Abstract
Background
Many people diagnosed with haematological malignancies experience anaemia, and red blood cell (RBC) transfusion plays an essential supportive role in their management. Different strategies have been developed for RBC transfusions. A restrictive transfusion strategy seeks to maintain a lower haemoglobin level (usually between 70 g/L to 90 g/L) with a trigger for transfusion when the haemoglobin drops below 70 g/L), whereas a liberal transfusion strategy aims to maintain a higher haemoglobin (usually between 100 g/L to 120 g/L, with a threshold for transfusion when haemoglobin drops below 100 g/L). In people undergoing surgery or who have been admitted to intensive care a restrictive transfusion strategy has been shown to be safe and in some cases safer than a liberal transfusion strategy. However, it is not known whether it is safe in people with haematological malignancies.
Objectives
To determine the efficacy and safety of restrictive versus liberal RBC transfusion strategies for people diagnosed with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without a haematopoietic stem cell transplant (HSCT).
Search methods
We searched for randomised controlled trials (RCTs) and non‐randomised trials (NRS) in MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1982), Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 6), and 10 other databases (including four trial registries) to 15 June 2016. We also searched grey literature and contacted experts in transfusion for additional trials. There was no restriction on language, date or publication status.
Selection criteria
We included RCTs and prospective NRS that evaluated a restrictive compared with a liberal RBC transfusion strategy in children or adults with malignant haematological disorders or undergoing HSCT.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
We identified six studies eligible for inclusion in this review; five RCTs and one NRS. Three completed RCTs (156 participants), one completed NRS (84 participants), and two ongoing RCTs. We identified one additional RCT awaiting classification. The completed studies were conducted between 1997 and 2015 and had a mean follow‐up from 31 days to 2 years. One study included children receiving a HSCT (six participants), the other three studies only included adults: 218 participants with acute leukaemia receiving chemotherapy, and 16 with a haematological malignancy receiving a HSCT. The restrictive strategies varied from 70 g/L to 90 g/L. The liberal strategies also varied from 80 g/L to 120 g/L.
Based on the GRADE rating methodology the overall quality of the included studies was very low to low across different outcomes. None of the included studies were free from bias for all 'Risk of bias' domains. One of the three RCTs was discontinued early for safety concerns after recruiting only six children, all three participants in the liberal group developed veno‐occlusive disease (VOD).
Evidence from RCTs
A restrictive RBC transfusion policy may make little or no difference to: the number of participants who died within 100 days (two trials, 95 participants (RR: 0.25, 95% CI 0.02 to 2.69, low‐quality evidence); the number of participants who experienced any bleeding (two studies, 149 participants; RR:0.93, 95% CI 0.73 to 1.18, low‐quality evidence), or clinically significant bleeding (two studies, 149 participants, RR: 1.03, 95% CI 0.75 to 1.43, low‐quality evidence); the number of participants who required RBC transfusions (three trials; 155 participants: RR: 0.97, 95% CI 0.90 to 1.05, low‐quality evidence); or the length of hospital stay (restrictive median 35.5 days (interquartile range (IQR): 31.2 to 43.8); liberal 36 days (IQR: 29.2 to 44), low‐quality evidence).
We are uncertain whether the restrictive RBC transfusion strategy: decreases quality of life (one trial, 89 participants, fatigue score: restrictive median 4.8 (IQR 4 to 5.2); liberal median 4.5 (IQR 3.6 to 5) (very low‐quality evidence); or reduces the risk of developing any serious infection (one study, 89 participants, RR: 1.23, 95% CI 0.74 to 2.04, very low‐quality evidence).
A restrictive RBC transfusion policy may reduce the number of RBC transfusions per participant (two trials; 95 participants; mean difference (MD) ‐3.58, 95% CI ‐5.66 to ‐1.49, low‐quality evidence).
Evidence from NRS
We are uncertain whether the restrictive RBC transfusion strategy: reduces the risk of death within 100 days (one study, 84 participants, restrictive 1 death; liberal 1 death; very low‐quality evidence); decreases the risk of clinically significant bleeding (one study, 84 participants, restrictive 3; liberal 8; very low‐quality evidence); or decreases the number of RBC transfusions (adjusted for age, sex and acute myeloid leukaemia type geometric mean 1.25; 95% CI 1.07 to 1.47 ‐ data analysis performed by the study authors)
No NRS were found that looked at: quality of life; number of participants with any bleeding; serious infection; or length of hospital stay.
No studies were found that looked at: adverse transfusion reactions; arterial or venous thromboembolic events; length of intensive care admission; or readmission to hospital.
Authors' conclusions
Findings from this review were based on four studies and 240 participants.
There is low‐quality evidence that a restrictive RBC transfusion policy reduces the number of RBC transfusions per participant. There is low‐quality evidence that a restrictive RBC transfusion policy has little or no effect on: mortality at 30 to 100 days, bleeding, or hospital stay. This evidence is mainly based on adults with acute leukaemia who are having chemotherapy. Although, the two ongoing studies (530 participants) are due to be completed by January 2018 and will provide additional information for adults with haematological malignancies, we will not be able to answer this review's primary outcome. If we assume a mortality rate of 3% within 100 days we would need 1492 participants to have a 80% chance of detecting, as significant at the 5% level, an increase in all‐cause mortality from 3% to 6%. Further RCTs are required in children.
Plain language summary
Restrictive or liberal red blood cell transfusion policies for people with blood cancer
Review question
To determine the benefit and harm of a restrictive red blood cell transfusion strategy when compared with a liberal red blood cell transfusion strategy for people diagnosed with a blood cancer (for example leukaemia, lymphoma, myeloma) who were receiving intensive treatments for their disease (chemotherapy or stem cell transplantation).
Background
People with blood cancers often have anaemia (low haemoglobin level) due to their underlying cancer or its treatment (chemotherapy or a stem cell transplant. Haemoglobin is essential for carrying oxygen around the body.
A red blood cell transfusion is given to increase the haemoglobin level to prevent symptoms of anaemia occurring, or to treat symptoms of anaemia. The decision to give a red cell transfusion should balance its benefits with its potential risks (e.g. rash, fever, chills, developing breathing problems). These reactions are usually mild and easily treated, severe reactions to red blood cell transfusions are extremely rare. In high‐income countries the likelihood of getting an infection from a red blood cell transfusion is very low, however the risk is much higher in low‐income countries. The need for a red cell transfusion is usually guided by the haemoglobin level. In people with other conditions a transfusion is usually given if the haemoglobin level drops to around 70 g/L to 80 g/L (restrictive transfusion strategy). People with blood cancers may benefit from a higher haemoglobin level (100 g/L to 120g/L, liberal transfusion strategy), they may bleed less and have an improved quality of life. In people undergoing surgery or people who are admitted to intensive care units a restrictive transfusion strategy has been shown to be as safe as, or safer than a liberal transfusion strategy.
Study characteristics
We searched for randomised and prospective non‐randomised trials. Six studies met our inclusion criteria, four are completed and two are still ongoing. An additional study is awaiting classification. The completed studies were conducted between 1997 and 2015 and included 240 participants. One study included children receiving a stem cell transplant and it was stopped early due to safety concerns (six children), the other three studies only included adults, 218 adults with acute leukaemia receiving chemotherapy, and 16 with a blood cancer receiving a stem cell transplant. Three studies were randomised controlled trials and the fourth was a non‐randomised study. The haemoglobin threshold of the restrictive strategies varied across the studies.
The sources of funding were reported in all four studies. One study was industry sponsored.
Key results
The evidence is current to June 2016 and is mainly based on adults with acute leukaemia who are having chemotherapy.
A restrictive red blood cell transfusion policy may reduce the number of red blood cell transfusions received by an individual.
A restrictive red blood cell transfusion policy may have little or no effect on: whether an individual receives a red blood cell transfusion; death due to any cause; bleeding; or hospital stay.
We are uncertain whether a restrictive red blood cell transfusion policy affects quality of life, or the risk of developing a serious infection.
No studies were found that looked at: adverse reactions to transfusion; development of blood clots; length of stay in intensive care; or need to be readmitted to hospital.
There are two ongoing trials (planning to recruit 530 adults) that are due to be completed by January 2018 and will provide additional information for adults with blood cancers. There are no ongoing trials in children.
Quality of evidence
The overall quality of the evidence was very low to low as the included studies were at considerable risk of bias, the estimates were imprecise, and most of the evidence was only for adults with acute leukaemia.
Summary of findings
Background
Description of the condition
Approximately 30% to 100% of people diagnosed with haematological malignancies experience anaemia during the course of their disease and red blood cell (RBC) transfusions play an essential supportive role in their management (Knight 2004). Intensive chemotherapy administered to treat people with haematological malignancies often results in prolonged periods of myelosuppression necessitating frequent RBC transfusions, especially in the setting of haematopoietic stem cell transplantation (HSCT). Indeed, people with haematology‐oncology medical conditions are amongst the largest consumers of RBC transfusions (Borkent‐Raven 2010; Javadzadeh Shahshahani 2015; Tinegate 2016; Whitaker 2015).
Of an estimated 108 million blood donations collected worldwide, over 50% are collected in high‐income countries, which represent only 18% of the world’s population (WHO 2015). Blood availability, distribution and safety vary widely throughout the world. In developing countries, blood supply is inadequate and the most common source is family or paid blood donors (WHO 2015). The prevalence of transfusion‐transmissible infections (TTI) in blood donations is extremely low in high‐income countries but is significantly higher in low‐ and middle‐income countries (Table 3; Bolton‐Maggs 2014). Although all donated blood should be screened for infections before use, currently 25 countries are not able to screen for one or more of the minimum mandatary blood screening infections: human immunodeficency virus (HIV), hepatitis B (HBV), hepatitis C (HCV) and syphilis (Table 4; WHO 2015). Irregular supply of test kits is one of the most commonly reported barriers to screening blood donations (WHO 2015). As well as the risk from infection, the risk of immune modulation, alloimmunisation, and iron overload are not uncommon events associated with blood transfusions (Bolton‐Maggs 2014; Vamvakas 2007). The limited supply and increasing cost of blood has placed additional emphasis on appropriate transfusion practice (Murphy 2011; Shander 2011).
1. Important complications of transfusion and their approximate frequency in the UK extrapolated from data from the Serious Hazards of Transfusion (SHOT) scheme 2014 (Bolton‐Maggs 2014) compared with low income countries (WHO 2015).
| Transfusion Risk | Frequency in the UK (units transfused) ( Bolton‐Maggs 2014) | Frequency in low income countries ( WHO 2015 ) |
| ABO incompatible red cell transfusion | 3.7 in 1 million (10 cases per 2663488 transfusions) | unknown |
| Transfusion‐related acute lung injury | 0.4 in 1 million (10 cases per 2663488 transfusions) | unknown |
| Transfusion associated circulatory overload | 34.1 in 1 million (91 cases per 2663488 transfusions) | unknown |
2. Frequency of infection in donated blood in high and low income countries (WHO 2015).
| Transfusion transmitted infection | Frequency in high income countries | Frequency in low income countries |
| HIV | 2 in 100,000 (IQR 0.4 in 100,000 to 20 in 100,000) | 850 in 100,000 (IQR 480 in 100,000 to 2000 in 100,000) |
| HBV | 20 in 100,000 (IQR 8 in 100,000 to 24 in 100,000) | 3590 in 100,000 (IQR 2010 in 100,000 to 6080 in 100,000) |
| HCV | 20 in 100,000 (IQR 0.4 in 100,000 to 22 in 100,000) | 1070 in 100,000 (IQR 630 in 100,000 to 1690 in 100,000) |
IQR=interquartile range
Description of the intervention
Marked progress has been made on developing strategies for RBC transfusion. For many years, based on the findings of Adam 1942, RBC transfusion was recommended when the haemoglobin level dropped below 100 g/L or the haematocrit was below 30%. Although originally recommended to improve outcomes in people undergoing surgery with poor anaesthetic risk, this perioperative threshold was adopted in many other areas of clinical practice. This was challenged by the Transfusion Requirements In Critical Care (TRICC) trial, a landmark randomised controlled trial (RCT) that assessed the rates of death and severity of organ dysfunction using restrictive versus liberal transfusion strategies in the critical care setting (Hébert 1999). The restrictive strategy was to maintain the haemoglobin between 70 g/L to 90 g/L with a trigger for transfusion when the haemoglobin dropped below 70 g/L, whereas the liberal transfusion strategy was to maintain the haemoglobin between the range of 100 g/L to 120 g/L, with a threshold for transfusion when the haemoglobin dropped below 100 g/L. The restrictive transfusion strategy was seen to be as safe as the liberal transfusion strategy with similar 30‐day overall mortality rates. In a recent systematic review that assessed a restrictive versus liberal RBC transfusion strategies (31 trials, 9813 participants), the restrictive strategy was associated with a reduction in the number of RBC units transfused, with no differences in mortality and overall morbidity compared to a liberal strategy (Holst 2016). Moreover, results from a Cochrane review (19 trials, 6264 participants) also highlighted that restrictive transfusion strategies are safe, with no impact on mortality, cardiac events, myocardial infarction, stroke, pneumonia and thromboembolism when compared with liberal strategies (Carson 2016).
The current NICE guidelines recommend implementing restrictive RBC transfusion thresholds of a haemoglobin level after transfusion of between 70 g/L to 90 g/L, when major haemorrhage, acute coronary syndrome and chronic anaemia are absent (NICE 2015). In addition, the transfusion of a single RBC unit is also recommended for adults who do not have active bleeding.
Carson 2016 indicated that on average, restrictive transfusion strategies reduced the risk of receiving a transfusion by 39% compared to the liberal transfusion strategy. Similar findings were also seen from a retrospective cohort study in 139 people with haematological malignancies receiving intensive chemotherapy, this also Implementing a single‐unit transfusion policy that saved 25% of RBC units and, in addition to reducing the risks associated with allogeneic blood transfusions (Berger 2012).
People receiving intensive therapy for haematological malignancy have different physiological requirements to the critical care population as they are generally more physically active as well as experiencing more profound periods of erythropoietic suppression. Great uncertainty remains about whether it is safe to 'withhold' blood for patients with haematological malignancy until a lower threshold level is reached, and about the impact this poses on mortality, bleeding and adverse events, in addition to quality of life (Valeri 1998).
How the intervention might work
The aim of RBC transfusion is generally to improve oxygen delivery to the organs ‐ which could minimise morbidity, improve anaemia symptoms and enhance quality of life. However, unnecessary and unsafe blood transfusion practices could expose recipients to the risk of serious adverse transfusion reactions and TTI (WHO 2015).
The traditional transfusion threshold is a haemoglobin level of 100 g/L. This level has been lowered to haemoglobin levels between 60 g/L to 80 g/L. The Transfusion Requirement in Critical Care (TRICC) trial was the first to suggest that a 70 g/L was a safe threshold and perhaps safer than the traditional 100 g/L threshold (Hébert 1999). Several later trials assessed transfusion strategies in different clinical areas concluding that a restrictive transfusion strategy would trigger transfusion of a certain number of RBC units below a defined haemoglobin concentration (Carson 2011; Colomo 2008; Hajjar 2010). A liberal transfusion strategy would adopt a higher haemoglobin threshold and patients would tend to receive more blood components. However, the liberal transfusion strategy is still in use, especially when treating coronary artery syndromes (Carson 2013). A Cochrane review on this topic including 19 RCTs with over 6000 trial participants enrolled from many different clinical settings ‐ mainly surgical, trauma and critical care (Carson 2016) ‐ concluded that restrictive transfusion strategies did not appear to impact the rate of adverse events or the 30‐day mortality compared to liberal transfusion strategies. It was actually associated with a significant decline in hospital mortality.
In the recent systematic review on this topic (Holst 2016), only one RCT specifically included people with haematological malignancies. This study recruited 60 people with leukaemia undergoing chemotherapy or HSCT (Webert 2008). This multicentre, single‐blinded pilot RCT assessed the feasibility of a larger trial to identify the effect of the haemoglobin concentration on bleeding risk using higher transfusion thresholds and determined that a larger RCT was feasible.
Why it is important to do this review
Both anaemia and transfusion are associated with increased morbidity and mortality and national clinical guidelines on the appropriate use of blood transfusion and systems to monitor its safety are not currently in place throughout the world (WHO 2015). The optimal RBC transfusion strategy remains unclear in patients with haematological malignancy receiving intensive treatment. Due to the lack of available good quality evidence, no clear transfusion strategies are recommended in national guidelines for haematology patients or for those receiving chemotherapy (Carson 2012; Gunn 2012; McCelland 2010). Therefore, great variability exists at a local level. In Canada, the majority of centres have adopted a transfusion trigger of 80 g/L with a general reluctance to use a lower trigger of 70 g/L in all but one of 15 centres surveyed (Tay 2011). In the UK, a national audit also showed great variability in practice (Tinegate 2016).
In a variety of patient populations, including critical care patients, RBC transfusions have been associated with increased mortality and morbidity including more frequent infections, poor wound healing and increased arterial and venous thrombotic events (Blajchman 2005; Hébert 1999; Khorana 2008). The accumulated evidence showed restrictive RBC transfusion could improve outcomes for critically ill people (Salpeter 2014), or people with acute upper gastro‐intestinal bleeding (Villanueva 2013). Therefore, there is growing evidence to suggest that a restrictive RBC transfusion strategy is more beneficial than the liberal strategy in many clinical situations. However, this has not been systematically addressed in people with haematological malignancies.
A low haemoglobin level has been associated with an increased bleeding time (Ho 1998; Valeri 1998), and RBC transfusions have been shown to improve haemostasis in people with anaemia who are thrombocytopenic (Ho 1996) or uraemic (Livio 1982). Therefore, in people with haematological malignancies, a restrictive RBC transfusion policy may increase their risk of bleeding further in addition to their increased risk due to thrombocytopenia.
Objectives
To determine the efficacy and safety of restrictive versus liberal red blood cell (RBC) transfusion strategies for people diagnosed with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell transplantation (HSCT).
Methods
Criteria for considering studies for this review
Types of studies
As it was likely that the number of randomised controlled trials (RCTs) that could be included in this review were small given the authors' tacit knowledge of the subject, we included data from non‐randomised studies (NRS) for all outcome evaluations. We only included NRS which were prospective in design and included a rigorous definition of the interventions and outcomes measured as an attempt to assess as wide a data volume as possible. Such studies included quasi‐randomised, non‐RCTs and prospective cohort studies. We excluded retrospective and uncontrolled studies (e.g. case series) due to the potential for unacceptable bias. Despite the inferiority of study quality that most NRS offered, a review of eligible NRS might identify a need for further RCTs and prove informative to the design of such a trial.
Types of participants
We included studies conducted on people with haematological malignancies requiring a red blood cell (RBC) transfusion whilst receiving intensive chemotherapy or radiotherapy, or both, with or without HSCT. We Included studies on participants of all ages, with the exception of neonates (up to 28 days old). We accepted the individual study's definition of intensive chemotherapy or radiotherapy, sufficient to cause myelosuppression. The number of potential intensive chemotherapy or radiotherapy regimens, or both was broad. We listed some examples, but these were not limited to, chemotherapeutic regimes listed on public oncology web sites such as:
Public Health England, Systemic anti‐cancer therapy Chemotherapy Dataset;
Cancer Care Ontario Drug Formulary;
Macmillan Cancer Support Combination Chemotherapy Regimen;
BC Cancer Agency Lymphoma and Myeloma Chemotherapy Protocols;
Royal Surrey County Hospital NHS Foundation Trust Haematology Chemotherapy.
We included studies that involved participants at all stages of treatment. We included studies on people with high risk myelodysplasia (refractory anaemia with excess of blasts (RAEB), and RAEB in transformation into acute leukaemia, (RAEB‐t)) if intensive chemotherapy or radiotherapy, or both was received. We excluded studies on people with a haematological malignancy requiring transfusion support only.
Where a study included mixed populations of participants, we planned to only use data relevant to haematological malignancies. There were no studies with mixed populations of participants with haematological and solid tumours or non‐intensive and intensive chemotherapy. In future updates of this review, when a study includes mixed populations of participants with haematological and solid tumours, we will only use data relevant to haematological malignancies. If the data are not available within the published literature or through direct author contact, we will exclude the study if fewer than 80% of the study population had haematological malignancies or high‐risk myelodysplasia. In future updates of this review, when studies include participants receiving intensive and non‐intensive chemotherapy or radiotherapy for haematological malignancies, we will only include data from the former group. If these data are not available, we will exclude the study if fewer than 80% of the study population were receiving intensive chemotherapy or radiotherapy for haematological malignancies.
Types of interventions
We included allogeneic red blood cell (RBC) transfusion strategies defined as 'restrictive' and 'liberal'. We expected that such definitions varied between studies, however, we accepted the individual study definitions of these strategies such that the restrictive (intervention) group in all included studies should receive a transfusion of allogeneic RBC units below a certain 'trigger' or 'threshold' haemoglobin or haematocrit level. The control group should receive a transfusion of RBC in accordance with a more liberal transfusion policy such as when a higher haemoglobin or haematocrit threshold was reached. For the purposes of this Cochrane review, we applied a standard published equation to convert haematocrit to haemoglobin: Haemoglobin (g/dL) = Haematocrit (%)/3 (Quintó 2006).
Types of outcome measures
We reported the outcomes from RCTs and NRS separately (Reeves 2011).
Primary outcomes
All‐cause mortality: all deaths (undefined time period); deaths during a defined period: short‐ (zero to seven days), medium‐ (eight to 30 days), and long‐term intervals (31 to 100 days) where day zero is the start of the follow‐up period or randomisation, or both.
