Abstract
Background
Hemeoxygenase-1 (HO-1) has recently been identified as a major driver of metaflammation and obesity-related insulin resistance (IR). Drug-induced IR increases cardiovascular risk within the HIV-1-infected population receiving antiretroviral therapy (ART). We therefore investigated a possible role of HO-1 in ART-induced IR.
Methods
Effects of HIV-1 protease inhibitor ritonavir and integrase inhibitor raltegravir on expression levels of HO-1 and proinflammatory cytokines including interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor-α (TNFα), Chemokine (C-C motif) ligand 5 (CCL5) and monocyte chemotactic protein 1 (MCP-1) were studied in monocyte and hepatocyte cell lines. Plasma levels of HO-1 and inflammatory markers were measured in insulin-resistant and insulin-sensitive HIV-1-infected patients under ART and seronegative controls.
Results
We show that, in contrast to raltegravir, ritonavir treatment significantly increases mRNA expression levels of HO-1, IL-8, TNFα, CCL5 and MCP-1 in vitro in a dose dependent manner. HO-1 plasma levels were significantly higher in insulin-resistant compared to insulin-sensitive patients on ritonavir-boosted ART (LPV/r group: 3.90 ± 1.15 vs 2.56 ± 1.07 ng/ml, P<0.005 and DRV/r group: 3.16 ± 1.37 vs 2.28 ± 1.23 U/ml, P<0.05) and were correlated with expression levels of TNFα in individuals on ritonavir-boosted ART (LPV/r group: r2=0.108, P<0.05 and DRV/r group: r2=0.221, P<0.05) but not in HIV-1-infected individuals receiving raltegravir or in seronegative controls.
Implications
HIV-1-infected patients on stable ART are often faced with non-AIDS related metabolical comorbidities increasing their individual cardiovascular risk. Here, we provide insight into a novel mechanism of ritonavir-induced IR involving proinflammatory properties of HO-1. Our initial observations might also provide prognostic value in the future in order to identify patients at risk for the development Type 2 Diabetes Mellitus.
Keywords: HIV-1, insulin resistance, protease inhibitors, integrase inhibitors, ritonavir, hemeoxygenase-1
Introduction
Human immunodeficiency virus (HIV) infection still constitutes a pandemy with more than 35 million people infected globally.1 Anti-retroviral therapy (ART) has transformed early diagnosed and treated HIV-1 infection from a fatal illness to a chronic disease with an almost normal life expectancy.2 Nevertheless, the observed decline in morbidity and mortality has been accompanied by a number of non-AIDS related comorbidities, mostly arising from drug-induced derangements in lipid and glucose metabolism.3;4 There is growing concern regarding ART-associated metabolic complications and their potential long-term risk for cardiovascular disease (CVD), potentially resulting in reduced survival rates and quality of life.5 Antiretroviral drugs most frequently associated with these adverse side effects are HIV protease inhibitors (PIs) such as ritonavir, lopinavir or indinavir,6 and several mechanistic pathways are being discussed.7 However, in contrast to other first generation PIs, ritonavir is still widely used as a boosting agent for modern PIs, due to its inhibitory effect on the drug metabolizing enzyme cytochrome CYP3A4.8
In 2007, the integrase inhibitor raltegravir was introduced as a member of a new class of anti-retroviral agents. Raltegravir demonstrated potent efficacy against multidrug-resistant HIV-1 strains and was initially approved for the management of treatment-experienced patients.9 Currently, raltegravir is also listed as one of the preferred drug regimens for treatment-naïve patients according to the DHHS panel’s recommendations.10 Importantly, HIV-1-infected subjects treated with raltegravir show fewer adverse side effects, a more favorable metabolic profile and in contrast to PIs, raltegravir has not been associated with fat redistribution such as visceral fat accumulation or lipohypertrophy during short- and long-term treatment.11;12 Furthermore, chronic untreated HIV-1 infection itself is also associated with persistent inflammation and ART initiation improves endothelial function in patients with low CVD risk.13 Taken together, ART treatment of patients with chronic HIV-1 infection and the rising obesity prevalence in developed countries make early recognition of metabolic risk preeminent for the management of HIV-1 infected patients.
Previous studies have clearly demonstrated that exposure to ritonavir induces disturbances in glucose homeostasis in HIV-1-infected patients and HIV-seronegative, healthy humans using oral glucose tolerance and hyperinsulinemic/euglycemic clamp procedures.14;15 Research on diet-induced insulin resistance (IR) led to a new concept termed “metaflammation”, characterizing a chronic inflammatory process mediated by the immigration of pro-inflammatory macrophages into adipose tissue depots.16 These macrophages aggregate around dead adipocytes, forming characteristic ring patterns referred to as crown-like structures (CLS) and release proinflammatory cytokines including tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), IL-6 and monocyte chemotactic protein 1 (MCP-1), which can directly interfere with insulin signaling.17;18 The pro-inflammatory, M1-type macrophages perturb normal adipocyte function and are necessary and sufficient to generate systemic insulin resistance in mouse models of obesity.19;20 Current studies in insulin-resistant, obese humans and in macrophage and hepatocyte conditional knock-out mice identified hemeoxygenase-1 (HO-1), one of the first and rate limiting enzymes in heme metabolism, as a major driver of metaflammation and diet-induced IR.21 Macrophage-specific HO-1 knock-out mice were characterized by reduced epididymal fat infiltration of pro-inflammatory (M1) macrophages and showed decreased expression of inflammatory cytokines in both myeloid cells and hepatocytes.21 Interestingly, ritonavir has previously been shown to potently induce HO-1 gene expression and apoptotic cell death in the DLD-1 colon-carcinoma cell line by activating the activator protein-1 (AP-1) signaling pathway.22 We aimed to investigate the role of HO-1 in ritonavir-induced IR by measuring effects of ritonavir treatment on expression levels of HO-1 and inflammatory cytokines in human monocytes/macrophages and hepatocytes. In addition, we evaluated the associations of HO-1 plasma levels with the homeostasis model assessment insulin resistance index (HOMA-IR) in a preliminary cohort analysis.
