Abstract
Objective
To evaluate the association between Cushing syndrome and hypercoagulability in children.
Study design
A prospective, observational study was performed of 54 patients with Cushing syndrome, 15.1 ± 3.9 years, treated at the National Institutes of Health Clinical Center. Coagulation profiles were taken before and 6-12 months after surgery and compared with 18 normocortisolemic children, 13.7 ± 3.6 years.
Results
At baseline, patients with Cushing syndrome had greater levels of the procoagulant factor VIII (FVIII) vs controls (145 IU/dL ± 84 vs 99 ± 47, P = .04); 6-12 months after surgery, FVIII levels decreased to 111 ± 47, P = .05. Patients with Cushing syndrome had greater levels of the antifibrinolytic α2-antiplasmin, 96 ± 17% vs 82 ± 26%, P = .015. After surgery, antifibrinolytic α2-antiplasmin levels decreased to 82 ± 24%, P < .001. Anticoagulants were greater in patients with Cushing syndrome vs controls at baseline, including protein C (138 ± 41% vs 84 ± 25%, P < .001), protein S (94 ± 19% vs 74 ± 19%, P = .001), and antithrombin III (96 ± 18% vs 77 ± 13%, P < .0001). The 24-hour urinary free cortisol levels correlated positively with FVIII levels, r = 0.43, P = .004.
Conclusion
Children with Cushing syndrome had elevated procoagulants, antifibrinolytics, and anticoagulants at baseline compared with controls; normalization of coagulation measures was seen after surgical cure. Despite the increase in anticoagulants, hypercortisolemia is associated with a hypercoagulable state in children, as is the case in adults. This finding has potential implications for prevention of venous thromboembolism in children with Cushing syndrome.
Cushing syndrome is a state of excess glucocorticoids characterized by signs and symptoms such as obesity, striae, growth deceleration, and hypertension.1 Cushing syndrome has been associated with hypercoagulability and thromboembolic events in adults. Data in pediatric patients are lacking.2 In a study by Stuijver et al,3 adults with Cushing syndrome were found to have a 10-fold increased risk of venous thromboembolism (VTE) compared with the normal population. In addition, this study showed a postoperative risk of VTE of 3.4% compared with 0% in patients who underwent operation for nonfunctional pituitary adenomas.
The hypercoaguable state in adults with Cushing syndrome is explained by enhanced coagulation factors and impaired fibrinolysis, as reflected by increased levels of fibrinogen, factor VIII (FVIII), von Willebrand factor (vWF), plasminogen activator inhibitor-1 (PAI-1), thrombin activatable fibrinolysis inhibitor, and α2-antiplasmin (A2AP).4 The 2015 Endocrine Society clinical practice guidelines state that use of perioperative prophylaxis for VTE in adult patients with Cushing syndrome undergoing surgery may be useful and is indicated specifically for bedridden or low-mobility patients or those with urinary-free cortisol (UFC) levels greater than 5-fold normal.5 No such guidelines, however, exist for children.
In this report, we describe 4 clinically significant thromboembolic events in children out of a total of 171 patients who underwent operation for Cushing syndrome at the National Institutes of Health (NIH) Clinical Center between 1997 and 2015. VTE is reported to occur in 0.07 of every 1000 children per year.6,7 More recent studies have reported a greater prevalence of pediatric VTE, ranging from 4.9 to 21.9 VTE per 10 000 hospital admissions.8,9 In comparison, the incidence of VTE in children after spinal fusion surgery is approximately 21 events per 10 000 spinal fusions per year.10 Even when highest quoted VTE prevalence of 21.9 per 10 000 hospital admissions is used, the rate is significantly lower than the 4 of 171 pediatric patients with Cushing syndrome at the NIH who have experienced thromboembolic events (P < .0001). To better understand this, we prospectively evaluated several key coagulation elements in children with Cushing syndrome before and after surgical intervention to see whether these findings are consistent with those found in adults.
Methods
All patients were seen under 3 protocols (95CH0059, 97CH0076, and 00CH160) that treat children with Cushing syndrome at the NIH Clinical Research Center (ClinicalTrials.gov: NCT00001595). All protocols have been approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and all patients and/or their parents signed proper consent and/or assent forms.
