Abstract
Purpose
To describe two unrelated families with multiple members demonstrating a less commonly recognized vortex pattern of corneal deposits confirmed to be granular corneal dystrophy type 1(GCD1) following identification of the p.(Arg555Trp) mutation in the transforming growth factor β-induced gene (TGFBI).
Methods
A slit lamp examination was performed on individuals from two families, one of Mexican descent and a second of Italian descent. Following DNA extraction from affected individuals and their unaffected relatives, TGFBI screening was performed.
Results
Eight of 20 individuals in the Mexican family and 20 of 55 in the Italian family demonstrated corneal stromal opacities. Seven of the eight affected individuals in the Mexican family and four of the 20 affected individuals in the Italian family demonstrated a phenotype characterized by a “sea fan” or vortex pattern of superficial stromal corneal deposits originating from the inferior aspect of the cornea. Screening of TGFBI in both families revealed a heterozygous missense mutation (p.(Arg555Trp)) in exon 12, confirming the diagnosis of GCD1.
Conclusion
Our findings demonstrate that GCD1 may present with a vortex pattern of anterior stromal deposits. Although this pattern of dystrophic deposits is not recognized by clinicians as a typical phenotype of GCD1, it is consistent with the production of the majority of the TGFBI protein by the corneal epithelium.
Keywords: granular corneal dystrophy, TGFBI, vortex, corneal epithelium
INTRODUCTION
Granular corneal dystrophy type 1 (GCD1), also known as Groenouw type I, is an autosomal dominant corneal stromal dystrophy caused by mutations in the TGFBI gene mapped to chromosome 5q31.1, 2 The classic form of GCD1 is characterized by bilateral, symmetric, axially distributed, discrete, greyish-white, anterior stromal opacities.3 These lesions usually appear in the first decade of life although as the intervening stroma remains clear, affected individuals are asymptomatic early in the course of the condition.4, 5 Progression of the dystrophy is characterized by an increase in the size and number of the deposits, which extend deeper into the stroma and spread further into the corneal mid-periphery.4, 5
A vortex pattern of corneal deposits has been described in individuals with presumed GCD1 in six prior reports.6-11 Only 1 family was confirmed to have GCD1 through identification of the p.(Arg555Trp) mutation in TGFBI, leaving the diagnosis in the other families uncertain.6 In the family with confirmed GCD1, the only two affected individuals examined both demonstrated a vortex or sea fan pattern of corneal deposits. As other family members were not available for examination, it is not known whether all affected family members demonstrated this phenotype, or if others demonstrated the classic GCD1 phenotype. Herein, we describe two unrelated families, one of Italian descent and one of Mexican descent, with affected members demonstrating both the classic and vortex phenotypes of GCD1, confirmed by identification of the p.(Arg555Trp) mutation in TGFBI. We propose that the description of the phenotype of GCD1 be expanded to include the vortex pattern of deposits to ensure that clinicians are able to accurately diagnose this variant of both primary and recurrent GCD1.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board/Ethics Committee of the Institute of Ophthalmology “Conde de Valenciana” in Mexico City. All patient samples were collected after the patients signed written informed consent, and clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Clinical Evaluation
A slit lamp examination was performed on individuals from two families, one of Mexican descent and a second of Italian descent
TGFBI screening
Mexican family
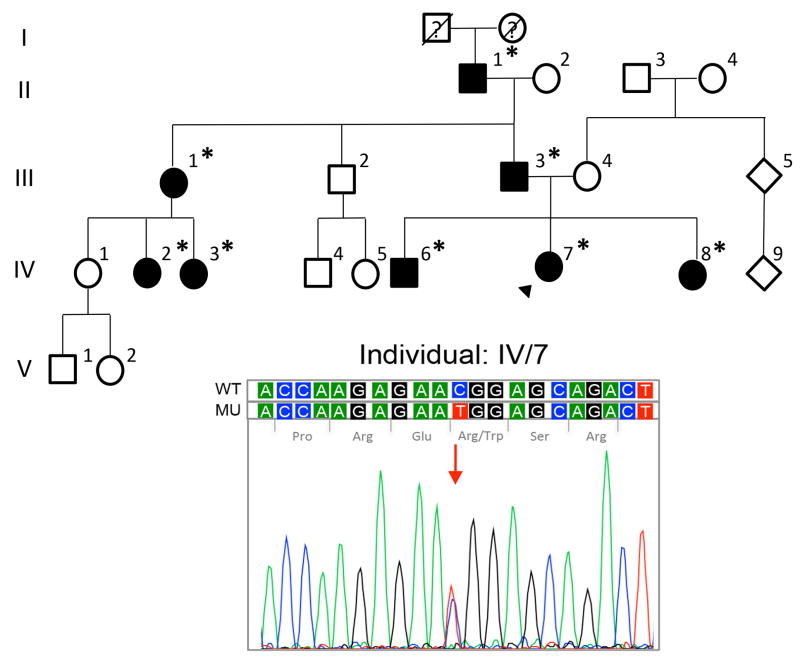
After informed consent was obtained, blood samples were collected from eight affected family members (Fig.1; individuals II-1, III-1, III-3, IV-2, IV-3, IV-6, IV-7, IV-8). Genomic DNA was extracted from peripheral blood leukocytes and PCR amplification of all 17 exons of TGFBI was performed on DNA collected from an affected individual (Fig.1; individual II-1) using previously described primers and conditions.12 Sanger sequencing was performed using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) and samples were analyzed in a 3130 Genetic Analyzer (Applied Biosystems). The wild type TGFBI transcript ID ENST00000305126 (www.ensembl.org) was used for sequence comparisons. DNA samples from the seven other affected family members were screened for the variants identified in individual II-1.