Methods
Cell culture studies
THP-1 cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St.Louis, USA) supplemented with 2 mM L-glutamine (Sigma-Aldrich), 10% FBS (Biochrom AG, Berlin, Germany) and 50μM 2-mercaptoethanol (Invitrogen, Carlsbad, CA, USA). HepG2 cells were cultured as described.23 Antiretrovirals were provided by the manufacturers: Ritonavir (AbbVie Limited, North Chicago, IL, USA); Raltegravir (Merck Sharp&Dome Limited, Kenilworth, NJ, USA). Both drugs were dissolved in DMSO, as recommended by the manufacturer (Selleckchem, Munich, Germany), prior to dilution into the serum free cell culture medium to the final concentrations between 10 and 50μg/ml. The final concentration of DMSO during cell culture experiments was never higher than 0.05% and did not affect HO-1 expression in the control experiments. The effects of PIs and of raltegravir were tested on cells for 8, 16 and 24 hours at the following concentrations. For raltegravir, since maximal plasma concentrations in healthy subjects range from 10 to 25μM (25-62 μg/ml) as assessed by HPLC-MS in pharmacokinetic studies,24 our in vitro concentrations were chosen between 10-50μg/ml. Antiviral activity of raltegravir in DMSO has been described previously.25 For ritonavir, if used as a sole protease inhibitor in humans, the recommended dose is 600mg every 12 hours, leading to a maximal plasma concentration of 15 +/-5μM according to the FDA label. Currently, it is usually used as a low-dose booster for protease inhibitors with 100 to 200mg per day. Further, in order to achieve comparable molar concentrations of both drugs, since ritonavir (MW=720) has a higher molecular weight than raltegravir (MW=444), ritonavir was studied between 12.5, 25 and 50 μg/ml. Viability of the cell lines was not affected by either ritonavir or raltegravir as assessed by growth characteristics and trypan blue exclusion.
Gene expression studies
Total RNA was isolated from THP-1 and HepG2 cells cultured in the absence or presence of antiretroviral drugs, as indicated, using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA samples were stored at -80°C and digested with Dnase I (Promega, Madison, WI, USA) prior to reverse transcription as described.26 Complementary DNA (cDNA) was synthesized using random hexamer primers and the SuperScript IV First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Transcript levels of hemeoxygenase-1 (Hs01110250_m1), IL-1β (Hs01555410_m1), IL-6 (Hs00985639_m1), IL-8 (Hs00174103_m1), TNFα (Hs01113624_m1), CCL5 (Hs00982282_m1) and MCP-1 (Hs00234140_m1) were quantified in triplicates using Taqman gene expression assays (Applied Biosystems, Foster City, CA, USA) listed in parenthesis. Constitutively expressed acidic ribosomal protein p0 (RPLP0) mRNA (4326314E) was used as an internal standard for normalization of mRNA abundance. Relative mRNA levels were calculated using the comparative threshold cycle method (ΔΔCT) and the iCycler iQ Multicolour Real-Time polymerase chain reaction Detector (BioRad, Hercules, CA, USA). The average interassay coefficient of variation of mRNA measurements ascertained by overlapping cDNA samples was ~12 %.
Study population
Plasma samples were recruited from HIV-1 infected individuals from an Austrian HIV outpatient clinic in Salzburg, which is part of the Austrian HIV Cohort (AHIVCOS) and the study has been approved by the ethics committee of the Salzburg Federal Government (No. 1159/2010). A sex-, age- and BMI-matched, HIV-seronegative control cohort from the same geographical area was selected from the biobank of the Medical University of Vienna. Written informed consent was given by the patients for their information to be used for research. Study subjects were required to be virologically suppressed (HIV-1 RNA <40 copies/mL) and on stable ART for >6 months. ART regimens included two nucleoside reverse transcriptase inhibitors (NRTIs) (tenofovir or abacavir and emtricitabine or lamivudine) plus a protease inhibitor or an integrase inhibitor. We calculated the sample size using an online power-and-sample size calculator (http://powerandsamplesize.com/Calculators/Compare-2-Means/2-Sample-Equality). The effect size was estimated based on a previous study describing drug effects on HO-1 plasma levels.27 For a sample size of N=28 the calculated power was 0.9.
The most commonly prescribed NRTI backbone was tenofovir in all 3 groups of HIV-1-infected patients. Participants on PI based regimens received either lopinavir/ritonavir (LPV/r), commercially available as Kaletra (Abbott Laboratories, Abbott Park, Illinois, USA), 200/50 mg two tablets twice daily (N=38) or darunavir/ritonavir (DRV/r) commercially available as Prezista (Janssen-Cilag International NV, Beerse, Belgium) 800/100 once daily or 600/100 mg twice daily (N=54). Participants on an integrase inhibitor regimen received 400 mg raltegravir (RAL) twice daily (N=35), commercially available as Isentress (Merck, Philadelphia, USA). HIV seronegative subjects served as a control group (N=28). Subjects with a BMI >35, Type 1 or Type 2 diabetes mellitus and individuals with current use or use within the last six months of androgen therapy or lipid lowering agents were excluded. There were no serious adverse clinical events during the course of the study.