Retrospective Data Analysis
One hundred seventy-one pediatric patients were treated for Cushing syndrome at the NIH Clinical Center between 1997 and 2015 (139 with adrenocorticotropic hormone [ACTH]-secreting pituitary tumors, 26 with primary adrenocortical Cushing syndrome, and 6 with ectopic ACTH- or corticotropin-releasing hormone [CRH]-secreting tumors). Four patients who had experienced a perioperative thromboembolic event were identified by electronic record review using the terms “thrombus,” “clot,” “deep-vein thrombosis (DVT),” and “embolism,” “stroke,” and “ischemia.”
Prospective Study
Coagulation measures were obtained in 54 consecutive children (33 female and 21 male), mean age of 15.1 ± 3.9 years, who were admitted to the NIH for surgical intervention of confirmed Cushing syndrome between January 2013 through August 2015. Fifty-two of the patients with Cushing syndrome had a pituitary adenoma, 1 had an ectopic CRH and ACTH co-secreting adenoma, and 1 had an adrenal adenoma. Methods used to evaluate children for hypercortisolemia have been described previously.11 Data also were collected from a control group (n = 18, 10 female, 8 male) of normocortisolemic children, mean age of 13.7 years, to use for comparison. Coagulation profiles were gathered on each patient before surgical intervention and at 6-12 months after surgery. Patients with Cushing syndrome underwent transsphenoidal surgery (n = 51), an adrenalectomy (n = 1), or removal of an ectopic ACTH-secreting pulmonary neuroendocrine tumor (n = 1). Coagulation profile included fibrinogen (reference range, 177-466 mg/dL), FVIII (41-184 IU/dL), vWF antigen (50-197 IU/dL), and PAI-1 plasma levels (3-86 IU/mL); antithrombin III (ATIII; 57-134%), protein C (59-144%), protein S (55-134%), and A2AP (75-132%) activities; and activated partial thromboplastin time (PTT) measurements (25.3-37.3 seconds).
Laboratory Methods
Antigen levels of FVIII, PAI-1, vWF:Ag, and fibrinogen were measured, whereas activity was measured for proteins C and S, A2AP, and ATIII. FVIII antigen levels were measured by correction of the activated partial thromboplastin time at various dilutions of patient sample mixed with FVIII-depleted plasma (George King Bio-Medical Inc, Overland Park, Kansas); vWF:Ag assays were done by immunoturbidometric measurements, fibrinogen measurements—by the method of Clauss; and protein C, protein S, ATIII, A2AP, and PAI-1 were analyzed by a chromogenic assays. All coagulation factors analyses were performed with a StaRevolution analyser (Diagnostica Stago Inc, Parsippany, New Jersey).
Statistical Analyses
Data were described with simple descriptive statistics and are presented as mean ± SD. Data were compared with t tests, or Wilcoxon rank-sum test, as appropriate, with SPSS 20 (IBM Inc, Armonk, New York). The relevant prevalence rates of VTE were compared with the z test for proportions. A 2-sided P value less than .05 was considered statistically significant.
Results
Retrospective Data Analysis: Identification of 4 Cases
Four children who had developed a perioperative thromboembolic event were identified. Case 1 was a 7-year-old girl who underwent transsphenoidal surgery. On postoperative day 6, she developed right shoulder pain with swelling around her central venous catheter line. An ultrasound scan detected an upper-extremity DVT in the right cephalic vein to the junction of the right subclavian vein. The central venous catheter was removed, and the patient was sent to the intensive care unit, where she was treated with unfractionated heparin and blood flow improved. Anticoagulation with low-molecular-weight heparin was continued 3 months after discharge. Follow-up at 6 months showed no residual pain or deficit.
The second case was a 16-year-old boy who underwent uncomplicated transsphenoidal surgery for removal of a pituitary adenoma. After uneventful recovery, he was discharged. Two weeks after surgery, he presented to the emergency department with persistent, severe headache and emesis. A computed tomography scan of the head revealed cerebral venous sinus thrombosis. He was admitted to the intensive care unit and was treated with enoxaparin therapy. Evaluation by hematology revealed an underlying factor V Leiden deficiency. At follow up 1-year after surgery, he had transitioned to daily aspirin therapy and had no residual sequelae.