Figure 1.
Pedigree of Mexican family with granular corneal dystrophy type I (GCD1). Screening of TGFBI exon 12 in the proband (arrowhead; individual IV-7) demonstrated the c.1663C>T (p.(Arg555Trp)) mutation associated with GCD1. Filled symbols, affected individuals; open symbols, unaffected individuals; asterisk, TGFBI screening performed.
Italian family
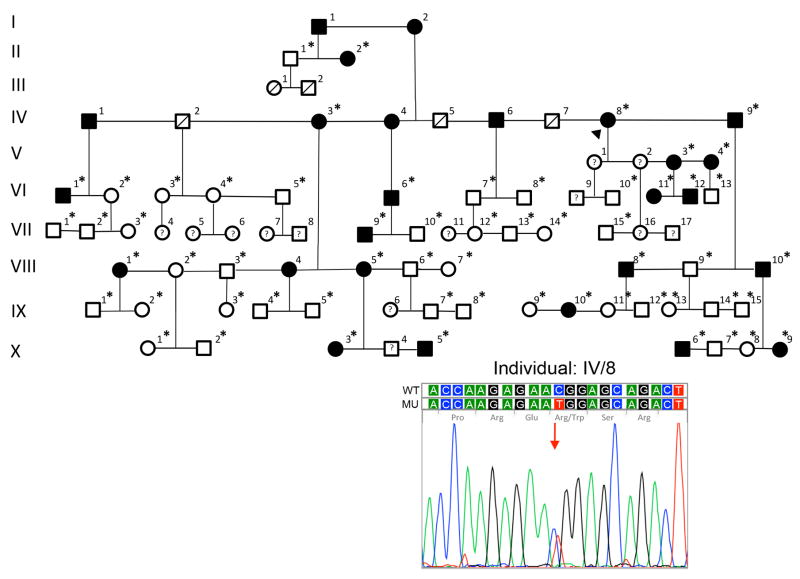
After informed consent was obtained, buccal swab samples were obtained from 55 family members (Fig. 2; individuals indicated with an asterisk). Genomic DNA was extracted and was subsequently used in a proprietary TaqMan quantitative PCR assay (Avellino Laboratories USA, Inc., Menlo Park, CA) to identify sequence variants in codons 124 and 555 of TGFBI. In addition, all 17 exons of TGFBI were screened by a commercial laboratory (Laragen, Inc., Culver City, CA) in two affected individuals, one with the classic (Fig. 2; IV-3) and one with the vortex (Fig. 2; VIII-10) pattern of dystrophic deposits. Sequence variants identified in one of these affected individuals were screened for in the DNA samples from the remaining affected individuals to identify variants that segregated with either the classic or vortex deposit phenotype.
Figure 2.
Pedigree of Italian family with GCD1. Screening of TGFBI exon 12 in the proband (arrowhead; individual IV-8) demonstrated the c.1663C>T (p.(Arg555Trp)) mutation associated with GCD1. Filled symbols, affected individuals; open symbols, unaffected individuals; asterisk, TGFBI screening performed.
