SUMMARY
Meniscal lesions are common problems in orthopaedic surgery and sports medicine, and injury or loss of the meniscus accelerates the onset of knee osteoarthritis. Despite a variety of therapeutic options in the clinics, there is a critical need for improved treatments to enhance meniscal repair. In this regard, combining gene-, cell-, and tissue engineering-based approaches is an attractive strategy to generate novel, effective therapies to treat meniscal lesions. In the present work, we provide an overview of the tools currently available to improve meniscal repair and discuss the progress and remaining challenges for potential future translation in patients.
Keywords: Meniscus, meniscal repair, gene therapy, cell therapy, tissue engineering
Principles of meniscal repair
Structure and function of the meniscus and of the meniscal roots
The menisci are semilunar fibrocartilage structures that transmit joint forces and increase stability, facilitate nutrition, provide lubrication for the articular cartilage, and promote knee proprioception (Fig. 1). Type-I collagen is the predominant collagen of the meniscal extracellular matrix (ECM)1. Collagen organization is paramount for meniscus mechanical function, with circumferential oriented bundles circumnavigating the c-shaped structure enabling efficient transfer of load in this direction. Additionally, interspersed radially oriented “tie” fibers interdigitate through this circumferential network and act to bind this primary collagen organization together2. Other components of the meniscus ECM chiefly include proteoglycans and glycoproteins. The major role of the proteoglycans is to retain water (about 65–75% of its total weight) within the meniscal tissue. The interactions between the macromolecular ECM and the water enable the important viscoelastic properties of the menisci3.
Fig. 1.
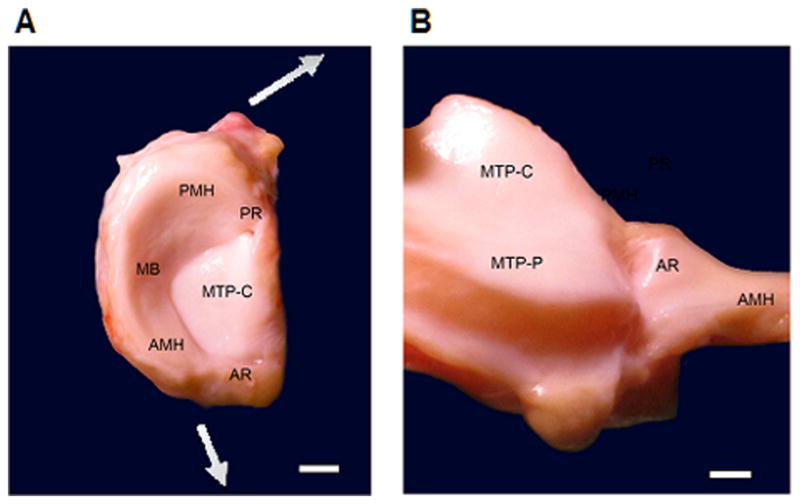
(A) Normal human left medial meniscus attached to the medial tibial plateau. The meniscal body (MB) and the anterior (AMH) and posterior horn (PMH) of the meniscus are anchored into the tibial intercondylar region by the meniscal roots. The anterior root (AR) of the medial meniscus inserts into the anterior intercondylar area while its posterior root (PR) inserts on the posterior medial intercondylar eminence of the tibia. The central part of the medial tibial plateau (MTP-C) is not covered by the meniscus. Radially directed forces are presented by the arrows. Scale bar: 1 cm. (B) Visualization of the anterior root (AR) of the medial meniscus and its insertion into the anterior intercondylar area by pulling the medial meniscus towards the right side of the picture. The tibial aspect of the anterior horn of the medial meniscus (AMH) is shown. The peripheral part of the anterior medial tibial plateau (MTP-P) is now exposed, together with the central part of the medial tibial plateau (MTP-C). Scale bar: 0.5 cm.
The inner (central) parts of the menisci are mainly comprised of fibrochondrocytes and contain a higher proportion of type-II collagen and proteoglycans, whereas the outer (peripheral) region contains more fibroblastic cells and contains type-I collagen and a lower proteoglycan content4. Meniscal vascular supply originating from branches of the geniculate arteries is restricted to the peripheral 10–25% of the meniscal tissue5. If a small tear is present in this “red zone”, natural repair may occur. The nerve fibers mostly follow these blood vessels, with the two horns of the meniscus being the most richly innervated. The central (inner) third of the meniscus, called the “white zone”, is avascular and has no nerve supply. Therefore, no spontaneous healing of tears in this region occurs, although meniscal repair may be attempted (e.g. simultaneously with the reconstruction of a torn anterior cruciate ligament) also in the intermediate red-white or white-white zone applying novel suture techniques and/or anabolic factors6.
The meniscal roots are four ligamentous structures that firmly anchor the anterior and posterior horns of the menisci into the tibial intercondylar region7. The strong attachment of the medial meniscus to the medial collateral ligament is the reason for its decreased mobility during joint motion compared with the lateral meniscus, which is not attached to the lateral collateral ligament nor to the joint capsule at the site of the popliteus tendon hiatus8.
The menisci play a critical biomechanical role, contributing to joint congruence, load distribution, and stability in the knee9. Importantly, damage to the meniscus following trauma or with degeneration is associated with altered joint function that often leads to progressive joint degeneration10. The critical biomechanical functions of the menisci are provided by their geometry, attachments, and the unique mechanical properties of the tissue. The geometry and physical attachment of the menisci within the joint provide important restraints that govern the overall mechanical response and movement of the menisci to applied forces and provide for the complex kinematics of the tibiofemoral joint during the activities of daily living.
Due to the unique geometry of the menisci, loading of the knee joint gives rise to a vertical and radially-directed force component, which tends to outwardly displace the menisci. However, the meniscal attachments restrain this displacement, and give rise to a large circumferentially-directed force, and associated tensile “hoop stress” within the tissue. Under normal physiological loading of the knee, the menisci (and cells within the menisci) experience a complex mechanical environment consisting of large tensile, compressive, and shear stresses. Significant structural differences exist between the medial and lateral femorotibial compartments in the knee, including both the tibial plateaus and the menisci.
