Abstract
Mutations in PNPLA1 have been identified as causative for autosomal recessive congenital ichthyosis in humans and dogs. So far, the underlying molecular mechanisms are unknown. In this study, we generated and characterized PNPLA1-deficient mice and found that PNPLA1 is crucial for epidermal sphingolipid synthesis. The absence of functional PNPLA1 in mice impaired the formation of omega-O-acylceramides and led to an accumulation of nonesterified omega-hydroxy-ceramides. As a consequence, PNPLA1-deficient mice lacked a functional corneocyte-bound lipid envelope leading to a severe skin barrier defect and premature death of newborn animals. Functional analyses of differentiated keratinocytes from a patient with mutated PNPLA1 demonstrated an identical defect in omega-O-acylceramide synthesis in human cells, indicating that PNPLA1 function is conserved among mammals and indispensable for normal skin physiology. Notably, topical application of epidermal lipids from wild-type onto Pnpla1-mutant mice promoted rebuilding of the corneocyte-bound lipid envelope, indicating that supplementation of ichthyotic skin with omega-O-acylceramides might be a therapeutic approach for the treatment of skin symptoms in individuals affected by omega-O-acylceramide deficiency.
Introduction
In humans, ichthyoses comprise a genetically and clinically heterogeneous group of Mendelian disorders of cornification that are primarily characterized by a generalized abnormal desquamation of the skin. Most of the known ichthyoses are nonsyndromic, and the clinical manifestation is restricted to the skin (Schmuth et al., 2013; Traupe et al., 2014). Among different ichthyosis types, the appearance of the skin is very similar, displaying a dry, thickened, scaly phenotype. Yet, the severity of cutaneous symptoms varies and ranges from the relatively mild and most common ichthyosis vulgaris (OMIM #146700) to life-threatening harlequin ichthyosis (OMIM #242500) (Traupe et al., 2014). To categorize ichthyoses, the different types are classified by clinical appearance (i.e., type and distribution of scales, time of onset) and the genetic cause (Oji et al., 2010). Ichthyosis with an autosomal recessive heritable skin defect diagnosed at birth is referred to as autosomal recessive congenital ichthyosis (ARCI). Positional cloning and homozygosity mapping of individuals and families suffering from ARCI identified eight different genes with disease-associated mutations (Eckl et al., 2013; Fischer, 2009; Grall et al., 2012; Radner et al., 2013). We originally identified PNPLA1 (OMIM *612121) as an ARCI-associated gene in a spontaneous mutant dog model (Grall et al., 2012). In this golden retriever breed, we found a homozygous insertion-deletion mutation in PNPLA1 that leads to a premature stop codon. Thus, affected dogs developed lamellar ichthyosis resembling human ARCI. Furthermore, we and others described PNPLA1 mutations in patients affected by ARCI (OMIM #615024) demonstrating the involvement of PNPLA1 in human ichthyoses (Ahmad et al., 2015; Fachal et al., 2014; Grall et al., 2012; Lee et al., 2016). However, the enzymatic function of PNPLA1 is unknown.
To gain insight into the role of PNPLA1 in skin metabolism and the development of ichthyosis, we studied mutant mice that globally lack PNPLA1 and examined lipid metabolism in primary keratinocytes derived from a patient with functional PNPLA1 deficiency due to a PNPLA1 nonsense mutation (Grall et al., 2012). We found that PNPLA1 is an essential factor for the generation of omega-O-acylceramides (ω-O-AcylCers) and the development of a functional skin barrier. Additionally, we provide experimental evidence that topical application of ω-O-AcylCers might represent a therapeutic strategy for the treatment of skin symptoms of patients that are associated with ω-O-AcylCer deficiency, such as those reported for neutral lipid storage disease with ichthyosis (also known as Chanarin-Dorfman syndrome, OMIM #275630) or certain types of ARCIs.
Results
Tissue-specific expression of Pnpla1 in mice
First, we analyzed Pnpla1 mRNA expression in different mouse tissues using quantitative real-time reverse transcriptase-PCR (qRT-PCR). Consistent with an important function in the skin (Grall et al., 2012), Pnpla1 mRNA was mainly expressed in surface lining tissues such as the epidermis, dermis, and tongue (Supplementary Figure S1 online). Interestingly, isolated dermal primary fibroblasts from mice did not show Pnpla1 mRNA expression (Supplementary Figure S1), indicating that cell types other than fibroblasts express Pnpla1 in the dermis (e.g., cells of sebaceous glands or sweat glands, endothelial cells, or immune cells). Low but detectable Pnpla1 mRNA expression was also observed in gastrointestinal epithelial tissue such as in the stomach and small intestine. In all other tissues examined, Pnpla1 mRNA was present only in trace amounts or undetectable.
Generation of PNPLA1-deficient mice
To investigate the physiological role of PNPLA1 in vivo, we generated mice lacking functional PNPLA1. Using the bacteriophage-derived Cre-loxP recombination system, we removed Pnpla1 exon 1 that encodes the translation start codon and the putative lipase GXSXG consensus sequence (Supplementary Figure S2a online). The Southern blot analysis of genomic DNA confirmed disruption of the Pnpla1 gene (Supplementary Figure S2b). Pnpla1 mRNA, as determined by qRT-PCR, was completely absent in the epidermis of PNPLA1-deficent (Pnpla1−/−) mice, whereas mice heterozygous for the targeted Pnpla1 allele (Pnpla1+/−) exhibited an approximately 50% reduced Pnpla1 mRNA expression level in the epidermis as compared with wild-type littermates (Supplementary Figure S2c). Immunoblotting analysis using a polyclonal rabbit anti-PNPLA1 antiserum detected PNPLA1 protein at a molecular weight of approximately 73 kDa in the epidermis of wild-type and Pnpla1+/− mice, but not in that of Pnpla1−/− mice (Supplementary Figure S2d).
