Abstract
The majority of aggressive lymphomas is characterized by an up regulated glycolytic activity, which enables the visualization by F-18 FDG-PET/CT. One-stop hybrid FDG-PET/CT combines the functional and morphologic information, outperforming both, CT and FDG-PET as separate imaging modalities. This has resulted in several recommendations using FDG-PET/CT for staging, restaging, monitoring during therapy, and assessment of treatment response as well as identification of malignant transformation. FDG-PET/CT may obviate the need for a bone marrow biopsy in patients with Hodgkin's lymphoma and diffuse large B-cell lymphoma. FDG-PET/CT response assessment is recommended for FDG-avid lymphomas, whereas CT-based response evaluation remains important in lymphomas with low or variable FDG avidity. The treatment induced change in metabolic activity allows for assessment of response after completion of therapy as well as prediction of outcome early during therapy. The five point scale Deauville Criteria allows the assessment of treatment response based on visual FDG-PET analysis. Although the use of FDG-PET/CT for prediction of therapeutic response is promising it should only be conducted in the context of clinical trials. Surveillance FDG-PET/CT after complete remission is discouraged due to the relative high number of false-positive findings, which in turn may result in further unnecessary investigations. Future directions include the use of new PET tracers such as F-18 fluorothymidine (FLT), a surrogate biomarker of cellular proliferation and Ga-68 CXCR4, a chemokine receptor imaging biomarker as well as innovative digital PET/CT and PET/MRI techniques.
Keywords: PET, PET/CT, PET/MRI, FDG, FLT, Ga-68 CXCR4, Lymphoma, Leukemia, Hematologic Malignancies
Introduction
Positron emission tomography (PET) is a functional imaging technique developed in the late 1950s. A number of biological molecules have been labelled with positron emitting radionuclides offering a variety of specific tissue information including receptor expression, cell proliferation, and cellular viability. The radiolabeled glucose analogue Fluorine-18 fluorodeoxyglucose (F-18 FDG) is the most frequently used PET tracer. It allows visualization of the cellular uptake of glucose, which is often up regulated in malignant neoplasms including lymphoma. FDG-PET has completely replaced Gallium-67 scintigraphy, which was previously used to assess the extent and viability of lymphoma1, 2. An important advantage of FDG-PET is the ability to quantify the level of FDG uptake based on normalizing the measured tissue activity from PET images to the injected dose and patients' body weight resulting in a standardized uptake value (SUV). In clinical practice the maximum activity within a lymphoma lesion is generally being reported.
The development of combined PET/CT was a major breakthrough in the clinical acceptance of FDG-PET. The CT component of PET/CT provides co-registered anatomical information for optimal localization and characterization of tissue metabolic activity. The combination of CT and PET results in a decrease in the number of false-positive and false-negative PET findings. In addition, PET/CT allows for shorter PET scanning time since the information about tissue densities derived from CT is used for the attenuation correction of PET photons.
There are some variations in the PET imaging protocols pertaining to injected activity of F-18 FDG (ranging from 150-700 MBq), the uptake time (ranging from 40 to >120 min), duration of PET acquisition per “bed position” (ranging from 1-3 minutes), addition of intravenous and oral CT contrast agents as well as the use of respiratory and cardiac gating. The American Society of Clinical Oncology (ASCO) has published guidelines for diagnosis, staging, response evaluation at interim and at the end of treatment3, 4. Likewise, the European Society for Medical Oncology (ESMO)5 together with the National Comprehensive Cancer Network (NCCN)6 and the International Working Group (IWG)7 have published recommendations regarding assessment of relapse and follow-up using FDG-PET/CT in lymphoma patients, as well as in patients with other hematologic malignancies.
Lymphoma
Until the early 2000s, the staging of lymphoma patients was essentially based on CT as the main imaging modality along with results from clinical examination and bone marrow biopsy8. CT offered structural anatomical information, which was limited in detecting disease in normal-sized lymph nodes and in identifying diffuse splenic, hepatic or bone marrow involvement9, 10. A number of studies since have shown that combined FDG-PET/CT is superior to FDG-PET or contrast-enhanced CT as separate imaging procedures in the staging of lymphoma 1, 9, 11-14. The use of FDG-PET/CT can change the stage of disease in 10-30% of patients, frequently resulting in an upstaging, although FDG-PET/CT infrequently has significant impact on management or overall outcome15. According to two recent meta-analyses, FDG-PET/CT is also superior to magnetic resonance imaging (MRI) as a single procedure in the staging of lymphoma, little data exist so far on the use of combined FDG-PET/MRI16, 17.