Meniscal lesions
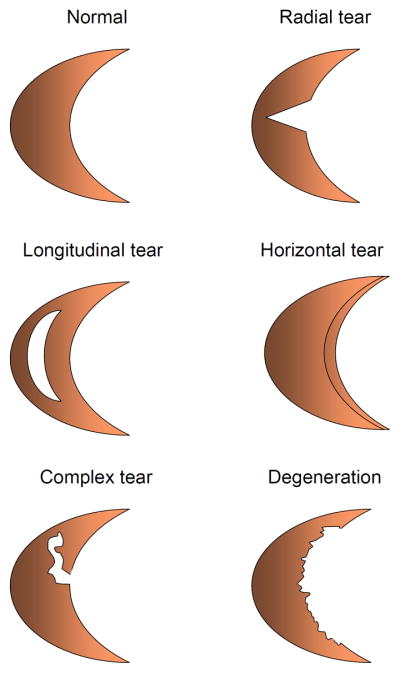
The meniscus can tear either from trauma or as a consequence of age-related degenerative changes11. Meniscal tears include radial, longitudinal, horizontal, complex and degenerative tears (Fig. 2–5). They are the most prevalent intraarticular knee injuries and the most frequent cause of orthopaedic surgical procedures, representing a significant risk factor for the development of knee osteoarthritis (OA)12. While traumatic tears can occur at any age (even in children), patients over the age of 50–60 are more susceptible to degenerative meniscal tears. Traumatic tears have a better prognosis for reconstructive surgical options. Longitudinal and horizontal tears are amenable to repair, while symptomatic traumatic radial tears are often treated with partial meniscectomy. Of note, the extent of meniscal resection correlates with alterations in the stress distribution in the joint13, as well as the degree of OA development14. Medial meniscal injuries are much more common compared to those in the lateral meniscus15, potentially because of its lesser mobility16. However, lateral meniscus tears are associated more frequently with less favorable clinical outcomes than tears of the medial meniscus, including OA development and rapid chondrolysis14. As meniscal root tears disrupt the circumferential fibers that provide hoop strength, meniscal extrusion with altered biomechanical properties of the meniscus is a critical clinical consequence17–19. Importantly, recent studies have shown that meniscal extrusion may be closely associated with progressive cartilage loss and accelerated OA20. Furthermore, meniscal cysts may occur in both the lateral and medial sides. These are commonly associated with horizontal meniscal tears, and result in a void space within the meniscus21. The need for tailored tissue engineering-based approaches to enhance the healing of the meniscus is similar for all types of meniscal injuries.
Fig. 2.
Classification of meniscal tears. Meniscal tears include radial, longitudinal, horizontal, complex and degenerative tears.
Fig. 5.
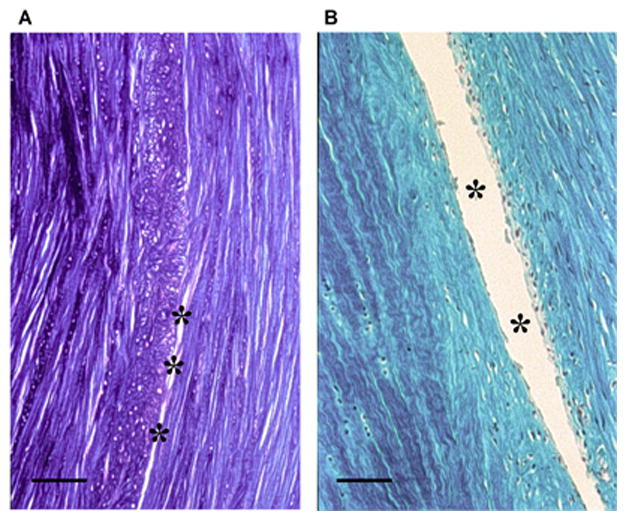
Histological evaluation of a complex traumatic canine meniscal tear model. A full-thickness circumferential tear was performed as described in Figure 4. Formalin-fixed, paraffin-embedded sections (5 μm) were taken in the axial plane (12 weeks) and stained with toluidine blue (A) and trichrome (B), highlighting the circumferentially aligned collagen fibers. Evidence of incomplete healing in the tear is demonstrated by the asterisks in the mid-substance region. Scale bar: 500 μm.
An important factor that may influence meniscal repair is the inflammatory environment within the joint22. In particular, inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), are elevated following injury or with OA in the knee joint23,24. Importantly, these cytokines can increase tissue degradation, with increased matrix metalloproteinase (MMP) activity, sulfated glycosaminoglycan (S-GAG) release, and the presence of pro-inflammmatory mediators, such as nitric oxide and prostaglandin E2. These cytokines have also been shown to strongly inhibit integrative meniscal repair in vitro, by decreasing cell accumulation and tissue formation at the meniscal repair interface, ultimately compromising the shear strength of repair25,26. Initial acute exposure to IL-1 for 1–3 days potently suppresses meniscal repair for at least 28 days26, suggesting that the initial inflammatory environment in a joint post-injury may have long-term degenerative effects. In addition, IL-1 and TNF-α activate other degradative and pro-inflammatory pathways in the meniscus and other joint tissues that contribute to joint degeneration27.
Natural repair and current surgical options
Preserving as much meniscal tissue as possible is the current approach when performing arthroscopic partial meniscectomy, as total meniscectomy alone inevitably leads to osteoarthritic degeneration in the long-term14,28,29. Partial meniscal resection is generally associated with a lesser degree of OA development, although it sometimes may occur in a rapid manner30. Increased knowledge on the anatomy and function of the meniscus has led to the development of sophisticated techniques for meniscal repair and reconstruction to preserve or restore meniscus function31,32. For the purpose of this review, we refer to meniscal repair when torn parts of the meniscus are put back and held in apposition, and refer to meniscal reconstruction when missing parts of the meniscus or the entire meniscus is replaced by an allograft or a meniscal substitute.
As a result of the differences in vascularization and blood supply, tears in the peripheral, vascularized portion of the meniscus may be successfully repaired using a variety of operative procedures, such as sutures33. Lesions in the central, avascular area that has a poor healing capacity34,35 may be treated by arthroscopic partial meniscectomy. Meniscal reconstruction is based on the use of allografts and substitutes. The use of meniscal allografts36,37 is a therapeutic option especially for young patients with tibiofemoral joint pain and a history of meniscectomy in a normally aligned, stable joint without severe degenerative changes of the articular cartilage.
Meniscal allograft transplantation improves pain and function in the short and intermediate term33. Meniscal substitutes have been shown to overcome the problems of allografting and to promote repair of segmental meniscal defects, for example, resulting from a partial meniscectomy38. These replacement devices include porous bovine type-I collagen/GAG matrices39 or polyurethane40. More recently, artificial biomaterials have shown initial success in restoring meniscal function and preventing degenerative changes following meniscectomy41.
Strategies to improve meniscal repair
Preservation of meniscal tissue is paramount for long-term joint function and therefore the decisive guiding principle in the treatment of meniscal defects33,42. While there are no established criteria for a successful meniscal replacement strategy, tests of stiffness in tension and compression are commonly used to assess the similarity of mechanical properties of repair tissue to the native tissue. Other outcomes may include pullout strength of meniscal attachment, cycles of loading to failure (fatigue), subsidence, and friction property. When mechanical integrity of meniscal repair strategies cannot be achieved, experimental approaches for replacement or regeneration of meniscal tissue are required. These may include the administration of cells or tissues, candidate factors or gene transfer vectors, and/or biocompatible materials (Fig. 6).
Fig. 6.
