Abstract
Several lines of evidence over the last few years have been important in ascertaining that the pedunculopontine nucleus (PPN) region could be considered as a potential target for deep brain stimulation (DBS) to treat freezing and other problems as part of a spectrum of gait disorders in Parkinson disease and other akinetic movement disorders. Since the introduction of PPN DBS, a variety of clinical studies have been published. Most indicate improvements in freezing and falls in patients who are severely affected by these problems. The results across patients, however, have been variable, perhaps reflecting patient selection, heterogeneity in target selection and differences in surgical methodology and stimulation settings. Here we outline both the accumulated knowledge and the domains of uncertainty in surgical anatomy and terminology. Specific topics were assigned to groups of experts, and this work was accumulated and reviewed by the executive committee of the working group. Areas of disagreement were discussed and modified accordingly until a consensus could be reached. We demonstrate that both the anatomy and the functional role of the PPN region need further study. The borders of the PPN and of adjacent nuclei differ when different brainstem atlases and atlas slices are compared. It is difficult to delineate precisely the PPN pars dissipata from the nucleus cuneiformis, as these structures partially overlap. This lack of clarity contributes to the difficulty in targeting and determining the exact localization of the electrodes implanted in patients with akinetic gait disorders. Future clinical studies need to consider these issues.
Keywords: Anatomy, Brainstem, Deep brain stimulation, Pedunculopontine nucleus, Parkinson disease
Gait disorders are a major source of disability in patients with akinetic movement disorders. Their anatomical basis and pathophysiology are poorly understood, and there is a need to further investigate the role of surgery in this field. The possible relevance of the pedunculopontine nucleus (PPN) region for movement disorders has been outlined by independent groups of investigators who demonstrated that there are degenerative changes in patients with advanced akinetic disorders such as Parkinson disease (PD), progressive supranuclear palsy and multiple systems atrophy [1–5]. More recent data indicate that cholinergic denervation due to degeneration of PPN neurons may underlie dopamine-nonresponsive gait and balance impairment in PD [6, 7].
Several lines of evidence over the last few years have been important in ascertaining that the PPN region could be considered as a potential target for deep brain stimulation (DBS) to treat freezing and other problems as part of the spectrum of gait disorders in PD. In 1986, Mitchell et al. [8] had shown increased 2-deoxyglucose uptake reflecting increased synaptic activity in the PPN region of a primate rendered parkinsonian after MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) injections. The PPN receives GABAergic projections from the medial pallidum [9, 10], and therefore it has been assumed that PPN activity was reduced in the parkinsonian brain [9, 11]. Yet, this concept has been challenged by the known pathophysiology that the subthalamic nucleus (STN), which is excitatory and hyperactive in PD, also projects to the PPN. This latter pathway might account for the hyperactivity of PPN neurons projecting to the STN in rats with unilateral lesion of the substantia nigra [12]. In normal primates, high-frequency stimulation, radiofrequency and excitotoxic PPN lesions induced akinesia [13–17]. In MPTP-treated parkinsonian primates, however, low-frequency stimulation (25 Hz) and microinjections of bicuculline, a GABA antagonist, into the PPN reversed akinesia [17–20]. Recording of the midlatency auditory evoked P50 potential in PD patients concluded that the PPN was overactive in PD, and that bilateral pallidotomy normalized PPN output [21, 22].