Most lymphoma types are characterized by an increase in metabolic activity with resultant increased FDG uptake on PET. Hodgkin's lymphoma (HL) and diffuse large B cell lymphoma (DLBCL) are “aggressive” and the most common lymphomas. In a review of 766 patients with newly diagnosed lymphoma, 100% of HL and 97% of DLBCL were hypermetabolic on FDG-PET/CT18.
HL usually spreads contiguously from an involved lymph node station to the nearest one, whereas non-Hodgkin lymphoma (NHL) disseminates haphazardly through different lymph node groups and commonly involves multiple organs and the bone marrow. In HL, the neoplastic Hodgkin and Reed-Sternberg cells represent only a minority of the cellular infiltrate, with a frequency ranging from 0.1-10%19. The majority of a HL lesion is comprised of a reactive infiltrate containing non-neoplastic small lymphocytes, eosinophilic and neutrophilic granulocytes, histiocytes, plasma cells and fibroblasts in varying proportions. One exception is the rare subtype of ‘lymphocyte-depleted classical Hodgkin lymphoma’ comprising less than 1% of all HL cases, which is characterized by a predominance of neoplastic cells in relation to the lymphocytic infiltrate19. The accumulation of FDG in activated lymphocytes, eosinophilic and neutrophilic granulocytes, histiocytes, and plasma cell is similar to what is seen in inflammatory lesions. FDG-PET in HL demonstrates increased glucose metabolism in entire tumor, which in turn implies that activated non-neoplastic cells contribute to a significant portion of the metabolic activity.
A recent study investigated the expression of glucose transporter 1 (GLUT1), a receptor involved in the regulation of glucose metabolism, in different subtypes of HL19. Membrane bound expression of GLUT1 was detected in 49% of neoplastic Hodgkin- and Reed-Sternberg cells with a broad variation between different subtypes. However, there was no correlation between GLUT1 expression in neoplastic cells and the level of tumor FDG-uptake19. Of note, a strong expression of GLUT1 was observed in reactive B cells within progressively transformed germinal centers and hyperplastic follicles, confirming that reactive inflammatory cells contribute significantly to the FDG uptake in Hodgkin lesions 19. Another study compared the expression of the GLUT1 and GLUT3 transport protein with the level of FDG uptake in 31 patients with HL and NHL. Although GLUT1 and GLUT3 expression was observed in all cases, only GLUT1 expression correlated with the level of FDG-uptake within entire tumor lesions 20. In 52% of tumors, only the non-lymphomatous cells expressed GLUT1 or GLUT3, again confirming the important role of the reactive infiltrate for metabolic visualization of lymphomas.
DLBCL constitutes 25-30% of adult NHL in western countries with a higher incidence in developing countries, occurring at a median age in the 7th decade 20. DLBCL, follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma and small lymphocytic lymphoma (SLL) are generally characterized by a majority of neoplastic lymphoid cells and a minority of reactive inflammatory cells. In contrast to HL, the neoplastic lymphoid cells of DLBCL NOS (not otherwise specified) constitute the majority of the cellular infiltrate, with variable amounts of admixed T-cells and histiocytes19 (Fig. 1).
Figure 1.
69 year old male with new right tonsillar diffuse large B cell lymphoma. Baseline PET/CT study shows hypermetabolic right tonsillar mass. Mild activity in the left tonsillar fossa is reactive. No additional site of lymphoma was identified on remainder of images.
Other FDG-avid aggressive lymphoma subtypes include Burkitt lymphoma, MCL and lymphomas of T-cell origin such as natural killer/T-cell lymphoma and anaplastic large cell lymphoma 18. FL are generally indolent and incurable and up to 95% present with increased FDG uptake18. More variable levels of metabolic activity have been found in SLL (50-83%), extranodal marginal zone lymphoma (54-67%) and cutaneous lymphomas 3, 18, 21, 22. The rare variant of T-cell/histiocyte-rich large B-cell lymphoma accounts for less than 10% of DLBCL, and is an exception as it presents with few scattered large neoplastic B-cells embedded in a background of abundant T-cells and histiocytes23 In marginal zone lymphoma, there is a neoplastic small B-cell infiltrate around reactive follicles, which expands into interfollicular areas. Although neoplastic cells dominate the cellular infiltrate, residual reactive follicles and interfollicular are as contribute to a variable amount of non-neoplastic lymphoid cells (Fig. 2).