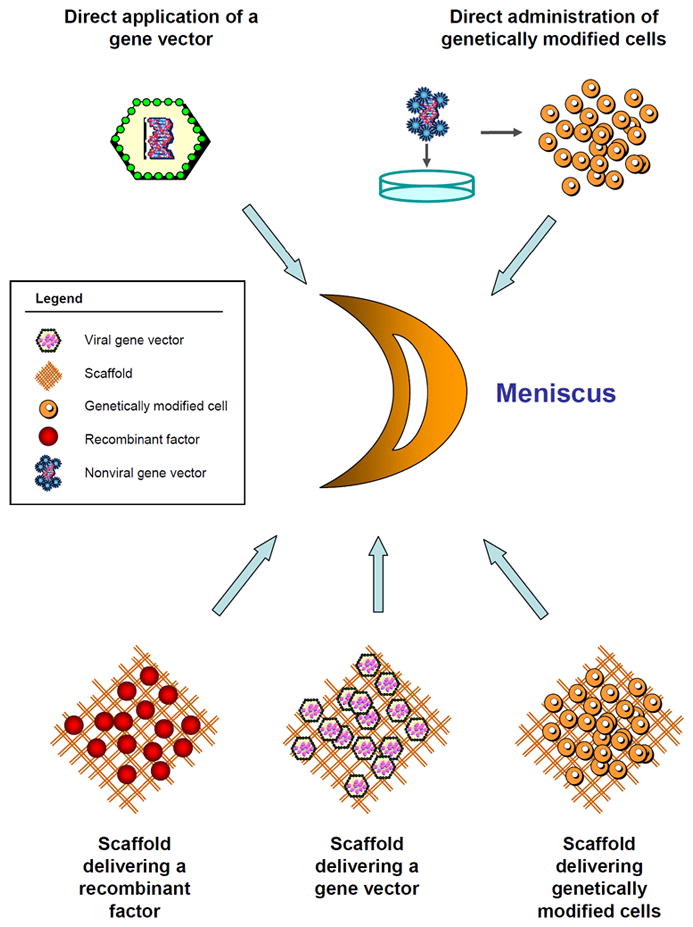
Principles of gene-, cell-, and tissue engineering-based approaches for meniscal repair. Schematic view of a bucket-handle meniscal tear. Gene-, cell-, and tissue engineering-based approaches for meniscal repair include the direct in vivo administration of a (viral) gene transfer vector or of ex vivo genetically modified cells within a meniscal lesion, and the implantation of biocompatible and bioactive materials in the form of scaffolds that deliver either a recombinant factor, gene transfer vector, or ex vivo genetically modified cells that may serve the dual role of providing the therapeutic factor and repopulating the lesion.
Target cells and tissues
Different cells or tissues may be targeted in approaches that aim at improving meniscal repair:
isolated meniscal fibrochondrocytes that may repopulate the injured meniscus or enhance integrative repair of a tear43–52,
progenitor cells that may be induced towards a fibrochondrocyte-like phenotype, the most studied being mesenchymal stem cells (MSCs) from the bone marrow, adipose tissue, synovium, periosteum, trabecular bone, umbilical cord blood, amniotic fluid, Wharton’s jelly, or skeletal muscle48,50,53–59; also notably, embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have recently become a focus of stem cell-based regenerative medicine60.
Candidate factors
Various factors have been identified that have a therapeutic potential to enhance meniscal repair by:
activating cell proliferation and survival: platelet-derived growth factor (PDGF)61, basic fibroblast growth factor (FGF-2)62, insulin-like growth factor I (IGF-I)63, transforming growth factor beta (TGF-β)22,64,65,
modulating cell migration: TGF-β22,65, stromal cell derived factor-1 (SDF-1)66,
stimulating anabolic pathways: PDGF67,68, TGF-β64,67, bone morphogenetic protein 7 (BMP-7)67, hepatocyte growth factor (HGF)68, FGF-269, IGF-I63,
inhibiting inflammatory and catabolic pathways: IL-1 receptor antagonist (IL-1Ra)25, TNF antibody25, inhibitors of MMPs70, TGF-β22,
activating biomechanical signalling pathways that are pro-anabolic or anti-catabolic71–73, and
combinations of such approaches: PDGF/HGF68.
Direct application of recombinant factors to meniscal lesions is generally hindered by their short pharmacologic half-lives, highlighting the necessity for improving treatment by using gene delivery procedures and/or biomaterials coated with such factors to allow for sustained therapeutic activities.
Gene transfer vectors
Different classes of gene vehicles (nonviral and viral vectors) are currently available to genetically modify relevant target cells and tissues, with specific advantages and limitations listed in Table 1.
Table 1.
Overview of currently used gene transfer vectors for meniscal repair
| Classes | Integration | Advantages | Shortcomings |
|---|---|---|---|
| NV | no | . nontoxic . large capacity |
. relatively low efficiency . short-term expression |
| AdV | no | . high efficiency . large capacity |
. possible replication . immunogenicity/toxicity . short-term expression |
| RV/LV | yes | . high efficiency . relatively large capacity . long-term expression |
. possible replication . insertional mutagenesis |
| HSV | no | . high efficiency . large capacity |
. possible replication . toxicity . short-term expression |
| rAAV | mostly episomal | . high efficiency . long-term expression . low immunogenicity/toxicity |
. difficult to produce . size limitation . serotype specificity |
AdV: adenoviral vectors; HSV: herpes simplex virus vectors; LV: lentiviral vectors; NV: nonviral vectors; rAAV: recombinant adeno-associated virus vectors; RV: retroviral vectors.
Nonviral vectors
Nonviral vectors are safe gene vehicles as they avoid the risk of acquiring replication competence inherent to viral vectors. These vectors can be repeatedly administered, but they mediate relatively low and short-term transgene expression (~10–38% transfection efficiency in meniscal cells and 14–25% in progenitor cells)43,50,54,60.
Adenoviral vectors
Adenoviral vectors allow for high transduction efficiencies and levels of transgene expression in vitro (~80–100% efficiency in meniscal and progenitor cells) but they are immunogenic in vivo and promote very short-term efficacy (1 to 2 weeks maximum)43,46,74.
Retro-/lentiviral vectors
Retroviral vectors allow for long-term maintenance of their transgenes by integration into the host cell genome. Nevertheless, such integration may lead to insertional mutagenesis and tumor gene activation. These vectors can only transduce dividing cells and at a relatively low efficacy (~20–30% efficiency before cell selection, reaching up to 90% upon selection)43,46,74.
Lentiviral vectors have certain advantages over other viral approaches as they can integrate into the genome of nondividing cells and show high levels of transduction efficacy (up to 90% in progenitor cells)55,56, but concerns remain for their potential for insertional mutagenesis.
Herpes simplex virus (HSV) vectors
HSV are large vectors that can deliver long transgenes in almost all known cell types, including nondividing cells (~70% transduction efficiency in meniscal cells). However, these vectors are relatively toxic and provide only transient transgene expression43.