Laboratory analyses
Blood was collected after an overnight fast and plasma was separated by centrifugation (1,100 g; 10 min) within 2-4h. Glucose, cholesterol, TG, HDL cholesterol, c-reactive protein, IL-6, aspartate transaminase, alanine transaminase, g-glutamyl transferase, alkaline phosphatase, amylase, and lipase were measured as described.23 Insulin and TNFα were measured by CMIA immunoassay using the architect platform (Abbott Laboratories, Abbott Park, IL, USA). Insulin resistance was estimated using the homeostatic model assessment HOMA-IR as described.21 HOMA-IR has been widely utilized as a surrogate marker of insulin resistance in clinical and epidemiological studies. At least to our knowledge there is no generally accepted HOMA-IR cut-off value, that absolutely distinguishes normoglycemic subjects from those with an impaired glucose tolerance, since this is a gradual process. According to the literature (see Supplemental Digital Content), we chose a HOMA-IR cut-off value of 2.5 (individuals were divided in two categories according to their HOMA-IR (insulin sensitive: HOMA-IR < 2.5; insulin resistant: HOMA-IR >2.5).28 HbA1c was measured by capillary electrophoresis using the ADAMS HA-8160 system (Menarini Diagnostic, Firence, Italy). HO-1 (Enzo Life sciences Inc., Farmingdale, NY, USA), IL-1β, IL-8, CCL5 and MCP-1 (R&D Systems Inc., Minneapolis, MN, USA) were measured by quantitative sandwich enzyme immunoassay. For determination of HIV-1 viral load, the Abbott m24sp automated sample preparation system was used for RNA isolation (Abbott Molecular, Des Plaines IL, USA). PCR amplification was performed using the Abbott m2000rt Real-Time PCR system and the Abbott RT HIV-1 assay with a dynamic range of 40 to 10.000.000 copies/mL.
Statistical analyses
Statistical analyses were performed using the SPSS for windows software package version 21.0 (SPSS Inc. Chicago, IL, USA). Data are presented as means ± SD unless otherwise indicated. Descriptive statistics were calculated for all demographic and clinical characteristics of the study groups. Group differences of continuous variables were ascertained by analysis of variance (ANOVA). Measurements were adjusted for the concomitant effects of age, sex and BMI, as indicated. Parametric data were analyzed by using Student t-test, to assess whether the mean values for normally distributed groups differed significantly. P-values of <0.05 were considered significant.
Results
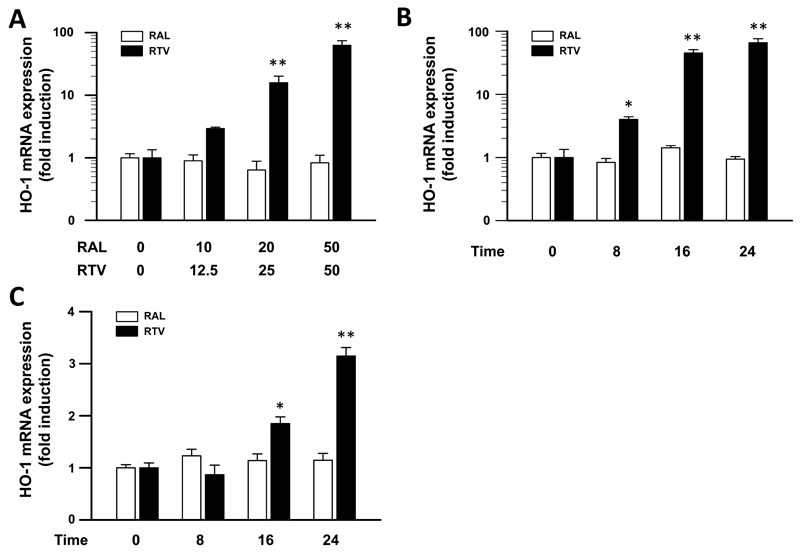
Previous studies suggested that ritonavir stimulates transcriptional regulation of the HO-1 gene in colon carcinoma cells.22 We therefore investigated its effects on HO-1 expression in the monocyte cell line THP-1 and in immortalized HepG2 hepatocytes. As shown in figure 1A, ritonavir exposure of THP-1 cells for 24 hours resulted in a dose-dependent increase of HO-1 mRNA expression levels, compared to unstimulated control cells. At ritonavir concentrations of 12.5, 25 and 50 μg/ml, HO-1 expression levels showed a 2.9±0.16-fold, 15.8±4.3-fold and 62.4±11.9-fold increase (P<0.01), respectively. In a time-course experiment, ritonavir enhanced HO-1 mRNA expression 4.0±0.41-fold, 45.3±5.7-fold and 68.8±10.3-fold (P<0.01) after 8, 16 and 24 h of exposure, respectively (Fig. 1B). In contrast, raltegravir treatment of THP-1 cells did not significantly alter steady-state levels of HO-1 mRNA at any drug concentration or time point studied (Fig. 1A and 1B). A less pronounced effect of ritonavir treatment was observed in HepG2 cells. Ritonavir treatment using 50 μg/ml for 16 and 24 hours resulted in a 1.85±0.13-fold and 3.15±0.17-fold increase in HO-1 mRNA expression (P<0.05) , respectively, whereas raltegravir treatment again did not significantly affect HO-1 expression levels (Fig. 1C).