Statistical Analysis
Minor allele frequencies were obtained for each identified variant from the 1000 Genomes and Exome Aggregation Consortium (ExAC) databases. The probability and corresponding 95% confidence interval (CI) of identified variants found in the two families were estimated based on the observed frequencies in the families, and the estimated probability was compared with specific probability value obtained from the observed frequency of same variants in the population described above assuming binomial distribution of allele frequencies.
RESULTS
Clinical Features
Mexican Family
In total, 20 family members were examined by slit lamp biomicroscopy, with eight observed to have corneal deposits at the level of the anterior stroma that were consistent with GCD1. Seven of the eight (87.5%) affected individuals demonstrated a vortex pattern of anterior stromal corneal deposits that appeared to radiate from the inferior paracentral cornea. The clinical features of a few of the affected individuals are presented below.
The proband is a 12-year-old girl (Fig. 1; individual IV-7) with a history of renal tubular acidosis diagnosed at 10 years of age who presented with decreasing visual acuity that started at 8 years of age. On examination, uncorrected visual acuity was 20/60 in right eye and 20/40 in left eye, improving with correction to 20/30 in each eye. Anterior segment examination revealed bilateral, greyish-white anterior stromal deposits that originated from the inferior paracentral cornea and radiated peripherally, creating a vortex or sea fan pattern (Fig. 3A).
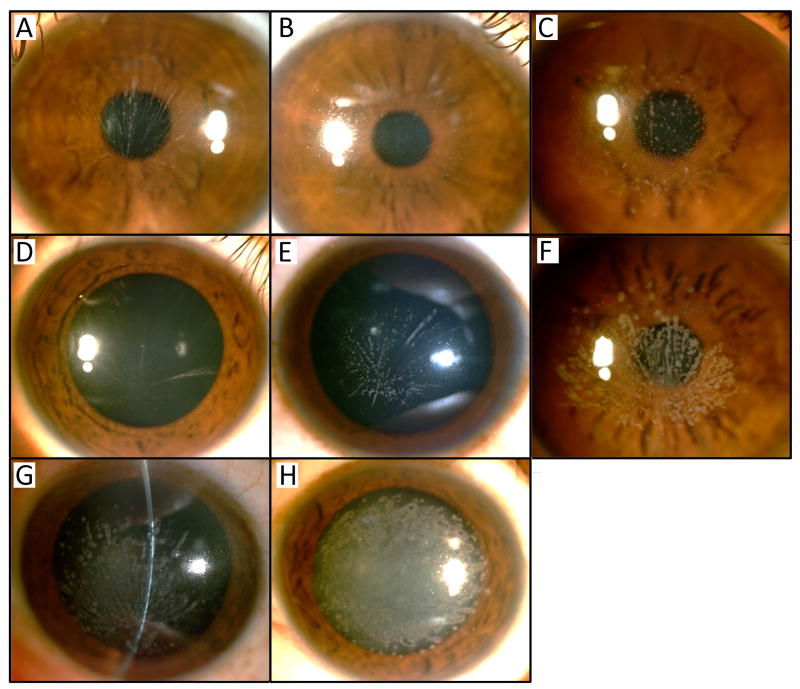
Figure 3.
Corneal opacities in Mexican family with GCD1. (A) A vortex pattern of deposits was observed in the 12 year-old female proband (IV-7), (B) the 9 year-old sister of the proband (IV-8), (C) the 22 year-old brother of proband (IV-6) and (D) the 8 year-old (IV-3) and (E) 15 year-old (IV-2) female cousins of the proband. Similar appearing but more numerous deposits are observed in (F) the 43 year-old father (III-3) and (G) 65 year-old grandfather (II-1) of the proband. (H) Confluent deposits are observed in the 45 year-old paternal aunt of the proband (III-1).
The 9-year-old sister of the proband (Fig. 1; individual IV-8) noted decreasing visual acuity since she was 5 years old. On examination, uncorrected visual acuity was 20/70 in the right eye and 20/50 in the left eye, improving to 20/30 in each eye with correction. Slit-lamp exam revealed a very fine vortex pattern in the central 5 mm of each cornea with small white oval anterior stromal deposits originating centrally. The intervening stroma and the periphery of the corneas were clear (Fig. 3B).
A presumptive diagnosis of granular corneal dystrophy was made in both the proband and her sibling. Family history revealed multiple other family members with declining visual function. Anterior segment examination of the proband’s brother (Fig. 1; individual IV-6; Fig. 3C), two cousins (Fig.1; individuals IV-2 and IV-3; Fig. 3D-E), father (Fig.1; individual III-3; Fig. 3F) and paternal grandfather (Fig. 1; individual II-1; Fig. 3G) revealed similar findings of symmetric deposition of round, grey-white, anterior corneal stromal opacities in a vortex pattern with varying degrees of density.