Since the introduction of the PPN area as a target for DBS in PD, a variety of studies have been published. Most indicate improvements in freezing and falls in patients that are severely affected by these problems. The results across patients, however, have been variable, perhaps reflecting patient selection, heterogeneity in target selection and differences in surgical methodology and stimulation settings. Therefore, we (E.M., J.K.K.) initiated an interdisciplinary task force in 2010 with the aim to explore in more detail the possible role of the PPN region as a target for DBS in PD. It was our goal to involve experts from different disciplines in the task force to reflect both diversity in opinion and in methodology. The task force became an official working group of the Movement Disorders Society (MDS) in 2012 with an executive committee consisting of three neurologists (E.M., B.R.B., M.S.O.) and three neurosurgeons (J.K.K., A.M.L., T.A.). In 2013, the president of the MDS at that time, Matthew Stern, and the president of the World Society for Stereotactic and Functional Neurosurgery (WSSFN), J.K.K., agreed that the working group would become a bisocietal endeavor, named the MDS Pedunculopontine Nucleus DBS Working Group in collaboration with WSSFN. The working group then planned to evaluate four different domains relevant to PPN DBS surgery in PD: preoperative selection of patients and available rating scales, clinical outcome and DBS programming, surgical anatomy, and surgical technique. It was decided that the first two papers would be submitted to a primarily neurological journal while the two latter papers would be submitted to a specialized neurosurgical journal. Here we outline both the accumulated knowledge and the domains of uncertainty in surgical anatomy and terminology. Issues relevant for surgical technique, side effects and postoperative imaging will be addressed in a companion paper.
Methodological Approach
In order to evaluate the details of both surgical anatomy and terminology of the PPN region relevant to DBS surgery, the executive committee formulated several questions during a consensus conference which were distributed to the co-authors of the paper. The PubMed database was searched using the following key words: pedunculopontine nucleus; deep brain stimulation; anatomy; physiology; surgery.
Specific topics were assigned to groups of authors, and this work was accumulated and reviewed by the executive committee of the working group. Areas of disagreement were discussed and modified accordingly until a consensus could be reached. The literature was continuously updated during that process.
Anatomy and Function of the PPN Region
The PPN, subcuneiform and cuneiform nuclei (CnF) comprise the mesencephalic locomotor region (MLR) [23–25]. This is a functional region from which electrical stimulation induces coordinated locomotion in decerebrate mammals [23, 24].
The PPN projects and receives projections from the STN, globus pallidus internus, and substantia nigra reticulata and compacta [26–28]. Further, it has afferent and efferent connections with the cerebellum, thalamus, cerebral cortex, and the spinal cord [9, 23].
The PPN is also connected to catecholaminergic systems in the brainstem, such as the noradrenergic locus coeruleus [23, 29] and to serotonergic neurons of the dorsal raphe nucleus [30–32].
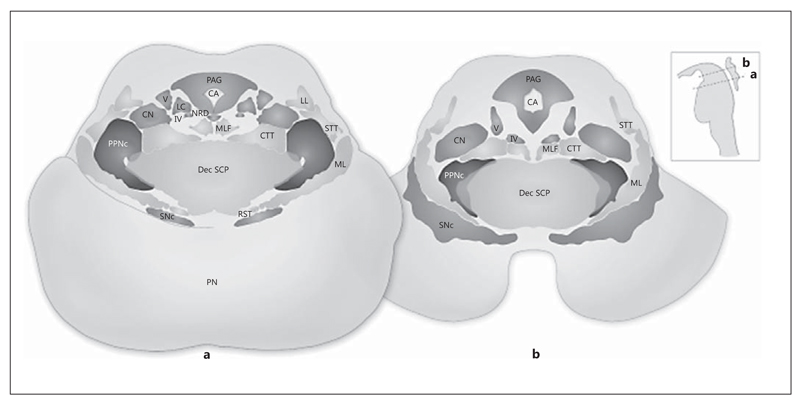
On the basis of its cytoarchitectural organization, the PPN has been subdivided into a pars dissipata and a pars compacta (fig. 1). Triple in situ hybridization studies determined that the profile of PPN neurons varies across its extent [33]. The PPN pars dissipata is located throughout the rostrocaudal extent of the PPN region and contains mainly small to medium-sized GABAergic neurons. The PPN pars compacta is located in the caudal half of the nucleus and contains mostly cholinergic and glutamatergic cells [9, 11, 23, 34, 35]. In addition, cholinergic neurons of the PPN are also known as the Ch5 cholinergic cell group according to the classification of Mesulam et al. [36]. Similar to the PPN, the subcuneiform and cuneiform nuclei do not contain homogenous neuronal populations. Neurons of the CnF are mainly comprised of nitrinergic and GABAergic cells. These nuclei have no clear boundaries. This has led to some confusion concerning the overlap of functionally or anatomically defined regions in this area.