Secondary outcomes
Mortality
-
Deaths due to:
Infection;
Bleeding;
Adverse transfusion reactions.
-
Death within 30 days of receiving:
Intensive radiotherapy;
Intensive chemotherapy;
HSCT.
Adverse events
-
Bleeding episodes
Any bleeding (e.g. World Health Organization (WHO) grades 1 to 4, National Cancer Institute's common terminology criteria for adverse events (CTCAE 2009), grades 1 to 5 or equivalent)
Clinically significant bleeding (e.g. WHO/CTCAE grades > 2 or equivalent)
Severe bleeding (e.g. WHO/CTCAE grade > 3 or equivalent)
Adverse transfusion reactions (e.g. TRALI, TACO, ABO incompatibility, TTI)
Serious infections (e.g. CTCAE > grade 3 or equivalent (CTCAE 2009))
Arterial or venous thromboembolic events
Toxicity score for HSCT recipients (e.g. Bearman Toxicity Score > grade 3 or equivalent (Bearman 1988))
Blood product requirements
RBC transfusion requirements and intervals
Platelet transfusion requirements and intervals
Other
Quality of life
Length of hospital admission
Length of intensive care admission
Hospital readmission
We tried to report secondary outcomes over meaningful periods common to as many studies as possible, such as the number and severity of bleeding episodes per person per day of study.
Search methods for identification of studies
The Information Specialist of the Systematic Review Initiative (CD) formulated the search strategies in collaboration with the Cochrane Haematological Malignancies Group. The searches for this review were performed in to phases the first phase was run until December 2012, thereafter the second phase was run until November 2015.
Electronic searches
Bibliographic databases
We searched for RCTs and NRS in the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library 2016, Issue 6 ‐Appendix 1);
MEDLINE (1946 to 15 June 2016‐ Appendix 2);
Embase (1974 to 15 June 2016 ‐ Appendix 3);
CINAHL (1982 to 15 June 2016 ‐ Appendix 4);
PubMed (epublications only ‐ Appendix 5);
Transfusion Evidence Library (www.transfusionevidencelibrary.com) (1980 to 15 June 2016 ‐ Appendix 6);
LILACS (1982 to 15 June 2016 ‐ Appendix 7);
IndMed (1986 to 15 June 2016 ‐ Appendix 8);
KoreaMed (1995 to 15 June 2016 ‐ Appendix 9);
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (Thomson Reuters, 1990 to 15 June 2016 ‐ Appendix 10).
We combined searches in MEDLINE with the Cochrane RCT highly sensitive search filter, as detailed in Lefebvre 2011 and with the SIGN observational studies filter for MEDLINE. Also, we merged searches in Embase and CINAHL with the relevant SIGN RCT and observational studies filters (www.sign.ac.uk/methodology/filters.html).
Ongoing studies databases
We also checked for ongoing RCTs and NRS in the following databases to 15 June 2016:
ClinicalTrials.gov (Appendix 11);
ISRCTN Register (Appendix 12);
WHO International Clinical Trials Registry (ICTRP) (Appendix 13);
EU Clinical Trials Register (EUDRACT) (Appendix 14).
Searches were not limited by date, language or publication status.
Searching other resources
We augmented database searching with the following.
Handsearching reference lists
We checked the reference lists of all included studies, relevant reviews, conference abstracts and current treatment guidelines to identify additional studies that were not retrieved through databases searches.
Personal contact
We contacted the lead authors of relevant studies, study groups and international experts working in this field to identify any unpublished material, or to gather information regarding ongoing studies.
Data collection and analysis
Selection of studies
We selected studies according to Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Systematic Review Initiative’s Information Specialist (CD) initially screened all search hits for relevance against the eligibility criteria and discarded all those that were clearly irrelevant. Thereafter, two review authors (LE, RM) independently screened all the remaining references for relevance against the full eligibility criteria. We retrieved full‐text papers for all references for which a decision on eligibility could not be made from title and abstract alone. We assessed study design features against the review inclusion criteria. Additional information was requested from study authors as necessary to assess the eligibility for inclusion of individual studies. Two review authors (LE, RM) discussed the results of study selection and resolved all disagreements by discussion without the need to consult a third review author (MM). We recorded the reasons why potentially relevant studies failed to meet the eligibility criteria. We reported the results of study selection using a PRISMA flow diagram (Moher 2009) (Figure 1).
1.
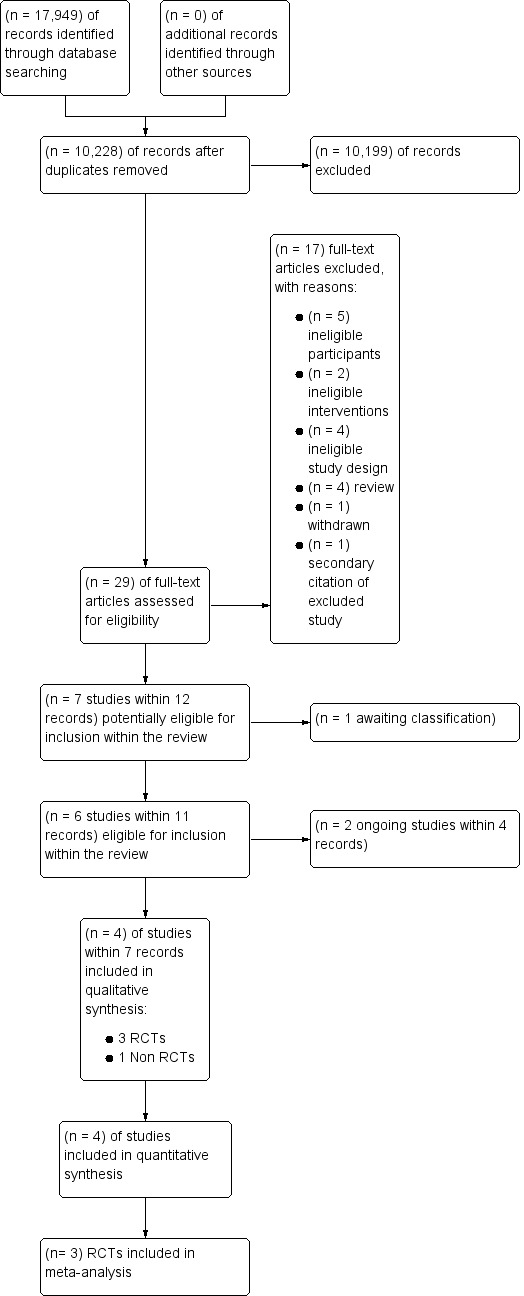
Study flow diagram for study selection
We initially assessed the RCTs. As there were not two or more well‐conducted eligible RCTs including at least 1000 patients, we extended the assessment and selection of studies to NRS.
Data extraction and management
As recommended in Higgins 2011a, two review authors (RM, LE) independently extracted data onto standardised forms and performed a cross‐check. The two review authors piloted the data extraction form for included RCTs and NRS. Any disagreements between the review authors were resolved by consensus. We were not blinded to the names of the study authors, institutions, journals or the study outcomes. If a trial had multiple publications we used one form only to extracted relevant data. When these sources provided insufficient information, we contacted the authors and study groups for additional details. One review author (RM) performed data entry into software, which a second review author (LE) checked for accuracy.
We extracted the following information for each study: 1. Source: Study ID; report ID; review author ID; date of extraction; ID of author checking extracted data; citation of paper; contact authors details; 2. Eligibility: Fate of study; reason for exclusion (as appropriate); 3. General study information: Publication type; study objectives; funding source; conflict of interest declared; other relevant study publication reviewed; 4. Study design: Was there a comparison; how were participants allocated to groups?; which part of the study were prospective?; on which variables was comparability between groups assessed?; type of study; 5. Study details and methods: Location; country; setting; number of centres; total study duration; recruitment dates; length of follow‐up; power calculation; primary analysis (and definition); stopping rules; method of sequence generation; allocation concealment; blinding (of clinicians, participants and outcome assessors); concerns regarding bias; inclusion & exclusion criteria; primary outcome(s); secondary outcomes; 6. Characteristics of interventions: Number of study arms; description of experimental arm; description of control arm; duration of red cell storage; frequency of minor ABO mismatched transfusions; other treatment (e.g. gamma irradiation); 7. Characteristics of participants: Age; gender; ethnicity; body surface area; primary diagnosis; stage of disease; category of intensive chemotherapy received; details of radiotherapy received; type of HSCT received; additional therapy received; risk of alloimmunisation; baseline haematology laboratory; subgroups evaluated; cofounders reported; 8. Participant flow: Total number screened for inclusion; total number recruited; total number excluded; total number allocated to each study arm; total number analysed (for primary outcome); number of allocated patients who received planned treatment; number of drop‐outs with reasons (percentage in each arm); protocol violations; missing data; 9. Outcomes: All cause mortality (undefined and within short, medium and long term periods); mortality due to infection, bleeding, TRALI, TACO, ABO incompatibility, TTI, other (with details); number and severity of bleeding episodes, adverse transfusion reactions, serious infections, toxicity score and quality of life scores; number and volume of red cell transfusion units received per patient; interval between red cell transfusions, number and volume of platelet doses received per patient; interval between platelet transfusion; duration of hospital admission; duration of intensive care admission; readmission to hospital during follow‐up period.
10. In the case of the NRS, we additionally extracted information on confounding factors, results from adjusted models and variables adjusted for.
Assessment of risk of bias in included studies
We assessed the quality of all included trials (RCTs) using the Cochrane risk of bias tool as described in Higgins 2011b. Two review authors (RM, LE) independently assessed each element of potential bias listed below as either 'high', 'low' or 'unclear risk of bias'. We also provided a brief description in the Characteristics of included studies table of the judgement statements upon which the review authors assessed potential bias. We reached a consensus on the degree of risk of bias through comparison of the review authors' statements and, where necessary, through consultation with a third author (SH).
To assess risk of bias, we addressed the following questions in the ’Risk of bias’ table for each included study.
Selection bias: Was the allocation sequence randomly generated? Was allocation adequately concealed prior to assignment?
Performance bias: Where possible, were the study participants and personnel adequately blinded?
Detection bias: Was blinding of the outcome assessors effective in preventing systematic differences in the way in which the outcomes were determined?
Attrition bias: Were incomplete outcome data adequately addressed for every outcome?
Reporting bias: Are reports of the study free of selective outcome reporting?
Other issues: Was the study apparently free of other problems that could put it at risk?
We assessed the quality of the NRS using an adapted Newcastle‐Ottawa scale (Wells 2013). We included an expansion of the comparability of cohorts section with the inclusion of a list of important confounding factors we felt should be addressed in the studies methods or analysis. Also, we included an additional question concerning equal follow‐up durations for mortality, the primary and secondary outcomes of this review. We scored the studies with reference to the Ottawa Hospital Research Institute's coding manual and scale guidelines. We provided judgement statements for each score in tabular form as for the 'Risk of bias' assessment of interventional trials. The score was reported out of a maximum of 10 stars using the following categories.
Selection (one star each, maximum four stars).
Representiveness of the exposed cohort
Selection of the non‐exposed cohort
Ascertainment of exposure
Demonstration that outcome of interest was not present at start of study
Comparability (maximum of two stars).
Recognition of at least 75% of the main potential confounding factors as listed below within study design or adjustment in analysis (two stars).
Primary diagnosis, separated by: haematological malignancy (acute leukaemia, high risk myelodysplasia, chronic lymphocytic leukaemia (CLL), myeloma, lymphoma) or natural history of primary diagnosis e.g. a) 'Indolent' (myeloma, low grade lymphoproliferative disorders (e.g. CLL, low grade lymphoma), acute lymphoblastic leukaemia/acute myeloid leukaemia in complete remission (CR1), chronic myeloid leukaemia in chronic phase, high risk myelodysplastic syndrome) or b) 'aggressive' (high grade lymphomas (e.g. diffuse large B‐cell lymphoma (DLBCL), Burkitt), all other stages of acute leukaemia, chronic myeloid leukaemia in accelerated or blast phase).
Age: variability in the age of patients included, e.g. paediatric (< 18 years) versus adult (> 18 years).
Gender: male to female ratio.
Previous severe bleeding (e.g. WHO/CTCAE grade > 3 or equivalent).
Use of anticoagulation during study.
Previous alloimmunisation.
Co‐existing cardiovascular disease.
Performance Status (e.g. ECOG, KPS).
HSCT: autograft versus allograft (allograft source: sibling, matched unrelated, cord donation; conditioning type: myeloablative including total body irradiation versus reduced intensity conditioning); harvest type: peripheral blood stem cell versus bone marrow harvest); ABO compatibility; cytomegalovirus (CMV) compatibility, stem cell dose received.
Radiation use in addition to intensive chemotherapy.
Recognition of 50% to 75% of the main potential confounding factors within study design or adjustment in analysis (one star).
Outcome (one star each, maximum of four stars).
Assessment of outcome.
Was follow‐up long enough for outcomes to occur?
Adequacy of follow‐up of cohorts.
Follow‐up equal between groups for primary and secondary outcomes?
We requested additional information from study authors as necessary to address the quality of individual studies. Two review authors discussed the results of the study quality assessment and try to resolve any discrepancies between themselves.
We planned to use the 'Risk of bias' assessment to explore statistical heterogeneity in each included study and to perform sensitivity analyses, but no meta‐analyses were performed. For both RCTs and NRS, we planned to exclude monocentric studies from a sensitivity analysis due to their higher likelihood of bias, but no meta‐analyses were performed.
Measures of treatment effect
We did not combine data retrieved from RCTs and NRS in a single meta‐analysis, but reported data separately (Reeves 2011).
We undertook quantitative assessments using Review Manager (RevMan) 2014.
Randomised controlled trials (RCTs)
For dichotomous outcomes, we recorded the number of outcomes in the treatment and control groups and estimated the treatment effect measures across individual studies as the relative effect measures (risk ratio with 95% confidence intervals (CIs)). Where the number of observed events was small (< 5% of sample per group), we used Peto odds ratio (Peto OR) method for analysis (Deeks 2011). For continuous outcomes using the same scale, we assessed the mean difference (MD) with 95% confidence intervals (CI), and continuous outcomes measured with different scales we planned to present the standard mean difference (SMD). No hazard ratios (HR) were reported, and we were unable to estimate the HR using the available data and a purpose built method based on the Parmar and Tierney tool (Parmar 1998; Tierney 2007).
Non‐randomised studies (NRS)
For the NRS, we planned to report the adjusted odds ratios (OR) with 95% confidence intervals (CIs), but none were reported. If the adjusted ORs were not available, we planned to make every effort before pooling to establish whether the groups were comparable at base‐line. No meta‐analyses were performed, we planned to use the random‐effects model for all analyses of NRS.
We were aware that after collecting the data and assessing the quality of the included studies, it might be necessary to alter the analysis plan based on methodological considerations. No changes were made because we only included one NRS.
All studies
We did not report the number needed to treat to benefit (NNTB) with CIs and the number needed to treat to harm (NNTH) with CIs because there were no differences between any of the outcomes. If we could not report the available data in any of the formats described above, we performed a narrative report.
Unit of analysis issues
There were no cluster‐randomised trials or cross‐over studies eligible for inclusion in this review.
We treated other unit of analysis issues in accordance with the advice given in Higgins 2011c. There was a unit of analysis issue for this review for the number of RBC or platelet transfusions reported in Webert 2008. Data were reported per participant day rather than per participant. However, the authors used a recurrent events analysis to take into account the repeated events data (Cook 1997).
Dealing with missing data
Where we identified data as missing or unclear in published literature, we contacted study authors directly. We recorded the number of patients lost to follow‐up for each study. Where possible, we planned to analyse data by intention‐to‐treat (ITT) but if insufficient data were available, we planned to present per protocol (PP) analyses (Higgins 2011c).
Assessment of heterogeneity
We only performed one meta‐analysis due to the small number of eligible studies and the lack of studies reporting similar outcomes.
We had planned to perform separate meta‐analyses for data extracted from RCTs and observational studies, if the clinical and methodological characteristics of studies were sufficiently homogeneous. We had also planned to assess the statistical heterogeneity of treatment effects between pooled studies using a Chi2 test with a significance level at P < 0.1. We would have used the I2 statistic to quantify the degree of potential heterogeneity and classify it as moderate if I2 > 30% or considerable if I2 > 75%.
We also anticipated that there would be at least moderate clinical and methodological heterogeneity within the included studies and the random‐effects model would be appropriate. If the heterogeneity was still considerable, we also planned to not report the overall summary statistic. In addition, we planned to assess potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
We did not perform a formal assessment of potential publication bias (small‐trial bias) (Sterne 2011), because the review included fewer than 10 trials.
Data synthesis
We performed analyses according to the recommendations of Deeks 2011 using aggregated data for analysis. For statistical analysis, we entered data into the Cochrane statistical package of Review Manager (RevMan) 2014. One review author (RM) entered the data into the software. A second author (LE) checked data for accuracy. We were only able to perform a limited number of meta‐analyses due to the small number of eligible studies and the perceived clinical and methodological heterogeneity between these studies. Aditionally, we provided a narrative quantitative summary for relevant outcome data retrieved from RCTs and NRS separately. If the qualitative assessment was not feasible, we therefore described outcome data extracted from each study individually and we also commented on any apparent trends.
We used the GRADE system to build a 'Summary of Findings' table, as suggested in Schünemann 2011a and Schünemann 2011b. In the review protocol we considered the following most relevant outcomes.
All‐cause mortality
Quality of life
Bleeding episodes
Serious infections
Length of hospital admission
Hospital readmission rate
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were performed because we only performed a limited number of meta‐analyses. We had planned to perform subgroup analysis for each outcome if data were available for the following.
Intensive chemotherapy versus radiotherapy.
Paediatric (less than 18 years) versus adult (18 years or older).
HSCT versus no HSCT.
-
For those that received HSCT:
autologous HSCT versus allogeneic HSCT;
reduced intensity transplant versus myeloablative HSCT.
-
For those that received intensive chemotherapy without HSCT:
induction versus consolidation chemotherapy (at least for acute leukaemia);
acute leukaemia versus non acute leukaemia.
We had also planned to undertake a subgroup analysis of results from studies including participants with pre‐existing cardiovascular diseases, if the relevant data were given separately in the study.
Sensitivity analysis
This was not feasible as we only performed a limited number of meta‐analyses and one of which was where data from the three included RCTs were combined. However, we originally planned to assess the robustness of our findings by performing the following sensitivity analyses where appropriate:
including only those studies with a 'low risk of bias' (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation);
including only those studies with less than a 20% dropout rate;
including only multicentric studies.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
See the PRISMA Flow Diagram (Figure 1). We identified a total of 17,949 potentially‐relevant records in the search conducted on 15 June 2016. There were 10,228 records after we removed duplicates. Two out of four reviewers (CB, LE, RM, JT) excluded 10,199 records on the basis of the abstract (two were review authors and two were protocol authors). The full texts of 29 articles were assessed for eligibility. Of those, 16 studies within 17 records were excluded with reasons; details were listed in the Characteristics of excluded studies table. We identified seven studies, within 12 records that met or may meet the review inclusion criteria. One of these is awaiting classification (NCT02099669), two of these are ongoing studies (Chantepie 2015; Tay 2011), three were randomised controlled trials (RCTs) (Robitaille 2013; Webert 2008; De Zern 2016) and one was a prospective non‐randomised study (NRS) (Jansen 2004).
We contacted the author of one potentially eligible for inclusion study (Mear 2014), as the study was only available as an abstract. We requested additional information related to the study design, study detailed data and whether the study authors were aware of any relevant ongoing or completed studies. The authors provided us with the requested information and thereafter we excluded the study as data were collected retrospectively.
We did not find any additional studies from other sources: checking lists of reference from the included studies and relevant reviews or by contacting experts in the field. All included studies were published in English.
Included studies
See Characteristics of included studies for full details of each study.
The four studies eligible for inclusion in this review were conducted between 1997 and 2015 and published between 2004 and 2016.
Three were RCTs, two were conducted in Canada (Robitaille 2013; Webert 2008), and one (De Zern 2016), in the USA. The remaining study was an NRS (Jansen 2004) conducted in the Netherlands.
Description of included RCTs
Study design and duration
We included three RCTs in this review, two were planned to be multicentre parallel RCTs (Robitaille 2013; Webert 2008) and De Zern 2016 was a single‐centre trial. Robitaille 2013 planned to recruit participants from six centres, however only one centre recruited participants prior to the trial's closure by the Data Safety Monitoring Board (DSMB). Two RCTs were open‐label trials (Robitaille 2013; De Zern 2016 ) and the other one was a single‐blinded trial (Webert 2008). Robitaille 2013 was closed after recruiting six participants because all three participants in the liberal transfusion arm were diagnosed with veno‐occlusive disease (VOD). The DSMB made their decision based on clinical observations, statistical comparison of the data in the two study arms, and statistical comparison of the data in the liberal transfusion arm with historical (non‐trial) data that used a restrictive threshold.
In Webert 2008, the mean follow‐up period was 25.9 days (standard deviation (SD), 8.4) in the restrictive group and 23.6 days (SD, 10.0) in the liberal group for a total of 1482 days of observation. In Robitaille 2013, all participants were followed up until day +100 from randomisation and in De Zern 2016, both groups had a similar follow‐up time, the median duration was 5.6 weeks for participants in the restrictive group and 6.1 weeks for the liberal group.