Of note, the proband’s 45-year-old paternal aunt (Fig.1; individual III-1) presented with decreasing visual acuity since childhood. She had presumed superficial keratectomies performed in each eye as well as a penetrating keratoplasty (PK) in her right eye 2 years prior to presentation. On examination, uncorrected visual acuity was 20/70 in the right eye and 20/1000 in the left eye, which did not improve with correction. Slit lamp examination revealed a clear graft in her right eye with no evidence of recurrent dystrophic deposits. The left eye demonstrated multiple, confluent grey-white deposits in the central 8 mm of the cornea, without a sea fan pattern, sparing the peripheral cornea (Fig. 3H).
Italian Family
Of the 55 family members that were examined by slit lamp biomicroscopy, 20 were observed to have corneal deposits at the level of the anterior stroma that were consistent with GCD1. Four of the 20 (20%) affected individuals demonstrated a vortex pattern of corneal deposits while the remaining 12 individuals demonstrated classic GCD1 patterns of corneal deposits.
The proband, an 80-year-old woman (Fig. 2; individual IV-8) with a prior clinical diagnosis of GCD1, underwent PK in both eyes, followed by repeated phototherapeutic keratectomies (PTK) procedures for recurrent GCD1 in the right eye. The vision in the left eye was limited by repeat corneal graft failure. Slit lamp examination of the right eye revealed small annular and punctate anterior and mid-stromal opacities in the graft (Fig. 4A).
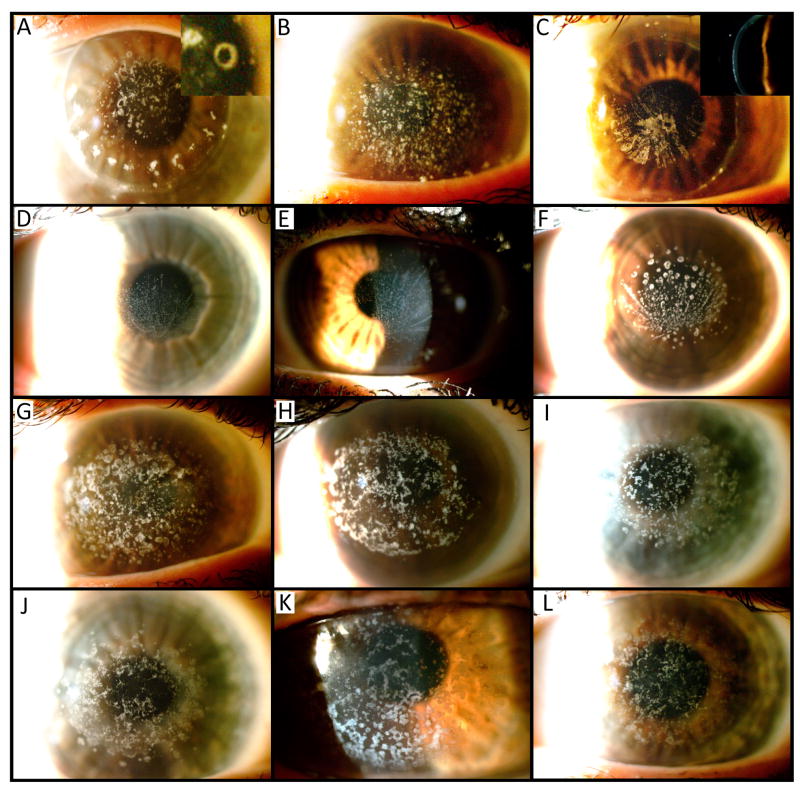
Figure 4.
Corneal opacities in Italian family with GCDI. (A) Following PK, annular stromal opacities were observed in the 80 year-old proband (IV-8). (B) The left cornea in a 49 year-old nephew of the proband (VIII-10) demonstrated punctate corneal opacities in the left eye representative of classic GCDI findings, while linear stromal opacities were observed radiating from the central cornea after PK in the right eye (C). A vortex pattern of anterior stromal deposits originating from the inferior paracentral cornea was observed in the proband’s great nieces, ages (D) 14 years (X-9), (E) 19 years (IX-10), and (F) 14 years (X-3). Along with the proband, the classic GCD1 pattern of corneal deposits was observed in individuals (G) VIII-8, (H) VIII-5, (I) VIII-1, (J) VI-12, (K) VI-1 and (L) V-3.