The third case was a 13-year-old boy with a metastatic thymic ACTH- and CRH-secreting neuroendocrine tumor. The patient developed dyspnea and hypertension, despite treatment with several medications to lower blood pressure. He developed a clot at the site of a left internal jugular venous line and failed to respond to thrombolytic treatment twice; he was then placed on Lovenox (Sanofi US, Bridgewater, New Jersey) for anticoagulation purposes. The patient had a median sternotomy with bilateral clamshell thoracotomy with placement of 4 chest tubes and an extensive neck dissection. During the operation, it was found that he had tumor infiltration at the cervical, paratracheal, and paraesophageal lymph nodes. The infiltration extended down into the proximal subclavian vein, resulting in right internal jugular venous thrombosis, while also clotting many other vessels.12 Interestingly, his family history is significant for maternal history of 2 strokes in her late 30s.
The fourth case was a 10-year-old boy who underwent transsphenoidal surgery. Two days after surgery, the patient reported feeling weakness in his lower left extremity and feelings of lethargy. Magnetic resonance imaging of the brain revealed acute ischemic infarction. These abnormalities were not present on the preoperative scans. Fibrinogen was elevated at 580 mg/dL with an upper limit of normal of 458. After a hematology workup, it was found that the patient was a carrier of a prothrombin 20210 G-to-A polymorphism, which confers a mildly increased thrombotic risk, but the remainder of the hypercoagulability workup was negative.13 The patient was administered heparin and aspirin therapy and discharged. At the time of his 8-month follow-up, he showed signs of full neurologic recovery.14 Based on a review of these cases and recent literature detailing the thromboembolic state in adults with Cushing syndrome, we pursued a prospective study of coagulation in children with Cushing syndrome.
Prospective Study of Coagulation Dynamics
Patient demographics and characteristics are summarized in Table I. The average midnight cortisol and mean 24-hour UFC levels were greater in patients than controls. As expected, our pediatric population with Cushing syndrome was overweight/obese.
Table I.
Demographic and clinical characteristics in pediatric patients
| Patient characteristics | Patients with Cushing syndrome (n = 54) |
Control patients (n = 18) |
|---|---|---|
| Females (%)/males (%) | 33 (61.11)/21 (38.89) | 10 (55.6)/8 (44.4) |
| Age at surgery, y | 15.11 ± 3.88 | 13.7 ± 3.6 |
| Race (%) | ||
| Asian | 4 (7.41) | 0 (0.0) |
| Black/African | 4 (7.41) | 1 (5.6) |
| White | 43 (79.63) | 15 (83.3) |
| Other/unknown | 3 (5.55) | 2 (11.1) |
| Ethnicity (%) | ||
| Latino or Hispanic | 11 (20.37) | 2 (11.1) |
| Non-Latino or Hispanic | 43 (79.63) | 16 (18.9) |
| Tumor type | ||
| Adrenal | 1 (1.85) | N/A |
| Ectopic | 1 (1.85) | N/A |
| Pituitary | 52 (96.30) | N/A |
| Midnight cortisol, μg/dL* | 17.45 ± 12.82 | 3.4 ± 2.4 |
| UFC, μg/m3† | 323.19 ± 558.44 | 18.3 ± 14.8 |
| BMI, kg/m2 | 30.72 ± 8.50 | 26.36 ± 6.08 |
| BMI z score | 2.06 ± 0.85 | 1.46 ± 0.96 |
N/A, not applicable
Midnight cortisol levels have a cut off value of ≥4.4 μg/dL.4
Normal UFC ranges for patients aged 3-8 years is 1.4-20 μg/24 h, patients aged 9-12 years is 2.6-37, patients aged 13-17 years is 4.0-56, and patients aged ≥18 years is 3.5-45.
The baseline coagulation profiles of patients with Cushing syndrome were compared with those of normocortisolemic patients, as summarized in Table II. At baseline, patients with Cushing syndrome had greater levels of procoagulant FVIII compared with controls. Patients with Cushing syndrome also had elevated levels of the antifibrinolytic A2AP. Lastly, the endogenous anticoagulants protein C, protein S, and ATIII were all elevated compared with control patients (Table II).
Table II.