Study size and setting
The number of participants recruited ranged from six (Robitaille 2013) to 90 (De Zern 2016). In Robitaille 2013, the six participants were recruited from paediatric transplant centres, and in both Webert 2008 and De Zern 2016, 150 participants were inpatients recruited from tertiary referral haematology centres.
Two trials were conducted in Canada (Robitaille 2013; Webert 2008) and the third (De Zern 2016) was conducted in the USA. The studies were conducted between 2000 and 2015.
Study participants
In total, 156 participants were randomised, and 155 were included in the analyses, one participant withdrew from the De Zern 2016 study prior to any study transfusion. One hundred and fifty participants were adults: 134 with acute leukaemia receiving chemotherapy, and 16 with a haematological malignancy receiving an allogeneic stem cell transplant (De Zern 2016; Webert 2008). Six were children (mean age 11.7 years): three with acute myeloid leukaemia, two with myelodysplasia, and one with immune deficiency; all children received an allogeneic bone marrow transplant (Robitaille 2013).
In Webert 2008, there was no significant difference in the baseline haemoglobin level between the two groups ( restrictive: 96.3 g/L, liberal: 96.5 g/L, P = 0.96), in De Zern 2016, the baseline haemoglobin levels were significantly lower in the restrictive group (restrictive: median 83 g/L, liberal: median 89 g/L, P = 0.03) . The baseline haemoglobin levels were not reported in Robitaille 2013.
Participants with active bleeding were included in Robitaille 2013, however they were excluded in both Webert 2008 and De Zern 2016.
Participants with a history of cardiovascular disease were included in Robitaille 2013, excluded in Webert 2008, and only participants with acute coronary syndrome were excluded in De Zern 2016.
Study Interventions
The restrictive red blood cell (RBC) transfusion trigger thresholds varied between the three studies (70 g/L in Robitaille 2013 and De Zern 2016) and 80 g/L in Webert 2008).
The liberal RBC transfusion trigger threshold was the same in two RCTs (120 g/L) (Webert 2008; Robitaille 2013), and it was much lower at 80g/L in De Zern 2016.
The amount of RBC components transfused in each study arm were the same if a transfusion was required (10 mL/kg to 15 mL/kg) in Robitaille 2013; and two units of RBC components in Webert 2008 and De Zern 2016.
One trial reported the mean length of RBC component storage and this did not differ between the two groups (Robitaille 2013).
All trials reported off‐protocol RBC transfusions. In Webert 2008, about a third of RBC transfusions were given in the restrictive group and liberal group when the haemoglobin level was above the study thresholds. In De Zern 2016, there were two off‐protocol deviations, one participant in each trial arm received a blood transfusion above the study haemoglobin thresholds. In Robitaille 2013, protocol deviations were recorded and none of the participants received off‐protocol transfusions.
In two trials (Robitaille 2013; Webert 2008), prophylactic platelets transfusions were given when the platelet count fell below 10 x 109/L. In Robitaille 2013, the platelet count threshold was increased to 20 x 109/L when infection or fever occurred, in Webert 2008 this depended on the protocol of the treating institution or physician. No information on when a platelet transfusion was given to participants was provided by De Zern 2016.
All other chemotherapeutic and other interventions were performed according to standard treatment protocols.
Study outcomes
In Webert 2008, no single primary ocutome was prioritised and all five determined outcomes were considered equally important for the study feasibility, these included: bleeding; proportions of days of thrombocytopenia; usage of RBC and platelet components and blood donor exposure, bleeding symptoms and severity.
In Robitaille 2013, time to neutrophil recovery was the study's primary outcome. Secondary outcomes included: time to platelet recovery, usage of RBC and platelet components, length of hospital stay, immune reconstitution, overall survival, transplant‐related mortality, relapse, acute and chronic graft versus host disease (GvHD) and chimerism (Robitaille 2013). Several of these outcomes, including the planned primary outcome were not reported. The assessment of all long‐term events was planned to be performed at one, two and five years follow‐up.
In De Zern 2016, the safety and tolerability of a restrictive transfusion strategy in comparison with a liberal strategy was the primary outcome of the study. Secondary outcomes included fatigue, bleeding, response to therapy, vital status on day 60, length of hospital stay and the number of RBC units transfused, the number of platelets transfused per participant and the feasibility of conducting a larger RCT. Some outcomes were planned in the trial registration but not reported in the published paper, these were treatment‐related mortality, end‐organ dysfunction, number of participants with Eastern Cooperative Oncology Group (ECOG) < 2 performance status, the incidence of cross‐over between the two groups due to symptomatic anaemia, and cost‐effective analysis. All outcomes were planned to be assessed at day 60 from the trial onset.
Study funding
All three included trials were publicly funded; De Zern 2016 was sponsored by Sidney Kimmel Comprehensive Cancer Center; Robitaille 2013 was funded by Fonds de la Recherche en Sante (grant 9967 and 24460) and C17 research network and Webert 2008 was funded by Canadian Blood Services & CIHR Canada Research Chair.
Description of included NRS
Study design and duration
We included one NRS with prospective data collection, the mean follow‐up period was 30 days for the restrictive group and 32 days for the liberal group (Jansen 2004). Data were collected as part of a previous RCT of chemotherapy agents in acute myeloid leukaemia.
Study size and setting
Eighty‐four participants were recruited from two inpatient haematology units within the department of haematology in Rotterdam (the Netherlands) from June 1997 to December 2001.
Study participants
Participants were aged 15 to 60 years with newly diagnosed acute myeloid leukaemia treated with induction chemotherapy (ARA‐C and Idarubicin) . Participants were assigned to one of the following transfusion strategies based on where they were treated for their acute leukaemia. The two groups were comparable with no significant differences with regard to age, gender or French America British (FAB) classification of acute myeloid leukaemia. Participants with active bleeding were included, but participants with severe cardiac dysfunction were excluded.
Study intervention
Restrictive transfusion strategy: (age dependent): participants aged less than 25 years received a RBC transfusion when their haemoglobin was less than 72 g/L; participants aged 25 to 50 years received a RBC transfusion when their haemoglobin was less than 80 g/L; participants aged 50 to 70 received a RBC transfusion when their haemoglobin was less than 88 g/L. They received one unit of RBCs.
Liberal transfusion strategy: participants received a RBC transfusion when their when haemoglobin level was less than 96 g/L. They received two units of RBCs at each transfusion.
Off‐protocol transfusion: No off‐protocol transfusion was reported, though the plan was to always transfuse blood regardless of the haemoglobin readings when signs and symptoms of decreased oxygen transportation capacity occurred.
Study outcomes
The study's primary outcome measures were to show the differences of the total number of RBC transfusions and the number of units of RBCs given per transfusion between the two groups. Secondary outcomes included: mortality, bleeding, infections, cardiac arrhythmia and cardiac dysfunction, response to chemotherapy and the myeloid:erythroid ratio of the bone marrow smears.
Study funding
The initial RCT (HOVON 29 study) was partially funded by pharmaceutical companies.
Ongoing studies
We found two ongoing RCTs, published within three records, eligible for inclusion in this review (Chantepie 2015; Tay 2011). Both included ongoing studies are open‐label parallel RCTs with two intervention groups. One is a single‐centre RCT (Chantepie 2015) conducted in France and the second RCT is a multicentre Canadian RCT (Tay 2011). A total of 530 adult participants are planned to be recruited across the two ongoing trials by 2018. See Characteristics of ongoing studies.
Excluded studies
See Characteristics of excluded studies for further details. Reasons for exclusion were mainly involving non‐haematological oncological participants, retrospective study design and reviews, and interventions that were not compatible with our inclusion criteria.
Five studies were conducted on non‐haematological participants (Almeida 2013; Bruun 2011; ISRCTN26088319; NTR2684; Yakymenko 2015).
Five studies were not RCTs or NRS with no prospective design (Bercovitz 2011; Lightdale 2012; Mear 2014; Paananen 2009; Patil 2013).
Two studies compared different interventions such as one versus two RBC units (Abels 1991; Berger 2012).
Four records were reviews (Bercovitz 2011; Carson 2014; Holst 2013; Prescott 2016).
Risk of bias in included studies
See Figure 2 and Figure 3 for visual representations of the ’Risk of bias’ assessments across all studies and for each item in the included studies. See the Characteristics of included studies section of the ’Risk of bias’ table for further information about the bias identified within the individual trials.
2.
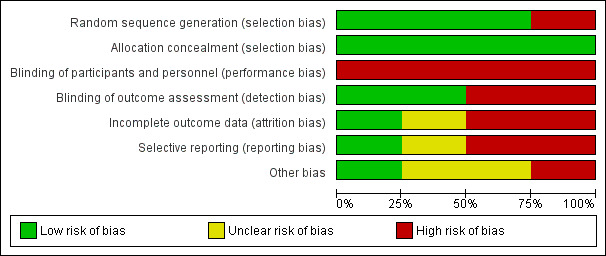
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
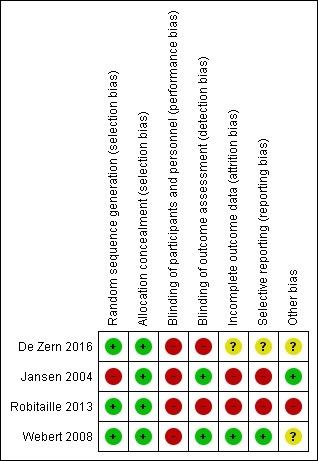
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias for included RCTs
Allocation
We assessed all three RCTS as low risk of selection bias due to adequate methods of sequence generation and allocation concealment.
Randomisation sequence generation:
All three trials provided information on method of randomisation. In Robitaille 2013 and De Zern 2016, randomisation was generated by computer and an Internet‐based randomisation web site was used to assign participants to either intervention or control in Webert 2008. In Webert 2008, participants were stratified by their treatment centre and diagnosis, and treatment allocation was scheduled by using a computer‐based generated random treatment allocation; this was developed with variable blocking factors and the size of the blocks selected randomly from a limited number of possibilities. In De Zern 2016, the random‐number sequence was generated using computer software.
Allocation concealment:
All three trials provided an adequate description of allocation concealment. Two trials used a web‐based randomisation system (Robitaille 2013; Webert 2008). One trial used sealed opaque sequentially numbered envelopes to allocate participants to either study arm (De Zern 2016).
Blinding
Blinding of participants and personnel:
We assessed all three trials as 'high risk' of performance bias. In Webert 2008, the study participants were aware of the intervention status and in both De Zern 2016 and Robitaille 2013 the study design was open‐label.
Blinding of clinical assessors:
We assessed Webert 2008, as 'low risk'' of detection bias, the personnel performing the clinical assessments were blinded to the participants' treatment allocation and to the haemoglobin levels.
We assessed De Zern 2016 and Robitaille 2013, as 'high risk'' of detection bias, both trials were open‐label design studies with no blinding.
Incomplete outcome data
We assessed one trial as 'low risk' of attrition bias (Webert 2008). All participants were accounted for within the study flow diagram and there were no withdrawals.
We assessed one trial as 'unclear risk' of attrition bias as the number of participants who withdrew from study was slightly higher from the restrictive group (De Zern 2016).
We assessed Robitaille 2013, as 'high risk' of attrition bias because all participants in the liberal transfusion arm were withdrawn from the study prior to day 100.
Selective reporting
We assessed Webert 2008 as 'low risk' of reporting bias as all study outcomes were prespecified by the authors and were provided for the study two groups.
We assessed De Zern 2016 as 'unclear risk' of reporting bias as some of the outcomes listed by the authors in the trial registration were not mentioned in the published paper. These outcomes were: mortality related to treatment, end‐organ dysfunction, number of participants with Eastern Cooperative Oncology Group (ECOG) performance status < 2, the incidence of treatment cross‐over because of symptomatic anaemia and cost‐effective analysis of restrictive RBC transfusion. The authors responded to our request for clarification by stating these outcomes would be the subject of a second paper.
We assessed Robitaille 2013 as 'high risk' of reporting bias due to the early discontinuing of the study, many of the planned outcomes were not reported or their analysis was not performed.
Other potential sources of bias
We assessed two trials as 'unclear risk' of other bias (De Zern 2016;Webert 2008). In De Zern 2016, the baseline haemoglobin level was significantly lower in the restrictive group, the analysis was adjusted for baseline haemoglobin, and a risk of contamination between the two groups reported as some participants crossed over from the restrictive to the liberal group. The two groups in Webert 2008 were not totally comparable as the number of study days after reaching the target haemoglobin levels differed between the groups, and it took longer for the liberal group to reach the threshold.
We assessed Robitaille 2013 as 'high risk' of other bias because the study was stopped early due to an increase incidence of VOD and therefore may substantially overestimate the risk of harm associated with a liberal RBC transfusion strategy.
Risk of bias for included NRS:
We used the Newcastle‐Ottawa Quality assessment scale (a star rating tool of risk of bias in three broad areas: selection of cohort, comparability cohort and outcomes) to assess the risk of bias for the one included NRS (Jansen 2004) . Details are presented in Table 5.
3. Jansen 2004: 'Risk of bias' assessment for observational studies using the Newcastle‐Ottawa Scale (Wells 2013).
| Risk of Bias | Assessment | Support for judgement |
| Selection (one star each, maximum four stars) | 2 stars | |
| Representativeness of the exposed cohort | 0 stars | This study only included participants with AML as opposed to all patients with haematological malignancies |
| Selection of the non‐exposed cohort | 0 star | Participants in the control were from a second haematology centre, but there was no information to reassure that this cohort was drawn from the same community as exposed cohort |
| Ascertainment of exposure | 1 star | Secondary analyses from HOVON 29, prospective randomised controlled trial |
| Demonstration that outcome of interest was not present at start of study | 1 stars | The primary outcome and other outcomes were defined and were based on events that occurred after the study started |
| Comparability of cohort on design and analyses (maximum of two stars) Recognition of at least 75% of the main potential confounding factors (2 stars) Recognition of 50% to 75% of the main potential confounding factors (1 star) | 0 stars | < 50% of potential cofounders considered and sex, age and AML type were adjusted for in the multiple regression model. There was no discussion on previous severe bleeding, use of anticoagulation, use of radiotherapy in addition to chemotherapy, previous cardiovascular disease, previous alloimmunisation or performance status |
| Outcome (one star each, maximum of four stars) | 2 stars | |
| Assessment of outcome | 0 stars | Not described |
| Was follow‐up long enough for outcomes to occur? | 1 star | Yes, 31 days from chemotherapy |
| Adequacy of follow‐up of cohorts | 0 star | Reported for all, except unclear for infection, mean Hb during follow‐up, total number of platelet/red blood cell units received |
| Follow‐up equal between groups for primary and secondary outcomes? | 1 star | Follow‐up not significantly different |
| Additional concerns | None | |
| Overall assessment | 4 stars |
AML = acute myeloid leukaemia Hb = haemoglobin
Selection of cohort
We awarded two out of a possible four stars for selection of cohort, one for the ascertainment of exposure as this study was part of a randomised controlled trial (HOVON 29), and the second star was given because the outcomes of interest were prespecified. No stars were awarded for the representative of the exposed cohort; only participants with acute myeloid leukaemia were included. Additionally, the selection of the non‐exposed cohort, was not clear with insufficient information to suggest that participants in the control group (non‐exposed), were drawn from the same community as the exposed cohort.
Comparability of the cohort
We gave zero out of a possible two stars for comparability of the cohort because the multiple regression model only adjusted for gender, age and acute myeloid leukaemia type. There was no considerations to other possible confounders such as severe bleeding, use of anticoagulation, use of radiotherapy in addition to chemotherapy, cardiovascular disease, previous alloimmunisation or performance status.
Assessment of outcomes
We awarded two out of a possible four stars for assessment of outcomes, one for adequate follow‐up time for the assessed outcomes and the second for the equal follow‐up between the two groups for both primary and secondary outcomes. We did not give any stars for assessment of outcomes. Hence, no information was given whether the outcomes were assessed blindly, and whether the follow‐up period was adequate. Some information was missing with regard to the incidence of infections, mean haemoglobin during follow‐up, total number of platelets and RBC units received.
Effects of interventions
for the main comparison.
| Restrictive compared with liberal for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support | ||||||
| Patient or population: people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support Setting: Hospitals, haematology centres Intervention: Restrictive Comparison: Liberal red blood cell transfusion RCTs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with liberal red blood cell transfusion RCTs | Risk with Restrictive | |||||
| All‐cause mortality at 31 to 100 days | Study population 61 per 1000 |
15 per 1000 (1 to 163) |
RR 0.25 (0.02 to 2.69) |
95 (2 RCTs) |
⊕⊕⊝⊝ LOW1 |
|
| Quality of life |
Liberal group: median 4.5 (IQR: 3.6 to 5) Restrictive: median 4.8 (IQR: 4 to 5.2)a |
89 (1 RCT) |
⊕⊕⊝⊝ VERY LOW2,3,4 |
|||
| Number of participants with any bleeding | Study population 639 per 1,000 |
595 per 1000 (467 to 754) | RR 0.93 (0.73 to 1.18) | 149 (2 RCTs) | ⊕⊕⊝⊝ LOW2,3 | |
| Number of participants with clinically significant bleeding | Study population 443 per 1,000 |
456 per 1000 (332 to 633) | RR 1.03 (0.75 to 1.43) | 149 (2 RCTs) | ⊕⊕⊝⊝ LOW2,3 | |
| Serious infections | Study population 400 per 1,000 |
492 per 1000 (296 to 816) |
RR 1.23 (0.74 to 2.04) | 89 (1 RCT) |
⊕⊝⊝⊝ VERY LOW2,3,4 |
|
| Length of hospital admission (days) |
Liberal: median 36 days (IQR: 29.2 to 44) Restrictive: median: 35.5 days (IQR: 31.2 to 43.8) |
89 (1 RCT) |
⊕⊕⊝⊝ LOW2,3 |
|||
| Hospital readmission rate ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The level of evidence was downgraded by 2 due to imprecision. 2The level of evidence was downgraded by 1 due to imprecision. 3 The level of evidence was downgraded by 1 due to indirectness, the studies only included adults. 4The level of evidence was downgraded by 1 due to risk of bias (unblinded study).
aThis is a ten‐point scale with a score of zero indicating no fatigue and a score of ten indicating the worst possible fatigue. The median fatigue scare was similar for both groups; P = 0.53.
Interquartile range: IQR
Summary of findings 2. Summary of findings of NRS.
| Restrictive compared with liberal for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support | ||||||
| Patient or population: people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support Setting: Department of haematology Intervention: Restrictive Comparison: Liberal red blood cell transfusion NRS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with liberal red blood cell transfusion RCTs | Risk with Restrictive | |||||
| All‐cause mortality at 31 to 100 days |
Liberal: 1 death (46 participants) Restrictive: 1 death (38 participants) |
84 (1 study) | ⊕⊝⊝⊝ VERY LOW1 | Mean 31 days follow‐up | ||
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ||
| Number of participants with any bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ||
| Number of participants with clinically significant bleeding |
Liberal: 8 (46 participants) Restrictive: 3 (38 participants) |
84 (1 study) | ⊕⊝⊝⊝ VERY LOW1 | The study authors reported that there was no significant difference between the two groups. | ||
| Serious infections ‐ not reported | ‐ | ‐ | ‐ | ‐ | ||
| Length of hospital admission ‐ not reported | ‐ | ‐ | ‐ | ‐ | ||
| Hospital readmission rate ‐ not reported | ‐ | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The level of evidence was downgraded by 1 due to imprecision.
We reported results from the three included RCTs and the one NRS individually in this review.
Effects of the interventions from included RCTs
Primary outcome: all‐cause mortality
All‐cause short‐term mortality, within zero to seven days from the study start
One of the three included RCTs provided data for this outcome (Robitaille 2013). No deaths occurred in either treatment arm; all six participants were alive at seven days.
All‐cause medium‐term mortality, within eight to 30 days from the study start
One of the three included RCTs provided data for this outcome (Robitaille 2013). No deaths occurred in either treatment arm; all six participants were alive at 30 days.
All‐cause long‐term mortality, within (31 to 100 days) from the study start
Two of the three included RCTs provided data for this outcome (De Zern 2016; Robitaille 2013). No deaths occurred in one of the studies (Robitaille 2013), and in De Zern 2016 one death occurred in the restrictive group and two deaths in the liberal group. There was no evidence for a difference in the number of participants who died between a restrictive and liberal RBC transfusion strategy (two studies; 95 participants; risk ratio (RR) 0.25, 95% CI 0.02 to 2.69, P = 0.26) (low‐quality evidence) (Analysis 1.1)
1.1. Analysis.
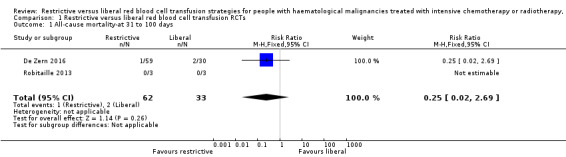
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 1 All‐cause mortality‐at 31 to 100 days.
The small number of children enrolled in Robitaille 2013 were followed up for two years with one death occurred at six months from the liberal group due to relapse from leukaemia.
Secondary outcomes
Mortality
Deaths due to: infection; bleeding; adverse transfusion reactions
One of the three included RCTs reported: death due to infection; death due to bleeding or death due to transfusion reactions (Robitaille 2013). No deaths occurred in either treatment arm; all six participants were alive at 100 days.
Deaths within 30 days of receiving: intensive radiotherapy; intensive chemotherapy; haematopoietic stem cell transplant (HSCT)
Death due to intensive radiotherapy
None of the three included RCTs looked at this outcome.