A 49-year-old male nephew of the proband (Fig. 2; individual VIII-10) was diagnosed with GCD1 at 13 years of age, and subsequently underwent PK in the right eye and PTK in the left eye twice. Slit-lamp evaluation of the left eye revealed discrete, greyish-white, anterior stromal opacities with clear intervening stroma and periphery (Fig. 4B). In contrast, the grey-white deposits in the right eye assumed a linear configuration, appearing to radiate inferiorly from the center of the corneal transplant (Fig. 4C).
This sea fan pattern of corneal deposits was also observed in several younger members of the family: a 14-year-old great niece of the proband (Fig. 2; individual X-9), a 19-year-old granddaughter of one of the proband’s siblings (Fig. 2; individual IX-10) and a 24-year-old granddaughter of one of the proband’s siblings (Fig. 2; individual X-3). On slit lamp examination, all three individuals showed a very fine vortex pattern of small white oval and round anterior stromal deposits originating just below the inferior pupillary border with the rays of the sea fan extending across the central cornea (Fig. 4D, 4E, and 4F). Apart from the vortex pattern, the characteristic clear intervening stroma and periphery were observed in these and all other affected individuals.
Multiple other family members demonstrated the classic GCD1 phenotype characterized by discrete and confluent, axially-distributed anterior stromal dystrophic corneal deposits that spared the corneal periphery (Fig. 4G-L).
Genetic analysis
Mexican Family
Screening of all 17 exons of TGFBI in one member of the family (Fig. 1; individual II-1) revealed the c.1663C>T variant in the heterozygous state in exon 12, predicted to result in the p.(Arg555Trp) missense mutation associated with GCD1 (Fig. 1). In addition, two common single nucleotide polymorphisms (SNPs) predicted to encode synonymous changes in the TGFBI protein, c.981A>G (p.Val327Val) (MAF: 0.32 (ExAC), 0.38 (1000 Genomes)) and c.1620T>C (p.Phe540Phe) (MAF=0.33 (ExAC), 0.42 (1000 Genomes)), were identified in the heterozygous state in exons 8 and 12, respectively. Screening of exon 12 in the seven other affected family members (Fig. 1; individuals III-1, III-3, IV-2, IV-3, IV-6, IV-7, IV-8) for the c.1663C>T variant revealed its presence in the heterozygous state in all seven affected individuals, confirming the diagnosis of GCD1. Each of the affected family members also demonstrated the c.981A>G and c.1620T>C variants, with the former present in the homozygous state in individuals III-1, IV-2 and IV-3 and the latter present in the homozygous state in individuals III-1, IV-2, IV-3, IV-6 and IV-7. The allele frequency of the c.981A>G variant in these eight affected individuals was therefore 11/16 (68.75%, 95% CI: 41.3% - 89.0%), significantly more common than the population allele frequencies of 32% (ExAC) and 38% (1000 Genomes) (p=0.002 and p=0.011, respectively). Similarly, the allele frequency of the c.1620T>C variant in these eight affected individuals was 13/16 (81.25%, 95% CI: 54.4% - 96.0%), significantly more common than the population allele frequencies of 33% (ExAC) and 42% (1000 Genomes) (p<0.0001 and p=0.002, respectively).
Italian Family
Screening of codon 124 in exon 4 and codon 555 in exon 12 of TGFBI was performed in 55 members of the Italian family (Fig. 2; indicated with an asterisk), of which 20 were clinically affected and 35 were unaffected. In the 20 affected individuals screened, genetic analysis revealed the c.1663C>T (p.(Arg555Trp) variant present in the heterozygous state, diagnostic of GCD1. The 35 unaffected individuals did not demonstrate a sequence variant in either codon 124 or codon 555. Screening of all 17 exons of TGFBI in two members of the family, one with a classic (Fig. 2; individual IV-3) and one with a vortex (Fig. 2; individual VIII-10) pattern of dystrophic deposits revealed a common single nucleotide polymorphism in exon 6, c.651G>C (p.Leu217Leu), (MAF: 0.42 (ExAC), 0.32 (1000 Genomes)), in the homozygous state in both individuals.