Figure 1.
HIV-1 protease inhibitor ritonavir treatment increases hemeoxygenase-1 (HO-1) mRNA expression in vitro. A, THP-1 cells, a human monocyte cell line, were incubated for 24 hours with increasing doses of HIV-1 integrase inhibitor raltegravir (RAL) or protease inhibitor ritonavir (RTV), respectively, as indicated (see Methods section). B, THP-1 cells were incubated for 8, 16 and 24 hours with 20 μg/ml RAL or 50 μg/ml RTV, respectively. C, HepG2, a human hepatocyte cell line, were incubated for 8, 16 and 24 hours with 20 μg/ml RAL or 50 μg/ml RTV, respectively. Relative HO-1 mRNA expression was quantified by real-time PCR using the comparative threshold cycle method. HO-1 abundance levels were normalized for the expression of acidic ribosomal protein p0 (RPLP0). Data are shown as mean ± SE of three samples each measured in triplicate. * P < 0.05, ** P < 0.01 for comparison to untreated control cells using the Student´s t-test.
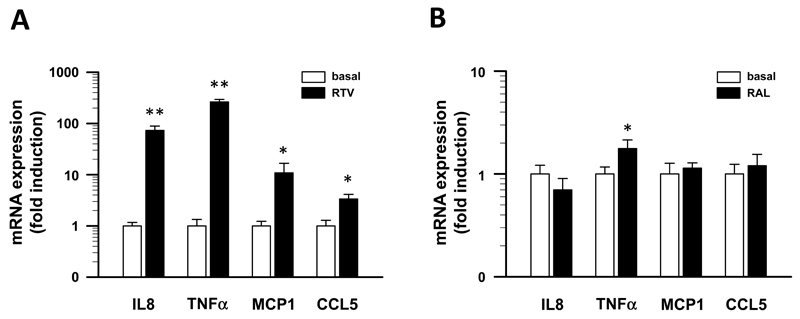
HO-1 exhibits pro-inflammatory properties that drive metabolically-triggered low grade, chronic inflammation (termed “meta-inflammation” or “cold inflammation”) and result in the development IR in obese humans21. As shown above, ritonavir potently induces HO-1 expression in THP-1 cells. Therefore, we studied possible effects of ritonavir exposure on the expression of proinflammatory cytokines in THP-1 cells. A 24 h exposure of THP-1 cells cultured in the presence of 50 mg/ml ritonavir significantly altered mRNA levels of proinflammatory cytokines. As shown in figure 2A, ritonavir increased the expression of IL-8 (73±15.8-fold, (P<0.01), TNFα (264±30.2-fold, (P<0.01), MCP1 (11±5.8-fold, (P<0.01) and CCL5 (3±0.8-fold, (P<0.01), whereas expression levels of IL-1β and IL-6 remained unaffected (data not shown). In contrast, incubation of THP-1 cells for 24 h with 20 μg/ml raltegravir had only minor effects on TNFα mRNA expression (1.8±0.3-fold, (P<0.05) and did not alter mRNA levels of IL-8, CCL5 and MCP-1 (Fig. 2B) as well as IL-1β and IL-6 (data not shown).
Figure 2.
HIV-1 protease inhibitor ritonavir treatment increases the expression of inflammatory cytokines in vitro. A, THP-1 cells, a human monocyte cell line, were cultured for 24 hours in the presence of 50 μg/ml RTV. B, THP-1 cells were cultured for 24 hours in the presence of 20 μg/ml RAL. Relative expression levels of interleukin-8 (IL-8), tumor necrosis factor α (TNFα), monocyte chemotactic protein 1 (MCP-1) and CC-chemokine ligand 5 (CCL5) were quantified by real-time PCR using the comparative threshold cycle method. Abundance levels were normalized for the expression of acidic ribosomal protein p0 (RPLP0). Data are shown as mean ± SE of three samples each measured in triplicate. * P < 0.05, ** P < 0.01 for comparison to untreated control cells using the Student´s t-test.