Death due to intensive chemotherapy
One of the three included RCTs reported death within 30 days of receiving intensive chemotherapy (De Zern 2016). We are very uncertain whether there is any difference in death due to intensive chemotherapy between the two groups (one study; 89 participants;RR 0.51, 95% CI 0.03 to 7.85) (Analysis 1.2).
1.2. Analysis.
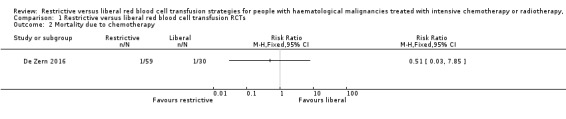
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 2 Mortality due to chemotherapy.
Death due HSCT
One of the three included RCTs reported death within 30 days of receiving a HSCT (Robitaille 2013), no deaths occurred in either treatment arm; all six participants were alive at 30 days.
Adverse Events
Bleeding episodes
Bleeding was reported in two of the three studies (De Zern 2016; Webert 2008).
Participants with any bleeding
There may be little or no difference in the number of participants who suffered from any bleeding between a restrictive versus liberal RBC transfusion strategies (two studies; 149 participants;RR 0.93, 95% CI 0.73 to 1.18, P = 0.54) (low‐quality evidence) (Analysis 1.3).
1.3. Analysis.
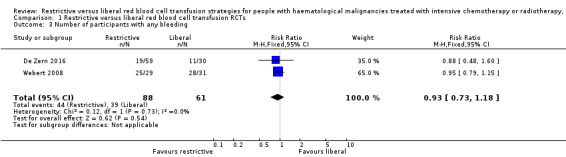
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 3 Number of participants with any bleeding.
Clinically significant bleeding (WHO grade 2 and above)
There may be little or no difference in the number of participants who experienced clinically significant bleeding between the restrictive and liberal groups (two studies; 149 participants; RR: 1.03, 95% CI 0.75 to 1.43, P = 0.85) (low‐quality evidence) (Analysis 1.4)
1.4. Analysis.
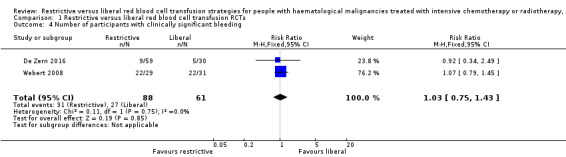
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 4 Number of participants with clinically significant bleeding.
Severe bleeding (WHO grade 3 and above)
We are very uncertain whether there is any difference between a restrictive versus liberal RBC transfusion strategies in the number of participants who suffered from severe bleeding (WHO grade 3 and above) (two studies; 149 participants; RR: 1.57, 95% CI 0.39 to 6.30) (Analysis 1.5) (Data from the Webert 2008 study was unpublished data provided by the study authors).
1.5. Analysis.
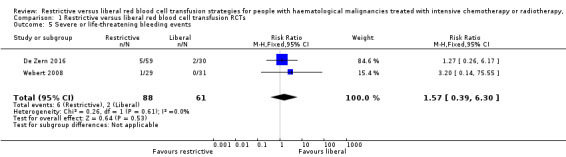
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 5 Severe or life‐threatening bleeding events.
Webert 2008 also reported number of days with bleeding (RR: 1.19, 95%CI 0.84 to 1.70, P = 0.323) and time to first bleeding episode (RR: 1.36, 95%CI 0.79 to 2.34, P = 0.27) with no difference between treatment arms was seen. (Authors' own data).
Adverse transfusion reactions: such as transfusion‐related acute lung injury (TRALI), transfusion‐related circulatory overload (TACO), ABO incompatibility or transfusion transmitted infection (TTI)
None of the included trials provided data for these outcomes.
Serious infections
De Zern 2016 reported the episodes of neutropenic fever. We are very uncertain whether there is any difference in the number of participants who had experienced fever with positive blood culture between the restrictive and liberal transfusion strategy groups (one study; 89 participants; RR: 1.23, 95% CI 0.74 to 2.04) (very low‐quality evidence) (Analysis 1.6) (unpublished data provided by the author).
1.6. Analysis.
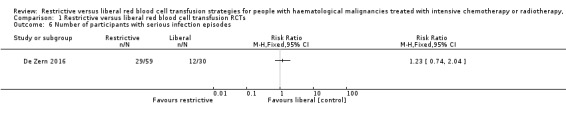
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 6 Number of participants with serious infection episodes.
Arterial or venous thromboembolic events
None of the included trials provided data for this outcome.
Toxicity score for HSCT recipients
No data were provided for a toxicity score from the two included RCTs that included HSCT recipients (Robitaille 2013; Webert 2008). However, Robitaille 2013, reported the occurrence of veno‐occlusive disease (VOD) (a complication of HSCT). There was an increase in the risk of VOD in the liberal transfusion arm (Peto OR 28.03, 95% CI 1.51 to 520.65; six participants) (Analysis 1.7).
1.7. Analysis.
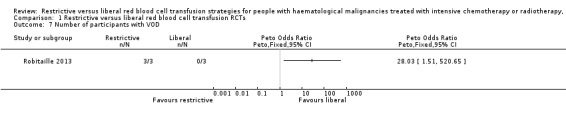
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 7 Number of participants with VOD.
Blood product utilisation
Red blood cell (RBC) transfusion requirements and intervals
All three RCTs reported RBC transfusion requirements (Robitaille 2013; Webert 2008; De Zern 2016 ); no RCTs reported RBC transfusion intervals (see Table 6 for details).
4. Review secondary outcome‐red blood cell transfusion.
| Study | No. of participants received RBC transfusion during the study period | Mean no. RBC units transfusions/participant during study period | Number of participant‐days with RBC transfusions | Proportion of participant‐days with RBC transfusions | Mean Hb within first +100 days: | Number of RBC units per transfusion |
Interval between RBC transfusions (mean) (days) |
| RCTs | |||||||
| De Zern 2016 |
Restrictive: 57/59 Liberal: 30/30 |
Restrictive: mean 8.2 (SD 4.2) Liberal: mean 11.3 (SD 5.4) |
NR | NR |
Restrictive: mean 33.6 (SD 1.4) Liberal: mean 33.2 (SD 2.0) |
NR | NR |
| Robitaille 2013 |
Restrictive: 3/3 Liberal: 3/3 |
Restrictive: (mean 1.3 [SD 0.58]; median 1 [range 1 to 2; SE 0.33]) Liberal: (mean 8.7 [SD 5.5]; median 9 [range 3 to 14; SE 3.2]) |
NR | NR |
Restrictive: [mean pre transfusion Hb 69 g/L (range 69 g/L to 70 g/L) no SD] Liberal: [mean pre transfusion Hb 106 g/L (range 118 g/L to 132 g/L) no SD] |
NR | NR |
| Webert 2008 |
Restrictive: 26/29 (24/29 from when Hb ≥80 g/L) Liberal: 29/31 (28/28 from when Hb ≥ 120g/L) |
NR |
Restrictive: 75 (70/467 days of observation once reached target Hb) Liberal: 113 g/L (72/410 days of observation once reached target Hb) |
Restrictive: 0.151 (0.150 after study Hb threshold reached) Liberal: 0.233 (0.176 after study Hb threshold reached) [RR 1.56; 95% CI 1.16 to 2.10] [RR 1.18: 95% CI 0.90 to 1.54; once study Hb threshold reached]a |
NR | NR | NR |
| NRS | |||||||
| Jansen 2004 | NR |
Restrictive: mean 9.6 (SD 3.9); Median 9 (SE 0.6); Range 3 to 21 Liberal: mean 10.8 (SD 2.9); Median 11.0 (SE 0.4); Range 6 to 21 |
NR | NR |
Restrictive: Age < 25 Hb 7.5 (n = 3); 25‐50 yrs Hb 8.0 (n = 22); Age 50‐70 years Hb 8.3 (n = 13) Liberal: Age < 25 years Hb 8.8 (n = 3); 25‐50 years Hb 9.3 (n = 32); 50‐70 years Hb 9.5 (n = 11) |
Restrictive: Mean 1.3 (SD 0.5); Median 1.0 (SE 0.03); range 1‐4 Liberal: Mean 1.82 (SD 0.4); Median 2 (SE 0.03); Range 1‐5 |
Restrictive: 3.1 days (No SD) Liberal: 3 days (No SD) |
CI = confidence interval Hb = haemoglobin RBC = red blood cell RR ‐ risk ratio SD = standard deviation SE = standard error
Trial HB: Restrictive ≥ 80g/L and Liberal ≥ 120g/L
aData analysed by study authors, reported as relative risks derived from adjusted Cox regression models, allowing for repeated measures.
Number of participants who received a RBC transfusion
All three trials reported the number of participants who received a RBC transfusion (De Zern 2016; Robitaille 2013; Webert 2008). There may be little or no difference in the number of participants who required RBC transfusions from study entry between the restrictive and liberal RBC transfusion groups (three studies; 155 participants: RR: 0.97, 95% CI 0.90 to 1.05) (Analysis 1.8). However, in the Webert 2008 study there was a reduction in the number of participants who required RBC transfusions in the restrictive RBC transfusion group after the study haemoglobin threshold was reached (one study; 57 participants: RR: 0.83, 95% CI 0.70 to 0.99) (Analysis 1.9).
1.8. Analysis.
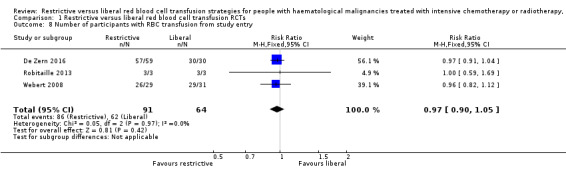
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 8 Number of participants with RBC transfusion from study entry.
1.9. Analysis.

Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 9 Number of participants with RBC Transfusion after reaching Hb >80 g/L for restrictive & Hb > 120 g/L for liberal.
Number of RBC transfusions per participant
We calculated the mean number of RBC transfusions per participant based on individual patient data provided in the study report (Robitaille 2013) (Table 6). There was a reduction in the mean number of transfusions in the restrictive RBC transfusion group (two studies; 95 participants; mean difference (MD) ‐3.58, 95%CI ‐5.66 to ‐1.49, P < 0.0001) (Analysis 1.10).
1.10. Analysis.
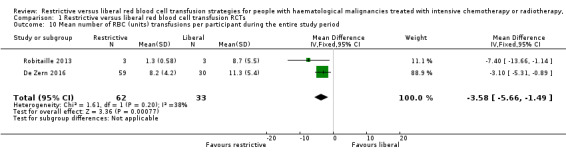
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 10 Mean number of RBC (units) transfusions per participant during the entire study period.
Proportion of participant days with a RBC transfusion
One trial reported the proportion of participant‐days with a RBC transfusion.There was a reduction in the proportion of participant‐days with a RBC transfusion in the restrictive RBC transfusion group from study entry ((Table 6). There was no evidence for a difference once the target haemoglobin levels were reached (Table 6).
Platelet transfusion requirements and intervals
Two trials provided data on platelet transfusion requirements (De Zern 2016; Webert 2008). See details in Table 7. No trials provided data on platelet transfusion intervals.
5. Review secondary outcomes ‐ platelet and red cell transfusions.
| Study ID | Number of PLTs transfused per participant | Number of participant‐days with PLT transfusions | Proportion of participant‐days with RBC transfusions | Interval between PLT transfusions (mean & SD)(days) | Number of PLT units per transfusion (mean & SD) (units) | Nmber of PLT transfusion per participant |
| RCTs | ||||||
| De Zern 2016 | NR | NR | NR | NR | NR |
Restrictive: median:9 (IQR: 5.5 to 12.5) Liberal; median: 9 (IQR: 7 to 12) |
| Robitaille 2013 | NR | NR | NR | NR | NR | NR |
| Webert 2008 |
Restrictive: 26/29 from study entry, 26 from when target Hb reached Liberal: 27/31 from study entry; 26 from when target Hb reached |
Restrictive: 124 from study entry; 122 from Hb 80 g/L to 100 g/L Liberal: 0.265 from study entry; 0.283 from when Hb > 120 g/L |
Restrictive: 0.249 from study entry; 0.261 from when Hb 8‐10 Liberal: 0.265 from study entry; 0.283 from when Hb > 12 [RR 1.06; 95% CI 0.74 to 1.52] [RR 1.02: 95% CI 0.73 to 1.44; once study Hb threshold reached]a |
NR | NR | NR |
| NRS | ||||||
| Jansen 2004 |
Restrictive: Mean 7.5 (SD 3.8); median 7 (SE 0.6); range 2 to 18 Liberal: Mean 8.5 (SD 4.9); median 7 (SE 0.7); range 2 to 30 P > 0.05 |
NR | NR |
Restrictive: 4 days (No SD) Liberal: 3.8 days (No SD) |
Restrictive: Mean 1.1 (SD 0.4); median 1 (SE 0.03); range 1 to 4 Liberal: Mean 1.2 (SD 0.5); Median 1 (SE 0.03); range 1 to 4 |
NR |
aData analysed by study authors, reported as relative risks derived from Cox regression models.
IQR = Interquartile range
Number of participants who received a platelet transfusion
There may be little or no difference in the number of participants who required platelets transfusions between the restrictive and liberal groups from study entry (one trial; 60 participants; RR:1.03, 95% CI 0.86 to 1.24) (Analysis 1.11).
1.11. Analysis.
Comparison 1 Restrictive versus liberal red blood cell transfusion RCTs, Outcome 11 Number of participants with PLT transfusions from the study entry.
Number of platelet transfusions per participant
In De Zern 2016, there may be little or no difference in the number of platelet transfusions per participant between the groups (restrictive group: median 9, Interquartile range (IQR) 5.5 to 12.5; liberal group: median 9, IQR 7 to 12) (Table 7) (Authors' own data).
Proportion of participant days with a platelet transfusion
In Webert 2008, there may be little or no difference in the proportion of participant days with platelet transfusions between the restrictive and liberal groups from study entry, or after the study haemoglobin threshold was reached (Table 7) (Authors' own data).
Quality of life
One RCT reported quality of life (De Zern 2016), and a fatigue‐self scoring tool was implemented (Mendoza 1999). This is a 10‐point scale with a score of zero indicating no fatigue and a score of 10 indicating the worst possible fatigue. The median fatigue scare was similar for both groups; for restrictive group: 4.8 (IQR: 4 to 5.2) and for the liberal group: 4.5 (IQR: 3.6 to 5), P = 0.53 (Authors’ own data).
Length of hospital admission
De Zern 2016 reported no difference in the length of hospital stay between the two groups; the median stay for restrictive group was 35.5 days (IQR; 31.2 to 43.8) compared with 36 days, IQR: 29.2 to 44, ( P = 0.53) (Authors's own data).
Length of intensive care admission
None of the included RCTs measured this outcome.
Hospital readmission
None of the included RCTs measured this outcome.
Effects of the interventions from included NRS
All the following outcomes were reported from the one included NRS (Jansen 2004).
Primary outcome: all‐cause‐mortality
All‐cause short‐term mortality, within zero to seven days and eight to 30 days from the study start
The Jansen 2004 study did not report these outcomes.
All‐cause long‐term mortality, within (31 to 100 days) from the study start
The Jansen 2004 study reported this outcome.Two deaths occurred over a mean 31 days follow‐up, one participant died from each group (very low‐quality evidence). No adjusted analysis was reported.
Secondary outcomes
Mortality causes
Mortality: death due to infection; bleeding; or adverse transfusion reactions
Jansen 2004, did not report on any of these outcomes.
Mortality within 30 days: due to intensive radiotherapy; or intensive chemotherapy
Jansen 2004, did not report on any of these outcomes.
Mortality within 30 days: due to HSCT
No participant in the Jansen 2004 study had a HSCT.
Adverse events
Bleeding episodes
Participants with any bleeding
Jansen 2004, did not report the number of participants with any bleeding.
Clinically significant bleeding (WHO grade 2 and above)
The authors did not report an adjusted analysis. Three participants had clinically significant bleeding in the restrictive group (38 participants) and eight participants had clinically significant bleeding in the liberal group (46 participants). The authors reported that there was no significant difference between the two groups.
Severe bleeding (WHO grade 3 and above)
Jansen 2004, did not report the number of participants with severe bleeding.
Adverse transfusion reactions: such as transfusion‐related acute lung injury (TRALI), transfusion‐related circulatory overload (TACO), ABO incompatibility or transfusion transmitted infection (TTI)
Jansen 2004, did not report on adverse transfusion reactions.
Serious infections
In Jansen 2004, it was stated that "No differences were found in incidence of infections and type of infective agents" between the two transfusion strategies. However, no data were provided to support this.
Arterial or venous thromboembolic events
Jansen 2004, did not report on arterial or venous thrombosis incidence.
Toxicity score for HSCT recipients
No participant in the Jansen 2004 study had a HSCT.
Blood product utilisation
Red blood cell (RBC) requirements and intervals
The total number of RBC transfusions was lower in the restrictive group compared to the liberal RBC transfusion group (unadjusted ratio of the geometric means 1.17, 95% CI 1.01 to 1.37: adjusted for age, sex and acute myeloid leukaemia type geometric mean 1.25; 95% CI 1.07 to 1.47 ‐ data analysis performed by the study authors)
The study authors stated that the number of RBC units given per transfusion was lower in the restrictive strategy group in comparison with the liberal strategy (Table 6). The study authors did not report an adjusted analysis.
The mean interval between RBC transfusions was 3.1 days in the restrictive group compared to 3.0 days in the liberal group (Table 6). The study authors did not report an adjusted analysis.
Platelet requirements and intervals
The study authors stated that there was no difference in the number of platelet transfusions per participant between the restrictive and liberal groups (Table 7). The study authors did not report an adjusted analysis.
The study authors stated that there was no difference in the number of platelet units per participant between the restrictive and liberal groups (Table 7). The study authors did not report an adjusted analysis.
The mean interval between platelets transfusions was 4.0 days in the restrictive group and 3.8 days in the liberal group (Table 7). The study authors did not report an adjusted analysis.
Other outcomes
Quality of life
This outcome was not assessed in Jansen 2004.
Length of hospital admission
This outcome was not assessed in Jansen 2004.
Length of intensive care admission
This outcome was not assessed in Jansen 2004.
Hospital readmission
This outcome was not assessed in Jansen 2004.
Discussion
Our main objective of conducting such a review was to compare participants safety and clinical outcomes of a restrictive blood transfusion strategy and a liberal blood transfusion strategy for people with haematological disorders undergoing myelosuppressive chemotherapy or stem cell transplantation. In recent years, a more conservative blood transfusion policy had been implemented in other populations.
Summary of main results
We identified four studies for inclusion in this review, three randomised controlled trials (RCTs) and one non‐randomised study (NRS) involving in total 240 participants.
We identified six studies eligible for inclusion in this review; five RCTs and one NRS. Three completed RCTs (156 participants), one completed NRS (84 participants), and two ongoing RCTs. The completed studies were conducted between 1997 and 2015. One study included children receiving a haematopoietic stem cell transplantation (HSCT) (six participants), the other three studies only included adults, 218 participants with acute leukaemia receiving chemotherapy, and 16 with a haematological malignancy receiving a HSCT. The restrictive strategies varied from 70 g/L to 90 g/L. The liberal strategies also varied from 80 g/L to 120 g/L. There is one additional study awaiting classification.
The follow‐up time from randomisation to outcome reporting was short, four to six weeks, in three studies (De Zern 2016; Jansen 2004; Webert 2008). In Robitaille 2013, mortality was assessed at two years from the initial enrolment day.
Summary of main results from included RCTs
A restrictive red blood cell (RBC) transfusion policy may reduce the number of RBC transfusions per participant (two trials; 95 participants; mean difference (MD) ‐3.58, 95%CI ‐5.66 to ‐1.49).
A restrictive RBC transfusion policy may make little or no difference to:
the number of participants who require RBC transfusions (three trials; 155 participants: RR: 0.97, 95% CI 0.90 to 1.05);
the number of participants who die within 100 days (two trials, 95 participants: RR: 0.25, 95% CI 0.02 to 2.69. low‐quality evidence);
the number of participants who experienced any bleeding (two studies, 149 participants; RR:0.93, 95% CI 0.73 to 1.18, low‐quality evidence), or clinically significant bleeding (two studies, 149 participants, RR: 1.03, 95% CI 0.75 to 1.43, low‐quality evidence);
the length of hospital stay (restrictive median 35.5 days (IQR: 31.2 to 43.8); liberal 36 days (IQR: 29.2 to 44)).
We are very uncertain whether the restrictive RBC transfusion strategy reduces:
the risk of developing any serious infection (one study, 89 participants, RR: 1.23, 95% CI 0.74 to 2.04, very low‐quality evidence).
quality of life (one trial, 89 participants, fatigue score: restrictive median 4.8 (interquartile range (IQR) 4 to 5.2); liberal median 4.5 (IQR 3.6 to 5)).
Summary of main results from included NRS
We are very uncertain whether the restrictive RBC transfusion strategy decreases:
the risk of death within 100 days (one study, 84 participants, restrictive 1 death; liberal 1 death);
the risk of clinically significant bleeding (one study, 84 participants, restrictive 3; liberal 8;very low‐quality evidence);
the number of RBC transfusions (adjusted for age, sex and acute myeloid leukaemia type geometric mean 1.25; 95% CI 1.07 to 1.47 ‐ data analysis performed by the study authors).
No NRS were found that looked at: quality of life; number of participants with any bleeding; serious infection; or length of hospital stay.