Recombinant adeno-associated virus (rAAV) vectors
rAAV vectors are derived from a replication-defective human parvovirus. These vectors are less immunogenic than adenoviral vectors and more effective than nonviral and retro-/lentiviral vectors for the transduction of both dividing and nondividing cells (up to 80% transduction efficiency in meniscal cells and up to 90% in progenitor cells), allowing for prolonged transgene expression. rAAV are maintained as stable, episomal constructs in their host, while permitting gene transfer in situ through the dense ECM due to their small size (20 nm)43,47,49,52,53. Of note, the use of trans-splicing rAAV systems has allowed for the enhancement of the size capacity of the vectors. For all these reasons, rAAV has become a vector of choice for clinical applications.
Biocompatible materials
Numerous tissue engineering strategies have emerged for the replacement of meniscal tissue75, based on the use of acellular76 or cell-seeded matrices77.
Several approaches for treating meniscal defects concentrate on meniscal replacement with acellular matrices78,79, avoiding possible risks associated with transplantation of human allografts, such as high failure rate or immunoreaction and disease transmission80. Different types of meniscal substitutes, such as autologous tissue81–83, decellularized allogenic and xenogenic grafts76,84,85, collagen grafts86, permanent synthetic scaffolds38, silk fibroin scaffolds87,88, and biodegradable scaffolds based on small intestine submucosa89, poly-lactic acid (PLA) or poly-glycolic acid (PGA)90,91, have been used in experimental and clinical studies.
However, after transplantation of acellular meniscal constructs into defects, the transplants are populated by synovial fibroblasts, resulting in a scar tissue with poor biomechanical properties92. Therefore, some tissue engineering approaches focus on additional cell-seeding techniques prior to transplantation38,93. Meniscal cells94, articular chondrocytes95, synovial fibroblasts96, and MSCs97 have been proposed as potential cell sources and have been cultivated in vivo and in vitro on various matrices. In addition, different environmental factors, such as growth factors, have been used to optimize cell proliferation in vitro98.
Of note, different biomaterials have been tested in experimental settings in vitro, in situ, and in vivo for meniscal repair applications concomitantly with the use of cell- and gene-based approaches, including alginate49,99, type-I collagen solutions74 or type-I collagen/GAG matrices48, and PGA scaffolds45.
Strategies
Gene therapy may be applied directly or in combination with various cell- or tissue engineering-based approaches for meniscal repair (Fig. 6):
directly injecting a gene transfer vector,
administrating genetically modified cells,
implanting a biocompatible material that delivers a recombinant factor,
applying autologous platelet-rich plasma or fibrin clots,
providing a biomaterial that delivers a gene transfer vector, or
transplanting a material seeded with cells that have been genetically modified.
Cell-free strategies are less invasive, but the presence of cells in the therapeutic composition might be necessary as an effective means to repopulate meniscal lesions, particularly given the scarcity of fibrochondrocytes in mature tissue.
Evidence for gene transfer in vitro
All of the gene transfer vectors mentioned above have been successfully employed to target most of the cells relevant for meniscal repair in vitro including meniscal fibrochondrocytes43–52,74 and various types of progenitor cells53,54,60, and provide varying gene transfer efficiencies in vitro:
nonviral vectors: ~ 9–38% efficiency in meniscal cells (over less than a week)43,99 and 14–25% in progenitor cells (bone marrow, perichondrium, umbilical cord blood) (between days 2 and 14)54,
adenoviral vectors: up to 100% in meniscal cells (less than a week)43,46,48,74,
retroviral vectors: up to 90% upon selection of meniscal cells43,44,46,74,
lentiviral vectors: 10–90% in progenitor cells (bone marrow, iPSCs) depending on the cell source and time points evaluated (2–15 days)60,
HSV vectors: ~ 70% in meniscal cells (less than a week)43,
rAAV vectors: ~ 80% in meniscal cells (only 2 days tested)47 and up to 90% in progenitor cells (bone marrow) (21 days)53.
Various pathways as described below have been targeted to enhance the reparative capacities of both cell types via therapeutic gene delivery approaches (Table 2). Stimulation of proliferative activities in meniscal and progenitor cells (bone marrow) has been demonstrated following gene transfer of IGF-I50, FGF-249,51, and TGF-β without52 or in a type-I collagen/GAG matrix48 for up to 21 days48,49,53 using nonviral50, adenoviral48, and rAAV vectors49,52,53. Successful activation of anabolic processes has been reported in meniscal and progenitor cells (bone marrow) upon gene transfer of IGF-I or TGF-β without biomaterial44,52 or with a type-I collagen/GAG matrix for up to 21 days48 using adenoviral48, retroviral44, and rAAV vectors52. One potential therapeutic approach for enhancing meniscal repair may be through the controlled inhibition of pro-inflammatory mediators, either through direct protein delivery25 or through gene therapy approaches. Successful co-delivery of different candidate genes has not been reported in meniscal or progenitor cells to date, but this might be achieved as evidenced in articular chondrocytes100.
Table 2.
Overview of current in vitro gene-, cell-, and tissue engineering approaches for meniscal repair
| Vector | Gene | Biomaterial | Cells | Effects | References |
|---|---|---|---|---|---|
| NV | IGF-I | - | meniscal cells | cell proliferation | 50 |
| FGF-2 | alginate | meniscal cells | cell proliferation | 51 | |
| AdV | TGF-β | type-I collagen/GAG matrix | meniscal and progenitor cells | cell proliferation matrix synthesis | 48 |
| RV | TGF-β | - | meniscal cells | matrix synthesis | 44 |
| rAAV | FGF-2 | - | meniscal and progenitor cells | cell proliferation | 49,53 |
| TGF-β | - | meniscal cells | cell proliferation matrix synthesis | 52 |
AdV: adenoviral vectors; FGF-2: basic fibroblast growth factor; GAG: glycosaminoglycans; IGF-I: insulin-like growth factor I; NV: nonviral vectors; PGA: poly-glycolic acid; rAAV: recombinant adeno-associated virus vectors; RV: retroviral vectors; TGF-β: transforming growth factor beta.
Evidence for gene transfer in situ and in vivo
Gene transfer in situ
Different gene transfer vectors have been tested via indirect (cell-based)48,51,74 and direct (cell-free)49,52,74 experimental procedures to target meniscal tissue, including:
nonviral vectors: ~ 10% transfection efficiency in meniscal cells transplanted in injured meniscal explants (8 days)51,
adenoviral vectors: ~ 80% in meniscal and progenitor cells (bone marrow) transplanted in injured meniscal explants using a type-I collagen/GAG matrix (21 days)48 and ~ 40% by direct injection in intact meniscal explants (several weeks)74,
retroviral vectors: ~ 30% in meniscal cells transplanted in intact meniscal explants using a type-I collagen solution (several weeks)74,
rAAV vectors: ~ 70–75% by direct injection in intact or injured meniscal explants (15 days)49,52.
Therapeutic gene transfer in situ has been attempted by transplanting meniscal and progenitor cells (MSCs) modified by a TGF-β rAAV vector52 or with an adenoviral vector using type-I collagen/GAG matrix in injured bovine meniscal explants48 or by direct injection of rAAV FGF-2 and TGF-β vectors in human meniscal lesions49,52 leading to an enhanced repair of the treated lesions over ~ 15–21 days (Table 3).
