressure. Invest Ophthalmol Vis Sci 54: 4851–4855. [DOI] [PubMed] [Google Scholar]
- Gagnon MM, Boisjoly HM, Brunette I, Charest M & Amyot M (1997): Corneal endothelial cell density in glaucoma. Cornea 16: 314–318. [PubMed] [Google Scholar]
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ & Schiffman JC (2007): Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow‐up. Am J Ophthalmol 143: 23–31. [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC & Tube Versus Trabeculectomy Study Group (2012a): Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow‐up. Am J Ophthalmol 153: 804–814. e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL & Tube versus Trabeculectomy Study Group (2012b): Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow‐up. Am J Ophthalmol 153: 789–803. e782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau S & Barton K (2009): Corneal complications of glaucoma surgery. Curr Opin Ophthalmol 20: 131–136. [DOI] [PubMed] [Google Scholar]
- Kim CS, Yim JH, Lee EK & Lee NH (2008): Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Experiment Ophthalmol 36: 142–147. [DOI] [PubMed] [Google Scholar]
- Kocabeyoglu M, Mocan MC, Irkec M & Karakaya J (2016): In Vivo Confocal Microscopic Evaluation of Corneas in Patients With Exfoliation Syndrome. J Glaucoma 25: 193–197. [DOI] [PubMed] [Google Scholar]
- Koo EB, Hou J, Han Y, Keenan JD, Stamper RL & Jeng BH (2015): Effect of glaucoma tube shunt parameters on cornea endothelial cells in patients with Ahmed valve implants. Cornea 34: 37–41. [DOI] [PubMed] [Google Scholar]
- Lee EK, Yun YJ, Lee JE, Yim JH & Kim CS (2009): Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2‐year follow‐up. Am J Ophthalmol 148: 361–367. [DOI] [PubMed] [Google Scholar]
- McDermott ML, Swendris RP, Shin DH, Juzych MS & Cowden JW (1993): Corneal endothelial cell counts after Molteno implantation. Am J Ophthalmol 115: 93–96. [DOI] [PubMed] [Google Scholar]
- Mendrinos E, Dosso A, Sommerhalder J & Shaarawy T (2009): Coupling of HRT II and AS‐OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye (Lond) 23: 1836–1844. [DOI] [PubMed] [Google Scholar]
- Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD & Singh K (2008): Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology 115: 1089–1098. [DOI] [PubMed] [Google Scholar]
- Stein JD, Ruiz D Jr, Belsky D, Lee PP & Sloan FA (2008): Longitudinal rates of postoperative adverse outcomes after glaucoma surgery among medicare beneficiaries 1994 to 2005. Ophthalmology 115: 1109–1116. e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr‐Paulsen A, Norregaard JC, Ahmed S, Storr‐Paulsen T & Pedersen TH (2008): Endothelial cell damage after cataract surgery: divide‐and‐conquer versus phaco‐chop technique. J Cataract Refract Surg 34: 996–1000. [DOI] [PubMed] [Google Scholar]
- Tan AN, De Witte PM, Webers CA, Berendschot TT, De Brabander J, Schouten JS & Beckers HJ (2014): Baerveldt drainage tube motility in the anterior chamber. Eur J Ophthalmol 24: 364–370. [DOI] [PubMed] [Google Scholar]
- Topouzis F, Coleman AL, Choplin N, Bethlem MM, Hill R, Yu F, Panek WC & Wilson MR (1999): Follow‐up of the original cohort with the Ahmed glaucoma valve implant. Am J Ophthalmol 128: 198–204. [DOI] [PubMed] [Google Scholar]
- Whikehart DR, Parikh CH, Vaughn AV, Mishler K & Edelhauser HF (2005): Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis 11: 816–824. [PubMed] [Google Scholar]