No studies were found that looked at: adverse transfusion reactions; arterial or venous thromboembolic events; length of intensive care admission; or readmission to hospital.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the effectiveness and safety of a restrictive RBC transfusion strategy compared to a liberal RBC transfusion strategy for people diagnosed with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without a haematopoietic stem cell transplant (HSCT).
There was low‐quality evidence that a restrictive RBC transfusion strategy has little or no effect on all‐cause mortality, any bleeding or clinically significant bleeding. These were the only meta‐analyses performed within this review
The results of these meta‐analyses should not be interpreted without considering the impact of the following factors.
The majority of participants in this review were adults with acute leukaemia treated with chemotherapy, the findings of this review may not be generalisable to children; people with other blood cancers; or people treated with a HSCT.
Data on the review’s primary outcome, all‐cause mortality, was not provided by one trial (Webert 2008) (60 participants), therefore data were missing for over one third of participants within the included RCTs (155 participants).
The recording of bleeding is subjective, and only one of the three studies that reported bleeding reported the method of assessing and grading bleeding (Webert 2008).
There was little or no difference in all‐cause mortality, this review's primary outcome, but the 95% confidence interval (0.25 to 2.69) demonstrates that a clinically important difference in the proportion of participants who died could have been missed. If we assume a mortality rate of 3% within 100 days (data from De Zern 2016). We would need 1492 participants to have a 80% chance of detecting, as significant at the 5% level, an increase in all‐cause mortality from 3% to 6%. The ongoing studies are planning to recruit 530 participants and therefore we may not be able to answer this review's primary outcome even when the ongoing studies have been completed.
The early termination of one trial after recruiting only six participants influenced the availability of results (Robitaille 2013).
Different restrictive triggers were tested in the RCTs; less than 70 g/L in both Robitaille 2013 and De Zern 2016, and less than 80 g/L in Webert 2008.
Different restrictive triggers were tested in the RCTs; less than 120 g/L in both Webert 2008 and Robitaille 2013, and a much lower Hb transfusion threshold 80 g/L in De Zern 2016.
The evidence of the safety of restrictive transfusion strategies among people with existing cardiovascular disease were also lacking as this subset of participants was excluded from the three of the included studies. Less stable participants were considered for inclusion in De Zern 2016 only.
Other important considerations in this review are:
not all endpoints from all the studies could be incorporated into a meta‐analysis due to differences in the ways the studies had reported the outcomes;
some review outcomes were not reported by any of the included studies, for example, none of the studies reported transfusion reactions.
Quality of the evidence
There was evidence from three RCTs and one NRS.
The NRS only reported one outcome (number of RBC transfusions) that had been adjusted for confounding factors, and this analysis adjusted for only some of the potentially important confounding factors (age, sex and acute myeloid leukaemia type). The evidence from the NRS was very low according to GRADE criteria.
All of the RCTs were prone to bias. In all three RCTs participants and personnel were unblinded to the intervention, and in only one of the trials were outcome assessors blinded (Webert 2008). This is likely to reflect the inherent difficulties with blinding RBC transfusion trials because medical staff caring for participants cannot be blinded to their patients’ blood results.
In De Zern 2016, some participants crossed over between the two transfusion strategies. Seven of 59 (11.9%; 95% CI, 4.91% to 22.93%) allocated to the restrictive transfusion strategy and two of 30 (6.7%; 95% CI, 0.82% to 22.07%) allocated to the liberal transfusion strategy crossed over during the study. Analysis was by intention‐to‐treat and therefore the ability to detect a difference between the two arms would have been reduced.
The ability to detect bias was limited by only two of the included studies having a trial registration or published protocol (De Zern 2016; Robitaille 2013).
We assessed the GRADE quality of evidence as low for:
all cause mortality (RCT);
bleeding episodes (RCT);
length of hospital admission (RCT).
We downgraded the quality of evidence due to: the imprecision of the estimates (small number of included participants and there was a low number of events) and the indirectness of the evidence (most of the included participants were adults with acute leukaemia). We did not downgrade the evidence due to risk of bias due to lack of blinding because all‐cause mortality is an objective outcome and the majority of the evidence for bleeding episodes was from a study in which outcome assessors were blinded (Webert 2008).
We assessed the GRADE quality of evidence as very low for:
serious infection (RCT);
quality of life (RCT);
all‐cause mortality (NRS);
bleeding episodes (NRS).
For serious infection and quality of life, we downgraded the quality of evidence due to: the imprecision of the estimates, indirectness of the evidence (included participants were adults with acute leukaemia), and risk of bias (unblinded study).
For evidence from the NRS, we downgraded the quality of the evidence due to imprecision of the estimates.
Potential biases in the review process
There were no obvious biases within the review process. We conducted a wide search, which included ongoing trial databases and contact with researchers in the field; we carefully assessed the relevance of each paper identified; and we made no restrictions for the language in which the paper was originally published or its publication status. We performed all screening and data extractions in duplicate.We prespecified all outcomes and subgroups prior to analysis. The numbers of included studies were insufficient for us to combine to complete a funnel plot in order to examine the risk of publication bias.
None of the review authors were involved in the included or excluded studies.
Agreements and disagreements with other studies or reviews
Several reviews support the implementation of a more restrictive RBC transfusion strategies in a range of clinical settings. This has been shown in another Cochrane systematic review with a meta‐analysis of 31 randomised controlled trials, involving 12,587 participants from mainly surgical and intensive‐care settings (Carson 2016). The authors stated that the restrictive transfusion strategies were as safe as the liberal strategies for all‐cause mortality, myocardial infarction, stroke, and thromboembolism. This review agreed with the findings from Carson 2016 regarding all‐cause mortality, but we could not comment on myocardial infarction, stroke, and thromboembolism because none of our included studies reported these outcomes. The Carson 2016 review did not include some of this review's secondary outcomes. These outcomes were bleeding (not due to surgical procedures) and quality of life, both of which are relevant to people with haematological disorders. Carson 2016 did not perform a subgroup analysis for people with cardiac disease.
Several reviews have shown that a restrictive transfusion strategy decreases the number or RBC units transfused and number of participants being transfused (Carson 2016; Holst 2015). Carson 2016 and Holst 2015 had similar inclusion criteria, and both excluded trials in neonates. Holst 2015 (31 trials, 9813 participants) included data on six trials not included in Carson 2016 (two trials in infants and children undergoing cardiac surgery (Cholette 2011; De Gast‐Bakker 2013), one trial in orthotopic liver transplantation reported as an abstract (Wu 2011), and three trials excluded from Carson 2016 for various reasons (Fortune 1987; Robertson 2014; Zygun 2009)). Carson 2016 included data from six trials not included in Holst 2015 (five more recent trials (De Zern 2016; Fan 2014; Jairath 2015; Murphy 2015; Nielsen 2014), and one older trial (Fisher 1956)). Our review agreed that a restrictive transfusion strategy reduced the number of transfusions a patient receives, but did not find a difference in the number of participants who received a RBC transfusion. This may be because the majority of trials in these other reviews are in people receiving surgery or who are in intensive care rather than people who have an underlying haematological disorder.
Docherty 2016 assessed the risk of a restrictive transfusion policy in people undergoing non‐cardiac surgery who have cardiovascular disease (11 trials, 3033 participants). The results show that it may not be safe to use a restrictive transfusion threshold of less than 80 g/L in patients with ongoing acute coronary syndrome or chronic cardiovascular disease, although some of the results were uncertain. In this review we were unable to comment about people with cardiovascular disease due to lack of data.
Rohde 2014 focused on the risk of healthcare‐associated infection such as pneumonia, wound infection, and sepsis (17 trials, 7593 participants). None of these trials were in people with haematological disorders. Rohde 2014 showed that a restrictive RBC transfusion strategy was associated with a significant reduction in the incidence of serious infection in hospital‐admitted participants. Rohde 2014's finding agreed with Salpeter 2014 (three trials, 2364 participants), but disagreed with Carson 2016, which showed no difference in the risk of infection. The reviews differed, Rohde 2014 included unpublished data from four studies and data from neonatal studies; Salpeter 2014 only included published data for trials that used a restrictive threshold of 70 g/L in people in critical care or bleeding, and Carson 2016 included data from seven recently published trials not included in Rohde 2014 or Salpeter 2014. In this review, due to the lack of data we were very uncertain whether there was any difference in the risk of infection.
A possible association between the liberal transfusion strategy and a high incidence of developing veno‐occlusive disease (VOD) was highlighted in Robitaille 2013, the study was stopped after enrolment of only six children because of this concern. In contrast, results from another RCT, on critically ill children reported no differences in the survival or adverse events between the two strategies (Lacroix 2007). However, the trigger for blood transfusion in the liberal group was below 95 g/L in comparison to 120 g/L for the liberal group in Robitaille 2013. Increased blood viscosity due to a high RBC level is one of the mechanisms known to increase the risk of VOD (Beck 1998).
Authors' conclusions
Implications for practice.
There is insufficient evidence to suggest that a restrictive red blood cell (RBC) transfusion strategy is superior to a liberal strategy in managing anaemia in people with haematological malignancies. Although restrictive blood transfusion strategies could potentially reduce blood use and lead to less exposure to blood products in this population.
The safest haemoglobin threshold for this population is still uncertain.
Implications for research.
The low‐quality evidence in this review is mainly based on adults with acute leukaemia who are having chemotherapy. The two ongoing studies (530 participants) are due to be completed by January 2018 and will provide additional information for adults with haematological malignancies. Adverse events related to transfusion should be reported in future studies. Further randomised controlled trials (RCTs) are required in children.
Acknowledgements
We thank the National Institute of Health Research (NIHR). This review was part of a series of reviews that have been funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme. The views expressed were those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health (DoH).
We thank the editorial base of the Cochrane Haematological Malignancies Review Group.
We thank the authors of the protocol: Alan Tinmouth, Susan Brunskill, Caroline Butler and Jason Tay.
We thank Caroline Butler and Jason Tay for assisting in screening of the search results.
We also thank David Burke for help in screening of studies for inclusion in the review.
Appendices
Appendix 1. CENTRAL search strategy on the 15 June 2016
#1 MeSH descriptor: [Blood Transfusion] this term only
#2 MeSH descriptor: [Blood Component Transfusion] explode all trees
#3 MeSH descriptor: [Erythrocyte Transfusion] this term only
#4 (erythrocyte* or "red cell*" or blood or RBC*) near/5 (transfus* or unit*)
#5 (("red cell*" or RBC* or erythrocyte* or "red blood cell*" or "whole blood" or transfus*) near/5 (trigger* or level* or threshold* or rule* or target* or restrict* or liberal* or reduc* or limit*))
#6 (("red cell*" or blood) near/3 (management or sparing or support or strateg*))
#7 ("allogeneic blood" or (unit* near/2 blood) or "allogenic blood" or (blood near/2 exposure) or "donor blood" or "blood product" or "blood products" or "blood component" or "blood components" or "blood support")
#8 (h*emotransfus* or hypertransfus* or h*emotherap*)
#9 ("red cell*" or "red blood cell*" or RBC* or transfus*):ti
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Hematologic Neoplasms] explode all trees
#12 MeSH descriptor: [Hematologic Diseases] this term only
#13 MeSH descriptor: [Leukemia] explode all trees
#14 MeSH descriptor: [Lymphoma] explode all trees
#15 MeSH descriptor: [Neoplasms, Plasma Cell] explode all trees
#16 MeSH descriptor: [Anemia, Aplastic] explode all trees
#17 MeSH descriptor: [Bone Marrow Diseases] explode all trees
#18 MeSH descriptor: [Thrombocytopenia] explode all trees
#19 (thrombocytop*eni* or leuk*emi* or lymphom* or "aplastic an*emi*" or "refractory an*emi*" or myelodysplas* or myeloproliferat* or myelom* or plasmacytom*)
#20 (lymphogranulomato* or histiocy* or granulom* or thrombocyth*emi* or polycyth*emi* or myelofibros* or AML or CLL or CML or Hodgkin* or nonhodgkin* or reticulos* or reticulosarcom* or MDS or RAEB or "RAEB‐t")
#21 (burkitt* next (lymph* or tumo*r)) or lymphosarcom* or brill‐symmer* or sezary
#22 ((h*ematolog* or blood or "red cell*" or "white cell*" or lymph* or marrow or platelet*) near/3 (malignan* or oncolog* or cancer* or neoplasm* or carcinoma*))
#23 MeSH descriptor: [Antineoplastic Agents] explode all trees
#24 MeSH descriptor: [Remission Induction] explode all trees
#25 MeSH descriptor: [Antineoplastic Protocols] explode all trees
#26 MeSH descriptor: [Stem Cell Transplantation] explode all trees
#27 MeSH descriptor: [Bone Marrow Transplantation] this term only
#28 MeSH descriptor: [Radiotherapy] explode all trees
#29 MeSH descriptor: [Lymphatic Irradiation] this term only
#30 (("bone marrow" or "stem cell*" or "progenitor cell*") near/2 (transplant* or graft* or engraft* or rescu*))
#31 ((h*ematolog* or h*emato‐oncolog*) near/2 patients)
#32 (ASCT or ABMT or PBPC or PBSCT or PSCT or BMT or SCT or HSCT)
#33 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32
#34 ((consolidat* or induct* or maintenance or conditioning*) near/3 (therap* or treat* or regimen* or patient*))
#35 ((therap* or induc*) near/3 remission*)
#36 ((cytosta* or cytotox*) near/2 (therap* or treat* or regimen*))
#37 ((multimodal* or multi‐modal*) near/3 (treat* or therap*))
#38 (combi* near/2 modalit*)
#39 MeSH descriptor: [Transplantation Conditioning] explode all trees
#40 mini‐tra?splant*
#41 MeSH descriptor: [Transplantation, Homologous] explode all trees
#42 (allograft* or allo‐graft* or allotransplant* or allo‐transplant* or ((allogen* or allo‐gen*) near/5 (transplant* or trasplant* or graft* or rescue*)) or homograft* or homo‐graft* or homotransplant* or homo‐transplant* or homotrasplant* or homo‐trasplant*)
#43 MeSH descriptor: [Transplantation, Autologous] this term only
#44 (autograft* or auto‐graft* or autotransplant* or auto‐transplant* or autotra?splant* or auto‐tra?splant* or (autolog* near/5 (transplant* or graft* or trasplant* or rescu*)))
#45 #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44
#46 #33 or #45
#47 #10 and #46
#
Appendix 2. MEDLINE (Ovid) search strategy on the 15 June 2016
1. BLOOD TRANSFUSION/
2. *BLOOD COMPONENT TRANSFUSION/
3. ERYTHROCYTE TRANSFUSION/
4. ((erythrocyte* or red cell* or blood or RBC*) adj
5 (transfus* or unit*)).tw,kf. 5. ((red cell* or RBC* or erythrocyte* or red blood cell* or whole blood or transfus*) adj3 (trigger* or level* or threshold* or rule* or target* or restrict* or liberal* or reduc* or limit*)).tw,kf.
6. (allogeneic blood or (unit* adj2 blood) or allogenic blood or (blood adj2 exposure) or donor blood or blood product* or bl ood component* or blood support).tw,kf.
7. (h?emotransfus* or hypertransfus* or h?emotherap*).tw,kf.
9. (red cell* or erythrocyte* or transfus* or whole blood or RBC*).ti.
10. BLOOD COMPONENT TRANSFUSION/ not (EXCHANGE TRANSFUSION, WHOLE BLOOD/ or PLASMA EXCHANGE/ or PLATELET TRANSFUSION/ or exp LEUKOCYTE TRANSFUSION/)
11. ERYTHROCYTES/ or (red cell* or red blood cell* or erythrocyte* or RBC*).ti.
12. 10 and 11
13. or/1‐9,12
14. exp Hematologic Neoplasms/ or Hematologic Diseases/
15. exp Leukemia/ or exp Lymphoma/
16. exp Neoplasms, Plasma Cell/
17. exp Anemia, Aplastic/
18. exp Bone Marrow Diseases/
19. exp Thrombocytopenia/
20. (thrombocytopeni* or thrombocytopaeni* or leukemi* or leukaemi* or lymphom* or myelodysplas* or myeloproliferat* or myelom* or plasm??ytom*).tw,kf,ot.
21. (lymphogranulomato* or histiocy* or granulom* or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin* or nonhodgkin* or reticulosis or reticulosarcom* or MDS or RAEB or RAEB‐t).tw,kf,ot.
22. ((aplastic or refractory) adj an?emi*).tw,kf,t.
23. ((burkitt* adj (lymph* or tumo?r)) or lymphosarcom* or brill‐symmer* or sezary).tw,kf,ot.
24. ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) adj3 (malignan* or oncolog* or cancer* or neoplasm* or carcinoma*)).tw,kf,ot.
25. exp Antineoplastic Agents/ or exp Remission Induction/ or exp Antineoplastic Protocols/
26. exp Stem Cell Transplantation/ or Bone Marrow Transplantation/ or exp Radiotherapy/
27. exp Lymphatic Irradiation/
28. (chemotherap* or antineoplast* or anti‐neoplast* or radiotherap* or radio‐therap* or chemoradiotherap* or chemo‐radiotherap* or stem cell* or progenitor cell* or (bone marrow adj2 (transplant* or graft* or engraft* or rescu*))).tw,kf,ot.
29. (ASCT or ABMT or PBPC or PBSCT or PSCT or BMT or SCT or HSCT).tw,kf,ot.
30. ((h?ematolog* or h?ematooncolog* or h?emato‐oncolog*) adj3 patients).tw,kf,ot.
31. (h?ematooncolog* or h?emato‐oncolog*).ti.
32. or/14‐3133. exp Remission Induction/ 34. exp Antineoplastic Protocols/
35. ((consolidat* or induct* or maintenance or conditioning*) adj6 (therap* or treat* or regimen* or patient*)).tw,kf,ot. 36. ((therap* or induc*) adj3 remission*).tw,kf,ot.
37. ((cytosta* or cytotox*) adj2 (therap* or treat* or regimen*)).tw,kf,ot.
38. ((multimodal* or multi‐modal*) adj3 (treat* or therap*)).tw,kf,ot. 39. (combi* adj3 modalit*).tw,kf,ot.
40. or/33‐39
41. Transplantation Conditioning/
42. mini‐tra?splant*.tw.
43. exp Transplantation, Homologous/
44. (allograft* or allo‐graft* or allotransplant* or allo‐transplant* or ((allogen* or allo‐gen*) adj5 (transplant* or trasplant* or graft* or rescue*)) or homograft* or homo‐graft* or homolog* or homotransplant* or homo‐transplant* or homotrasplant* or homo‐trasplant*).tw,kf,ot.
45. Transplantation, Autologous/
46. (autograft* or auto‐graft* or autotransplant* or auto‐transplant* or autotra?splant* or auto‐tra?splant* or (autolog* adj5 (transplant* or graft* or trasplant* or rescue*))).tw,kf,ot.
47. or/41‐46
48. 32 or 40 or 47
49. 13 and 48
50. (201312* or 2014* or 2015*).dc,ed. or ("2013" or "2014" or "2015" or "2016").yr.
51. 49 and 5
Appendix 3. Embase (Ovid) search strategy on the 15 June 2016
1. *Blood Transfusion/
2. Blood Component Therapy/
3. Erythrocyte Transfusion/
4. ((erythrocyte* or red blood cell* or red cell* or blood or RBC*) adj5 (transfus* or unit*)).tw,kf.
5. ((red cell* or RBC* or erythrocyte* or red blood cell* or whole blood or transfus*) adj3 (trigger* or level* or threshold* or rule* or target* or restrict* or liberal* or reduc* or limit*)).tw,kf.
6. (allogeneic blood or (unit* adj2 blood) or allogenic blood or (blood adj2 exposure) or donor blood or blood product* or blood component* or blood support).tw,kf.
7. (h?emotransfus* or h?emotherap* or hypertransfus*).tw,kf.
8. (transfus* or red cell* or red blood cell* or RBC* or whole blood).ti.
9. or/1‐8
10. Hematologic Malignancy/
11. Lymphoma/
12. NonHodgkin Lymphoma/
13. Hodgkin Disease/
14. exp Myeloproliferative Disorder/
15. exp Aplastic Anemia/
16. exp Thrombocytopenia/
17. (thrombocytopeni* or thrombocytopaeni* or leukemi* or leukaemi* or lymphom* or aplast* anemi* or aplast* anaemi* or myelodysplas* or myeloproliferat* or myelom* or plasm??ytom*).tw,kf,ot.
18. (lymphogranulomato* or histiocy* or granulom* or thrombocythemi* or thrombocythaemi* or polycythemi* or polycythaemi* or myelofibros* or AML or CLL or CML or Hodgkin* or nonhodgkin* or reticulosis or reticulosarcom* or MDS or RAEB or RAEB‐t).tw,kf,ot.
19. ((burkitt* adj (lymph* or tumo?r)) or lymphosarcom* or brill‐symmer* or sezary).tw,kf,ot.
20. ((haematolog* or hematolog* or blood or red cell* or white cell* or lymph* or marrow or platelet*) adj3 (malignan* or oncolog* or cancer* or neoplasm* or carcinoma*)).tw,kf,ot.
21. exp Stem Cell Transplantation/
22. exp Bone Marrow Transplantation/
23. exp Chemotherapy/
24. exp Radiotherapy/
25. exp Antineoplastic Agent/
26. exp Immunosuppressive Treatment/
27. Remission/
28. (chemotherap* or antineoplast* or anti‐neoplast* or radiotherap* or radio‐therap* or chemoradiotherap* or chemo‐radiotherap* or stem cell* or progenitor cell* or (bone marrow adj2 (transplant* or graft* or engraft* or rescu*))).tw,kf,ot.