PNPLA1 deficiency in mice causes ichthyosis
Newborn Pnpla1−/− mice could be easily identified by the shinier and reddened appearance of their skin that lacked elasticity when compared with their wild-type or heterozygous littermates. Shortly after birth, the skin of Pnpla1−/− mice turned dry and wrinkled, and most of the Pnpla1−/− mice showed signs of a necrotic tail tip (Figure 1a). Similar to mutant mice suffering from other genetic forms of ichthyosis (Herrmann et al., 2003; Nakagawa et al., 2012; Radner et al., 2010; Vasireddy et al., 2007), newborn Pnpla1−/− mice were less active, markedly smaller, and weighed significantly less than their wild-type and heterozygous littermates (Supplementary Table S1 online). Notably, all of the investigated newborn Pnpla1−/− mice died prematurely within 12 hours after birth. Genotype analysis of 707 newborn mice from 85 heterozygous breedings demonstrated a normal Mendelian frequency of the mutant Pnpla1 allele (wild type n = 165, 24.3%; Pnpla1+/− n = 356, 52.5%; Pnpla1−/− n = 157, 23.2%; P < 0.388 by chi-square test), indicating that Pnpla1 disruption is not associated with a prenatal lethal phenotype in mice.
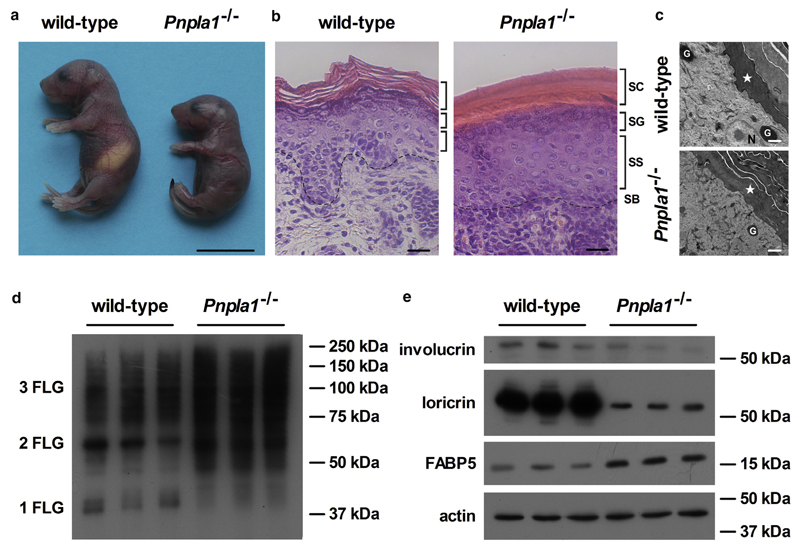
Figure 1. Ichthyosis phenotype of PNPLA1-deficient mice.
(a) Photograph of newborn wild-type and Pnpla1−/− mice taken 8–10 hours postpartum (scale bar = 10 mm). Skin sections of newborn wild-type and Pnpla1−/− mice stained with (b) hematoxylin and eosin for light microscopic analyses (SC, stratum corneum; SG, stratum granulosum; SS, stratum spinosum; SB, stratum basale; dashed lines indicate interphase between dermis and SB; scale bar = 25 μm) or (c) analyzed by transmission electron microscopy (asterisk, SC; N, nucleus; G, granule; scale bar = 1 μm). (d, e) Protein expression levels of filaggrin mono-, di-, and trimers (1, 2, and 3 FLG), involucrin, loricrin, and fatty acid binding protein 5 (FABP5) analyzed by immunoblotting in Pnpla1−/− and wild-type epidermis using actin as loading control. PNPLA1, patatin-like phospholipase domain-containing 1.
To examine whether the loss of PNPLA1 function affects metabolic parameters, we determined plasma levels of important lipid and carbohydrate energy substrates in newborn animals before and after their first suckling (Supplementary Table S2 online). Immediately after birth, plasma fatty acid (FA), triacylglycerol, and glucose concentrations were comparable among the different genotypes and significantly increased after first suckling in wild-type and heterozygous mice. In contrast, concentrations of circulating energy substrates remained low in PNPLA1-deficient mice even 8 hours postpartum, indicating that mutant mice do not suckle. This suckling failure is also evident from the absence of milk stripes in these animals (Figure 1a).
Histological analyses of PNPLA1-deficient skin sections by light or transmission electron microscopy revealed hyperkeratosis with a strongly condensed stratum corneum as well as acanthosis with an increased number of cell layers in the stratum spinosum, and reduced epidermal rete ridges (Figure 1b and c). To examine whether PNPLA1 deficiency affects the epidermal differentiation and cornification process, we analyzed the expression of selected terminal keratinization markers by immunoblot analyses of wild-type and mutant skin (Figure 1d and e). During cornification, profilaggrin undergoes proteolytic processing into its filaggrin units, to crosslink keratin intermediate filaments via disulfide bonds (Sandilands et al., 2009). In PNPLA1-deficient skin, this processing was disturbed as less monomeric, but much more di- and trimeric filaggrin units as well as other profilaggrin peptides of abnormal chain length were present than in skin samples of controls (Figure 1d). Consistent with this delay in differentiation and cornification, the expression levels of involucrin and loricrin, markers for the proper assembly of the corneocyte envelope, were reduced in mutant mice (Figure 1e). Furthermore, the expression level of FA-binding protein 5, which has been reported to be elevated in the epidermis of patients with psoriasis or ichthyosis due to abnormal keratinocyte differentiation and disturbance of the epidermal lipid metabolism (Amen et al., 2013; Siegenthaler et al., 1994), was increased in the Pnpla1−/− skin (Figure 1e). Taken together, these pathological alterations in the skin are characteristics of ichthyoses and indicate that PNPLA1 deficiency in mice results in a condition similar to those present in humans and dogs affected by PNPLA1 mutations.