Coagulation profile: patients at baseline vs control patients
| Patients with Cushing syndrome (n) |
Mean ± SD | Control patients (n) |
Mean ± SD | P value | |
|---|---|---|---|---|---|
| ATIII, % | 44 | 96.0 ± 17.9 | 16 | 75.5 ± 13.2 | <.001 |
| A2AP, % | 42 | 96.2 ± 16.6 | 16 | 81.2 ± 26.1 | .015 |
| Protein C, % | 44 | 138.2 ± 40.5 | 15 | 84.2 ± 24.5 | <.001 |
| Protein S, % | 44 | 94.2 ± 19.1 | 16 | 73.5 ± 18.9 | .001 |
| PAI-1, IU/mL | 39 | 18.8 ± 9.9 | 13 | 28.2 ± 14.9 | .012 |
| vWF, IU/dL | 45 | 106.3 ± 54.9 | 16 | 85.0 ± 44.7 | NS |
| FVIII, IU/dL | 45 | 145.2 ± 83.5 | 16 | 98.9 ± 46.7 | .04 |
| Fibrinogen, mg/dL | 48 | 331.5 ± 99.5 | 16 | 298.2 ± 59.8 | NS |
| PTT, s | 53 | 30.6 ± 8.1 | 17 | 32.5 ± 3.5 | NS |
NS, not significant.
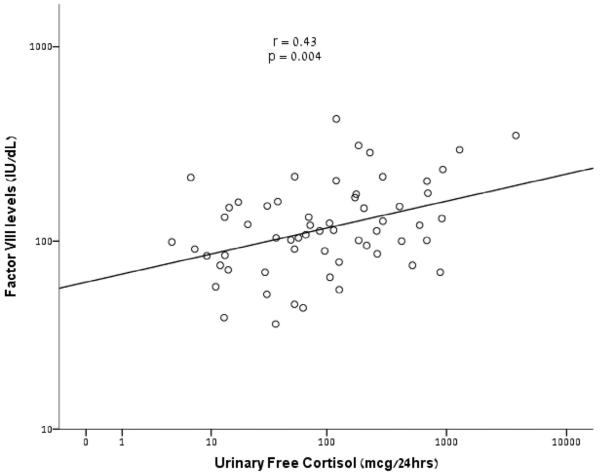
Changes in coagulation elements in patients with Cushing syndrome after surgery were then compared with preoperative findings (Table III). Follow-up after surgery was at a mean of 13.1 ± 6.5 months. All patients seen for postsurgical follow-up were in remission from Cushing syndrome. After surgery, FVIII levels normalized, and A2AP activity decreased. Endogenous anticoagulants were all lower after surgical intervention compared with patients with Cushing syndrome at baseline, including protein C activity, protein S activity, and ATIII activity (Table III). All other coagulation elements, including PAI-1, vWF, fibrinogen, and PTT, did not show any statistically significant changes postoperatively (Table III). It was observed that 24-hour UFC levels positively correlated with FVIII levels (r = 0.43, P = .004; Figure). Correlation analysis between either body mass index (BMI) and BMI z score with the coagulation profile elements revealed a mild positive correlation between BMI and PTT (r = 0.3, P = .02); however, no other correlations were found.
Table III.
Changes in coagulation elements before and after intervention in patients with Cushing syndrome
| n | Before, mean ± SD | After, mean ±SD | P value | |
|---|---|---|---|---|
| ATIII, % | 29 | 98.2 ± 18.0 | 77.0 ± 16.6 | <.001 |
| A2AP, % | 28 | 96.0 ± 16.4 | 82.1 ± 14.0 | <.001 |
| Protein C, % | 31 | 141.1 ± 37.8 | 87.8 ± 23.8 | <.001 |
| Protein S, % | 31 | 95.5 ± 17.5 | 66.2 ± 19.3 | <.001 |
| PAI-1, IU/mL | 24 | 17.4 ± 10.6 | 19.1 ± 9.4 | NS |
| vWF, IU/dL | 31 | 108.2 ± 59.5 | 92.3 ± 34.8 | NS |
| FVIII, IU/dL | 31 | 150.5 ± 92.5 | 111.3 ± 46.5 | .05 |
| Fibrinogen, mg/dL | 31 | 321.4 ± 108.7 | 348.6 ± 111.5 | NS |
| PTT, s | 43 | 30.5 ± 8.9 | 32.4 ± 3.0 | NS |
Figure.
Correlation analysis between 24-hour UFC levels and FVIII plasma levels among children with Cushing syndrome.