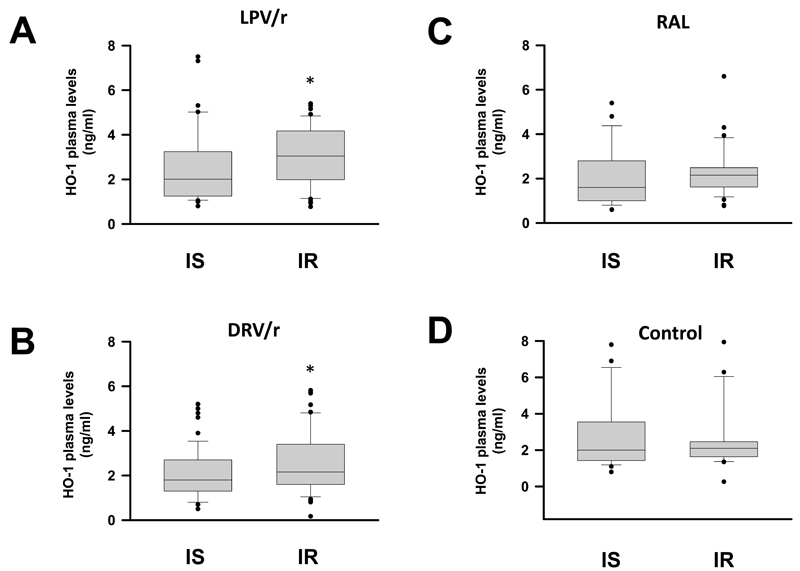
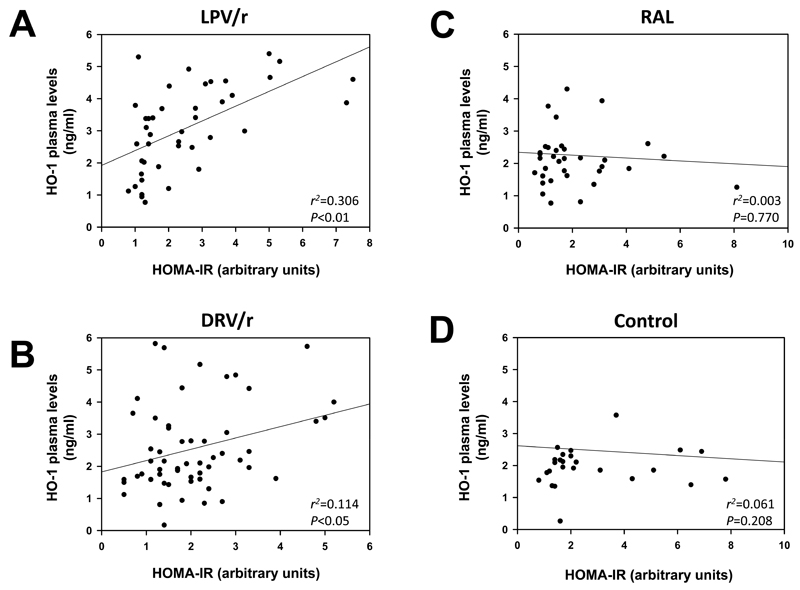
To investigate whether HO-1 plasma levels are associated with derangements of glucose homeostasis in HIV-1-infected subjects receiving ritonavir-boosted therapy regimens, we enrolled 155 study participants, that were assigned to four different groups. The study included 38 HIV-1- infected patients receiving lopinavir/ritonavir-based ART (LPV/r group), 54 HIV-1- infected patients receiving darunavir/ritonavir-based ART (DRV/r group), 35 HIV-1-infected patients on integrase inhibitor-based ART receiving raltegravir (RAL group) and 28 HIV seronegative subjects, that served as a control group. Table 1 summarizes the demographic and clinical characteristics of the study participants. Fasting HDL-cholesterol levels were significantly lower and triglyceride levels were significantly higher in protease inhibitor recipients (LPV/r and DRV/r groups) compared to HIV seronegative controls (Table 1). HIV-1-infected subjects receiving raltegravir had higher fasting HDL cholesterol and lower triglyceride levels in comparison to controls. Fasting glucose levels were elevated in all ART-receiving groups compared to HIV seronegative subjects. There were no significant differences in BMI, total cholesterol, LDL-cholesterol and HOMA-IR between the groups. As shown in Figure 3A, plasma HO-1 levels (mean values ± SD) were significantly higher in insulin-resistant (HOMA-IR >2.5), compared to insulin-sensitive (HOMA-IR <2.5) HIV-1-infected individuals in the LPV/r group (3.90 ± 1.15 vs 2.56 ± 1.07 ng/ml, P<0.005). A similar increase was observed in the DRV/r group (3.16 ± 1.37 vs 2.28 ± 1.23 U/ml, P<0.05; Fig. 3B), whereas no significant differences were noted in the RAL group (2.11 ± 0.80 vs 2.30 ± 1.19 U/ml, P=0.654; Fig. 3C) and in the non-HIV control group (2.52 ± 1.47 vs 2.45 ± 1.74 U/ml, P=0.904; Fig, 3 D). A significant correlation was found between HO-1 plasma levels and HOMA-IR in the LPV/r group and the DRV/r group but not in the RAL and non-HIV control group (Fig. 4). Furthermore, HO-1 levels were correlated with plasma levels of TNF-α in the LPV/r group (r2=0.108, P<0.05) and in the DRV/r group (r2=0.221, P<0.05) but not in the RAL group or in seronegative controls. No significant correlations of HO-1 with plasma levels of IL-1β, IL-6, IL-8, CCL5 or MCP1 were observed (data not shown).
Table 1. Characteristics of HIV-1-Infected and Seronegative Study Participants.