PNPLA1 deficiency in mice disrupts the skin permeability barrier
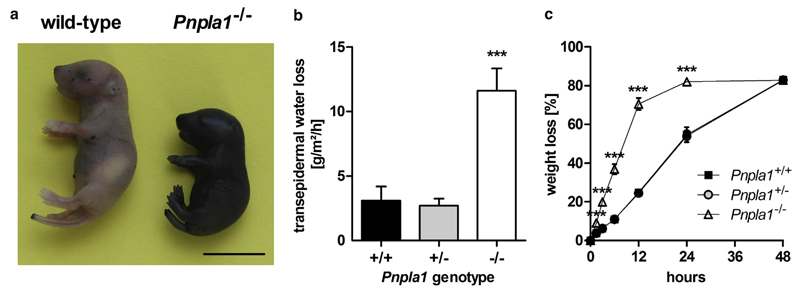
To assess whether the distinct morphological alterations in PNPLA1-deficient epidermis are associated with a loss of the skin permeability barrier function, we determined skin integrity and parameters of barrier function in mutant mice. First, we investigated the penetration of an aqueous toluidine blue solution into the epidermis of newborn mice. As depicted in Figure 2a, Pnpla1−/− mice showed massive staining when exposed to toluidine blue dye indicative of a leaky skin barrier. The defect in the skin barrier function in mutant mice resulted in an approximately 4-fold increased transepidermal water loss when compared with wild-type and heterozygous littermates (Figure 2b). An increased transepidermal water loss was further evident when desiccation of euthanized newborn mice was monitored at 60 °C over time. Consistent with a defect in the skin permeability barrier function, mutant mice desiccated much faster than their wild-type and heterozygous littermates (Figure 2c).
Figure 2. Defective skin permeability barrier function in mice lacking PNPLA1.
(a) Skin barrier function of newborn mice assayed by toluidine blue staining (scale bar = 10 mm). (b) Transepidermal water loss measured in newborn wild-type, Pnpla1+/−, and Pnpla1−/− mice (n = 5 mice/genotype). (c) Desiccation of euthanized newborn wild-type, Pnpla1+/−, and Pnpla1−/− mice monitored at 60 °C over time (n = 6 mice/genotype). Data symbols for heterozygous mice are hidden by that for wild-type mice. Data are presented as means ± SD. Statistically significant differences were determined by unpaired two-tailed Student’s t-test (***P < 0.001) in comparison to wild-type controls. PNPLA1, patatin-like phospholipase domain-containing 1.
PNPLA1-deficient skin lacks ω-O-AcylCers
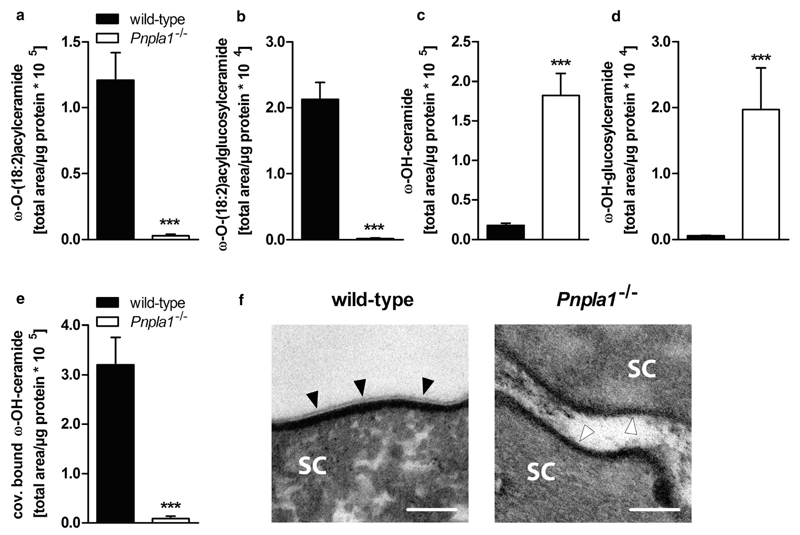
To investigate whether the skin barrier defect in PNPLA1-deficient mice is linked to changes in epidermal lipid composition, we measured epidermal concentrations of neutral and polar lipids in newborn mice by ultra-performance liquid chromatography-quadrupole time of flight (UPLC-qTOF) analyses. In line with normal triacylglycerol metabolism of in vitro differentiated keratinocytes from a patient harboring a PNPLA1 nonsense mutation (Grall et al., 2012), triacylglycerol content in PNPLA1-deficient mice did not differ from controls (Supplementary Figure S3a online). Likewise, epidermal phosphatidylcholine and phosphatidylethanolamine levels were not changed in Pnpla1−/− epidermis (Supplementary Figure S3b and c). Yet, the loss of functional PNPLA1 in the skin strongly affected epidermal ceramide homeostasis. ω-O-(18:2)AcylCers (for molecular structure see Supplementary Figure S4a online) as well as their glucosylated derivatives (i.e., ω-O-(18:2)acylglucosylceramides; for molecular structure see Supplementary Figure S4b), both of which are required for the formation of a functional skin permeability barrier (Uchida and Holleran, 2008), were barely detectable in mutant mice (Figure 3a and b; Supplementary Figure S5a and b online). In contrast, the proposed precursor lipids for ω-O-acyl(glucosyl)ceramide synthesis, free-extractable, ultralong chain ω-hydroxy-ceramides (ω-OH-Cers; for molecular structure see Supplementary Figure S4c) and the glucosylated derivatives (i.e., ω-OH-glucosylceramides, ω-OH-GlcCers; for molecular structure see Supplementary Figure S4d), respectively, substantially accumulated in PNPLA1-deficient epidermis (Figure 3c and d; Supplementary Figure S5c and d). Notably, the total amount of non-hydroxy-ceramides (for molecular structure see Supplementary Figure S4e) was not significantly different in PNPLA1-deficient epidermis compared with controls (Supplementary Figure S3d), indicating a functional turnover of noncomplex ceramide species.
Figure 3. Lack of ω-O-acylceramides in PNPLA1-deficient skin.