Table 3.
Overview of current gene-, cell-, and tissue engineering approaches for meniscal repair in situ and in vivo
| System | Vector | Gene | Biomaterial | Cells | Effects | References |
|---|---|---|---|---|---|---|
| In situ | AdV | TGF-β | type-I collagen/GAG matrix | meniscal and progenitor cells | repair of meniscal lesions | 48 |
| rAAV | FGF-2 | - | - | cell proliferation, contraction, repair of meniscal lesions | 49 | |
| TGF-β | - | - | cell proliferation, contraction, repair of meniscal lesions | 52 | ||
| In vivo | NV | IGF-I | alginate | progenitor cells | repair of meniscal lesions | 50 |
| AdV | HGF | PGA | meniscal cells | repair of meniscal lesions, vascularization | 45 |
AdV: adenoviral vectors; FGF-2: basic fibroblast growth factor; GAG: gylcosaminoglycan; HGF: hepatocyte growth factor; IGF-I: insulin-like growth factor I; NV: nonviral vectors; PGA: poly-glycolic acid; rAAV: recombinant adeno-associated virus vectors; TGF-β: transforming growth factor beta.
Gene transfer in vivo
In vivo most of the gene transfer vectors have also been applied by indirect46,50,74 and direct47,74 approaches to target the meniscus or to repair meniscal lesions, such as:
nonviral vectors: ~ 22% transfection efficiency in meniscal cells transplanted in meniscal lesions using alginate (2 days)99,
adenoviral vectors: ~ 40% by direct injection in meniscal lesions (several weeks)74,
retroviral vectors: ~ 20% in meniscal cells transplanted in meniscal lesions using a type-I collagen solution (several weeks)74 or ~ 50% by meniscal allograft transplantation (several weeks)46,
rAAV vectors: ~ 50% by direct injection in meniscal lesions (at least 20 days)47.
Therapeutic gene transfer in vivo has been performed by transplantation of meniscal cells modified by an HGF adenoviral vector using a PGA scaffold in an athymic nude mouse model45 or by progenitor cells (MSCs) modified with an IGF-I nonviral vector using alginate in goat meniscal lesions50, leading to an enhanced repair of the treated lesions for up to 16 weeks (Table 3). In general, more information will be needed on the possible deleterious effects of the different approaches especially when viral vectors are being manipulated in vivo for a safe future application in the patient.
Conclusions
Gene therapy - alone or in combination with cell or tissue engineering-based strategies - provides attractive approaches to enhance the repair of meniscal lesions in light of significant advances in experimental research in cell biology, molecular biology (therapeutic candidate factors and genes), biomaterials, and translational science. While there is an accumulating body of preclinical evidence showing the benefits of gene therapy to treat meniscal injury and various completed or ongoing clinical protocols using diverse scaffolds, implants, or biological compounds (www.clinicaltrials.gov), no trial of meniscal gene therapy has been initiated to the best of our knowledge. Among the open questions, the best suited candidate gene(s) and vector and the most adapted cell source and scaffold have to be clearly identified, as still relatively little information is available in experimental models in vivo. Furthermore, translational animal models need to specifically reflect the different lesion types, etiologies, and locations as seen in the clinical situation. It will also be essential to take into account that the composition will need to meet the challenging requirements of regulatory organizations prior to enrollment in a patient protocol. In this regard, combined efforts between scientists, clinicians, industry, and regulatory organizations will be necessary to tackle the question of treating such lesions in patients.
Fig. 3.
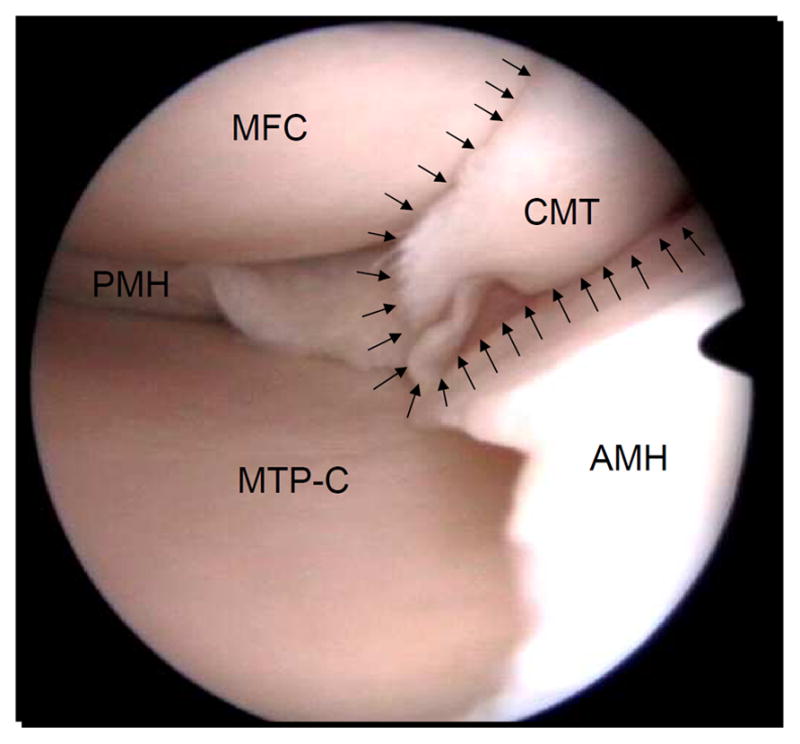
Traumatic meniscal tear. Arthroscopic view of a complex traumatic tear of the medial meniscus in a 29-year old man. The round medial femoral condyle (MFC) can be seen in the top left side of the picture, and the corresponding central part of the medial tibial plateau (MTP-C) on the bottom left side of the picture. Note the macroscopic good aspect of the articular cartilage in the medial femorotibial compartment. In the middle right, the complex rupture pattern of the meniscal tear (CMT) located in the pars intermedia can be appreciated (arrows), in part obscuring the view of the medial femoral condyle. The posterior horn of the medial meniscus (PMH) is shown on the left side of the picture and the anterior horn of the medial meniscus (AMH) extends on the right side of the picture.
Fig. 4.