29. (ASCT or ABMT or PBPC or PBSCT or PSCT or BMT or SCT or HSCT).tw,kf,ot.
30. ((haematolog* or hematolog* or haemato‐oncolog* or hemato‐oncolog*) adj2 patients).tw,kf,ot.
31. (malignan* or oncolog* or cancer*).ti.
32. ((consolidat* or induct* or maintenance or conditioning*) adj6 (therap* or treat* or regimen* or patient*)).tw,kf,ot.
33. ((therap* or induc*) adj3 remission*).tw,kf,ot.
34. ((cytosta* or cytotox*) adj2 (therap* or treat* or regimen*)).tw,kf,ot.
35. ((multimodal* or multi‐modal*) adj3 (treat* or therap*)).tw,kf,ot.
36. (combi* adj3 modalit*).tw,kf,ot.
37. Allotransplantation/
38. Autotransplantation/
39. mini‐tra?splant$.tw.
40. (allograft* or allo‐graft* or allotransplant* or allo‐transplant* or ((allogen* or allo‐gen*) adj5 (transplant* or trasplant* or graft* or rescue*)) or homograft* or homo‐graft* or homolog* or homotransplant* or homo‐transplant* or homotrasplant* or homo‐trasplant*).tw,kf,ot.
41. (autograft* or auto‐graft* or autotransplant* or auto‐transplant* or autotra?splant* or auto‐tra?splant* or (autolog* adj5 (transplant* or graft* or trasplant* or rescue*))).tw,kf,ot.
42. or/10‐41
43. 9 and 42
44. limit 43 to dd=20131201‐20151105
Appendix 4. CINAHL (EBSCOhost) search strategy on the 15 June 2016
S1 (MH "Hematologic Neoplasms+")
S2 (MH "Leukemia+")
S3 (MH "Lymphoma+")
S4 (MH "Multiple Myeloma")
S5 TI ( leukemi* or leukaemi* or lymphoma* or myeloma* or plasmacytoma or plasma cell dyscrasia or AML or CLL or CML or Hodgkin* or haematolymphoid* or hematolymphoid* ) OR AB ( leukemi* or leukaemi* or lymphoma* or myeloma* or plasma cell dyscrasia or AML or CLL or CML or Hodgkin* or haematolymphoid* or hematolymphoid* )
S6 TI ( (haematoncolog* or hematoncolog* or haemato‐oncolog* or hemato‐oncolog*) N5 patient* ) OR AB ( (haematoncolog* or hematoncolog* or haemato‐oncolog* or hemato‐oncolog*) N5 patient* )
S7 (MH "Myelodysplastic Syndromes")
S8 myelodysplas* or "bone marrow dysplasia" or preleukemi* or preleukaemi* or dysmyelopoietic or dysmyelopoiesis
S9 refractory N2 (anemia or anaemia)
S10 MDS or RAEB or RAEB‐t
S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10
S12 (MH "Blood Transfusion")
S13 (MH "Blood Component Transfusion")
S14 (MH "Erythrocyte Transfusion")
S15 (erythrocyte* or red cell* or red blood cell* or RBC*) N5 (transfus* or unit*)
S16 (transfus* or erythrocyte* or red cell* or red blood cell* or RBC*) N5 (trigger* or level* or target* or threshold* or rule* or restrict* or liberal*)
S17 red cell* management or red cell* sparing or red cell* support or red cell* strategy
S18 (red cell* N2 reduc*) or (red cell* N3 requirement*)
S19 hemotransfus* or haemotransfus* or hemotherap* or haemotherap*
S20 TI (red cell* or red blood cell* or erythrocyte* or RBC* or blood)
S21 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20
S22 S11 AND S21
Appendix 5. PubMed search strategy (epublications only) on the 15 June 2016
#1 (((erythrocyte*[TI] OR blood[TI]) AND (unit*[TI] AND trigger*[TI] OR level*[TI] OR threshold*[TI] OR rule*[TI] OR target*[TI] OR restrict*[TI] OR liberal*[TI] OR requir*[TI] OR reduc*[TI] OR limit*[TI])) OR (hemotransfus*[TI] OR haemotransfus*[TI] OR hemotherap*[TI] OR haemotherap*[TI] OR "red cell*"[TI] OR "red blood cell*"[TI] OR RBC*[TI] OR transfus*[TI]))
#2 ((random* OR blind* OR "control group" OR placebo* OR controlled OR cohort* OR nonrandom* OR observational OR retrospective* OR prospective* OR comparative OR comparator OR groups OR trial* OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]))
#3 (thrombocytopeni*[TI] OR thrombocytopaeni*[TI] OR leukemi*[TI] OR leukaemi*[TI] OR lymphom*[TI] OR "aplastic anemia"[TI] OR "aplastic anaemia"[TI] OR myelodysplas*[TI] OR myeloproliferat*[TI] OR myeloma[TI] OR lymphogranulomato*[TI] OR histiocy*[TI] OR granulom*[TI] OR thrombocythemi*[TI] OR thrombocythaemi*[TI] OR polycythemi*[TI] OR polycythaemi*[TI] OR myelofibros*[TI] OR AML[TI] OR CLL[TI] OR CML[TI] OR Hodgkin*[TI] OR burkitt*[TI] OR lymphosarcom*[TI] OR brill‐symmer*[TI] OR sezary[TI] OR ((haematolog*[TI] OR hematolog*[TI] OR blood[TI] OR red cell*[TI] OR white cell*[TI] OR marrow[TI] OR platelet*[TI]) AND (malignan*[TI] OR oncolog*[TI] OR cancer*[TI] OR neoplasm*[TI] OR carcinoma*[TI])) OR chemotherap*[TI] OR radiotherap*[TI] OR chemoradiotherap*[TI] OR "stem cell"[TI] OR "stem cells" OR "progenitor cell"[TI] OR "progenitor cells"[TI] OR bone marrow transplant*[TI] OR bone marrow graft*[TI] OR "bone marrow rescue"[TI] OR rituximab[TI] OR antineoplast*[TI] OR anti‐neoplast*[TI] OR ASCT[TI] OR ABMT[TI] OR PBPC[TI] OR PBSCT[TI] OR PSCT[TI] OR BMT[TI] OR SCT[TI] OR HSCT[TI] OR "haematology patients"[TI] OR "hematology patients"[TI] OR "haematological patients"[TI] OR "hematological patients"[TI] OR "hemato‐oncology patients"[TI] OR "haemato‐oncology patients"[TI] OR remission[TI] OR ((consolidat*[TI] OR induct*[TI] OR maintenance[TI] OR conditioning*[TI]) AND (therap*[TI] OR treat*[TI] OR regimen*[TI] OR patient*[TI])) OR ((cytosta*[TI] OR cytotox*[TI]) AND (therap*[TI] OR treat*[TI] OR regimen*[TI])) OR ((multimodal*[TI] OR multi‐modal*[TI]) AND (treat*[TI] OR therap*[TI])) OR (combi*[TI] AND modalit*[TI]) OR (allograft*[TI] OR allo‐graft*[TI] OR allotransplant*[TI] OR allo‐transplant*[TI] OR ((allogen*[TI] OR allo‐gen*[TI]) AND (transplant*[TI] OR trasplant*[TI] OR graft*[TI] OR rescue*)) AND TI OR homograft*[TI] OR homo‐graft*[TI] OR homolog*[TI] OR homotransplant*[TI] OR homo‐transplant*[TI] OR homotrasplant*[TI] OR homo‐trasplant*[TI]) OR (autograft*[TI] OR auto‐graft*[TI] OR autotransplant*[TI] OR auto‐transplant*[TI] OR mini‐transplant*[TI]) OR (autolog*[TI] AND (transplant*[TI] OR graft*[TI] OR trasplant*[TI] OR rescu*[TI]))
#4 #1 AND #2 AND #3
Appendix 6. Transfusion Evidence Library on the 15 June 2016
Clinical Specialty: Haematology, Malignant
Subject Area: Red Cells
Appendix 7. LILACS search strategy on the 15 June 2016
db:("LILACS") AND type_of_study:("cohort" OR "clinical_trials" OR "case_control" OR "systematic_reviews") AND ((leukemi* OR leukaemi* OR lymphoma* OR myeloma* OR plasmacytoma OR plasma cell dyscrasia OR AML OR CLL OR CML OR Hodgkin* OR haematolymphoid* OR hematolymphoid* OR haematolog* OR hematolog* OR ((haematopoietic OR hematopoietic) AND (malignan* OR oncolog* OR cancer* OR neoplas*)) OR haematooncolog* OR hematooncolog* OR haemato‐oncolog* OR hemato‐oncolog* OR myelodysplas* OR bone marrow OR stem cell* OR preleukemi* OR preleukaemi* OR dysmyelopoietic OR dysmyelopoiesis OR refractory anemia OR refractory anaemia OR MDS OR RAEB*) AND (transfus* OR ((erythrocyte* OR red cell* OR red blood cell* OR RBC*) AND (unit* OR trigger* OR level* OR target* OR threshold* OR rule* OR restrict* OR liberal* OR requir* OR reduc* OR limit*)) OR hemotransfus* OR haemotransfus* OR hemotherap* OR haemotherap*))
Appendix 8. INDMED search strategy on the 15 June 2016
(leukemia OR leukaemia OR lymphoma OR myeloma OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR haematology OR hematology OR myelodysplasia OR marrow OR stem cell OR preleukemia OR preleukaemia OR dysmyelopoietic OR dysmyelopoiesis) AND (transfusion OR red cells OR red blood cells OR RBC OR RBCs OR hemotransfusion OR haemotransfusion OR hemotherapy OR haemotherapy OR hypertransfusion) AND (randomized OR randomised OR randomly OR blind OR blinded OR trial OR cohort OR observational OR control OR controlled OR groups)OR((haematopoietic OR hematopoietic OR haematological OR hematological) AND (malignancyOR oncology OR cancer OR neoplasm)) AND (transfusion OR red cells OR red blood cells OR RBC OR RBCs OR hemotransfusion OR haemotransfusion OR hemotherapy OR haemotherapy OR hypertransfusion) AND (randomized OR randomised OR randomly OR blind OR blinded OR trial OR cohort OR observational OR control OR controlled OR groups)
Appendix 9. KOREAMED search strategy on the 15 June 2016
Limits: ("Clinical Trial" [PT] OR "Comparative Study" [PT] OR "Evaluation Studies" [PT] OR "Meeting Abstract" [PT] OR "Meta‐Analysis" [PT] OR "Multicenter Study" [PT] OR "Practice Guideline" [PT] OR "Randomized Controlled Trial" [PT] OR "Review" [PT] ) AND "Blood Transfusion" [MH] OR transfus* [TI] OR "red cell*" [TI] OR "red blood cell*" [TI
Appendix 10. Web of Science (CPCI‐S) search strategy on the 15 June 2016
#1 TI=(leukemi* OR leukaemi* OR lymphoma* OR myeloma* OR plasmacytoma OR "plasma cell dyscrasia" OR AML OR CLL OR CML OR Hodgkin* OR haematolymphoid* OR hematolymphoid* OR haematolog* OR hematolog* OR haematooncolog* OR hematooncolog* OR haemato‐oncolog* OR hemato‐oncolog* OR myelodysplas* OR "bone marrow" OR "stem cell" OR "stem cells" OR preleukemi* OR preleukaemi* OR dysmyelopoietic OR dysmyelopoiesis OR "refractory anemia" OR "refractory anaemia" OR MDS OR RAEB*) OR TS=(leukemi* OR leukaemi* OR lymphoma* OR myeloma* OR plasmacytoma OR "plasma cell dyscrasia" OR AML OR CLL OR CML OR Hodgkin* OR haematolymphoid* OR hematolymphoid* OR haematolog* OR hematolog* OR haematooncolog* OR hematooncolog* OR haemato‐oncolog* OR hemato‐oncolog* OR myelodysplas* OR "bone marrow" OR "stem cell" OR "stem cells" OR preleukemi* OR preleukaemi* OR dysmyelopoietic OR dysmyelopoiesis OR "refractory anemia" OR "refractory anaemia" OR MDS OR RAEB*)
#2 TI=((haematopoietic OR hematopoietic) AND (malignan* OR oncolog* OR cancer* OR neoplas*)) OR TS=((haematopoietic OR hematopoietic) AND (malignan* OR oncolog* OR cancer* OR neoplas*))
#3 #1 OR #2
#4 TI=(erythrocytes OR red cells OR red blood cells OR RBCs OR transfus* OR hemotransfus* OR haemotransfus* OR hemotherap* OR haemotherap*) OR TS=(erythrocytes OR red cells OR red blood cells OR RBCs OR transfus* OR hemotransfus* OR haemotransfus* OR hemotherap* OR haemotherap*)
#5 TI=(randomi* OR blind* OR trial OR cohort* OR observational* OR control* OR groups) OR TS=(randomi* OR blind* OR trial OR cohort* OR observational* OR control* OR groups)
#6 #3 AND #4 AND #5
) #9 #7 AND #8
Appendix 11. ClinicalTrials.gov search strategy on the 15 June 2016
Study Type: All
Conditions: blood cancers OR hematological OR nonhodgkin OR leukemia OR lymphoma OR myeloma OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR myelodysplasia OR preleukemia OR dysmyelopoietic OR dysmyelopoiesis OR "refractory anemia" OR aplastic
Interventions: "red cell transfusion" OR "blood transfusion" OR "red cells" OR RBCs OR "red blood cells"
OR Search Terms: stem cell transplantation OR stem cells OR bone marrow transplantation OR blood cancers OR hematological OR nonhodgkin OR leukemia OR lymphoma OR myeloma OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR myelodysplasia OR preleukemia OR dysmyelopoietic OR dysmyelopoiesis OR "refractory anemia" OR aplastic Study
Type: All Interventions: "red cell transfusion" OR "blood transfusion" OR "red cells" OR RBCs OR "red blood cells"
Appendix 12. ISRCTN search strategy on the 15 June 2016
("red cell transfusion" OR "blood transfusion" OR "red blood cell transfusion") AND (leukemia OR leukaemia OR lymphoma OR myeloma OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR haematology OR hematology OR myelodysplasia)
Appendix 13. WHO ICTRP search strategy on the 15 June 2016
Title OR interventions: transfusion OR red cells OR red blood cells OR RBCs
Conditions: blood cancers OR haematological OR nonhodgkin OR leukemia OR lymphoma OR myeloma OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR myelodysplasia OR preleukemia OR dysmyelopoietic OR dysmyelopoiesis OR refractory anemia OR aplastic anemia
Recruitment Status: ALL
Appendix 14. EUDRACT search strategy on the 15 June 2016
(transfusion OR red cell OR red blood cell) AND (leukemia OR lymphoma OR myeloma OR hematology OR haematology OR haematological OR haematological OR plasmacytoma OR Hodgkin OR haematolymphoid OR hematolymphoid OR myelodysplasia OR preleukemia OR dysmyelopoietic OR dysmyelopoiesis OR refractory anemia)
Data and analyses
Comparison 1. Restrictive versus liberal red blood cell transfusion RCTs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality‐at 31 to 100 days | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.02, 2.69] |
| 2 Mortality due to chemotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of participants with any bleeding | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.18] |
| 4 Number of participants with clinically significant bleeding | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.43] |
| 5 Severe or life‐threatening bleeding events | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.39, 6.30] |
| 6 Number of participants with serious infection episodes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Number of participants with VOD | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Number of participants with RBC transfusion from study entry | 3 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.05] |
| 9 Number of participants with RBC Transfusion after reaching Hb >80 g/L for restrictive & Hb > 120 g/L for liberal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Mean number of RBC (units) transfusions per participant during the entire study period | 2 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐3.58 [‐5.66, ‐1.49] |
| 11 Number of participants with PLT transfusions from the study entry | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Zern 2016.
| Methods |
Type of Study: Open‐labelled single‐centre parallel‐arm randomised controlled trial Type of publication: Full Setting and country: USA Number of centres: single Recruitment dates (start and end): April 15, 2014 to July 23 2015 Median follow‐up duration: 5.9 weeks restrictive 6.1 weeks liberal Was a power calculation performed? |
|
| Participants |
Inclusion criteria: acute leukaemia patients (AML, ALL, APL, high grade MDS) admitted with plans for inpatient myelosuppressive chemotherapy (with standard of care or protocol regimens) Exclusion criteria:
Number screened: 162 eligible, only 112 approached due to resource limitations, 22 declined to participate Number recruited: 90 (1 participant withdrew prior to any transfusion) Age: restrictive: median 56 years (IQR 45.5 to 67): liberal: median 62.5 years (IQR 55.2 to 67.8) Gender: Male 49 (restrictive 33; liberal 16); Female 40 (restrictive 26; liberal 14) Ethnicity: not reported Diagnosis: AML (excluding APL) 73 (restrictive 50; liberal 23); APL 2 (restrictive 2; liberal 0); ALL 7 (restrictive 7; liberal 7) Stage of disease: not reported Baseline haemoglobin level: restrictive: median 83 g/L (IQR 75 to 89), liberal: median 89 g/L (IQR 81 to 92) Treatment: all received induction chemotherapy Number analysed for primary outcome: 89 participants (59 restrictive transfusion policy and 30 liberal transfusion policy) Were participants with active bleeding explicitly excluded? Yes Were participants with a history of myocardial ischaemia/infarction explicitly excluded? No, only participants with acute coronary syndrome where excluded |
|
| Interventions |
Restrictive RBC transfusion group: participants will receive blood transfusion with transfusion threshold of 70 g/L Hb Liberal RBC transfusion group: participants will receive blood transfusion with threshold of 80 g/L Hb. Off‐protocol transfusions: There were two protocol deviations, one per arm, where patients were transfused before reaching their preset trigger accidentally. Red cell component: Standard leucocyte‐reduced and irradiate red‐cell units irradiated and prepared in additive solutions. Duration of red cell storage: Not reported. |
|
| Outcomes |
Primary Outcome: Safety of a restrictive transfusion threshold of 70 g/L compared to a standard transfusion threshold of 80 g/L at 60 days. Seondary Outcomes:
|
|
| Assessment of Bleeding | Bleeding was graded using the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. "The patients were assessed for bleeding and fatigue daily by the treating providers and documented in the daily progress notes. This was prospectively planned at the start of the trial and is the standard protocol for these patients at our institution. The bedside nurses as well as the physicians are required to document bleeding and fatigue daily. There were no adjudicators." |
|
| Quality of Life Assessment | Fatigue was assessed by the National Cancer Institue Fatigue Scale (Mendoza 1999). It is a rapid assessment of the fatigue severity in people with cancer. It is a numeric 10‐point scale, with a score of 0 indicating no fatigue, a score of 5 interpreting as moderate fatigue and the maximum score of 10 is an indicate of the worst possible fatigue.This numeric scale was reported by the participants in the trial and the scoring form was completed by the clinical staff. | |
| Notes |
Trial Register ID: National Institute of Health, Clinicaltrials.gov registry NCT02086773 Supported by: Sidney Kimmel Comprehensive Cancer Center Conflicts‐of‐interest statement: No conflict of interest was disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random‐number sequence was generated using computer software (JMP Version 9.0, SAS Institute). Treatment assignment was done with a 2:1 ratio, for the LOW:HIGH Hb trigger groups, respectively. Blocking was used to specify a 2:1 ratio of treatment groups for each group of 18 consecutive patients." |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque sequentially numbered envelopes were opened upon determination of inclusion for each patient in the trial. The randomization sequence and creation and numbering of the envelopes was performed by an investigator who did not enroll or consent patients for the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "...the study, by its nature, was not blinded and both the patients and their providers were aware of the treatment assignment groups." "There was initial inherent bias among nurses and physicians who were concerned about withholding transfusions from patients who need them, which may have increased the incidence of crossovers from the LOW to the HIGH group." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Lack of blinding could theoretically have influenced some outcome measures. For example, the fatigue scores may have been falsely low in the LOW group, resulting in an overestimation of fatigue difference between groups" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "One patient randomized to the LOW arm was not treated on study as the patient withdrew consent before any transfusions performed.All 89 patients randomly assigned and treated on the study protocol were included in the analysis. The patients who were approached and declined cited reasons for refusal as lack of willingness to participate in a clinical trial (seven patients), refusal for randomization (12 patients), and expressed concern of withholding of standard of care transfusion threshold (three patients)." "Both patient and clinician decisions to withdraw from study were slightly higher in the LOW arm: patient decision two of 59 (3.4%; 95% CI, 0.41%‐11.71%) versus zero of 30 (0%; 95% CI, NA‐11.57%), and clinician decision five of 59 (8.5%; 95% CI, 2.81%‐18.68%) versus one of 30 (3.3%; 95% CI, 0.08%‐17.22%). The two patient reasons for withdrawal of consent were both noted as decreased performance status or fatigue that they believed would improve after transfusion to a higher Hb. Upon subsequent query, both patients believed that they did feel better off the trial. The clinician withdrawals of consent were an inpatient fall attributed to anemia resulting in a head laceration (one patient), sepsis and goal of improved perfusion with higher Hb (two patients), inability to follow trial trigger due to extensive alloantibodies and the requirement to transfusion only when blood was available (one patient), and a decreased patient performance status or fatigue perceived by the provider as related to anemia (one patient)." |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes reported in the trial registration but not reported in the published paper were:
We contacted the study authors who said that these outcomes would be the basis of a second publication. |
| Other bias | Unclear risk | "Baseline Hb levels were somewhat lower in the LOW threshold group, a median of 8.3 g/dL compared to 8.9 g/dL in the HIGH group (Wilcoxon p = 0.03)." "When the mean number of RBC units transfused was compared between arms of the study, adjusting for baseline Hb, the LOW arm was transfused 8.0 (95% CI, 6.9‐9.1) units per patient while the HIGH arm patients received 11.7 (95% CI, 10.1‐13.2) units for an estimated difference (LOW minus HIGH) of 23.7 (95% CI, 25.6 to 21.7) units per patient, analysis of covariance p = 0.0003." "The incidence of crossover was also similar in the two study arms: seven of 59 (11.9%; 95% CI, 4.91%‐22.93%) in the LOW arm and two of 30 (6.7%; 95% CI, 0.82%‐22.07%) in the HIGH (chi‐square, p = 0.44)." "The primary objective was the feasibility of conducting a larger randomized trial, which was defined a priori as achieving the following four criteria: 1) more than 50% of the eligible patients consented, 2) more than 75% of the patients randomized to the 7 g/dL arm tolerated the transfusion trigger, 3) fewer than 15% of patients crossed over from the lower transfusion threshold arm to the higher transfusion threshold arm, and 4) no indications for the need to pause the study for safety concerns." All these criteria were met but the upper limit of the 95% CI was higher than the prespecified 15% limit for cross‐over of participants. |
Jansen 2004.