Fig. 1.
The PPN region at the level of the decussation of the superior cerebellar peduncles and the inferior colliculus (a) and at the level of the trochlear nucleus and the intercollicular area (b). The main nuclei are labeled on the left and the long fiber tracts on the right side. STT = Spinothalamic tract; CA = cerebral aqueduct; CN = cuneiform nucleus; CTT = central tegmental tract; Dec SCP = decussation of the superior cerebellar peduncles; LC = locus coeruleus; LL = lateral lemniscus; ML = medial lemniscus; MLF = medial longitudinal fasciculus; NRD = nucleus raphe dorsalis; PAG = periaqueductal gray; PN = pontine nuclei; PPNc = pedunculopontine nucleus pars compacta; PPNd = pedunculopontine nucleus pars dissipata; SNc = substantia nigra pars compacta; RST = rubrospinal tract; IV = trochlear nucleus; V = mesencephalic nucleus of the trigeminal nerve. Adapted from Fournier-Gosselin et al. [35], with permission.
Experimental studies have shown that the PPN receives dopaminergic input from the substantia nigra compacta and ventral tegmental area [30, 37, 38]. Such inputs are modulated by NMDA, AMPA and GABAB receptors.
The PPN output controls the striatal loop, i.e. the STN, globus pallidus internus, and substantia nigra pars compacta. Other projections reach the intralaminar nuclei of the thalamus and nuclei of the lower brainstem. As such, the PPN occupies a strategic position between the limbic and striatal loops, and while it is mainly involved in locomotor activity [9, 11, 23, 34, 39–42], it is also potentially relevant in other domains including cognition and sleep. Together with the thalamic intralaminar nuclei, the PPN is part of the ‘ascending reticular activating system’ [9, 23, 43].
Both PPN and CnF have been suggested to influence muscle tone during the initiation of locomotion [44, 45]. Rodent studies have shown that MLR injections of the GABAA agonist muscimol completely abolish stepping. Because muscimol solely acts on neuronal cell bodies and not on passing axons, these results suggest that cells around the injection site (i.e. CnF and PPN) may be responsible for MLR-induced stepping [41]. A recent rodent study has shown that stimulation of the MLR markedly improves hind limb function in rats with incomplete spinal cord injury [46]. The integrative role of the PPN in human gait was demonstrated also by intraoperative and postoperative neurophysiological studies [47].
Considerations about the Terminology Used in PPN Anatomy
Since the description of the PPN by Jacobsohn [48] in 1909, the terminology used to label this nucleus has varied continuously. For instance, it has been labeled as ‘nucleus tegmenti pedunculopontinus’ [48], ‘pedunculopontine tegmental nucleus’ [49], ‘nucleus reticularis pedunculopontinus’ [50], ‘nucleus pedunculopontinus’ [51], ‘nucleus tegmenti pedunculopontinus’ [52, 53], ‘the area U’ [53–55], ‘the n nucleus’ [56], and ‘the PPTn of Kölliker’ [52] (the latter must be distinguished from the Kölliker-Fuse nucleus which now refers to a subnucleus of the parabrachialis nucleus). Since there are so many differences in nomenclature, the terminology used across studies has not been consistent.
Anatomical Localization of the PPN in Brainstem Atlases
There is variability concerning the exact anatomical localization of the PPN across different brainstem atlases, in particular with regard to its borders [49, 50, 52, 53, 57]. It is commonly accepted that the PPN is bordered medially by the superior cerebellar peduncle (and its decussation) as well as the central tegmental tract (fig. 2). Anterior and lateral to the PPN is the lemniscal system, and caudal and rostral are the retrorubral field and substantia nigra reticulata, respectively. The posterior aspect of the PPN is contiguous with the lateral portion of the CnF.