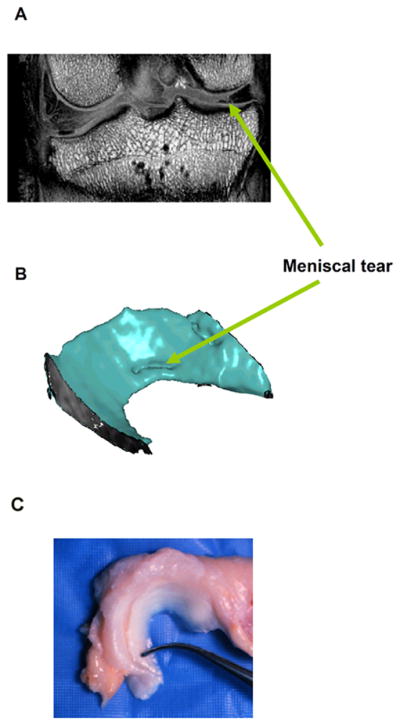
Magnetic resonance imaging (MRI) of a complex traumatic canine meniscal tear model. A full-thickness circumferential tear (1/3 of the circumferential length) was initiated at at the posterior aspect of the meniscus and at 2–3 mm from the menisco-synovial junction (12 weeks). (A) A coronal MRI demonstrates the meniscal tear in the central (white-white) zone. Excised menisci were placed within a birdcage RF transmitter-receiver coil for imaging at 9.4 T (Oxford Instrument). The scan volume was 125 cm3 with a resolution of 0.02 cm/pixel. Serial MRI sections were processed in MATLAB to generate a three-dimensional reconstruction of the meniscus as shown. (B) Three-dimensional (3-D) MRI reconstruction of the meniscal tear. High resolution, 3-D MRI scans were performed on intact canine joints following sacrifice. Joints were imaged within a 7.1 T (300 MHz, 85 gauss/cm2) at up to 5123 isotropic resolution. (C) Normal canine meniscus.
Acknowledgments
Research described in this review was supported in part by grants from the German Research Society (Deutsche Forschungsgemeinschaft), the German Osteoarthritis Foundation (Deutsche Arthrose-Hilfe), National Institutes of Health Grants (AR50245, AR48852, AG15768, AG46927, AR48182, AR047442, AR065956, and AR056624), the Nancy Taylor Foundation for Chronic Diseases, the Collaborative Research Center, AO Foundation, Davos, Switzerland, the Arthritis Foundation, and a VA Rehabilitation Research Service Award.
Footnotes
Author contributions
All authors were fully involved in the preparation of this manuscript and approved the final version.
Competing interest statement
The authors have no potential conflict of interest to disclose.
References
- 1.Bullough PG, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52:564–7. [PubMed] [Google Scholar]
- 2.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990:8–18. [PubMed] [Google Scholar]
- 3.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13:548–60. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DE, Arnoczky SP, Warren RF. Meniscal repair. Clin Sports Med. 1991;10:529–48. [PubMed] [Google Scholar]
- 6.James EW, LaPrade CM, Feagin JA, LaPrade RF. Repair of a complete radial tear in the midbody of the medial meniscus using a novel crisscross suture transtibial tunnel surgical technique: a case report. Knee Surg Sports Traumatol Arthrosc. 2015;23:2750–5. doi: 10.1007/s00167-014-3089-z. [DOI] [PubMed] [Google Scholar]
- 7.Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188:W446–50. doi: 10.2214/AJR.06.0509. [DOI] [PubMed] [Google Scholar]
- 8.Ullrich K, Krudwig WK, Witzel U. Posterolateral aspect and stability of the knee joint. I. Anatomy and function of the popliteus muscle-tendon unit: an anatomical and biomechanical study. Knee Surg Sports Traumatol Arthrosc. 2002;10:86–90. doi: 10.1007/s00167-001-0268-5. [DOI] [PubMed] [Google Scholar]
- 9.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664–70. [PubMed] [Google Scholar]
- 11.Englund M, Lohmander LS. Patellofemoral osteoarthritis coexistent with tibiofemoral osteoarthritis in a meniscectomy population. Ann Rheum Dis. 2005;64:1721–6. doi: 10.1136/ard.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Bedi A, Kelly N, Baad M, Fox AJ, Ma Y, Warren RF, et al. Dynamic contact mechanics of radial tears of the lateral meniscus: implications for treatment. Arthroscopy. 2012;28:372–81. doi: 10.1016/j.arthro.2011.08.287. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 15.Lengsfeld M, Rudig L, von Issendorff WD, Koebke J. Significance of shape differences between medial and lateral knee joint menisci for functional change of position. Unfallchirurgie. 1991;17:309–15. doi: 10.1007/BF02588301. [DOI] [PubMed] [Google Scholar]
- 16.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193:161–78. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papalia R, Vasta S, Franceschi F, D’Adamio S, Maffulli N, Denaro V. Meniscal root tears: from basic science to ultimate surgery. Br Med Bull. 2013;106:91–115. doi: 10.1093/bmb/ldt002. [DOI] [PubMed] [Google Scholar]
- 18.Bloecker K, Wirth W, Hunter DJ, Duryea J, Guermazi A, Kwoh CK, et al. Contribution of regional 3D meniscus and cartilage morphometry by MRI to joint space width in fixed flexion knee radiography--a between-knee comparison in subjects with unilateral joint space narrowing. Eur J Radiol. 2013;82:e832–9. doi: 10.1016/j.ejrad.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Emmanuel K, Quinn E, Niu J, Guermazi A, Roemer F, Wirth W, et al. Quantitative measures of meniscus extrusion predict incident radiographic knee osteoarthritis - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24:262–9. doi: 10.1016/j.joca.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloecker K, Wirth W, Guermazi A, Hunter DJ, Resch H, Hochreiter J, et al. Medial meniscal extrusion relates to cartilage loss in specific femorotibial subregions- data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2015;67:1545–52. doi: 10.1002/acr.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowden CH, 3rd, Barber FA. Meniscal cysts: treatment options and algorithm. J Knee Surg. 2014;27:105–11. doi: 10.1055/s-0033-1353995. [DOI] [PubMed] [Google Scholar]
- 22.McNulty AL, Guilak F. Integrative repair of the meniscus: lessons from in vitro studies. Biorheology. 2008;45:487–500. [PMC free article] [PubMed] [Google Scholar]
- 23.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001:S108–15. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 24.McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31:1039–45. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum. 2007;56:3033–42. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 26.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res. 2008;26:504–12. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Berg WB, Joosten LA, Kollias G, van De Loo FA. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999;58(Suppl1):I40–8. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salata MJ, Gibbs AE, Sekiya JK. A systematic review of clinical outcomes in patients undergoing meniscectomy. Am J Sports Med. 2010;38:1907–16. doi: 10.1177/0363546510370196. [DOI] [PubMed] [Google Scholar]
- 29.Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515–24. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- 30.Sonnery-Cottet B, Archbold P, Thaunat M, Carnesecchi O, Tostes M, Chambat P. Rapid chondrolysis of the knee after partial lateral meniscectomy in professional athletes. Knee. 2014;21:504–8. doi: 10.1016/j.knee.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Barber-Westin SD, Noyes FR. Clinical healing rates of meniscus repairs of tears in the central-third (red-white) zone. Arthroscopy. 2014;30:134–46. doi: 10.1016/j.arthro.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Grant JA, Miller BS, Mirza FM, Gagnier JJ. A systematic review of the psychometric properties of patient-reported outcome instruments for use in patients with rotator cuff disease. Am J Sports Med. 2015;43:2572–82. doi: 10.1177/0363546514565096. [DOI] [PubMed] [Google Scholar]