Epidermal (a) ω-O-(18:2)acylceramide, (b) ω-O-(18:2)acylglucosylceramide, (c) ω-OH-ceramide, (d) ω-OH-glucosylceramide, and (e) covalently bound ω-OH-ceramide content quantified by UPLC-qTOF analyses in lipid extracts from newborn wild-type and Pnpla1−/− mice. Peak areas of lipid species were combined and normalized to epidermal protein content. Data are presented as means + SD (n = 3–4 mice/genotype) and representative for two independent experiments. Statistically significant differences were determined by unpaired two-tailed Student’s t-test (***P < 0.001) between genotypes. (f) Stratum corneum (SC) of newborn wild-type and Pnpla1−/− mice analyzed by transmission electron microscopy (scale bar = 100 nm). Filled arrow heads indicate the corneocyte-bound lipid envelope (CLE) in wild-type mice. The absence of the CLE in PNPLA1-deficient pups is indicated by opened arrow heads. PNPLA1, patatin-like phospholipase domain-containing 1; UPLC-qTOF, ultra-performance liquid chromatography-quadrupole time of flight.
In the late steps of cornification, ω-O-AcylCers are transferred to the stratum corneum and react with structural proteins (Marekov and Steinert, 1998). This process generates a monolayer of covalently bound ω-OH-Cers, the so-called corneocyte-bound lipid envelope (CLE), which is critical for the maintenance of the skin permeability barrier homeostasis (Behne et al., 2000; Elias et al., 2014; Feingold and Elias, 2014; Swartzendruber et al., 1987). Importantly, covalently bound ω-OH-Cers (for molecular structure see Supplementary Figure S4f) were almost absent in PNPLA1-deficient epidermis as compared with controls (Figure 3e; Supplementary Figure S5e), indicative of a defective CLE. Likewise, electron microscopic analyses of the stratum corneum from neonatal mice demonstrated the absence of the CLE in Pnpla1−/− epidermis (Figure 3f). Thus, the skin permeability barrier dysfunction in PNPLA1-deficient mice derives from the lack of ω-O-acyl(glucosyl)ceramides that consequently interferes with the generation of a functional CLE.
PNPLA1 deficiency in mice and humans impairs ω-O-AcylCer generation
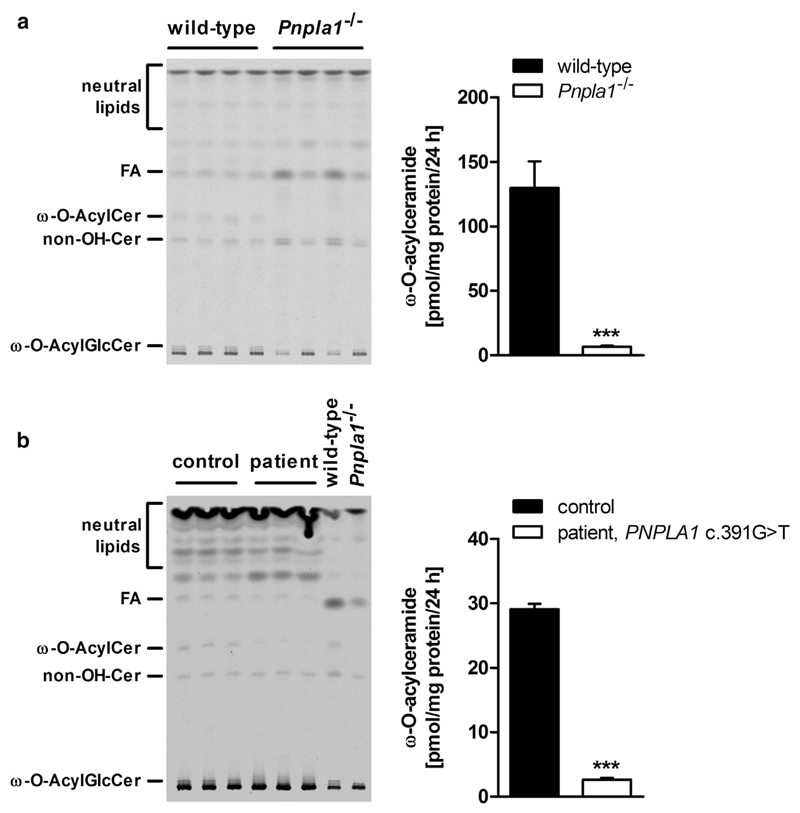
To determine whether ω-O-AcylCer deficiency in PNPLA1-deficient epidermis originates from defect in the synthesis of these lipid species, we incubated epidermal explants from mutant mice and in vitro differentiated keratinocytes from a patient suffering from functional PNPLA1 deficiency (Grall et al., 2012) with [1-14C]-labeled linoleic acid as tracer (Uchida and Holleran, 2008). After 24 hours, epidermal lipids were separated by thin-layer chromatography and radioactivity in the ω-O-AcylCer fraction was measured by liquid scintillation counting. Under these conditions, Pnpla1−/− epidermis and the patient’s keratinocytes exhibited 95% and 91% reduced ω-O-AcylCer synthesis activity, respectively, as compared with their controls (Figure 4a and b). This indicates that adequate ω-O-AcylCer generation requires functional PNPLA1 in both human and murine skin.
Figure 4. Impaired ω-O-acylceramide formation in PNPLA1-deficient epidermal explants and human primary keratinocytes.
Thin-layer chromatography (TLC, left panel) of ex vivo [1-14C] linoleic acid-labeled lipids from (a) epidermal explants of newborn wild-type and Pnpla1−/− mice or (b) in vitro differentiated primary human keratinocytes derived from a healthy individual and a patient carrying a PNPLA1 nonsense mutation (c.391G>T) leading to a premature stop codon and truncated protein (p.Glu131*). TLC plates were exposed to PhosphorImager screens and obtained autoradiography signals were analyzed by a Storm scanner. Output images were adjusted for low signal intensities. Radioactivity contained in the ω-O-AcylCer bands was quantified by scintillation counting (right panel). Data are presented as means + SD (n = 4 mice or 3 independent culture dishes/genotype) and representative for three independent experiments. Statistically significant differences were determined by unpaired two-tailed Student’s t-test (***P < 0.001) between genotypes. ω-O-AcylCer, ω-O-acylceramide; ω-O-AcylGlcCer, ω-O-acylglucosylceramide; FA, fatty acid; non-OH-Cer, non-hydroxy ceramide; PNPLA1, patatin-like phospholipase domain-containing 1.