Discussion
Several coagulation elements were found to be elevated among patients with Cushing syndrome compared with normocortisolemic control patients. We observed elevated levels of procoagulant FVIII, antifibrinolytic A2AP, and activity of the anticoagulants protein C, protein S, and ATIII. Although it may seem counterintuitive that the levels of anticoagulants are elevated in addition to the procoagulants, this pattern has been seen in adults. In 2 separate studies, adults with Cushing syndrome had greater levels of protein S and ATIII compared with controls.2,3 The clinical significance of these findings is unclear. It has been hypothesized that increases in endogenous anticoagulants reflect a compensatory protective mechanism to the hypercoaguable state.8 One may speculate that elevated anticoagulants in children with Cushing syndrome may play a role in part to explain why children with Cushing syndrome have a lower incidence of VTE than adults. Levels of 24-hour UFC were found to correlate positively with the level of the procoagulant FVIII. Although this increased hypercoagulable state is related in part to hypercortisolemia, there are likely additional contributing risk factors in pediatric Cushing syndrome, including obesity, hyperlipidemia, and inflammation.
Obesity is a hallmark of Cushing syndrome; obese patients have elevated levels of procoagulants and their BMI positively correlates with the hypercoagulable state.15 Patients with obesity have an increased production of inflammatory factors, including increased levels of FVIII, thrombin activity, and circulating monocyte tissue factor procoagulant compared with their counterparts of normal weight.16 This increased inflammatory response can lead to hypercoagulability, insulin resistance, and hepatic dysfunction.16 Similarly, hyperlipidemia also is associated with Cushing syndrome, yet another risk factor for VTE.15,17
Our group has identified low-density lipoprotein elevation in children with Cushing syndrome, contributing further to the hypercoagulable state.18 The prevalence of thromboembolic events that we have observed in our cohort of children with Cushing syndrome is much greater than in the general population of children. Importantly, 3 of the 4 children in our cohort with VTE were found to have either a genetic condition associated with hypercoagulability (2) or a family history of coagulopathy (1). Many of the risk factors that are observed in these children with Cushing syndrome align directly with the factors of the Virchow triad, (hypercoagulable state, stasis, and endothelial injury), leading to an increased risk of VTE. Surgery and indwelling intravenous catheters are linked to vascular wall injury; muscle weakness and postsurgical status contribute to immobility, thus affecting circulatory stasis; and lastly, the patient’s laboratory profile is consistent with a hypercoagulable state. All of these risk factors may predispose patients to thrombus formation.
In a study by Boscaro et al,19 adults with Cushing syndrome were observed to have decreased morbidity and mortality rates in relation to VTE when treated with postoperative antithromboembolic prophylaxis. This idea is further supported by current Cushing syndrome treatment guidelines for adults.5 In combination with our findings, one may argue that perhaps VTE prophylaxis may be indicated in children with Cushing syndrome. The risks and benefits of such an intervention likely differ in children compared with adults; however, because of the rarity of pediatric Cushing syndrome, a randomized controlled trial would be extremely difficult to undertake.
It is important to point out a significant difference in our pediatric experience with DVT compared with the adult literature. Whereas in adults DVTs occur before surgery, the rare instances of DVT in our patients have occurred after surgery. Stasis in the Virchow triad is a modifiable risk factor; at our institution all children, including those with lumbar drains, are mobilized on postoperative day 1, which may in part lead to the lower incidence of postoperative DVT in children compared with adults. Our results point to a need to raise awareness among health care professionals, patients, and their families so they can recognize signs and symptoms of these clots for early treatment and to emphasize early mobilization of children after surgery. Our laboratory and clinical findings show that children with Cushing syndrome are vulnerable to the hypercoagulable state; however, further research is needed to develop evidence-based treatment guidelines for VTE prevention in pediatric Cushing syndrome.
Acknowledgments
Supported by the Intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Glossary
- A2AP
α2-antiplasmin
- ACTH
Adrenocorticotropic hormone
- ATIII
Antithrombin III
- BMI
Body mass index
- CRH
Corticotropin-releasing hormone
- DVT
Deep-vein thrombosis
- FVIII
Factor VIII
- NIH
National Institutes of Health
- PAI-1
Plasminogen activator inhibitor-1
- PTT
Partial thromboplastin time
- UFC
Urinary-free cortisol
- VTE
Venous thromboembolism
- vWF
von Willebrand factor
Footnotes
The authors declare no conflicts of interest.