- 33.Heckmann TP, Barber-Westin SD, Noyes FR. Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome. J Orthop Sports Phys Ther. 2006;36:795–814. doi: 10.2519/jospt.2006.2177. [DOI] [PubMed] [Google Scholar]
- 34.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11:131–41. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 35.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnoczky SP, Warren RF, McDevitt CA. Meniscal replacement using a cryopreserved allograft. An experimental study in the dog. Clin Orthop Relat Res. 1990:121–8. [PubMed] [Google Scholar]
- 37.Kohn D, Verdonk R, Aagaard H, Seil R, Dienst M. Meniscal substitutes--animal experience. Scand J Med Sci Sports. 1999;9:141–5. doi: 10.1111/j.1600-0838.1999.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 38.van Tienen TG, Hannink G, Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28:143–56. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79:1770–7. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 40.de Groot JH, de Vrijer R, Pennings AJ, Klompmaker J, Veth RP, Jansen HW. Use of porous polyurethanes for meniscal reconstruction and meniscal prostheses. Biomaterials. 1996;17:163–73. doi: 10.1016/0142-9612(96)85761-9. [DOI] [PubMed] [Google Scholar]
- 41.Zur G, Linder-Ganz E, Elsner JJ, Shani J, Brenner O, Agar G, et al. Chondroprotective effects of a polycarbonate-urethane meniscal implant: histopathological results in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2011;19:255–63. doi: 10.1007/s00167-010-1210-5. [DOI] [PubMed] [Google Scholar]
- 42.Noyes FR, Heckmann TP, Barber-Westin SD. Meniscus repair and transplantation: a comprehensive update. J Orthop Sports Phys Ther. 2012;42:274–90. doi: 10.2519/jospt.2012.3588. [DOI] [PubMed] [Google Scholar]
- 43.Gerich TG, Ghivizani S, Fu FH, Robbins PD, Evans CH. Gene transfer into the patellar tendon of rabbits: a preliminary study of locoregional expression of growth factors. Wien Klin Wochenschr. 1997;109:384–9. [PubMed] [Google Scholar]
- 44.Goto H, Shuler FD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a TGFbeta(1)gene. Osteoarthritis Cartilage. 2000;8:266–71. doi: 10.1053/joca.1999.0300. [DOI] [PubMed] [Google Scholar]
- 45.Hidaka C, Ibarra C, Hannafin JA, Torzilli PA, Quitoriano M, Jen SS, et al. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng. 2002;8:93–105. doi: 10.1089/107632702753503090. [DOI] [PubMed] [Google Scholar]
- 46.Martinek V, Usas A, Pelinkovic D, Robbins P, Fu FH, Huard J. Genetic engineering of meniscal allografts. Tissue Eng. 2002;8:107–17. doi: 10.1089/107632702753503108. [DOI] [PubMed] [Google Scholar]
- 47.Madry H, Cucchiarini M, Kaul G, Kohn D, Terwilliger EF, Trippel SB. Menisci are efficiently transduced by recombinant adeno-associated virus vectors in vitro and in vivo. Am J Sports Med. 2004;32:1860–5. doi: 10.1177/0363546504265189. [DOI] [PubMed] [Google Scholar]
- 48.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13:2227–37. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 49.Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009;16:1363–72. doi: 10.1038/gt.2009.91. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin Orthop Relat Res. 2009;467:3165–74. doi: 10.1007/s11999-009-0921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HP, Rey-Rico A, Cucchiarini M, Madry H. Nonviral gene transfer into human meniscal cells. Part II: effect of three-dimensional environment and overexpression of human fibroblast growth factor 2. Int Orthop. 2014;38:1931–6. doi: 10.1007/s00264-014-2405-z. [DOI] [PubMed] [Google Scholar]
- 52.Cucchiarini M, Schmidt K, Frisch J, Kohn D, Madry H. Overexpression of TGF-beta via rAAV-mediated gene transfer promotes the healing of human meniscal lesions ex vivo on explanted menisci. Am J Sports Med. 2015;43:1197–205. doi: 10.1177/0363546514567063. [DOI] [PubMed] [Google Scholar]
- 53.Cucchiarini M, Ekici M, Schetting S, Kohn D, Madry H. Metabolic activities and chondrogenic differentiation of human mesenchymal stem cells following recombinant adeno-associated virus-mediated gene transfer and overexpression of fibroblast growth factor 2. Tissue Eng Part A. 2011;17:1921–33. doi: 10.1089/ten.TEA.2011.0018. [DOI] [PubMed] [Google Scholar]
- 54.Elsler S, Schetting S, Schmitt G, Kohn D, Madry H, Cucchiarini M. Effective, safe nonviral gene transfer to preserve the chondrogenic differentiation potential of human mesenchymal stem cells. J Gene Med. 2012;14:501–11. doi: 10.1002/jgm.2644. [DOI] [PubMed] [Google Scholar]
- 55.Brunger JM, Huynh NP, Guenther CM, Perez-Pinera P, Moutos FT, Sanchez-Adams J, et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc Natl Acad Sci U S A. 2014;111:E798–806. doi: 10.1073/pnas.1321744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glass KA, Link JM, Brunger JM, Moutos FT, Gersbach CA, Guilak F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35:5921–31. doi: 10.1016/j.biomaterials.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage. 2015;23:1007–17. doi: 10.1016/j.joca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Adesida AB, Jomha NM. Meniscus repair using mesenchymal stem cells - a comprehensive review. Stem Cell Res Ther. 2015;6:86–95. doi: 10.1186/s13287-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozeki N, Muneta T, Matsuta S, Koga H, Nakagawa Y, Mizuno M, et al. Synovial mesenchymal stem cells promote meniscus regeneration augmented by an autologous Achilles tendon graft in a rat partial meniscus defect model. Stem Cells. 2015;33:1927–38. doi: 10.1002/stem.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:19172–7. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB) J Orthop Res. 1995;13:201–7. doi: 10.1002/jor.1100130208. [DOI] [PubMed] [Google Scholar]
- 62.Webber RJ, Harris MG, Hough AJ., Jr Cell culture of rabbit meniscal fibrochondrocytes: proliferative and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3:36–42. doi: 10.1002/jor.1100030104. [DOI] [PubMed] [Google Scholar]
- 63.Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. J Bone Joint Surg Br. 2004;86:1077–81. doi: 10.1302/0301-620x.86b7.13747. [DOI] [PubMed] [Google Scholar]
- 64.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3:127–38. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 65.Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: influences on meniscal cell proliferation and migration. Arthritis Res Ther. 2011;13:R187–206. doi: 10.1186/ar3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen C, Yan J, Erkocak OF, Zheng XF, Chen XD. Nitric oxide inhibits autophagy via suppression of JNK in meniscal cells. Rheumatology (Oxford) 2014;53:1022–33. doi: 10.1093/rheumatology/ket471. [DOI] [PubMed] [Google Scholar]