PNPLA1-deficient skin is capable of rebuilding the CLE after topical application of epidermal lipids from wild-type mice
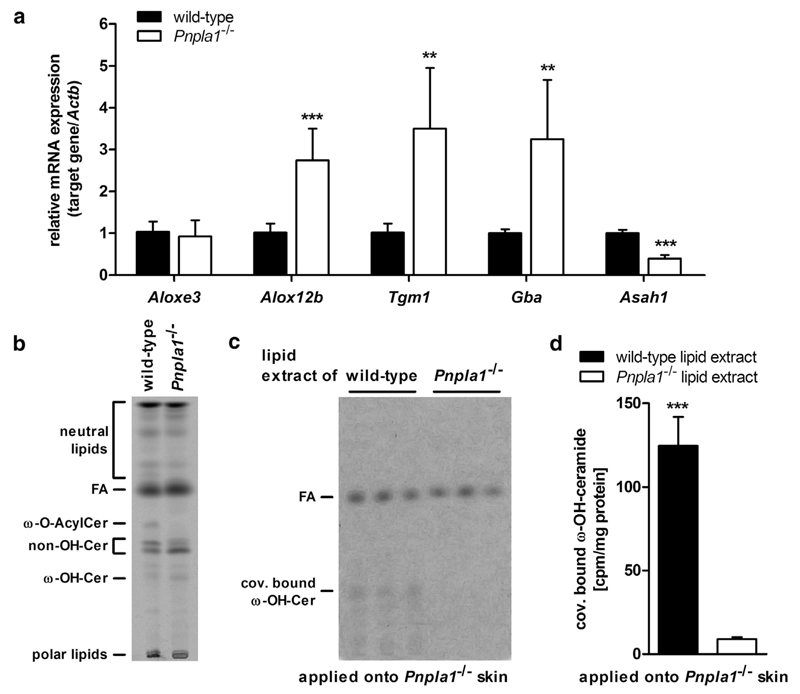
The covalent linkage of ω-O-AcylCers to corneocyte proteins involves several enzymes acting consecutively in a multistep process forming the CLE (Elias et al., 2014; Uchida and Holleran, 2008). Because PNPLA1-deficient mice lacked a functional CLE, we assessed whether their skins express these enzymes. Although the mRNA level of Aloxe3 was similar in PNPLA1-deficient and wild-type mice, mRNA expression of other genes coding for enzymes essential for covalent binding of ω-OH-Cers was induced in PNPLA1-deficient mice (Figure 5a). For example, Alox12b, Tgm1, and Gba expression was 2.7-, 3.5-, and 3.3-fold higher, respectively, in PNPLA1-deficient epidermis than that of controls. In contrast, Asah1, which encodes acidic ceramidase responsible for the degradation of covalently bound ω-OH-Cers to sphingosine and covalently bound ω-OH-FAs, showed a 60% reduced mRNA expression level in Pnpla1−/− epidermis (Figure 5a). This suggests that changes in expression of genes involved in CLE formation may be compensatory for the low abundance of covalently bound ω-OH-Cers in PNPLA1-deficient skin.
Figure 5. Corneocyte-bound lipid envelope (CLE) formation in PNPLA1-deficient skin.
(a) Relative mRNA expression levels of CLE target genes analyzed by qRT-PCR (n = 6 mice/genotype). Thin-layer chromatography (TLC) of (b) ex vivo radiolabeled epidermal lipids ([1-14C] palmitic acid as tracer) from wild-type and Pnpla1−/− mice or (c) CLE lipids from PNPLA1-deficient mice after topical application of radiolabeled lipid extracts from experiment shown in b with subsequent (d) quantification of radioactivity contained in the covalently bound ω-OH-Cer bands (n = 3 mice/lipid extract). TLC images were adjusted for low signal intensities. Data are presented as means + SD and representative for two independent experiments. Statistically significant differences were determined by unpaired two-tailed Student’s t-test (**P < 0.01, ***P < 0.001) between genotypes. ω-O-AcylCer, ω-O-acylceramide; ω-OH-Cer, ω-hydroxy ceramide; ACTB, actin beta; ALOXE3, arachidonate lipoxygenase 3; ALOX12b, arachidonate 12-lipoxygenase, 12R type; ASAH1, N-acylsphingosine amidohydrolase 1; FA, fatty acid; GBA, glucosylceramidase beta; non-OH-Cer, non-hydroxy-ceramide; PNPLA1, patatin-like phospholipase domain-containing 1; qRT-PCR, quantitative real-time reverse transcriptase-PCR; TGM1, transglutaminase 1.
To test whether PNPLA1-deficient skin is capable of forming a CLE, we used an in situ approach by applying epidermal lipids from wild-type and Pnpla1−/− mice onto PNPLA1-deficient skins, followed by determination of covalently bound ω-OH-Cers. For this purpose, we cultured epidermal sheets from Pnpla1−/− and wild-type mice in medium supplemented with [1-14C] palmitic acid. This procedure radiolabeled both the sphingoid backbone and the N-acylated FA of ceramides (Figure 5b). Subsequently, epidermal lipids were extracted and applied onto the skin of newborn PNPLA1-deficient mice. After 6 hours, we isolated the lipids of the CLE and analyzed them by thin-layer chromatography/autoradiography (Figure 5c) and liquid scintillation counting (Figure 5d). Mutant skins treated with wild-type epidermal lipids exhibited a 14-fold increased level of covalently bound ω-OH-Cers than mutant skins treated with epidermal lipids from PNPLA1-deficient mice (Figure 5c and d). In summary, these findings indicate that PNPLA1-deficient epidermis expresses metabolically active enzymes, which are capable of forming the CLE in the presence of ω-O-AcylCers.