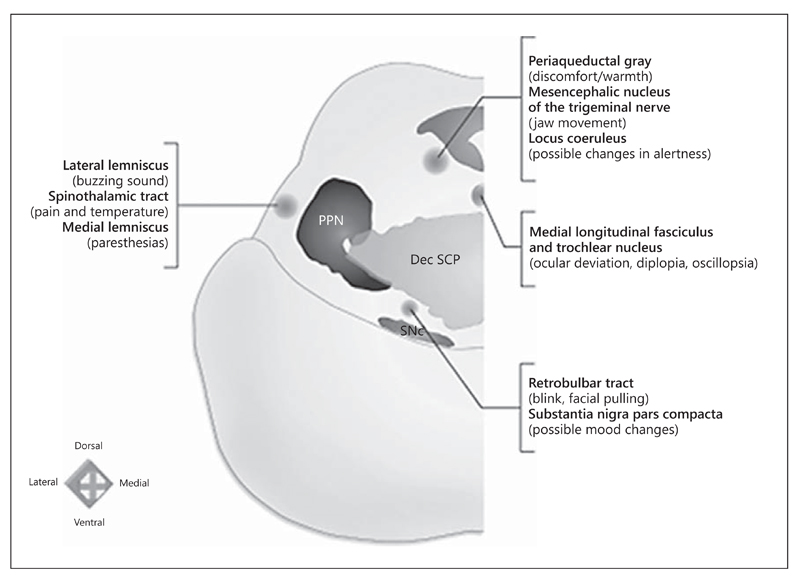
Fig. 2.
Functional mapping of PPN region: correlations between the structures in the vicinity of the PPN and their potential stimulation effects. An electrode positioned lateral to the PPN might be revealed by buzzing sounds (lateral lemniscus), unpleasant painful sensation and/or change in temperature sensation (spinothalamic tract), or paresthesias (medial lemniscus) when trial stimulation is applied. An anteromedial position could elicit the sensation of contralateral facial pulling or blinking (rubrobulbar tract) and/or mood changes (substantia nigra). A mediodorsal location could lead to oscillopsia, diplopia, or ocular deviation toward the side stimulated (medial longitudinal fascicle and trochlear nucleus), mandating careful inspection of extraocular movements. An electrode positioned dorsally could lead to a sensation of discomfort (periaqueductal gray), mandating that the stimulation be done with caution in the vicinity of the PPN. A dorsally placed electrode could also be noticed by jaw movements subjectively felt as pulling of masticatory muscles (mesencephalic nucleus of the trigeminal nerve). A nonspecific altered level of alertness (locus coeruleus) might also be observed. PPN = Pedunculopontine nucleus; Dec SCP = decussation of the superior cerebellar peduncles; SNc = substantia nigra pars compacta. Adapted from Fournier-Gosselin et al. [35], with permission.
Considering the cytoarchitectural characteristics of the pontomesencephalic reticular formation, the precise anatomical distinction between pontine and mesencephalic structures has always been open to debate. As a result, to define the rostrocaudal extent of the PPN some investigators have relied on the pontomesencephalic junction (PMJ), a line that connects the inferior aspect of the quadrigeminal plate (frenulum veli) posteriorly with the foramen caecum of the interpeduncular fossa anteriorly.
Reviewing different brainstem atlases with a focus on the PMJ as well as on the orientation of the slices in the transverse plane may provide a landmark for the rostrocaudal extent of the PPN in the human brainstem.