Figure 2.
55 year old female with gastric MALT lymphoma with accompanying splenic involvement. Baseline PET/CT images (a-d) show diffuse gastric wall thickening and hypermetabolic acitivity and hypermetabolic splenomegaly.
Imaging recommendations for staging of lymphoma
The initial staging of primary nodal lymphomas is performed according to the revised Ann Arbor classification, which broadly classifies patients in limited stage (I or II, nonbulky) or advanced stage (III or IV) disease 4. The staging considers the sites of involvement, type of involvement, particularly if nodal or extranodal, and the distribution of disease (Table 1)11, 24. Stage II bulky disease is considered limited or advanced as determined by histology and a number of different prognostic factors. Definition of tumor bulk is disease, stage, and treatment specific. Tumor bulk is a negative prognostic factor in early-stage HL and in DLBCL25. The designation “E” for extranodal disease is relevant only for limited extranodal disease in the absence of nodal involvement (IE) or in patients with stage II disease and direct extension to a non-nodal site. Extranodal disease is not relevant for patients with advanced-stage disease. The sub classification A and B is based respectively on the absence or the presence of disease-related symptoms: fevers to greater than 38.3°C and/or weight loss within the last 6 months, and is only applied to HL. Currently, the Ann Arbor staging system is included in most prognostic indices26-29. Imaging procedures for staging of lymphoma remain relevant for risk stratification of patients and play an important role in determining the best treatment regimen as well as to assess therapeutic outcome.
Table 1. Modified Ann-Arbor criteria for Primary Nodal Lymphomas.
| Stage | Nodal Involvement | Extranodal involvement (E) | |
|---|---|---|---|
| Limited | I | One node or a group of adjacent nodes | Only single extranodal lesions |
| II | Two or more nodal groups on the same side of the diaphragm | Stage I or II by nodal extent with limited contiguous extranodal involvement | |
| II bulky* | II as above with “bulky” disease | Not applicable | |
| Advanced | III | Nodes on both sides of the diaphragm; nodes above the diaphragm with spleen involvement | |
| IV | Additional noncontiguous extralymphatic involvement | ||
Note: extent of the disease is determined by PET/CT for FDG-avid lymphomas and CT for nonavid histologies
Tonsils, Waldeyer's ring, and spleen are considered nodal tissue
(E) Extranodal inolvement refers to extralymphtaic tissue, excluding liver and bone marrow
II bulky may be treated as limited or advanced desease based on histology and a number of prognostic factors
In 2007, the IWG updated the International Harmonization Project (IHP) to integrate FDG-PET/CT as standard of care imaging for staging and assessment of treatment response criteria for lymphoma7, 30. In June 2011, a workshop held at the 11th International Conference on malignant lymphoma in Lugano, Switzerland, provided recommendations for staging of lymphomas with primarily nodal involvement and primary extranodal DLBCL 3, 4. Separate criteria have been proposed for primary extranodal31, 32 and cut aneous 33 lymphomas.
Accordingly, the IHP recommends the use of FDG-PET before start of treatment in FDG-avid, potentially curable lymphomas (e.g., DLBCL, HL) to better delineate the extent of disease. Staging of FDG-avid lymphomas is recommended using visual assessment of PET and PET/CT images ideally scaled to a fixed parametric SUV display. Focally increased metabolic activity in nodal or extranodal sites is typical for aggressive lymphoma in addition to diffusely increased FDG uptake within the spleen, liver, or bone marrow4 (Fig. 3). The CT portion of PET/CT provides important information about the size of disease involvement and is helpful in equivocal findings and for the definition of treatment response. In individual patients, FDG-PET may be useful to select the best site for tissue biopsy3, 34, 35.
Figure 3.