- 67.Lietman SA, Hobbs W, Inoue N, Reddi AH. Effects of selected growth factors on porcine meniscus in chemically defined medium. Orthopedics. 2003;26:799–803. doi: 10.3928/0147-7447-20030801-19. [DOI] [PubMed] [Google Scholar]
- 68.Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In Vitro Cell Dev Biol Anim. 2005;41:305–10. doi: 10.1290/0503018.1. [DOI] [PubMed] [Google Scholar]
- 69.Tumia NS, Johnstone AJ. Promoting the proliferative and synthetic activity of knee meniscal fibrochondrocytes using basic fibroblast growth factor in vitro. Am J Sports Med. 2004;32:915–20. doi: 10.1177/0363546503261710. [DOI] [PubMed] [Google Scholar]
- 70.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467:1557–67. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. 2010;18:830–8. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nerurkar NL, Sen S, Baker BM, Elliott DM, Mauck RL. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011;7:485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNulty AL, Guilak F. Mechanobiology of the meniscus. J Biomech. 2015;48:1469–78. doi: 10.1016/j.jbiomech.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goto H, Shuler FD, Lamsam C, Moller HD, Niyibizi C, Fu FH, et al. Transfer of lacZ marker gene to the meniscus. J Bone Joint Surg Am. 1999;81:918–25. doi: 10.2106/00004623-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Setton LA, Guilak F, Hsu EW, Vail TP. Biomechanical factors in tissue engineered meniscal repair. Clin Orthop Relat Res. 1999:S254–72. doi: 10.1097/00003086-199910001-00025. [DOI] [PubMed] [Google Scholar]
- 76.Sandmann GH, Eichhorn S, Vogt S, Adamczyk C, Aryee S, Hoberg M, et al. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res A. 2009;91:567–74. doi: 10.1002/jbm.a.32269. [DOI] [PubMed] [Google Scholar]
- 77.Muller-Rath R, Mumme T, Miltner O, Andereya S, Schneider U. Meniscus replacement: current aspects in the field of tissue engineering. Z Orthop Ihre Grenzgeb. 2004;142:540–5. doi: 10.1055/s-2004-832362. [DOI] [PubMed] [Google Scholar]
- 78.Arnoczky SP. Building a meniscus. Biologic considerations. Clin Orthop Relat Res. 1999:S244–53. [PubMed] [Google Scholar]
- 79.Steadman JR, Rodkey WG. Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy. 2005;21:515–25. doi: 10.1016/j.arthro.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am. 2000;82-A:1071–82. doi: 10.2106/00004623-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Chang HC, Nyland J, Caborn DN, Burden R. Biomechanical evaluation of meniscal repair systems: a comparison of the Meniscal Viper Repair System, the vertical mattress FasT-Fix Device, and vertical mattress ethibond sutures. Am J Sports Med. 2005;33:1846–52. doi: 10.1177/0363546505278254. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi Y, Yasuda K, Kondo E, Katsura T, Tanabe Y, Kimura M, et al. Implantation of autogenous meniscal fragments wrapped with a fascia sheath enhances fibrocartilage regeneration in vivo in a large harvest site defect. Am J Sports Med. 2010;38:740–8. doi: 10.1177/0363546509350749. [DOI] [PubMed] [Google Scholar]
- 83.Ozeki N, Muneta T, Koga H, Katagiri H, Otabe K, Okuno M, et al. Transplantation of Achilles tendon treated with bone morphogenetic protein 7 promotes meniscus regeneration in a rat model of massive meniscal defect. Arthritis Rheum. 2013;65:2876–86. doi: 10.1002/art.38099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749–56. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stabile KJ, Odom D, Smith TL, Northam C, Whitlock PW, Smith BP, et al. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthroscopy. 2010;26:936–48. doi: 10.1016/j.arthro.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20:104–11. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- 87.Mandal BB, Park SH, Gil ES, Kaplan DL. Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials. 2011;32:639–51. doi: 10.1016/j.biomaterials.2010.08.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gruchenberg K, Ignatius A, Friemert B, von Lubken F, Skaer N, Gellynck K, et al. In vivo performance of a novel silk fibroin scaffold for partial meniscal replacement in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2015;23:2218–29. doi: 10.1007/s00167-014-3009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cook JL, Tomlinson JL, Kreeger JM, Cook CR. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27:658–65. doi: 10.1177/03635465990270051901. [DOI] [PubMed] [Google Scholar]
- 90.Klompmaker J, Jansen HW, Veth RP, de Groot JH, Nijenhuis AJ, Pennings AJ. Porous polymer implant for repair of meniscal lesions: a preliminary study in dogs. Biomaterials. 1991;12:810–6. doi: 10.1016/0142-9612(91)90066-j. [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi M, Toguchida J, Oka M. Development of an artificial meniscus using polyvinyl alcohol-hydrogel for early return to, and continuance of, athletic life in sports persons with severe meniscus injury. II: animal experiments. Knee. 2003;10:53. doi: 10.1016/s0968-0160(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 92.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–17. [PubMed] [Google Scholar]
- 93.Marsano A, Vunjak-Novakovic G, Martin I. Towards tissue engineering of meniscus substitutes: selection of cell source and culture environment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3656–8. doi: 10.1109/IEMBS.2006.259748. [DOI] [PubMed] [Google Scholar]
- 94.Chiari C, Koller U, Kapeller B, Dorotka R, Bindreiter U, Nehrer S. Different behavior of meniscal cells in collagen II/I,III and Hyaff-11 scaffolds in vitro. Tissue Eng Part A. 2008;14:1295–304. doi: 10.1089/ten.tea.2007.0341. [DOI] [PubMed] [Google Scholar]
- 95.Gunja NJ, Athanasiou KA. Effects of co-cultures of meniscus cells and articular chondrocytes on PLLA scaffolds. Biotechnol Bioeng. 2009;103:808–16. doi: 10.1002/bit.22301. [DOI] [PubMed] [Google Scholar]
- 96.Tan Y, Zhang Y, Pei M. Meniscus reconstruction through coculturing meniscus cells with synovium-derived stem cells on small intestine submucosa--a pilot study to engineer meniscus tissue constructs. Tissue Eng Part A. 2010;16:67–79. doi: 10.1089/ten.TEA.2008.0680. [DOI] [PubMed] [Google Scholar]
- 97.Yamasaki T, Deie M, Shinomiya R, Yasunaga Y, Yanada S, Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs. 2008;32:519–24. doi: 10.1111/j.1525-1594.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 98.Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333:439–47. doi: 10.1007/s00441-008-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang HN, Leng P, Wang YZ, Zhang J. Treating human meniscal fibrochondrocytes with hIGF-1 gene by liposome. Clin Orthop Relat Res. 2009;467:3175–82. doi: 10.1007/s11999-009-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cucchiarini M, Terwilliger EF, Kohn D, Madry H. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009;13:2476–88. doi: 10.1111/j.1582-4934.2008.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]