| Non-HIV (N=28) | RAL (N=35) | P* | LPV/r (N=38) | P † | DRV/r (N=54) | P ‡ | |
|---|---|---|---|---|---|---|---|
| Sex (% male) | 57.1 | 45.7 | n.a. | 65.7 | n.a. | 87.0 | n.a. |
| Age (years) | 42.14 ± 14.7 | 45.4 ± 13.7 | n.a. | 49.1 ± 11.7 | n.a. | 43.7 ± 12.4 | n.a. |
| BMI (kg/m2) | 25.2 ± 5.1 | 24.6 ± 4.9 | 0.311 | 24.2 ± 4.6 | 0.364 | 24.9 ± 4.1 | 0.449 |
| CD4 cell count (x106/l) | n.d. | 603.4 ± 242.4 | n.a. | 678.7 ± 264.5 | n.a | 654.0 ± 289.4 | n.a. |
| Total cholesterol (mg/dl) | 203.3 ± 42.5 | 192.0 ±35.9 | 0.697 | 217.7 ± 43.3 | 0.349 | 197.1 ± 37.1 | 0.652 |
| HDL-cholesterol (mg/dl) | 58.8 ± 17.6 | 62.2 ± 19.6 | <0.001 | 54.3 ± 13.3 | <0.001 | 50.5 ± 12.8 | <0.001 |
| LDL-cholesterol (mg/dl) | 124.3 ± 38.2 | 108.8 ± 35.0 | 0.172 | 122.9 ± 33.9 | 0.957 | 118.9 ± 35.5 | 0.384 |
| Triglyceride (mg/dl) | 115.6 ± 78.1 | 104.6 ± 57.7 | 0.055 | 188.5 ± 154.5 | 0.033 | 170.1 ± 100.2 | 0.007 |
| Fasting Glucose (mg/dl) | 90.6 ± 9.3 | 94.9 ± 13.2 | 0.006 | 92.5 ± 10.6 | 0.005 | 98.4 ± 14.0 | <0.001 |
| Fasting Insulin (mU/l) | 11.9 ± 7.6 | 8.4 ± 4.9 | 0.077 | 11.4 ± 8.3 | 0.897 | 10.3 ± 6.1 | 0.043 |
| HOMA-IR (a.u.) | 2.4 ± 1.9 | 2.1 ± 1.6 | 0.134 | 2.3 ± 1.4 | 0.165 | 2.1 ± 1.1 | 0.056 |
Numbers represent means ± SD; a.u., arbitrary units; RAL, raltegravir; LPV/r, lopinavir/ritonavir; DRV/r, darunavir/ritonavir; P, ANOVA, analysis of variance; adjusted for age and sex; P*, RAL vs. Non-HIV; P†, LPV/r vs. Non-HIV; P‡, DRV/r vs. Non-HIV;
Figure 3.
HO-1 plasma levels in HIV-1-infected subjects on ART and seronegative controls. HO-1 plasma levels in insulin sensitive (IS: HOMA-IR<2.5) compared to insulin resistant (IR: HOMA-IR>2.5) HIV-1-infected subjects receiving either A: lopinavir/ritonavir (LPV/r), B: darunavir/ritonavir (DRV/r) or C: raltegravir (RAL). D: A group of seronegative individuals served as control. Data are shown as box and whisker plots, with medians, interquartile ranges and ranges. * P<0.05 (ANOVA adjusted for sex, age and BMI).
Figure 4.
Correlations among HO-1 plasma levels and insulin resistance in ART-treated HIV-1-infected subjects and seronegative controls. Graphs show data dispersion of HO-1 plasma levels (ng/ml) and degree of insulin resistance as estimated using the homeostasis model assessment insulin resistance index (HOMA-IR) in the Cartesian axes, linear regression curves with r2 values P values obtained by the Spearman correlation test. Correlation analyses for HO-1 plasma levels and HOMA-IR in HIV-1-infected subjects receiving either A: lopinavir/ritonavir (LPV/r), B: darunavir/ritonavir (DRV/r) or C: raltegravir (RAL). D: A group of HIV seronegative individuals served as control.
Discussion
Previous studies argue for a prominent role of ritonavir in precipitating metabolic abnormalities observed in HIV-1-infected subjects.29 We provide evidence for a novel mechanism involving HO-1 as a driver of chronic inflammation in ritonavir-induced adipose tissue IR. The occurrence of immune cell infiltration in adipose tissue accompanied by the expression of inflammatory cytokines has been well established as a major hallmark in diet-induced IR.16 Although HO-1 has been recognized primarily as an anti-inflammatory enzyme in the current bulk of literature over the years,30;31 Jais et al. recently provided strong evidence for a pro-inflammatory role of HO-1, that drives the development of IR in obese humans. They demonstrated that HO-1 is involved in the priming of macrophage polarity towards a M1-like, pro-inflammatory phenotype.21
We show that ritonavir potently induces HO-1 expression in the human myeloid THP-1 cell line in a time- and dose-dependent manner. Ritonavir was chosen for this study since it is the most commonly used protease inhibitor booster in antiretroviral therapy. However, in our current study, we used ritonavir as a single drug and could observe an induction of HO-1 expression starting at a concentration of 12.5μg/ml. Further, we have to remain aware that even low-dose ritonavir is usually used continually in patients for months or years. In THP-1 cells, HO-1 induction is accompanied by a strong upregulation of the expression of major proinflammatory cytokines including IL-8, TNFα, CCL5 and MCP-1. These observations are in line with previous studies demonstrating that distinct PIs increase oxidative stress and alter cytokine secretion in THP-1 cells.32 Furthermore, Martinez et al. reported a significant decline in plasma levels of cardiovascular biomarkers including TNFα and MCP-1 in HIV-1-infected patients participating in the SPIRAL study, that were switched from ritonavir-boosted PIs to raltegravir.33 Effects of ritonavir exposure on the expression of proinflammatory cytokines were also described in 3T3-L1 cells.34
In HIV-infection the role of continued, low-grade inflammation, that is strongly associated with an increased risk for cardiovascular disease, has received much attention in the past.35 Persistent immune activation may result from multiple factors including coinfections,36;37 low-level viremia,38 enterocyte damage leading to microbial translocation39;40 or ART itself.41 Here, we propose a role for HO-1 in chronic inflammation in ritonavir-treated HIV-1-infected patients. Additional studies are required to elucidate the effects of distinct antiretroviral regimens on chronic inflammation during HIV-1 infection.
Hepatic IR is considered to be another important component in the pathogenesis of fasting hyperglycemia. Interestingly, it has been demonstrated that HO-1 serves as a negative regulator of insulin signaling in the liver via impairment of mitochondrial respiratory function, which leads to the induction of protein-tyrosine phosphatase 1B (PTP1), the key upstream inhibitor of the insulin receptor IRS1.21 We demonstrate, that ritonavir treatment significantly increases HO-1 expression in a hepatoma cell line (HepG2) and provide initial evidence, that ritonavir-induced IR might also involve HO-1-mediated impairments in the regulation of intrahepatic insulin receptor availability.