Cytoarchitecture of the Human Brainstem: Olszewski and Baxter
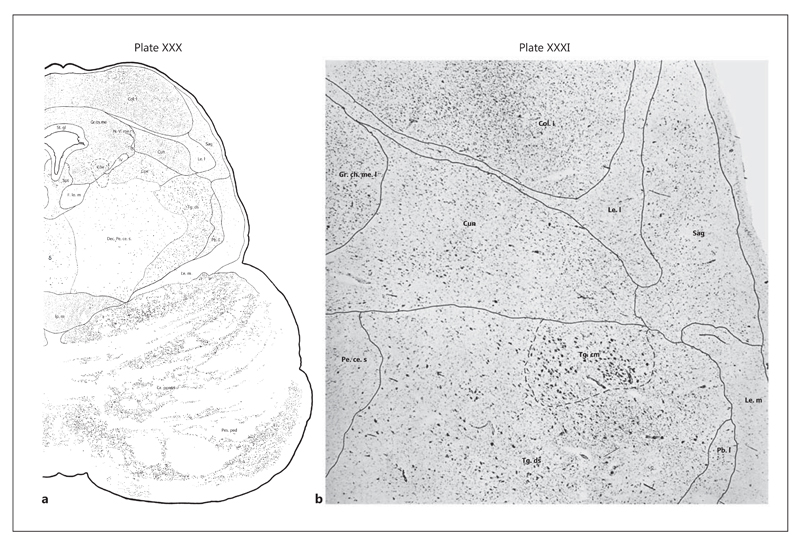
This atlas of brainstem structures provides an accurate description of the PPN and its subregions [52]. Caveats from a surgical perspective include that it is not based on stereotactic coordinates and that the transverse angle of sections is not exactly parallel to the PMJ. Two subdivisions of the PPN are distinguished: the pars dissipata and pars compacta. The PMJ slice is in plate No. XXVIII (cross-section No. 801) with no reference made to the PPN or the CnF. The next plate consists of a section 3 mm or 150 slices rostral (plate No. XXX; cross-section No. 651) that crosses the brainstem at the mid-portion of the inferior colliculus (IC) (fig. 3). In this section, the PPN pars dissipata and CnF are clearly delineated. Of interest, plate No. XXXI (cross-section No. 601), located 1 mm (50 slices) rostral to plate No. XXX and 4 mm rostral from the PMJ, contains the PPN pars compacta and dissipata, which is in contact with the CnF posteriorly. In this atlas, the PPN extends for 8 mm in the rostrocaudal axis, its most rostral extension reaching the level of the caudal border of the red nucleus (plate No. XXXIV, cross-section No. 401). The CnF and its subnuclei are represented as a large structure that extends from the PMJ (or just above it) to the level of the red nucleus.
Fig. 3.
Plates XXX and XXXI of Cytoarchitecture of the Human Brain Stem published by J. Olszewski and D. Baxter [52]. The PPN pars dissipata is labeled as Tg. ds, and the PPN pars compacta is labeled as Tg. cm. The CnF is labelled as Cun. a Plate XXX: representation of cross-section 651. The plate contains the most caudal aspect of the PPN observable in the atlas. The posterior aspect of the brainstem is clearly located in the mesencephalon due to the presence of the IC. According to the distances between cross-sections, the posterior aspect is located 3 mm rostral to the PMJ (plate XXVIII in cross-section 801). b Plate XXXI: photomicrograph of cross-section 601 located 1 mm rostral to plate XXX. The plate contains the PPN pars compacta. Reprinted from Olszewski and Baxter [52], with permission.
Atlas of the Human Brainstem: Paxinos and Huang
Plates in this brainstem atlas are numbered based on distance from the obex [49]. The PMJ is represented in its figures 48 (obex + 31 mm) and 49 (obex + 32 mm), which contain the nucleus of the trochlear nerve, fibers of the trochlear nerve, their decussation, and the frenulum veli, just caudal to the infracollicular recess. The caudal aspect of the PPN pars dissipata is shown in its figure 48 (obex + 31 mm). The pars compacta is shown in its figure 50 (obex + 33 mm), which also shows the pars dissipata at the level of the IC. According to the atlas, the pars compacta extends 4 mm rostrally but does not extend beyond the level of the IC. The PPN extends to the rostral pole of the IC (obex + 36 mm). Just above the PMJ, the CnF is located posterior to the PPN and extends to the caudal aspect of the superior colliculus. We note that in recent work, the authors have considerably changed this nomenclature [58].
Atlas for Stereotaxy of the Human Brain: Schaltenbrand and Wahren
In this atlas, the PPN is labeled as the nucleus tegmenti pedunculopontinus [53]. It is represented in coronal slices perpendicular to the anterior-posterior commissural plane at the level of the superior aspect of the superior cerebellar peduncle (plate 29). Axial slices of the rostral brainstem are presented in plate 57. The PPN per se is not labeled in those plates. Nevertheless, it may be included in the diffuse area labeled as griseum circumflexum brachii conjunctivi, which extends from the caudal mesencephalon to a region 5 mm below the PMJ.