DISCUSSION
A vortex pattern of anterior corneal dystrophic deposits in individuals with presumed granular corneal dystrophy was first reported by Bücklers in 1938.10 Almost 50 years later, Weidle and Lisch associated the vortex pattern of corneal opacities with the earliest of three clinical stages of GCD1 and Lyons and colleagues described a vortex pattern of recurrent dystrophic deposits following PK in individuals with clinically diagnosed (not genetically confirmed) GCD1.8, 11 In 2003, six years following the identification of the TGFBI gene, we reported the first genetically confirmed GCD1 associated with a vortex pattern of corneal deposits in two of four affected individuals in a family.6 This was followed by a report that described an individual with genetically confirmed GCD1 with dystrophic deposits in a vortex pattern who, following PK, developed recurrent corneal deposits in a pattern consistent with classic GCD1.7
In the families that we report, the (p.(Arg555Trp)) TGFBI mutation was identified in the heterozygous states in all affected individuals, many of whom demonstrated a vortex pattern of both primary and recurrent dystrophic deposits.2 In both families, this vortex pattern was observed in both young and elderly individuals as well as in both genders. The size and number of the corneal opacities that made up the vortex pattern seemed to progress with age in both families, as has been previously described with the classic GCD1 phenotype.14 A majority (88%) of the affected individuals demonstrated the vortex pattern in the Mexican family, while only 20% in the Italian family had the vortex phenotype among those affected. While the difference in the prevalence of the vortex phenotype between the two families is likely caused by differences in genetic background and/or distinct environmental factors, intra-family variation (including observed phenotypic switch after surgical intervention) is indicative of a more complex cause.15
The development of the vortex phenotype of GCD1 may be explained by the presumed pathogenesis of other corneal disorders associated with vortex patterns of corneal deposits. Fabry disease is characterized by a pigmented opacification of the inferior corneal epithelial in a vortex pattern, termed cornea verticillata.16 This phenotype, also seen with amiodarone use, is thought to arise as a consequence of deposition of intralysosomal lipid inclusion bodies in the corneal epithelium in conjunction with the centripetal migration of corneal epithelial cells (limbus to central cornea).16, 17 As the majority of the TGFBI protein is produced by the corneal epithelial cells, it is reasonable to suggest that the vortex pattern observed in some GCD1 individuals is also a consequence of the migration pattern of the corneal epithelium.14, 18, 19 However, unlike in Fabry disease, the vortex phenotype in GCD1 is observed in a minority of GCD1 individuals. Moreover, not all of the GCD1 individuals who undergo PK develop a recurrent phenotype consistent with the original phenotype. These observations suggest a more complex mechanism in the pathogenesis of the two distinct phenotypes observed for GCD1.
As we and other investigators have reported that the presence of a second variant in TGFBI can alter the phenotype associated with the first, we screened all 17 exons of TGFBI in affected individuals from each family.20, 21 In the Mexican family, two common SNPs were identified in all affected individuals screened, with an allele frequency significantly higher than that in the population. However, as these SNPs were identified in the homozygous state in individuals with both the classic and vortex patterns of dystrophic deposits, these common sequence variants do not appear to be associated with the determination of the affected phenotype in this family. Similarly, as a common SNP was the only TGFBI coding region variant other than the c.1663C>T mutation identified in affected individuals with both the classic and vortex patterns of dystrophic deposits in the Italian family, second TGFBI coding region variants were not associated with the determination of the corneal phenotype in either of these families.
For the primary (i.e., prior to surgery) presentation of dystrophic TGFBI protein deposition, we propose that the appearance of either one of the phenotypes (classic or vortex) is dependent on both the timing of the expression of TGFBI protein by the corneal epithelium and the establishment of the centripetal migration pattern of the corneal epithelial cells, events that occur late in vertebrate development.22-27 If the timing of these two events in humans is typically such that expression of TGFBI protein occurs prior to the establishment of centripetal migration of the corneal epithelial cells, TGFBI protein deposition will occur in a “random” pattern, reflecting the random migration of corneal epithelial cells, and will lead to the classic phenotype. Alternatively, if centripetal epithelial cell migration is established prior to TGFBI protein expression, then TGFBI protein will deposit in a manner consistent with the centripetal migration of the corneal epithelium, leading to the variant vortex phenotype.