Discussion
Survival in a terrestrial environment requires the development of a skin permeability barrier preventing excessive water loss through the body surface to minimize desiccation. In ichthyotic skin, this barrier is impaired due to defects in metabolic processes essential for terminal keratinocyte differentiation and desquamation. Despite extensive studies on affected patients and characterization of corresponding animal models, the exact molecular mechanisms underlying the pathogenesis of several ichthyoses are still poorly understood. In this study, we investigated the impact of PNPLA1 deficiency on murine and human skin physiology. Using a knockout mouse model and human primary keratinocytes, we show that PNPLA1 is involved in the synthesis of ω-O-AcylCers for the maintenance of cutaneous integrity and barrier function in human and murine skin.
Similar to humans and dogs with mutations in PNPLA1, we found that lack of PNPLA1 in mice also resulted in an ichthyotic phenotype with abnormal terminal keratinocyte differentiation. Pnpla1-mutant mice exhibited a defective skin barrier function characterized by elevated transepidermal water loss and premature death of the animals within few hours after birth. This neonatal lethal phenotype is also observed in other mouse models displaying ichthyoses (e.g., ABHD5-, CERS3-, or DGAT2-deficient mice) (Jennemann et al., 2012; Radner et al., 2010; Stone et al., 2004) and suggests that mice are more sensitive to skin barrier defects than humans or dogs. Indeed, mice do have a disadvantageous ratio of body volume to skin surface that might promote increased dehydration when the water permeability barrier is disturbed.
Our functional analyses of epidermis from PNPLA1-deficient mice and primary keratinocytes from a patient with mutated PNPLA1 indicate a defect in ω-O-AcylCer synthesis in the absence of PNPLA1. As a consequence, the amounts of ω-O-acyl(glucosyl)ceramides and protein-bound ω-OH-Cers were drastically reduced, whereas the precursor lipids for ω-O-acyl(glucosyl)ceramide synthesis, unbound ω-OH-(Glc)Cers, accumulated in the epidermis. These alterations in ceramide processing led to a severely impaired CLE formation and a dysfunctional permeability barrier. The observed cutaneous barrier abnormalities resemble those reported for human diseases and corresponding mouse models such as FA-elongase 4 (OMIM #614457) (Aldahmesh et al., 2011; Vasireddy et al., 2007), CERS3 (OMIM #615023) (Eckl et al., 2013; Jennemann et al., 2012; Radner et al., 2013), or ABHD5 deficiency (OMIM #275630) (Radner et al., 2010; Uchida et al., 2010). The skin of these patients and mutant mice also did not exhibit detectable levels of ω-O-AcylCers and lack the CLE, which is accompanied by an abnormal terminal keratinocyte differentiation and a defect in the water permeability barrier.
Our observation that unbound ω-OH-Cers and their corresponding glucosylated derivatives massively accumulate in PNPLA1-deficient skin indicates that PNPLA1 is directly or indirectly involved in the acylation reaction of these ceramide species, thereby generating ω-O-acyl(glucosyl) ceramides. Although ω-O-acyltransferase activity has been described in the epidermis, the corresponding gene and protein remain elusive (Takagi et al., 2004). Given that other members of the PNPLA family act as lipid acyltransferases or transacylases (Kienesberger et al., 2009; Kumari et al., 2012), we speculate that PNPLA1 itself exhibits ω-O-acyltransferase/transacylase activity. Moreover, the very similar epidermal sphingolipid composition in humans and mice with functional ABHD5 (also named CGI-58, *604780) deficiency (Radner and Fischer, 2014; Uchida et al., 2010) suggests that both proteins act in the same metabolic pathway. It is conceivable that ABHD5 coactivates PNPLA1, similarly as shown for PNPLA2 (also known as ATGL, OMIM *609059) (Lass et al., 2006). Using epidermal lysates from Pnpla1−/− mice and wild-type littermates, however, we did not detect ω-O-esterification activity (data not shown) under reported assay conditions (Takagi et al., 2004). The fact that we observed ω-O-AcylCer synthesis activity in murine wild-type skin explants as well as in differentiated primary keratinocytes from a healthy subject but not in in vitro assays suggests that acylation of ω-OH-(Glc)Cers may require cellular integrity, compartmentation, and/or additional proteins or factors, which are not present in cell lysates.
To date, there are few approaches for definitive treatment of congenital ichthyoses (Kiritsi et al., 2014; Paller et al., 2011). Here, we show that in the absence of PNPLA1 the skin lacks the CLE, despite normal or even increased expression of metabolically active enzymes involved in CLE formation. Consequently, epidermal lipid extracts from wild-type mice topically applied onto the skin of Pnpla1-mutant pups resulted in the covalent binding of ω-OH-Cers to corneocyte proteins, indicative for rebuilding of the CLE. This topical replenishment of skin lipids would represent a simple and inexpensive therapy strategy for human ichthyoses associated with epidermal lipid deficiency such as ABHD5 and FA-elongase 4 deficiency, or certain types of ARCIs (Aldahmesh et al., 2011; Eckl et al., 2013; Ohno et al., 2015; Radner et al., 2013; Uchida et al., 2010). The treatment may also be beneficial for other conditions where skin lipid concentrations are decreased, such as in aged skin (Farwick et al., 2008; Rogers et al., 1996). Future experiments will be required using chemically synthesized ω-O-AcylCers to demonstrate the efficacy of these lipid species for ichthyosis therapies.