Duvernoy’s Atlas of the Human Brainstem and Cerebellum: Naidich et al
This book [50] consists of a multimodal atlas of the brainstem based on magnetic resonance imaging. The PPN and CnF are described in several axial slices (P 84–89; P 329–330). Assumptions on the rostrocaudal extent of the PPN are difficult to conclude since the sections are not parallel to the PMJ.
The fact that most atlases rely on cytoarchitectural features may underestimate the extent of the PPN, since it is a diffuse reticular nucleus with indistinct borders. Using choline acetyltransferase immunohistochemistry, for example, Mesulam et al. [59] studied the extent of PPN cholinergic cells. They indicated that the caudal aspect of the PPN extends far below the level of the IC, whereas the PPN pars compacta could be observed as rostral as the decussation of the superior cerebellar peduncle. These findings were confirmed in a later study which provided more detail about the cholinergic cell group Ch5 within the PPN [1].
Two postmortem human studies found that sagittal sections were the most reliable for identifying the PPN in three dimensions. Labeling of cholinergic neurons showed that the pars compacta was located immediately dorsal to the brachium conjunctivum, with cells of the pars dissipata scattered within the brachium conjunctivum. The pars compacta was localized anterior/ventral to the posterior half of the IC, while the pars dissipata extended posteriorly and anteriorly below the PPN pars compacta [60, 61].
Conclusions
The PPN is a reticular nucleus with indistinct anatomical boundaries. Its long axis straddles the PMJ from the inferior collicular level to reach the rostral pons. The majority of its neurons lie lateral to the superior cerebellar peduncle and its decussation and the central tegmental tract, and medial to the curved band of the lemniscal system. It is evident that both the anatomy and the function of the PPN area will require further clarification. The extent of both the PPN and CnF does not exactly coincide when different brainstem atlases and atlas slices are compared. It is difficult to provide a precise delineation between the PPN pars dissipata and the CnF, as these two structures partially overlap in the mesencephalic reticular formation.
The indistinct boundaries of this nucleus and the general lack of consensus in the field are important overall limitations, particularly with regard to PPN DBS surgery. This lack of clarity contributes to the difficulty in targeting and determining the exact localization of the electrodes implanted in human subjects suffering from neurodegenerative disorders.
We suggest that authors may use commonly accepted anatomical terms in future studies and reports. We advise also that reference should be made to the source which has been used to delineate the PPN. While the term ‘PPN’ would be appropriate when the nuclear core can be clearly identified, ‘PPN region’ might be used when this is not the case. To allow easier comparison of surgical results, we recommend to indicate whether the rostral or the caudal portion of the PPN is used for neuromodulation.
It is important to consider these issues when planning future studies in PPN DBS surgery, in particular with regard to developing a commonly accepted study protocol.
Footnotes
Disclosure Statement
The MDS Pedunculopontine Nucleus DBS Working Group in collaboration with the WSSFN was supported by an unrestricted grant from Medtronic.