Meanwhile, numerous molecular mechanisms of ritonavir-induced glycemic dysregulation, that are mainly related to defects in insulin action and secretion, have been well established. The acute development of glucose intolerance even after short term treatment42 or large single doses of ritonavir14 has been attributed to a reversible inhibition of the glucose transporter GLUT 4 in peripheral tissues43 and/or inhibition of glucose sensing and glucose-stimulated insulin release, as has been shown in cultured pancreatic β-cells and in murine models.44 But also a long-term reduction in insulin sensitivity, significantly increasing the risk for the development of T2DM and cardiovascular disease, has been well established in antiretroviral-naïve and in PI-experienced HIV-1-infected subjects in numerous clinical studies.45;46 Our observations suggest that ritonavir-mediated upregulation of HO-1, which is accompanied by an increased expression of proinflammatory cytokine, including IL-8, TNFα, CCL5 and MCP-1, might exert an additional, indirect effect on glucose uptake by GLUT4. It has previously been demonstrated that WAT macrophage-derived TNFα blocks insulin action in adipocytes via downregulation of GLUT4 and IRS-1, leading to a decrease in Akt phosphorylation and impaired insulin-stimulated GLUT4 translocation to the plasma membrane.47 Other proposed mechanisms for the development of PI-induced IR include direct effects of ritonavir on adipocyte differentiation48 and function,32 β-cell apoptosis,49 and on ER stress accompanied by an induction of the unfolded protein response.50 Our initial observations in cell cultures and HIV-infected humans do not allow to form a reasonable estimate of the relative contribution of ritonavir-induced HO-1 induction to systemic IR in comparison to other known actions of the drug. However, due to the significant role of chronic inflammation in the etiology of IR, HO-1 might contribute significantly to ritonavir-induced IR.
HO-1 expression levels in liver and adipose tissue predict the extent of systemic insulin resistance in obese humans independently from age, sex and BMI.21 In our study we describe a significant correlation of HO-1 plasma levels and HOMA-IR, a surrogate marker for insulin resistance, in ritonavir-treated HIV-1-infected patients but not in patients on a raltegravir-based regimen or in seronegative, non-HIV control subjects, respectively. Furthermore, we show that lopinavir/ritonavir treatment had a stronger effect on HO-1 levels compared to darunavir/ritonavir treatment. Studies in 3T3-L1 preadipocytes revealed that lopinavir in contrast to darunavir, promotes PTP1 expression, via a yet unknown mechanism, thereby interfering with insulin signaling.51 An association of HO-1 plasma concentrations with impaired glucose tolerance has also been described in a non-diabetic cohort of Chinese subjects.52 Furthermore, significantly elevated HO-1 levels were observed in type 2 diabetic Chinese subjects in comparison to normoglycemic controls.53 Our data show that a careful evaluation of the predictive power of HO-1 plasma levels for the early detection of impairments in glucose tolerance is warranted in larger populations of treated HIV-1-infected subjects.
At least to our knowledge, this study demonstrates for the first time a strong correlation between insulin resistance and HO-1 in HIV-1-infected subjects on ritonavir-boosted ART and identifies HO-1 as a putative novel player in ritonavir-induced IR. Interestingly, previous studies demonstrated that plasma HO-1 levels were also raised in stable coronary artery disease and were increased in subjects suffering from acute coronary syndromes compared to healthy controls.54 Since traditional prediction tools such as the Framingham equation may underestimate the cardiovascular risk in HIV-1 patients, novel biomarkers are needed that allow an early recognition of metabolic derangements. Furthermore, in consideration of the long-term consequences of ART-induced chronic inflammation, a better knowledge of the underlying causes might lead to the development of safer treatment regimens, that attenuate the effects of immune activation in people living with chronic HIV-1 infection.
Supplementary Material
Acknowledgements
The authors are grateful to H. Schnaitl and S. Eder for their technical assistance. This work was supported by grants from Paracelsus Medical University Salzburg to N. Taylor (R-14/04/064-TAY) and H. Oberkofler (E-09/09/050-OBE). This work was supported by grants from Paracelsus Medical University Salzburg to N. Taylor (R-14/04/064-TAY), H. Oberkofler (E-09/09/050-OBE) and L. Kenner (FWF_P 26011).