| Methods |
Type of study: Non‐randomised controlled study (data collected prospectively for another study (HOVON 29), study designed retrospectively) Type of publication: Full Setting and country: Rotterdam, the Netherlands, Europe Number of centres: Two inpatient haematology units within a university hospital in Rotterdam (Erasmus Medical Centre & Daniel den Hoed Kliniek) Recruitment dates (start and end): June 1, 1997 to December 7, 2001 Mean follow‐up duration: Approximately one month (mean follow‐up duration 30 days for restrictive, 32 days for liberal group) Was a power calculation performed? Not reported |
|
| Participants |
Inclusion criteria: participants with newly diagnosed acute myeloid leukaemia (AML) treated with combination chemotherapy (ARA‐C then Idarubicin) during first cycle of induction chemotherapy. Aged 15 to 60 years Exclusion criteria: A concurrent active malignancy, except stage I cervical carcinoma or basal cell carcinoma. Previous treatment with chemotherapy. Leukaemia following from a documented myelodysplasia with a duration of more than 6 months. Blastic crisis of chronic myeloid leukaemia or leukaemia developing from myeloproliferative diseases. Renal or liver function abnormalities. HIV positive serology. Severe cardiac, pulmonary or neurological disease (Information from HOVON 29 protocol). Number screened: unknown Number recruited: 84 ( all included in analysis) Age: mean 42.8 years (SD 11.0) Gender: Male 39 (restrictive 18; liberal 21); Female 45 (restrictive 20; liberal 25) Ethnicity: not reported Diagnosis: acute myeloid leukaemia. (FAB Classification) M0 = 4 (restrictive 2; liberal 2); M1 = 22 (restrictive 11; liberal 11); M2 = 20 (restrictive 9; liberal 11); M3 = 8 (restrictive 3; liberal 5); M4 = 13 (restrictive 9; liberal 4); M5 = 15 (restrictive 2; liberal 13); M6 = 1 (restrictive 1; liberal 0) Stage of disease: all newly diagnosed. Risk category of disease according to HOVON leukaemia protocol: good risk (N = 13 (restrictive 7; liberal 6)); intermediate risk (N = 47 (restrictive 19; liberal 28)); poor risk (N = 21 (restrictive 11; liberal 10)); unknown (N = 3 (restrictive 1; liberal 2)) Baseline haemoglobin level: not reported Treatment: all received induction chemotherapy [ARA‐C 200mg/m2 (days 0 to 6) and Idarubicin 12mg/m2 (days 0 to 3)] Number analysed for primary outcome: 84 participants (38 restrictive transfusion policy and 46 liberal transfusion policy) Were participants with active bleeding explicitly excluded? No Were participants with a history of myocardial ischaemia/infarction explicitly excluded? Yes, participants with severe cardiac disease where excluded |
|
| Interventions | Participants were assigned to one of the following transfusion strategies (based on where they were treated for their acute leukaemia): i) Restrictive Policy (Erasmus Medical Centre): If aged < 25 years red cell transfusion when Hb < 72 g/L; 25 to 50 years red cell transfusion when Hb < 80 g/L; aged 50 to 70, red cell transfusion when Hb < 88 g/L. Transfused one RBC unit at a time ii) Liberal Policy (Daniel den Hoed Kliniek): 2 units of RBCs given per transfusion when Hb < 96 g/L (transfusions given above threshold if signs/symptoms of decreased oxygen transportation capacity) Off‐protocol transfusions: Not reported. Mean pre transfusion Hb 75 g/L (n = 3) for patients < 25 years when the restrictive strategy was to transfuse below 72 g/L Red cell component: leucocyte‐reduced red‐cell concentrates with a leucocyte count of less than 11x106/L, suspended in a 110 mL SAG‐M solution, with a haematocrit of 0.60 to a total volume of 265 mL. Duration of red cell storage: not reported. |
|
| Outcomes |
Primary: The number of RBC transfusions and the number of units given per transfusion. Secondary: Clinical outcomes planned to be reported in the methods section included: mortality, bleeding, infections, cardiac dysrhythmias, cardiac dysfunction (WHO CTC grade 2 or higher), response to chemotherapy and myeloid:erythroid (M:E) ratio of the bone marrow smears. Additional transfusion outcomes reported include the number of platelet transfusions received, mean number of platelet adult therapeutic doses per transfusion; mean interval between RBC and platelet transfusions. (These transfusion outcomes were not listed in the methods section). Potential confounding variables taken into account: sex, age and AML type |
|
| Assessment of Bleeding | Not reported | |
| Quality of Life Assessment | Not assessed | |
| Notes |
Trial registration: No Source(s) of funding: Pharmaceutical, including Amgen, CKTO, Johnson & Johnson, Roche Nederland BV,Novartis Pharma B.V, as part of the HOVON 29 study. Funding of this study not reported. Conflicts‐of‐interest statement: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A prospective cohort study not randomised. (Please see Table 5) |
| Allocation concealment (selection bias) | Low risk | This an observational cohort "cluster" study. As such there was no concealment of intervention, intervention depended on the site at which the participant was treated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not randomised. This an observational "cluster" study.There was no information if the status of each site was known to the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes assessed were blind to the participants' allocation status. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is difficult to ascertain in this case, where all potential cases were accounted for in this study. The number of participants was not reported for all outcomes in the paper. |
| Selective reporting (reporting bias) | High risk | Other pertinent end‐points were not reported e.g. incidence of infection, infection agents post transplant complications. |
| Other bias | Low risk | Multiple regression analysis was used and adjusted for potential confounding factors such as sex, age and AML type. |
Robitaille 2013.
| Methods |
Type of study: Planned as a multicentre randomised clinical trial Type of publication: Full Setting and country: Canada, North American Number of centres: Planned for six, only one centre recruited prior to trial closure by the Data Safety Monitoring Board Recruitment dates (start and end): study start date: June 2009; planned study completion date: June 2015. Study stopped in August 2010 due to safety concerns Mean follow‐up duration: Randomised on Day 0 with follow‐up to Day +100 post‐stem cell transplantation. Planned long‐term events to be recorded at 1, 2, and 5 years of follow‐up. Was a power calculation performed? Yes. The trial originally planned to recruit 62 participants |
|
| Participants |
Inclusion criteria: children aged 1 to 18 years who were undergoing an allogeneic BMT for a malignant or benign disease (except sickle cell disease) Exclusion criteria: children receiving autologous bone marrow transplant, cord blood transplant or peripheral stem cell transplant, sickle cell disease (since higher Hb level increases blood viscosity and puts these patients at risk for stroke); haematopoietic growth factor (G‐CSF, GM‐CSF, stem cell factor, erythropoietin) planned before transplantation (post‐transplant decision of haematopoietic growth factors administration as required by the patient's condition were accepted); presence of an allo‐antibody directed against RBC antigens. Number screened: unknown (all consecutive BMT patients) Number recruited: six were randomised prior to early stopping (all included in analyses). Age: mean 11.7 years (range 5.9 to 15.9 years) Gender: male 2 (restrictive 1; liberal 1); female 4 (restrictive 2; liberal 2) Ethnicity: not reported Stage of disease: not reported Diagnosis: AML 3 (restrictive 1; liberal 2); MDS 2 (restrictive 1; liberal 1); immune deficiency 1 (restrictive 1) Treatment: myeloablative (busulfan‐cyclophosphamide) allogeneic BMT; HLA‐matched sibling 4 (restrictive 1; liberal 3); HLA‐matched unrelated 1 (restrictive 1); HLA‐mismatched related 1 (restrictive 1). GvHD prophylaxis: methotrexate and cyclosporin 4 (restrictive 1; liberal 3); methotrexate, cyclosporin & ATG 2 (restrictive 2) Baseline haemoglobin level: not reported Number analysed for primary outcome: N = 6 (3in restrictive group and 3 in liberal group) Were participants with active bleeding explicitly excluded? No Were participants with a history of myocardial ischaemia/infarction explicitly excluded? No |
|
| Interventions | Patients were randomised to one of the following transfusion strategies: i) Restrictive: Maintain Hb > 70 g/L. Transfused 10 to 15 mL/kg unless the clinical condition dictated otherwise. ii) Liberal: Maintain Hb > 120 g/L. Transfused 10 to 15 mL/kg unless the clinical condition dictated otherwise. Off‐protocol transfusions: None received. 3 non‐compliant days in the liberal group recorded due to lack of transfusion during a 24 hr period where Hb < 120 g/L Red cell component: leucocyte‐reduced red‐cell concentrates. Duration of red cell storage: mean length of storage was not significantly different between study groups (restrictive 24.6 days; liberal 26.6 days). |
|
| Outcomes |
Primary: Time to neutrophil recovery (defined as the time from transplantation to the first of 3 consecutive days with a neutrophil count ≥ 0.5 x 109/L) Secondary: 1) Time to platelet recovery (from transplant to the first of three consecutive days with platelets count ≥ 20 x 109/L, without need for platelet transfusions in last 7 days), 2) number of RBC and platelet transfusions, 3) length of hospitalisation, 4) immune reconstitution, 5) overall survival, 6) transplantation‐related mortality, 7) relapse, 8) acute and chronic GvHD, and 9) chimerism. Long‐term events and follow‐up at 1, 2 and 5 years was part of the study plan. |
|
| Assessment of Bleeding | Not reported | |
| Quality of Life Assessment | Not assessed | |
| Notes |
Trial registration: NCT00937053; Canadian Blood and Marrow Transplant Group CBMTG 0603 Source(s) of funding: Fonds de la Recherche en Sante (grant 9967 and 24460) and C17 research network (public). Conflicts‐of‐interest statement: the authors declared no conflict of interest. Stopping of trial: The study was stopped after recruiting only 6 participants because of safety concerns. All 3 participants in the liberal transfusion arm developed VOD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation sytem " Randomization of patients was done on the first day of their conditioning regimen using a web‐based randomization system" |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation sytem " Randomization of patients was done on the first day of their conditioning regimen using a web‐based randomization system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study, no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study not complete, as it was stopped early by the study's DSMB after recruiting 6 participants. All participants in the liberal transfusion arm were withdrawn from the study prior to day 100. "A patient found the RBC transfusions bothering and withdrew from the trial for this reason on day +10. Another patient was withdrawn at day +25 by the treating physician because of the occurrence of VOD as well as 2 episodes of minor allergic reactions to the transfusion. The last patient was withdrawn on day +8 when VOD occurred." All participants in the restrictive arm continued on the planned transfusion policy until day 100. All participants recruited were followed up until day 100 |
| Selective reporting (reporting bias) | High risk | Study not complete, as it was stopped early by the study's DSMB. The statistical analysis for the study's primary outcome was not performed. Secondary outcomes not reported were: time to platelet recovery; length of hospitalisation; immune reconstitution; relapse; and chimerism. |
| Other bias | High risk | The early stopping of the trial could substantially overestimate the results with only six participants enrolled. |
Webert 2008.
| Methods |
Type of study: A multicentre, single‐blinded pilot randomised‐controlled trial Type of publication: Full paper Setting and country: Canada, North America Number of centres: 4 tertiary haematology centres Recruitment dates (start and end): 01/03/2003 to 31/10/2004 [20 months] Mean follow‐up duration: 25.9 days (SD 8.4) in the restrictive group, 23.6 days (10.0 SD) in the liberal group. 1482 days of observation. The study observation periods started on the day after randomisation and ended when one of the following criteria was met: Participant's platelet count was greater than 20 x 109/L for 7 days without a platelet transfusion or when it was not possible to perform daily bleeding assessments or when participant's physician requested removal from the study or when death occurred. Was a power calculation performed?: No |
|
| Participants |
Inclusion criteria: adults (age >16 years) with acute leukaemia admitted for induction or re‐induction chemotherapy or adult patients admitted to receive conditioning for HLA antigen–matched myeloablative allogeneic SCT for a haematologic malignancy. Exclusion criteria: acute promyelocytic leukaemia (French‐American British M3); aplastic anaemia; history of myocardial infarction or angina in the past 6 months; refusal to receive blood transfusions; history of inherited or acquired coagulation disorders; known haemolytic disease; international normalized ratio (INR) of greater than 1.5 (uncorrected by the administration of vitamin K); evidence of significant acute bleeding during the first 12 hours after admission to the hospital (defined as evidence of ongoing blood loss accompanied by a decrease in the Hb concentration of at least 30 g/L during the first 12 hours after admission or a requirement of at least 3 units of RBCs during the same period); presence of an alloantibody that could limit compatible blood supply; previous enrolment in this trial; and unwillingness or inability to give informed consent. Number screened: 84 (9 ineligible; 15 declined to participate) Number recruited: 60 (all included in analyses) Age: mean 47.9 years (range 18 to 77 years) Gender: Male 32 (restrictive 18; liberal 14); Female 28 (restrictive 11; liberal 17) Ethnicity: not reported Diagnosis: AML 39 (restrictive 19; liberal 20); ALL 5 (restrictive 3; liberal 2); haematological malignancy (unspecified) 16 (restrictive 7; liberal 9) Stage of disease: newly diagnosed AML 30 (restrictive 15; liberal 15); relapsed AML 9 (restrictive 4; liberal 5); unknown 21 (restrictive 10; liberal 11) Treatment: chemotherapy 44 (restrictive 22; liberal 22); SCT 16 (restrictive 7; liberal 9) Baseline haemoglobin level: restrictive: 96.3 g/L, liberal: 96.5 g/L Number analysed for across outcomes: For bleeding 60 (29 restrictive and 31 liberal), for platelets and RBC and Hb levels 57(29/28). Were participants with active bleeding explicitly excluded? Yes. Participants with acute bleeding during the first 12 hours after admission were excluded. Were participants with a history of myocardial ischaemia/infarction explicitly excluded? Yes, participants with a history of myocardial infarction or angina in the last 6 months. |
|
| Interventions | Patients were randomised to one of the following transfusion strategies: i) Restrictive: two units of RBC transfused when Hb below 80 g/L ii) Liberal: two units of RBC transfused when Hb below 120 g/L Off‐protocol transfusions: 36.4% of transfusions received in restrictive group when Hb > 80g/L; 29.8% of transfusions received when Hb > 120g/L in the liberal group Red cell component: leucocyte‐reduced before storage by Canadian blood services. volume 240 mL to 340 mL per unit, suspended in AS‐3, estimated haematocrit of 0.55 to 0.65. Duration of storage: not reported |
|
| Outcomes |
Primary and secondary: All five outcomes reported in the study considered relevant to the study feasibility and no single primary outcome was selected. The outcomes of the study included; 1) bleeding (the occurrence graded by the WHO score, the time to the first bleed and number of bleeding days); 2) proportion of days of thrombocytopenia where the Hb level was within the targeted range; 3) blood product utilisation (number of RBC and platelet units) and blood donor exposure; 4) the ability to document bleeding symptoms and bleeding severity; and 5) participant number enrolled. |
|
| Assessment of Bleeding | Clinical assessment of bleeding was performed each morning and reported according to the WHO scale. Each morning Monday to Friday a trained blinded assessor performed the observation. Saturday and Sunday assessments were performed retrospectively by reviewing the participant's chart. All bleeding episodes were independently reviewed by an adjudication committee who were blinded to the participants' treatment assignments and Hb levels. Bleeding categorised by the committee by severity: no bleeding, non‐clinically significant bleeding, and clinical significant bleeding. |
|
| Quality of Life Assessment | Not assessed | |
| Notes |
Trial registration: Unknown. Source(s) of funding: Canadian Blood Services & CIHR Canada Research Chair (Public source) Conflicts‐of‐interest statement: Unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated "A computer‐generated random treatment allocation schedule was developed with a variable blocking factor with the size of the blocks selected in a random fashion from a limited number of possibilities". |
| Allocation concealment (selection bias) | Low risk | Computer generated "patients were randomly assigned with an Internet‐based randomisation web site" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded, a member of the study group would assess clinical records to check if any bleeding episodes were missed. Bleeding reviews at weekend performed on Monday through review of patient's chart (not all bleeding episodes may have been recorded). Other reported outcomes are "hard" and/or "utilisation" outcomes, which could be argued to less influenced by un‐blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up until study completion. |
| Selective reporting (reporting bias) | Low risk | No missing data identified. The analysis is assumed be intention‐to‐treat but neither explicitly confirmed in the report. |
| Other bias | Unclear risk | Number of study days after target Hb levels reached differed were variable between groups (467 days restrictive; 410 days liberal), as it took longer for liberal group to reach the Hb threshold. Due to the way that bleeding assessed some bleeding episodes might had been missed. |
ALL = acute lymphocytic leukaemia AML = acute myeloid leukaemia APL = acute promyelocytic leukaemia CML: Chronic myelogenous leukaemia BMT = bone marrow transplant DSMB = Data Safety Monitoring Board G‐CSF = granulocyte‐colony stimulating factor GM‐CSF = Granulocyte‐macrophage colony‐stimulating factor GvHD = graft versus host disease Hb = haemoglobin IQR = interquartile range MDS = myelodysplastic syndrome RBC(s) = red blood cell(s) SCT = stem cell transplant SD = standard deviation VOD = veno‐occlusive disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abels 1991 | Participants: Oncology participants Intervention: transfusion rates studied not different triggers. |
| Almeida 2013 | Participants: non‐haematological oncological participants included. An RCT involving adults with cancer who were undergoing abdominal surgery. |
| Bercovitz 2011 | Study design: review |
| Berger 2012 | Study design: Ambidirectional cohort study Intervention: one versus two units studied as opposed to a haemoglobin trigger. |
| Bruun 2011 | Participants: non‐haematological oncological participants included |
| Carson 2014 | A review including RCTs conducting on people with myocardial infarction, brain injury, stroke, or haematological disorders. |
| Holst 2013 | Review |
| ISRCTN26088319 | An RCT involving people with myelodysplastic syndrome. |
| Lightdale 2012 | Study design: cohort study using retrospective control. |
| Mear 2014 | Study design: cohort study using historical control. |
| NCT00202371 | Study withdrawn prior to enrolment |
| NTR2684 | Participants: participants receiving chemotherapy were excluded |
| Paananen 2009 | Study design: no prospective element. |
| Patil 2013 | Study design: no prospective element. |
| Prescott 2016 | Review. |
| Yakymenko 2015 | Participants: non‐haematological oncological participants included. An RCT involving adults with cancer. |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02099669.
| Methods | Red Blood Cell Transfusion Thresholds and QOL in MDS (EnhanceRBC): a Pilot, Feasibility Study Open‐label, parallel, randomised controlled trial |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
|
| Outcomes |
Primary Outcome Percentage compliance of fortnightly HB The percentage compliance of fortnightly Hb being within or above the target range of the RBC transfusion threshold assigned (after the 4 week run‐in at study start as defined above). We will consider this study feasible and worthy of future development into a larger randomised trial (powered for QOL difference) if compliance is ≥70%. A compliance rate of 50% to 70%, would not exclude going forward with such an RCT but only after careful discussion and statistical planning Secondary Outcomes
|
| Notes |
Planned recruitment: 30 adults Sponsor: Sunnybrook Health Sciences Centre Trial registration: NCT02099669 on 26 March 2014 Location of trial: Canada Number of study centres: 1 |
ECOG = Eastern Cooperative Oncology Group ESA(s) = erythropoiesis‐stimulating agent(s) Hb = haemoglobin MDS = myelodysplastic syndrome pRBC(s) = packed red blood cell(s) QOL = quality of life RBC(s) = red blood cell(s) RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Chantepie 2015.