References
- 1.Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, German DC. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. mNeuroscience. 1999;89:759–770. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- 2.Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol. 1989;26:41–46. doi: 10.1002/ana.410260106. [DOI] [PubMed] [Google Scholar]
- 3.Zweig RM, Whitehouse PJ, Casanova MF, Walker LC, Jankel WR, Price DL. Loss of pedunculopontine neurons in progressive supranuclear palsy. Ann Neurol. 1987;22:18–25. doi: 10.1002/ana.410220107. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA. 1987;84:5976–5980. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger K. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1988;51:540–543. doi: 10.1136/jnnp.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabli D, Karachi C, Folgoas E, Monfort M, Tande D, Clark S, Civelli O, Hirsch EC, François C. Gait disorders in parkinsonian monkeys with pedunculopontine nucleus lesions: a tale of two systems. J Neurosci. 2013;33:11986–11993. doi: 10.1523/JNEUROSCI.1568-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell IJ, Cross AJ, Sambrook MA, Crossman AR. Neural mechanisms mediating 1-methyl-4-phenyl-1,2,3,6-tetrahydropyri-dine-induced parkinsonism in the monkey: relative contributions of the striatopallidal and striatonigral pathways as suggested by 2-deoxyglucose uptake. Neurosci Lett. 1986;63:61–65. doi: 10.1016/0304-3940(86)90013-3. [DOI] [PubMed] [Google Scholar]
- 9.Pahapill PA, Lozano AM. The pedunculo-pontine nucleus and Parkinson’s disease. Brain. 2000;123:1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 10.Shink E, Sidibe M, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey. II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol. 1997;382:348–363. [PubMed] [Google Scholar]
- 11.Hamani C, Stone S, Laxton A, Lozano AM. The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation. Parkinsonism Relat Disord. 2007;13(suppl 3):S276–S280. doi: 10.1016/S1353-8020(08)70016-6. [DOI] [PubMed] [Google Scholar]
- 12.Orieux G, François C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2000;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 13.Kojima J, Yamaji Y, Matsumura M, Nambu A, Inase M, Tokuno H, Takada M, Imai H. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett. 1997;226:111–114. doi: 10.1016/s0304-3940(97)00254-1. [DOI] [PubMed] [Google Scholar]
- 14.Aziz TZ, Davies L, Stein J, France S. The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br J Neurosurg. 1998;12:245–249. doi: 10.1080/02688699845078. [DOI] [PubMed] [Google Scholar]
- 15.Munro-Davies L, Winter J, Aziz TZ, Stein J. Kainate acid lesions of the pedunculopontine region in the normal behaving primate. Mov Disord. 2001;16:150–151. doi: 10.1002/1531-8257(200101)16:1<150::aid-mds1026>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci. 2002;9:170–174. doi: 10.1054/jocn.2001.0943. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24:319–328. doi: 10.1002/mds.22189. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a parkinsonian monkey. Neuroreport. 2004;15:2621–2624. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- 19.Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125:2418–2430. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- 20.Nandi D, Jenkinson N, Stein J, Aziz T. The pedunculopontine nucleus in Parkinson’s disease: primate studies. Br J Neurosurg. 2008;22(suppl 1):S4–S8. doi: 10.1080/02688690802448350. [DOI] [PubMed] [Google Scholar]
- 21.Teo C, Rasco L, Skinner RD, Garcia-Rill E. Disinhibition of the sleep state-dependent P1 potential in Parkinson’s disease – improvement after pallidotomy. Sleep Res Online. 1998;1:62–70. [PubMed] [Google Scholar]
- 22.Teo C, Rasco L, al-Mefty K, Skinner RD, Boop FA, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Mov Disord. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987;18:731–738. doi: 10.1016/0361-9230(87)90208-5. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Rill E, Simon C, Smith K, Kezunovic N, Hyde J. The pedunculopontine tegmental nucleus: from basic neuroscience to neurosurgical applications: arousal from slices to humans: implications for DBS. J Neural Transm. 2011;118:1397–1407. doi: 10.1007/s00702-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983;217:187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- 27.Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tracttracing methods. J Comp Neurol. 1994;344:210–231. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- 28.Aravamuthan BR, McNab JA, Miller KL, Rushworth M, Jenkinson N, Stein JF, Aziz TZ. Cortical and subcortical connections within the pedunculopontine nucleus of the primate Macaca mulatta determined using probabilistic diffusion tractography. J Clin Neurosci. 2009;16:413–420. doi: 10.1016/j.jocn.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus 𢀓 auditory input, arousal and pathophysiology. Prog Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- 30.Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- 31.Honda T, Semba K. Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res. 1994;647:299–306. doi: 10.1016/0006-8993(94)91329-3. [DOI] [PubMed] [Google Scholar]