In summary, our study assigns an essential role of PNPLA1 in the synthesis of ω-O-AcylCers in human and murine skin. The loss of PNPLA1 led to ω-O-AcylCer deficiency. As a consequence, the skin of PNPLA1-deficient mice lacked protein-bound ω-OH-Cers and exhibited a severely impaired cutaneous barrier function. Our findings of the in situ treatment of PNPLA1-deficient skin with wild-type epidermal lipids suggest that the topical application of a lotion supplemented with ω-O-AcylCers might be a promising therapeutic approach to treat skin symptoms of individuals affected by defects in the ω-O-AcylCer pathway.
Material and Methods
Generation of PNPLA1-deficient mice
Mice lacking PNPLA1 were generated by targeted disruption of Pnpla1 exon 1 as outlined in the Supplementary Material and Methods online. All animal studies were approved by the Ethics committee of the University of Graz and the Austrian Federal Ministry of Science, Research and Economy.
Southern blot analysis and genotyping
Methods are described in the Supplementary Material and Methods.
Isolation of RNA and analysis of mRNA expression by qRT-PCR
The epidermis was separated from the dermis using ammonium thiocyanate (Sigma-Aldrich, St. Louis, MO) as described (Trost et al., 2007). Dermal fibroblasts were isolated from adult C57BL/6J mice and cultivated as described (Huschtscha et al., 2012). Extraction of total RNA and subsequent analysis of mRNA expression of target genes by qRT-PCR was performed as described (Jaeger et al., 2015) using primer pairs listed in Supplementary Table S3 online.
Preparation of epidermis
The epidermis was separated from the dermis by incubation in phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) containing 10 mM EDTA for 45 minutes at 37 °C.
Immunoblot analysis
Immunoblot analyses were performed according to standard protocols using antibodies listed in Supplementary Table S4 online.
Blood chemistry
Blood samples were collected from euthanized newborn mice using a Microvette CB 300 (Sarstedt, Nü mbrecht, Germany). Plasma FA and triacylglycerol concentrations were determined using commercial kits (Wako Chemicals, Neuss, Germany; Thermo Fisher Scientific, Waltham, MA). Blood glucose levels were measured using blood glucose strips and the Wellion CALLA Classic glucometer (MED TRUST GmbH, Marz, Austria).
Skin histology and ultrastructural analysis
Skin histological analyses by light or transmission electron microscopy were conducted as described (Radner et al., 2010), see Supplementary Material and Methods.
Skin permeation assay and measurement of transepidermal water loss
Skin permeation was assessed by testing the epidermal penetration of an aqueous toluidine blue solution as described (Radner et al., 2010). Transepidermal water loss was measured on mouse dorsal skin using an evaporimeter (MPA 6, Courage and Khazaka Electronic GmbH, Köln, Germany).
Epidermal lipid analysis
Epidermal lipids were extracted and analyzed by UPLC-qTOF as described in the Supplementary Material and Methods.
Measurement of in vivo ω-O-AcylCer synthesis in human primary keratinocytes and murine epidermal explants
ω-O-AcylCer synthesis was measured in cultured human primary keratinocytes and murine epidermal explants using 20 μM [1-14C] linoleic acid (55 mCi/mmol; GE Healthcare, Buckinghamshire, UK) as described (Radner et al., 2010); see Supplementary Material and Methods.
In vivo transfer of murine epidermal lipids
CLE formation in PNPLA1-deficient epidermis was assayed by ex vivo radiolabeling of epidermal lipids from wild-type and Pnpla1-mutant mice with 90 μM [1-14C] palmitic acid (55 mCi/mmol; Hartmann Analytics GmbH, Braunschweig, Germany) and subsequent topical application of these lipids onto Pnpla1 mutants followed by the analysis of protein-bound lipids by thin-layer chromatography/autoradiography and liquid scintillation counting; see Supplementary Material and Methods.
Statistics
Statistical significant differences were determined by Student’s unpaired two-tailed t-test. Group differences were considered significant for P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
Supplementary Material
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2016.08.036.
Acknowledgments
We would like to thank H.-Christian Theussl and Jacek Wojciechowski for their help with blastocyst injections as well as Monika Oberer and Georg Waeg for their help preparing the anti-PNPLA1 antiserum. We are also grateful for animal care to Astrid Hermann and for animal genotyping to Birgit Juritsch and Eszter Fabianne-Kirilly. We thank Jasmin Paar, Lisa Pichler, and Benedikt Kien for their excellent technical assistance as well as Gabriele Schoiswohl for critical comments on the manuscript. This work was supported by FWF Projects P25944 (to FPWR), P28039 (to SD), P24944 (to GH), P25193 (to AL), and SFB Lipotox F3002 (to RZ and GH) funded by the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (FWF).