| Trial name or title | Randomized trial of two transfusion strategies in patients hospitalized for acute leukemia induction chemotherapy or hematopoietic stem cells with medico‐economic evaluation (1 VERSUS 2 CGR) |
| Methods | Open‐labelled single‐centre parallel‐arm randomised controlled trial |
| Participants |
Inclusion criteria 18 years or older with either acute leukaemia diagnosis receiving intensive chemotherapy or autologous transplantation for lymphoma, allogeneic stem cell transplantation. Exclusion criteria
|
| Interventions |
Two packed red blood cells Transfusion group (liberal): Two RBC packs will be transfused to the study participants with anaemia defined as a Hb level below 80 g/L. Clinical and biological monitoring will be carried out later each day. If the Hb is < 80 g/L, two new pRBCs are transfused and so on. Single red blood packed cells Transfusion group (restrictive): Single RBC pack will be administered in patient with anaemia defined as a Hb level below 80 g/L. Clinical and biological monitoring will be carried out later each day. If the Hb is < 80 g /L, a single unit is transfused and so on. |
| Outcomes |
Primary outcome measure:
(This includes: stroke, transient ischaemic attack, acute coronary syndrome, heart failure, arrhythmias or conduction cardiac disease, deep vein thrombosis, pulmonary embolism, elevated troponin, transfer to intensive care unit, death from any cause, new or progressive radiographic infiltrates, infections related to transfusion). Secondary outcome measures: All will be assessed up to 1 month after the last day of hospitalisation, these will include:
|
| Starting date |
Study Start Date: January 2016 Estimated Study Completion Date: January 2018 Estimated Primary Completion Date: January 2018 (Final data collection date for primary outcome measure) |
| Contact information | DR Sylvain P Chantepie, University Hospital, Caen, France chantepie‐s@chu‐caen.fr |
| Notes |
Planned recruitment: 230 adults Sponsor: University Hospital, Caen Trial registration: NCT02461264 on 3 June 2015 Location of trial: Caen, France Number of study centres: 1 |
Tay 2011.
| Trial name or title | Transfusion of red cells in hematopoietic stem cell transplantation (TRIST) |
| Methods | Open‐labelled multicentre parallel‐arm randomised controlled trial |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Liberal Strategy (Red cell transfusion trigger of 90 g/L): Participants will receive 2 units of packed RBCs if the Hb level is < 90 g/L, based the day’s CBC to target a Hb level of 90 to 110 g/L Restrictive Red cell Transfusion Strategy (Red cell transfusion trigger of 70 g/L): Participants will receive 2 units of pRBCs if the Hb level is < 70 g/L, based on the day’s CBC to target a Hb level of 70 g/L to 90 g/L |
| Outcomes |
Primary Outcome: Quality of Life by FACT‐BMT Secondary Outcomes: 1) Transfusion requirements (red cells, platelets and plasma), 2) Transplant‐related mortality, 3) Maximum grade of GvDH, 4) Veno‐occlusive disease, 5) Serious infections, 6) Bearman Toxicity Score, 7) Bleeding, 8) Quality of life by EQ 5D, FACT‐Anemia, 9) Number of Hospitalisations, 10) Number of ICU admissions |
| Starting date | March 2011 |
| Contact information | Dr Jason Tay, The Ottawa Hospital Blood and Marrow Programme, The Ottawa Hospital, Ottawa, Canada. jtay@ottawahospital.on.ca |
| Notes |
Planned recruitment: 300 adults Estimated study completion date: June 2016 Trial Register ID: National Institute of Health, Clinicaltrials.gov registry NCT01237639 Supported by: Canadian Blood Services (R&D Intramural Grants Competition 2010) and Canadian Institute of Health Research Location of trial: Canada Number of study centres: multiple |
ALT = alanine transaminase AST = aspartate transaminase GvHD = graft versus host disease HSCT = haematopoietic stem cell transplant ICU = intensive care unit pRBC(s) = packed red blood cell(s) RBC(s) = red blood cell(s)
Differences between protocol and review
Aspects listed in the review protocol (Butler 2014) that were not implemented due to lack of data. Publication bias: We did not perform a formal assessment of potential publication bias (small‐trial bias) because we included fewer than 10 trials within this review (Sterne 2011). With only a few studies were included this was not applicable.
Review outcome and reporting results: We performed meta‐analyses when appropriate. However, due to the small number of studies included in this review and the heterogeneity across these studies this was not always possible. We therefore, summarised and discussed results.
Subgroup analyses: We could not perform any of the subgroup analyses that we initially planned, this was because of absence of data and ultimately we were only able to conduct one meta‐analysis involving the three included randomised controlled trials (RCTs).
Meta‐regression: This was not possible as this was advised with a subgroup that contained more than 10 studies (Deeks 2011)
Sensistivity analyses: This was not possible as we were only able to combine relevant data extracted from the three included studies in one meta‐analysis.
Summary of findings: We listed the most important clinical outcomes for the comparison of the two transfusion methods in the review protocol. However, the hospital rate admission outcome was not reported in any of the included studies.
Contributions of authors
Lise Estcourt: protocol development, searching, selection of studies, eligibility and quality assessment, data extraction and analysis, and content expert.
Reem Malouf: searching, selection of studies, eligibility and quality assessment, data extraction and analysis, and drafting.
Marialena Trivella: protocol development and statistical expert.
Dean Fergusson: a content and methodological expert for this review and contributed to the preparation of the protocol and the proposed analysis.
Sally Hopewell: protocol development and methodological expert.
Mike Murphy: protocol development and content expert
Sources of support
Internal sources
NHS Blood and Transplant, Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
Lise Estcourt is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest.
Reem Malouf is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest.
Marialena Trivella is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest.
Sally Hopewell is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest.
Dean A Fergusson: None known
Mike Murphy: None known.
New
References
References to studies included in this review
De Zern 2016 {published data only}
- DeZern AE, Williams K, King K, Hand W, Levis M, Smith BD, et al. Liberal vs. restrictive transfusion thresholds in leukemia patients: a feasibility pilot study. The American Society of Hematology (ASH) the 57th Annual meeting & exposition Orlando. 2015.
- DeZern AE, Williams K, Zahurak M, Hand W, Stephens RS, King KE, et al. Red blood cell transfusion triggers in acute leukemia: a randomized pilot study. Transfusion 2016;56(7):1750‐7. [DOI: 10.1111/trf.13658] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02086773. Prospective randomized clinical feasibility study of red cell transfusion goals in patients with acute leukemias. clinicaltrials.gov/ct2/show/NCT02086773 (accessed 14 January 2016).
Jansen 2004 {published data only}
- Jansen AJ, Caljouw MA, Hop WC, Rhenen DJ, Schipperus MR. Feasibility of a restrictive red‐cell transfusion policy for patients treated with intensive chemotherapy for acute myeloid leukaemia. Transfusion Medicine 2004;14(1):33‐8. [DOI] [PubMed] [Google Scholar]
Robitaille 2013 {published data only}
- NCT00937053. Maintaining a higher level of haemoglobin: effect on the white cells after bone marrow transplantation in children. clinicaltrials.gov/ct2/show/NCT00937053 (accessed 14 January 2016).
- Robitaille N, Lacroix J, Alexandrov L, Clayton L, Cortier M, Schultz KR, et al. Excess of veno‐occlusive disease in a randomized clinical trial on a higher trigger for red blood cell transfusion after bone marrow transplantation: a Canadian blood and marrow transplant group trial. Biology of Blood and Marrow Transplantation 2013;19(3):468‐73. [DOI] [PubMed] [Google Scholar]
Webert 2008 {published data only}
- Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicentre pilot‐randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukaemia or stem cell transplantation . Transfusion 2008;48(1):81‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abels 1991 {published data only}
- Abels R, Gordon D, Nelson R, Krantz K, Ageeb M, Goon B, et al. Transfusion practice in advanced cancer patients. Blood 1991; Vol. 78:474a.
Almeida 2013 {published data only}
- Almeida JP, Galas F, Osawa E, Fukushima J, Moulin S, Park C, et al. Transfusion requirements in surgical oncology patients (TRISOP):a randomized, controlled trial. Critical Care 2013;17 Suppl 2:36410.1186/cc12302. [Google Scholar]
Bercovitz 2011 {published data only}
- Bercovitz RS, Dietz AC, Magid RN, Zantek ND, Smith AR, Quinones RR. What is the role of red blood cell transfusion threshold on number of transfusions in pediatric patients after hematopoietic stem cell transplant?. Blood 2011;118(21):1263. [Google Scholar]
Berger 2012 {published data only}
- Berger MD, Gerber B, Arn K, Senn O, Schanz U, Stussi G. Significant reduction of red blood cell transfusion requirements by changing from a double‐unit to a single‐unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica 2012;97(1):116‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruun 2011 {published data only}
- Bruun KH, Norgaard A, Johansson P, Daugaard G, Sorensen M. HaemOPtimal: Randomized feasibility study of the optimal haemoglobin trigger for red blood cell transfusion (RBC) of anaemic cancer patients (PTS) treated with chemotherapy (CT). Transfusion Alternatives in Transfusion Medicine 2011;12(1):19‐20. [Google Scholar]
Carson 2014 {published data only}
- Carson JL, Strair R. Transfusion strategies in hematologic and nonhematologic disease. Hematology, the American Society of Hematology Education Program 2014;5(1):548‐52. [DOI] [PubMed] [Google Scholar]
Holst 2013 {published data only}
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta‐analysis and trial sequential analysis. BMJ 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN26088319 {published data only}
- ISRCTN26088319. A trial looking at blood transfusions and quality of life in people with myelodysplastic syndrome (REDDS). controlled‐trials.com/ISRCTN26088319 (accessed 14 Jan 2016).
Lightdale 2012 {published data only}
- Lightdale JR, Randolph AG, Tran CM, Jiang H, Colon A, Houlahan K, et al. Impact of a conservative red blood cell transfusion strategy in children undergoing hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2012;18(5):813‐7. [DOI] [PubMed] [Google Scholar]
Mear 2014 {published data only}
- Mear JB, Chantepie S, Gac AC, Bazin A, Reman O. A restrictive strategy reduces the number of transfused packed red blood cells in allograft recipients. Blood 2014;124(21):5016. [Google Scholar]
NCT00202371 {published data only}
- NCT00202371. Transfusion effects in myelodysplastic patients: limiting exposure. clinicaltrials.gov/ct2/show/NCT00202371 (accessed 14 January 2016).
NTR2684 {published data only}
- NTR2684. Transfusion as supportive care for the improvement of the quality of life. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2684 (accessed 14 January 2016).
Paananen 2009 {published data only}
- Paananen P, Arola MO, Pelliniemi T, Salmi TT, Lahteenmaki PM. Evaluation of the effects of different transfusion trigger levels during the treatment of childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology/Oncology 2009;31:745‐9. [DOI] [PubMed] [Google Scholar]
Patil 2013 {published data only}
- Patil NR, Marques M, Mineishi S, Rudolph S, Vaughan W, Innis‐Shelton R, et al. A restrictive red cell transfusion approach does not adversely affect day 100 or 1 year survival in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation 2013;19:S268. [Google Scholar]
Prescott 2016 {published data only}
- Prescott L, Taylor J, Lopez‐Olivo M, Munsell M, VonVille H, Lairso D, et al. How low should we go: A systematic review and meta‐analysis of the impact of restrictive red blood cell transfusion strategies in oncology. Cancer Treatment Reviews 2016;46:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yakymenko 2015 {published data only}
- NCT01116479. Optimal transfusion in anaemic cancer patients treated with chemotherapy (HaemOPtimal) (HaemOPtimal). https://clinicaltrials.gov/ct2/show/NCT01116479 Accessed 27 June 2016.
- Yakymenko D, Frandsen KB, Christensen IJ, Norgaard A, Johansson P, Daugaard G, et al. Haemoptimal: Randomized feasibility study of the optimal hemoglobin trigger for red blood cell transfusion to anemic cancer patients treated with chemotherapy. Supportive Care in Cancer 2015;23(Supp 1):S275. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02099669 {published data only}
- NCT02099669. Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC). clinicaltrials.gov/ct2/show/NCT02099669 (accessed 14 Jan 2016).
References to ongoing studies
Chantepie 2015 {published data only}
- Chantepie SP, Mear JB, Guittet L, Dervaux B, Marolleau JP, Jardin F, et al. Transfusion strategy in hematological intensive care unit: study protocol for a randomized controlled trial. Trials 2015;16:533. [DOI: 10.1186/s13063-015-1057-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02461264. Randomized trial of two transfusion strategies in patients hospitalized for acute leukemia induction chemotherapy or hematopoietic stem cells with medico‐economic evaluation (1 VERSUS 2 CGR). https://clinicaltrials.gov/ct2/show/study/NCT02461264 ( accessed 28 January 2016).
Tay 2011 {published data only}
- NCT01237639. Study of red blood cell transfusion triggers in patients undergoing hematopoietic stem cell transplantation (TRIST). clinicaltrials.gov/ct2/show/NCT01237639 (accessed 14 January 2016).
- Tay J, Tinmouth A, Fergusson D, Allan D. Transfusion of red cells in hematopoietic stem cell transplantation (TRIST): study protocol for a randomized controlled trial. Trials 2011;12:207. [DOI: 10.1186/1745-6215-12-207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adam 1942
- Adam RC, Lundy JS. Anesthesia in cases of poor risk. Some suggestions for decreasing the risk. Surgery, Gynecology & Obstetrics 1942;74:1011‐101. [Google Scholar]
Bearman 1988
- Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen‐related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology 1988;6(10):1562‐8. [DOI] [PubMed] [Google Scholar]
Beck 1998
- Beck C, Dubois J, Grignon A, Lacroix J, David M. Incidence and risk factors of catheter‐related deep vein thrombosis in a pediatric intensive care unit: a prospective study. Journal of Pediatrics 1998;133:237‐41. [DOI] [PubMed] [Google Scholar]
Blajchman 2005
- Blajchman MA. Transfusion‐related immunomodulation or TRIM: what does it mean clinically?. Hematology 2005;10 Suppl 1:208‐14. [DOI] [PubMed] [Google Scholar]
Bolton‐Maggs 2014
- Bolton‐Maggs PHB (Ed), Thomas D, Cohen H, Watt A, Poles D, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report (2014). http://www.shotuk.org/wp‐content/uploads/report‐2014.pdf (accessed 29 January 2016):8‐197.
Borkent‐Raven 2010
- Borkent‐Raven BA, Janssen MP, Poel CL, Schaasberg WP, Bonsel GJ, Hout BA. The PROTON study: profiles of blood product transfusion recipients in the Netherlands. Vox Sanguinis 2010;99(1):54‐64. [DOI] [PubMed] [Google Scholar]
Carson 2011
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GR, et al. Liberal or restrictive transfusion in high‐risk patients after hip‐surgery. New England Journal of Medicine 2011;365(26):2453‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2012;157(1):49‐58. [DOI] [PubMed] [Google Scholar]
Carson 2013
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American Heart Journal 2013;165(6):964‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2016
- Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD002042.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cholette 2011
- Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single‐ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red‐cell transfusion strategy. Pediatric Critical Care Medicine 2011;12(1):39‐45. [DOI] [PubMed] [Google Scholar]
Colomo 2008
- Colomo A, Hernandez‐Gea V, Muniz‐Diaz E, Madoz P, Aracil C, Alvarez‐Urturi C, et al. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology 2008;48(S1):413A. [DOI: 10.1002/hep.22641] [DOI] [Google Scholar]
Cook 1997
- Cook RJ, Lawless JF. Marginal analysis of recurrent events and a terminating event. Statistics in Medicine 1997;16(8):911‐24. [DOI] [PubMed] [Google Scholar]
CTCAE 2009
- National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0.3. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf (accessed 23 July 2013):3‐194.
De Gast‐Bakker 2013
- Gast‐Bakker DH, Wilde RBP, Hazekamp MG, Sojak V, Zwaginga JJ, Wolterbeek R, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients:a randomized controlled trial. Intensive Care Medicine 2013;39:2011‐9. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statsitical Methods Group (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Docherty 2016
- Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst LB, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non‐cardiac surgery setting: systematic review and meta‐analysis. BMJ 2016;352:i1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2014
- Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Archives of Gerontologyand Geriatrics 2014;59(1):181‐5. [DOI] [PubMed] [Google Scholar]
Fisher 1956
- Fisher MR, Topley ET. The illness of trauma. British Journal of Clinical Practice 1956;10(11):770‐6. [PubMed] [Google Scholar]
Fortune 1987
- Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. Journal of Trauma 1987;27(3):243‐9. [DOI] [PubMed] [Google Scholar]
Gunn 2012
- Gunn S, Davis K, Ekert H, Ireland S, Thompson A, on behalf of the National Blood Authority (Australia). Patient blood management guidelines: module 3 medical. http://www.blood.gov.au/system/files/documents/pbm‐module3_0.pdf (accessed 17 February 2013); Vol. 1:36.
Hajjar 2010
- Hajjar LA, Vincent JL, Galas FRGB, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304(14):1559‐67. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 1996
- Ho CH. The hemostatic effect of adequate red cell transfusion patients with anaemia and thrombocytopenia. Transfusion 1996;36(3):290. [DOI] [PubMed] [Google Scholar]
Ho 1998
- Ho CH. The hemostatic effect of packed red cell transfusion in patients with anemia. Transfusion 1998;38:1011‐4. [DOI] [PubMed] [Google Scholar]
Holst 2015
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for guiding red blood cell transfusion: systematic review of randomised trials with meta‐analysis and trial sequential analysis. BMJ 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holst 2016
- Holst LB. Benefits and harms of red blood cell transfusions in patients with septic shock in the Intensive Care Unit. Danish Medical Journal 2016;63(2):1‐18. [PubMed] [Google Scholar]
Jairath 2015
- Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic,open‐label, cluster randomised feasibility trial.. Lancet 2015;386(9989):137‐44. [DOI] [PubMed] [Google Scholar]
Javadzadeh Shahshahani 2015
- Javadzadeh Shahshahani H, Hatami H, Meraat N, Savabieh S. Epidemiology of blood component recipients in hospitals of Yazd, Iran. Transfusion Medicine 2015;25(1):2‐7. [DOI] [PubMed] [Google Scholar]
Khorana 2008
- Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusion, thrombosis, and mortality in hospitalized patients with cancer. Archives of Internal Medicine 2008;168(21):2377‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2004
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anaemia in cancer: a systematic review of the literature. American Journal of Medicine 2004;116(7 Suppl 1):11‐26. [DOI] [PubMed] [Google Scholar]
Lacroix 2007
- Lacroix J, Hébert P, Hutchison J, Hume H, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine 2007;356(16):1609‐19. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Livio 1982
- Livio M, Gotti E, Marchesi D, Mecca G, Remuzzi G, Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet 1982;320:1013‐5. [DOI] [PubMed] [Google Scholar]
McCelland 2010
- McCelland DBL, Pine E, Fraklin IM, for the EU Optimal Use of Blood Project Partners. Manual of Optimal Blood Use. Support for safe, clinically effective and efficient use of blood in Europe. http://www.optimalblooduse.eu (accessed 17 February 2013); Vol. 1:34.
Mendoza 1999
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186‐96. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9, W64. [DOI] [PubMed] [Google Scholar]
Murphy 2011
- Murphy MF, Stanworth SJ, Yazer M. Transfusion practice and safety: current status and possibilities for improvement. Vox Sanguinis 2011;100(1):46‐59. [DOI] [PubMed] [Google Scholar]
Murphy 2015
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. New England Journal of Medicine 2015;372:997‐1008. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Blood transfusion. (NICE guideline 24.) 2015. www.nice.org.uk/guidance/NG24 ( Accessed 14 January 2016).
Nielsen 2014
- Nielsen K, Johansson PI, Dahl B, Wagner M, Frausing B, Børglum J, et al. Perioperative transfusion threshold and ambulation after hip revision surgery‐‐a randomized trial. BMC Anesthesiology 2014;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Quintó 2006
- Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, Espasa M, et al. Relationship between haemoglobin and haematocrit in the definition of anaemia. Tropical Medicine and International Health 2006;11(8):1295‐302. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA, on behalf of the Cochrane Non‐Randomised Studies Methods Group. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Inverventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Review Manager (RevMan) 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2014
- Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014;312(1):36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohde 2014
- Rohde J, Dimcheff D, Blumberg N, Saint S, Langa K, Kuhn L, Hickner A, Rogers M. Health care–associated infection after red blood cell transfusion: a systematic review and meta‐analysis. JAMA 2014;311(13):1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salpeter 2014
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta‐analysis and systematic review. American Journal of Medicine 2014;127:124‐31. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH, on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinegate 2016
- Tinegate H, Pendry K, Murphy M, Babra P, Grant‐Casey J, Hopkinson C, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139‐45. [DOI] [PubMed] [Google Scholar]
Valeri 1998
- Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has a sin of commission now become a sin of omission?. Transfusion 1998;38(6):602‐10. [DOI] [PubMed] [Google Scholar]
Vamvakas 2007
- Vamvakas EC, Blajchman MA. Transfusion‐related immunomodulation (TRIM): an update. Blood Reviews 2007;21(6):327‐48. [DOI] [PubMed] [Google Scholar]
Villanueva 2013
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New England Journal of Medicine 2013;368(1):11‐21. [10.1056/NEJMoa1211801.] [DOI] [PubMed] [Google Scholar]
Wells 2013
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 01 January 2013).
Whitaker 2015
WHO 2015
- World Health Organizaition (WHO). Blood safety and availability, fact sheet N°279. http://www.who.int/mediacentre/factsheets/fs279/en/ (accessed 01 February 2016).
Wu 2011
- Wu JF, Guan XD, Chen J, Ou‐Yang B. A randomized, controlled clinical trial of transfusion of requirement in patients after orthotopic liver transplantation. ESICM LIVES 2011, 24th Annual Congress 2011;37:Abstract. [Google Scholar]
Zygun 2009
- Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Critical Care Medicine 2009;37(4):1074‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Butler 2014
- Butler C, Tay J, Doree C, Brunskill SJ, Trivella M, Fergusson DA, et al. Restrictive versus liberal red blood cell transfusion strategies for patients with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011305] [DOI] [Google Scholar]