Portions of the study were presented as a presidential poster at the meeting of the Pediatric Academic Societies, April 30-May 3, 2016, Baltimore, MD.
Trial registration ClinicalTrials.gov: NCT00001595
References
- 1.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386:913–27. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 2.van der Pas R, de Bruin C, Leebeek FW, de Maat MP, Rijken DC, Pereira AM, et al. The hypercoagulable state in Cushing’s disease is associated with increased levels of procoagulant factors and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab. 2012;97:1303–10. doi: 10.1210/jc.2011-2753. [DOI] [PubMed] [Google Scholar]
- 3.Stuijver DJ, van Zaane B, Feelders RA, Debeij J, Cannegieter SC, Hermus AR, et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Metab. 2011;96:3525–32. doi: 10.1210/jc.2011-1661. [DOI] [PubMed] [Google Scholar]
- 4.van der Pas R, Leebeek FW, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf) 2013;78:481–8. doi: 10.1111/cen.12094. [DOI] [PubMed] [Google Scholar]
- 5.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:2807–31. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spentzouris G, Scriven RJ, Lee TK, Labropoulos N. Pediatric venous thromboembolism in relation to adults. J Vasc Surg. 2012;55:1785–93. doi: 10.1016/j.jvs.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7. [PubMed] [Google Scholar]
- 8.Erem C, Kocak M, Nuhoglu I, Yilmaz M, Ucuncu O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin Endocrinol (Oxf) 2010;73:502–7. doi: 10.1111/j.1365-2265.2009.03752.x. [DOI] [PubMed] [Google Scholar]
- 9.Erem C, Ucuncu O, Yilmaz M, Kocak M, Nuhoglu I, Ersoz HO. Increased thrombin-activatable fibrinolysis inhibitor and decreased tissue factor pathway inhibitor in patients with hyperthyroidism. Endocrine. 2009;36:473–8. doi: 10.1007/s12020-009-9271-2. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Karas DJ, Skolasky RL, Sponseller PD. Thromboembolic complications in children after spinal fusion surgery. Spine. 2014;39:1325–9. doi: 10.1097/BRS.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 11.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–86. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 12.Karageorgiadis AS, Papadakis GZ, Biro J, Keil MF, Lyssikatos C, Quezado MM, et al. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab. 2015;100:141–8. doi: 10.1210/jc.2014-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schobess R, Junker R, Auberger K, Munchow N, Burdach S, Nowak-Gottl U, et al. G1691A and prothrombin G20210A in childhood spontaneous venous thrombosis – evidence of an age-dependent thrombotic onset in carriers of factor V G1691A and prothrombin G20210A mutation. Eur J Pediatr. 1999;158(Suppl 3):S105–8. doi: 10.1007/pl00014335. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen JH, Lodish MB, Patronas NJ, Ugrasbul F, Keil MF, Roberts MD, et al. Extensive and largely reversible ischemic cerebral infarctions in a prepubertal child with hypertension and Cushing disease. J Clin Endocrinol Metab. 2009;94:1–2. doi: 10.1210/jc.2008-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415–22. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruf W, Samad F. Tissue factor pathways linking obesity and inflammation. Hamostaseologie. 2015;35:279–83. doi: 10.5482/HAMO-14-11-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho MC, Santos CV, Vieira Neto L, Gadelha MR. Adverse effects of glucocorticoids: coagulopathy. Eur J Endocrinol. 2015;173:M11–21. doi: 10.1530/EJE-15-0198. [DOI] [PubMed] [Google Scholar]
- 18.Libuit LG, Karageorgiadis AS, Sinaii N, Nguyen May NM, Keil MF, Lodish MB, et al. A gender-dependent analysis of Cushing’s disease in childhood: pre- and postoperative follow-up. Clin Endocrinol (Oxf) 2015;83:72–7. doi: 10.1111/cen.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boscaro M, Sonino N, Scarda A, Barzon L, Fallo F, Sartori MT, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87:3662–6. doi: 10.1210/jcem.87.8.8703. [DOI] [PubMed] [Google Scholar]