While the above reasonably explains the appearance of the primary phenotype, it does not explain the appearance of the recurrent phenotype following keratoplasty, which may appear in the form of the classic or vortex pattern of GCD1. It is well known that when recurrence occurs after PK in lattice corneal dystrophy, also a TGFBI dystrophy, the recurrent corneal deposits typically do not have the same branching appearance of the primary dystrophic deposits.28, 29 Penetrating and deep anterior lamellar keratoplasty result in replacement of the host keratocytes, which produce mutant TGFBI protein (albeit at lower levels than the corneal epithelial cells), with donor keratocytes that produce wild type TGFBI protein. This alters the interaction between the epithelial cells and keratocytes that has been shown to play an important role in the rate, location and morphology of dystrophic deposition, as observed in the aggregation of dystrophic deposits in the lamellar corneal interface following LASIK surgery and along corneal suture tracts in individuals with TGFBI dystrophies.30-32 It is likely that alterations in the interaction between epithelial cells and keratocytes, as well as potential differences in the composition of constitutively expressed and induced TGFBI protein, contribute to the altered morphology of the dystrophic deposits following keratoplasty.
Herein, we reported the first two large pedigrees with genetically confirmed GCD1 in which multiple affected members demonstrate a vortex pattern of dystrophic deposits. We propose that the clinical description of GCD1 be expanded to include this variant vortex form to ensure that clinicians are familiar with this less common manifestation of GCD1 and are therefore able to accurately diagnose affected patients with primary and recurrent GCD1.
Acknowledgments
The authors thank Avellino Laboratories for performing the TGFBI screening and creating the original family pedigree for the Italian family, which we modified for publication. We also thank Drs. Denise Loya-Garcia and Daniela Zapata-Gomez for referral and clinical assessment of the Mexican family.
Funding: National Eye Institute grants R01EY022082 (A.J.A.) and P30EY000331 (core grant to the Stein Eye Institute), an unrestricted grant from Research to Prevent Blindness (Stein Eye Institute), “Conde de Valenciana” foundation.
Footnotes
Conflicts of interest: None
References
- 1.Stone EM, Mathers WD, Rosenwasser GO, et al. Three autosomal dominant corneal dystrophies map to chromosome 5q. Nat Genet. 1994;6:47–51. doi: 10.1038/ng0194-47. [DOI] [PubMed] [Google Scholar]
- 2.Weiss JS, Moller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies--edition 2. Cornea. 2015;34:117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 3.Moller HU. Inter-familial variability and intra-familial similarities of granular corneal dystrophy Groenouw type I with respect to biomicroscopical appearance and symptomatology. Acta Ophthalmol (Copenh) 1989;67:669–677. doi: 10.1111/j.1755-3768.1989.tb04400.x. [DOI] [PubMed] [Google Scholar]
- 4.Mannis MJ, De Souza LB, Gross R. The stromal dystrophies. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. St Louis: Mosby; 1997. pp. 1043–1062. [Google Scholar]
- 5.Waring GO, 3rd, Rodrigues MM, Laibson PR. Corneal dystrophies. I. Dystrophies of the epithelium, Bowman's layer and stroma. Surv Ophthalmol. 1978;23:71–122. doi: 10.1016/0039-6257(78)90090-5. [DOI] [PubMed] [Google Scholar]
- 6.Aldave AJ, Yellore VS, Hwang DG. Atypical vortex pattern of corneal deposits in granular corneal dystrophy. Cornea. 2003;22:754–759. doi: 10.1097/00003226-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Han X, Huang D, et al. Analysis of TGFBI gene mutations in Chinese patients with corneal dystrophies and review of the literature. Mol Vis. 2010;16:1186–1193. [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons CJ, McCartney AC, Kirkness CM, et al. Granular corneal dystrophy. Visual results and pattern of recurrence after lamellar or penetrating keratoplasty. Ophthalmology. 1994;101:1812–1817. doi: 10.1016/s0161-6420(94)31096-7. [DOI] [PubMed] [Google Scholar]
- 9.Williams TD, Lyle WM. Granular corneal dystrophy: slitlamp biomicroscopic appearances in three generations of patients. Optom Vis Sci. 2001;78:79–84. doi: 10.1097/00006324-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bücklers M. Die erblichen Hornhautdystrophien. Bücherei des Augenarztes. 1938;3:1–143. [Google Scholar]
- 11.Weidle EG, Lisch W. Various forms of opacities of granular corneal dystrophy. Klin Monbl Augenheilkd. 1984;185:167–173. doi: 10.1055/s-2008-1054592. [DOI] [PubMed] [Google Scholar]