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Lee GA, Rao MN, Grunfeld C. The effects of HIV protease inhibitors on carbohydrate and lipid metabolism. Curr HIV /AIDS Rep. 2005;2:39–50. doi: 10.1007/s11904-996-0008-z. [DOI] [PubMed] [Google Scholar]
- 4.Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis. 2013;13:964–975. doi: 10.1016/S1473-3099(13)70271-8. [DOI] [PubMed] [Google Scholar]
- 5.Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep. 2008;10:61–70. doi: 10.1007/s11883-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Galescu O, Bhangoo A, Ten S. Insulin resistance, lipodystrophy and cardiometabolic syndrome in HIV/AIDS. Rev Endocr Metab Disord. 2013;14:133–140. doi: 10.1007/s11154-013-9247-7. [DOI] [PubMed] [Google Scholar]
- 8.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 10.Pandey KK. Raltegravir in HIV-1 infection: Safety and Efficacy in Treatment-naive Patients. Clin Med Rev Ther. 2011;2012:13–30. doi: 10.4137/CMRT.S5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockstroh JK, Lennox JL, Dejesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53:807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 12.Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29:256–265. doi: 10.1089/aid.2011.0275. [DOI] [PubMed] [Google Scholar]
- 13.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GA, Rao M, Mulligan K, et al. Effects of ritonavir and amprenavir on insulin sensitivity in healthy volunteers. AIDS. 2007;21:2183–2190. doi: 10.1097/QAD.0b013e32826fbc54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noor MA, Parker RA, O'Mara E, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004;18:2137–2144. doi: 10.1097/00002030-200411050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patsouris D, Li PP, Thapar D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jais A, Einwallner E, Sharif O, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhl H, Paulukat J, Hofler S, et al. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br J Pharmacol. 2004;143:890–898. doi: 10.1038/sj.bjp.0706023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberkofler H, Linnemayr V, Weitgasser R, et al. Complex haplotypes of the PGC-1alpha gene are associated with carbohydrate metabolism and type 2 diabetes. Diabetes. 2004;53:1385–1393. doi: 10.2337/diabetes.53.5.1385. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83:293–299. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 25.Kassahun K, McIntosh I, Cui D, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos. 2007;35:1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 26.Oberkofler H, Pfeifenberger A, Soyal S, et al. Aberrant hepatic TRIB3 gene expression in insulin-resistant obese humans. Diabetologia. 2010;53:1971–1975. doi: 10.1007/s00125-010-1772-2. [DOI] [PubMed] [Google Scholar]
- 27.Rizzo M, Abate N, Chandalia M, et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J Clin Endocrinol Metab. 2015;100:603–606. doi: 10.1210/jc.2014-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hruz PW. HIV protease inhibitors and insulin resistance: lessons from in-vitro, rodent and healthy human volunteer models. Curr Opin HIV AIDS. 2008;3:660–665. doi: 10.1097/COH.0b013e3283139134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 31.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z Gastroenterol. 2012;50:34–40. doi: 10.1055/s-0031-1282046. [DOI] [PubMed] [Google Scholar]
- 32.Lagathu C, Eustace B, Prot M, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 33.Martinez E, D'Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26:2315–2326. doi: 10.1097/QAD.0b013e328359f29c. [DOI] [PubMed] [Google Scholar]
- 34.Lagathu C, Bastard JP, Auclair M, et al. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir Ther. 2004;9:911–920. [PubMed] [Google Scholar]
- 35.Erlandson KM, Campbell TB. Inflammation in Chronic HIV Infection: What Can We Do? J Infect Dis. 2015;212:339–342. doi: 10.1093/infdis/jiv007. [DOI] [PubMed] [Google Scholar]
- 36.Crane M, Avihingsanon A, Rajasuriar R, et al. Lipopolysaccharide, immune activation, and liver abnormalities in HIV/hepatitis B virus (HBV)-coinfected individuals receiving HBV-active combination antiretroviral therapy. J Infect Dis. 2014;210:745–751. doi: 10.1093/infdis/jiu119. [DOI] [PubMed] [Google Scholar]
- 37.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chun TW, Nickle DC, Justement JS, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 40.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis. 2015;212:345–354. doi: 10.1093/infdis/jiv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee GA, Lo JC, Aweeka F, et al. Single-dose lopinavir-ritonavir acutely inhibits insulin-mediated glucose disposal in healthy volunteers. Clin Infect Dis. 2006;43:658–660. doi: 10.1086/505974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vyas AK, Koster JC, Tzekov A, et al. Effects of the HIV protease inhibitor ritonavir on GLUT4 knock-out mice. J Biol Chem. 2010;285:36395–36400. doi: 10.1074/jbc.M110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koster JC, Remedi MS, Qiu H, et al. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–1700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Carr A, Ritzhaupt A, Zhang W, et al. Effects of boosted tipranavir and lopinavir on body composition, insulin sensitivity and adipocytokines in antiretroviral-naive adults. AIDS. 2008;22:2313–2321. doi: 10.1097/QAD.0b013e328315a7a5. [DOI] [PubMed] [Google Scholar]
- 47.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dowell P, Flexner C, Kwiterovich PO, et al. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem. 2000;275:41325–41332. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Carper MJ, Lei X, et al. Protease inhibitors used in the treatment of HIV+ induce beta-cell apoptosis via the mitochondrial pathway and compromise insulin secretion. Am J Physiol Endocrinol Metab. 2009;296:E925–E935. doi: 10.1152/ajpendo.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker RA, Flint OP, Mulvey R, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005;67:1909–1919. doi: 10.1124/mol.104.010165. [DOI] [PubMed] [Google Scholar]
- 51.Kitazawa T, Yoshino Y, Suzuki S, et al. Lopinavir inhibits insulin signaling by promoting protein tyrosine phosphatase 1B expression. Exp Ther Med. 2014;8:851–855. doi: 10.3892/etm.2014.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao W, Rong S, Zhang M, et al. Plasma heme oxygenase-1 concentration in relation to impaired glucose regulation in a non-diabetic Chinese population. PLoS One. 2012;7:e32223. doi: 10.1371/journal.pone.0032223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao W, Song F, Li X, et al. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One. 2010;5:e12371. doi: 10.1371/journal.pone.0012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idriss NK, Lip GY, Balakrishnan B, et al. Plasma haemoxygenase-1 in coronary artery disease. A comparison with angiogenin, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and vascular endothelial growth factor. Thromb Haemost. 2010;104:1029–1037. doi: 10.1160/TH09-11-0802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.