Abbreviations
- ABHD5
α/β hydrolase domain containing 5
- ω-O-AcylCers
ω-O-acylceramides
- ARCI
autosomal recessive congenital ichthyosis
- CLE
corneocyte-bound lipid envelope
- FA
fatty acid
- ω-OH-(Glc)Cers
ω-hydroxy-(glucosyl)ceramides
- PNPLA1
patatin-like phospholipase domain-containing 1
- qRT-PCR
quantitative real-time reverse transcriptase-PCR
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Ahmad F, Ansar M, Mehmood S, Izoduwa A, Lee K, Nasir A, et al. A novel missense variant in the PNPLA1 gene underlies congenital ichthyosis in three consanguineous families [e-pub ahead of print] J Eur Acad Dermatol Venereol. 2015 doi: 10.1111/jdv.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89:745–50. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen N, Mathow D, Rabionet M, Sandhoff R, Langbein L, Gretz N, et al. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Hum Mol Genet. 2013;22:4164–79. doi: 10.1093/hmg/ddt264. [DOI] [PubMed] [Google Scholar]
- Behne M, Uchida Y, Seki T, de Montellano PO, Elias PM, Holleran WM. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114:185–92. doi: 10.1046/j.1523-1747.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- Eckl K-M, Tidhar R, Thiele H, Oji V, Hausser I, Brodesser S, et al. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J Invest Dermatol. 2013;133:2202–11. doi: 10.1038/jid.2013.153. [DOI] [PubMed] [Google Scholar]
- Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, et al. Formation and functions of the corneocyte lipid envelope (CLE) Biochim Biophys Acta. 2014;1841:314–8. doi: 10.1016/j.bbalip.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachal L, Rodríguez-Pazos L, Ginarte M, Carracedo A, Toribio J, Vega A. Identification of a novel PNPLA1 mutation in a Spanish family with autosomal recessive congenital ichthyosis. Br J Dermatol. 2014;170:980–2. doi: 10.1111/bjd.12757. [DOI] [PubMed] [Google Scholar]
- Farwick M, Santonnat B, Lersch P, Rawlings AV, Grether-Beck S, Medve-Koenigs K, et al. An aquaporin-inspired lipid concentrate for mature skin. Cosmet Toilet Mag. 2008;123:69–74. [Google Scholar]
- Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Fischer J. Autosomal recessive congenital ichthyosis. J Invest Dermatol. 2009;129:1319–21. doi: 10.1038/jid.2009.57. [DOI] [PubMed] [Google Scholar]
- Grall A, Guaguère E, Planchais S, Grond S, Bourrat E, Hausser I, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet. 2012;44:140–7. doi: 10.1038/ng.1056. [DOI] [PubMed] [Google Scholar]
- Herrmann T, van der Hoeven F, Grone H-J, Stewart AF, Langbein L, Kaiser I, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–15. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huschtscha LI, Napier CE, Noble JR, Bower K, Au AY, Campbell HG, et al. Enhanced isolation of fibroblasts from human skin explants. Biotechniques. 2012;53:239–44. doi: 10.2144/0000113939. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Schoiswohl G, Hofer P, Schreiber R, Schweiger M, Eichmann TO, et al. Fasting-induced G0/G1 switch gene 2 and FGF21 expression in the liver are under regulation of adipose tissue derived fatty acids. J Hepatol. 2015;63:437–45. doi: 10.1016/j.jhep.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50(Suppl):S63–8. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritsi D, Schauer F, Wölfle U, Valari M, Bruckner-Tuderman L, Has C, et al. Targeting epidermal lipids for treatment of Mendelian disorders of cornification. Orphanet J Rare Dis. 2014;9:33. doi: 10.1186/1750-1172-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3:309–19. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lee E, Rahman OU, Khan MT, Wadood A, Naeem M, Kang C, et al. Whole exome analysis reveals a novel missense PNPLA1 variant that causes autosomal recessive congenital ichthyosis in a Pakistani family. J Dermatol Sci. 2016;82:46–8. doi: 10.1016/j.jdermsci.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Marekov LN, Steinert PM. Ceramides are bound to structural proteins of the human foreskin epidermal cornified cell envelope. J Biol Chem. 1998;273:17763–70. doi: 10.1074/jbc.273.28.17763. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Yamamoto M, Imai Y, Sakaguchi Y, Takizawa T, Ohta N, et al. Knocking-in the R142C mutation in transglutaminase 1 disrupts the stratum corneum barrier and postnatal survival of mice. J Dermatol Sci. 2012;65:196–206. doi: 10.1016/j.jdermsci.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Nakamichi S, Ohkuni A, Kamiyama N, Naoe A, Tsujimura H, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci USA. 2015;112:7707–12. doi: 10.1073/pnas.1503491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Sorze 2009. J Am Acad Dermatol. 2010;63:607–41. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Paller AS, van Steensel MA, Rodriguez-Martín M, Sorrell J, Heath C, Crumrine D, et al. Pathogenesis-based therapy reverses cutaneous abnormalities in an inherited disorder of distal cholesterol metabolism. J Invest Dermatol. 2011;131:2242–8. doi: 10.1038/jid.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner FP, Fischer J. The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim Biophys Acta. 2014;1841:409–15. doi: 10.1016/j.bbalip.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Radner FP, Marrakchi S, Kirchmeier P, Kim G-J, Ribierre F, Kamoun B, et al. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 2013;9:e1003536. doi: 10.1371/journal.pgen.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–11. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288:765–70. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Martinz V, Janecke AR, Fauth C, Schossig A, Zschocke J, et al. Inherited ichthyoses/generalized Mendelian disorders of cornification. Eur J Hum Genet. 2013;21:123–33. doi: 10.1038/ejhg.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler G, Hotz R, Chatellard-Gruaz D, Didierjean L, Hellman U, Saurat JH. Purification and characterization of the human epidermal fatty acid-binding protein: localization during epidermal cell differentiation in vivo and in vitro. Biochem J. 1994;302:363–71. doi: 10.1042/bj3020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–76. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- Swartzendruber DC, Wertz PW, Madison KC, Downing DT. Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol. 1987;88:709–13. doi: 10.1111/1523-1747.ep12470383. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nakagawa H, Matsuo N, Nomura T, Takizawa M, Imokawa G. Biosynthesis of acylceramide in murine epidermis: characterization by inhibition of glucosylation and deglucosylation, and by substrate specificity. J Invest Dermatol. 2004;122:722–9. doi: 10.1111/j.0022-202X.2004.22307.x. [DOI] [PubMed] [Google Scholar]
- Traupe H, Fischer J, Oji V. Nonsyndromic types of ichthyoses—an update. J Dtsch Dermatol Ges. 2014;12:109–21. doi: 10.1111/ddg.12229. [DOI] [PubMed] [Google Scholar]
- Trost A, Bauer JW, Lanschuetzer C, Laimer M, Emberger M, Hintner H, et al. Rapid, high-quality and epidermal-specific isolation of RNA from human skin. Exp Dermatol. 2007;16:185–90. doi: 10.1111/j.1600-0625.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Cho Y, Moradian S, Kim J, Nakajima K, Crumrine D, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of Dorfman-Chanarin syndrome. J Invest Dermatol. 2010;130:2497–9. doi: 10.1038/jid.2010.145. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, Uchida Y, Salem N, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–82. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.