- 32.Kayama Y, Koyama Y. Control of sleep and wakefulness by brainstem monoaminergic and cholinergic neurons. Acta Neurochir Suppl. 2003;87:3–6. doi: 10.1007/978-3-7091-6081-7_1. [DOI] [PubMed] [Google Scholar]
- 33.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- 35.Fournier-Gosselin MP, Lipsman N, Saint-Cyr JA, Hamani C, Lozano AM. Regional anatomy of the pedunculopontine nucleus: relevance for deep brain stimulation. Mov Disord. 2013;28:1330–1336. doi: 10.1002/mds.25620. [DOI] [PubMed] [Google Scholar]
- 36.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 37.Ichinohe N, Teng B, Kitai ST. Morphological study of the tegmental pedunculopontine nucleus, substantia nigra and subthalamic nucleus, and their interconnections in rat organotypic culture. Anat Embryol (Berl) 2000;201:435–453. doi: 10.1007/s004290050331. [DOI] [PubMed] [Google Scholar]
- 38.Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 39.Inglis WL, Olmstead MC, Robbins TW. Pedunculopontine tegmental nucleus lesions impair stimulus-reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci. 2000;114:285–294. doi: 10.1037//0735-7044.114.2.285. [DOI] [PubMed] [Google Scholar]
- 40.Winn P, Brown VJ, Inglis WL. On the relationships between the striatum and the pedunculopontine tegmental nucleus. Crit Rev Neurobiol. 1997;11:241–261. doi: 10.1615/critrevneurobiol.v11.i4.10. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 42.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 44.Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain. 2011;134:11–23. doi: 10.1093/brain/awq322. [DOI] [PubMed] [Google Scholar]
- 45.Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28:161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 46.Bachmann LC, Matis A, Lindau NT, Felder P, Gullo M, Schwab ME. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med. 2013;5:208ra146. doi: 10.1126/scitranslmed.3005972. [DOI] [PubMed] [Google Scholar]
- 47.Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, Grabli D, Karachi C. The integrative role of the pedunculopontine nucleus in human gait. Brain. 2015;138:1284–1296. doi: 10.1093/brain/awv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobsohn LU. Über die Kerne des menschlichen Hirnstamms (Medulla oblongata, Pons und Pedunculus cerebri) Berlin: Preussische Akademie der Wissenschaften; 1909. [Google Scholar]
- 49.Paxinos G, Huang XF. Atlas of the Human Brainstem. San Diego: Academic Press; 1995. [Google Scholar]
- 50.Naidich TP, Duvernoy HM, Delman BM, Sorensen AG, Kollias SS, Haacke EM. Duvernoy’s Atlas of the Human Brain Stem and Cerebellum. Wien: Springer; 2007. [Google Scholar]
- 51.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System: A Synopsis and Atlas. 4 Berlin: Springer; 2007. [Google Scholar]
- 52.Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. 2 Basel: Karger; 1982. [Google Scholar]
- 53.Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. Chicago: Book Medical Publishers; 1978. [Google Scholar]
- 54.Riley HA. An Atlas of the Basal Ganglia, Brain Stem and Spinal Cord. Baltimore: Williams & Wilkins; 1943. [Google Scholar]
- 55.Ziehen GT. Makroskopische und mikroskopische Anatomie des Gehirns. Jena: Fischer; 1903. [Google Scholar]
- 56.Kölliker A. Handbuch der Gewebelehre des Menschen. Leipzig: Engelmann; 1896. [Google Scholar]
- 57.Afshar F, Watkins ES, Yap JC. Stereotaxic Atlas of the Human Brainstem and Cerebellar Nuclei. New York: Raven Press; 1978. [Google Scholar]
- 58.Paxinos G, Huang X, Sengul G, Watson C. The Human Nervous System. Amsterdam: Elsevier Academic Press; 2012. Organization of Brainstem Nuclei; pp. 260–327. [Google Scholar]
- 59.Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–633. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- 60.Karson CN, Garcia-Rill E, Biedermann J, Mrak RE, Husain MM, Skinner RD. The brain stem reticular formation in schizophrenia. Psychiatry Res. 1991;40:31–48. doi: 10.1016/0925-4927(91)90027-n. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, Karson CN. Mesopontine neurons in schizophrenia. Neuroscience. 1995;66:321–335. doi: 10.1016/0306-4522(94)00564-l. [DOI] [PubMed] [Google Scholar]