- 12.Zenteno JC, Correa-Gomez V, Santacruz-Valdez C, et al. Clinical and genetic features of TGFBI-linked corneal dystrophies in Mexican population: description of novel mutations and novel genotype-phenotype correlations. Exp Eye Res. 2009;89:172–177. doi: 10.1016/j.exer.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Munier FL, Korvatska E, Djemai A, et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997;15:247–251. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- 14.Han KE, Choi SI, Kim TI, et al. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog Retin Eye Res. 2016;50:67–88. doi: 10.1016/j.preteyeres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Aldave AJ. The genetics of the corneal dystrophies. Dev Ophthalmol. 2011;48:51–66. doi: 10.1159/000324077. [DOI] [PubMed] [Google Scholar]
- 16.Sodi A, Ioannidis A, Pitz S. Ophthalmological manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. [PubMed] [Google Scholar]
- 17.D'Amico DJ, Kenyon KR. Drug-induced lipidoses of the cornea and conjunctiva. Int Ophthalmol. 1981;4:67–76. doi: 10.1007/BF00139581. [DOI] [PubMed] [Google Scholar]
- 18.Escribano J, Hernando N, Ghosh S, et al. cDNA from human ocular ciliary epithelium homologous to beta ig-h3 is preferentially expressed as an extracellular protein in the corneal epithelium. J Cell Physiol. 1994;160:511–521. doi: 10.1002/jcp.1041600314. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar S, Meek KM, Ridgway AE, et al. Deposits and proteoglycan changes in primary and recurrent granular dystrophy of the cornea. Arch Ophthalmol. 1999;117:310–321. doi: 10.1001/archopht.117.3.310. [DOI] [PubMed] [Google Scholar]
- 20.Yamada N, Kawamoto K, Morishige N, et al. Double mutation (R124H, N544S) of TGFBI in two sisters with combined expression of Avellino and lattice corneal dystrophies. Mol Vis. 2009;15:974–979. [PMC free article] [PubMed] [Google Scholar]
- 21.Ann L, Abbouda A, Frausto R, et al. Variant lattice corneal dystrophy associated with compound heterozygous mutations in the TGFBI gene. Br J Ophthalmol (In Press) 2016 doi: 10.1136/bjophthalmol-2015-307602. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Zhao J, Chen L, et al. Abnormal epithelial homeostasis in the cornea of mice with a destrin deletion. Mol Vis. 2008;14:1929–1939. [PMC free article] [PubMed] [Google Scholar]
- 23.Schorderet DF, Menasche M, Morand S, et al. Genomic characterization and embryonic expression of the mouse Bigh3 (Tgfbi) gene. Biochem Biophys Res Commun. 2000;274:267–274. doi: 10.1006/bbrc.2000.3116. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi Y, Call MK, Liu CY, et al. Monoallelic expression of Krt12 gene during corneal-type epithelium differentiation of limbal stem cells. Invest Ophthalmol Vis Sci. 2010;51:4562–4568. doi: 10.1167/iovs.10-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson JW, Mikesh MF, Wheeler EF, et al. Developmental expression patterns of Beta-ig (betaIG-H3) and its function as a cell adhesion protein. Mech Dev. 2003;120:851–864. doi: 10.1016/s0925-4773(03)00165-5. [DOI] [PubMed] [Google Scholar]
- 26.Endo M, Zoltick PW, Chung DC, et al. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Ther. 2007;15:579–587. doi: 10.1038/sj.mt.6300092. [DOI] [PubMed] [Google Scholar]
- 27.Collinson JM, Morris L, Reid AI, et al. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- 28.Snead DR, Mathews BN. Differences in amyloid deposition in primary and recurrent corneal lattice dystrophy type 1. Cornea. 2002;21:308–311. doi: 10.1097/00003226-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Meisler DM, Fine M. Recurrence of the clinical signs of lattice corneal dystrophy (type I) in corneal transplants. Am J Ophthalmol. 1984;97:210–214. doi: 10.1016/s0002-9394(14)76092-1. [DOI] [PubMed] [Google Scholar]
- 30.Lemp MA. The surface of the corneal graft: in vivo color specular microscopic study in the human. Trans Am Ophthalmol Soc. 1989;87:619–657. [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TI, Roh MI, Grossniklaus HE, et al. Deposits of transforming growth factor-beta-induced protein in granular corneal dystrophy type II after LASIK. Cornea. 2008;27:28–32. doi: 10.1097/ICO.0b013e318156d36d. [DOI] [PubMed] [Google Scholar]
- 32.Han KE, Kim TI, Chung WS, et al. Clinical findings and treatments of granular corneal dystrophy type 2 (avellino corneal dystrophy): a review of the literature. Eye Contact Lens. 2010;36:296–299. doi: 10.1097/ICL.0b013e3181ef0da0. [DOI] [PubMed] [Google Scholar]