26 year old female with nodular sclerosis type HL. Baseline PET/CT images (a, b) show large hypermetabolic anterior mediastinal masses, hypermetabolic splenic foci, and periportal adenopathy. Mild marrow hyperplasia was also noted at baseline, bone marrow biopsy was negative. Interim PET/CT (c, d) images show resolution of hypermetabolic activity in the mediastinal mass, spleen and abdominal adenopathy indicating treatment response (Deauville scale 2)
FDG-PET is of limited utility within the brain due to the high physiologic FDG uptake within the gray matter and MRI remains the modality of choice for suspected CNS involvement of lymphomas3 (Fig. 4). Currently, the IHP has no recommendation for FDG-PET/CT imaging in MCL, indolent lymphomas including FL and those with variable FDG avidity such as marginal zone lymphoma, chronic lymphocytic leukemia/SLL, lymphoplasmacytic lymphoma/Waldenstrom's macroglobulinemia and mycosis fungoides3, 4. Although use of FDG-PET/CT is encouraged even in these subtypes for staging in clinical trials4, lack of clear recommendations, high cost and limited availability prevent it from being widely implemented in clinical practice across the world 3, 4
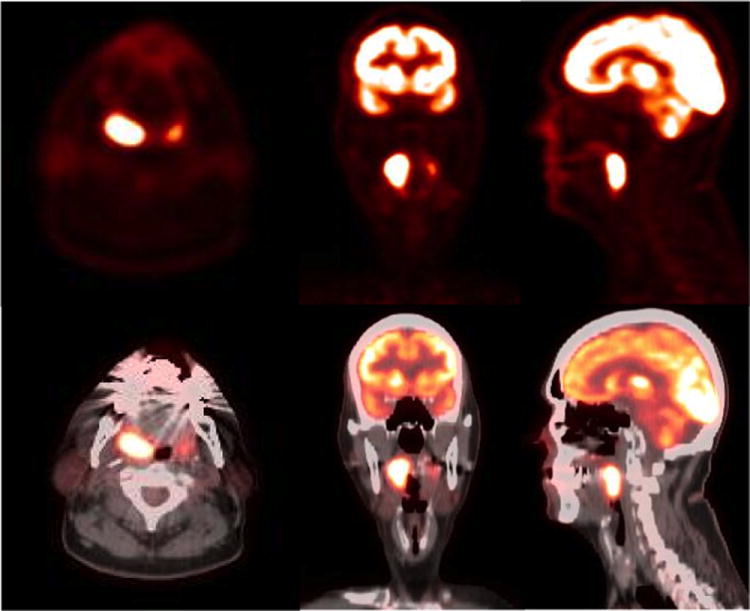
Figure 4.
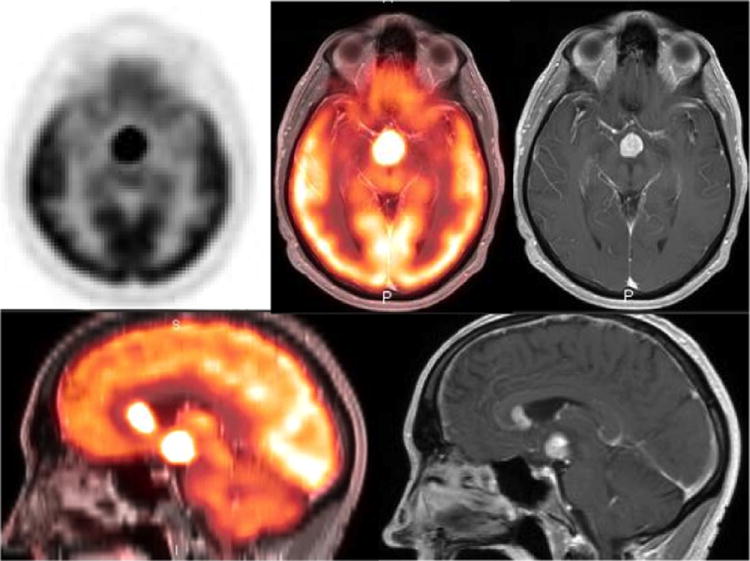
54 year old male with acute onset headache and confusion. Axial and sagittal PET images of brain fused with contrast enhanced T1 weighted MRI images show an intensely hypermetabolic mass in the suprasellar region. Additional hypermetabolic enhancing nodule is identified in the left frontal horn of lateral ventricle. Histopathology analysis confirmed the diagnosis of large B cell lymphoma.
Assessment of Bone Marrow Involvement
FDG-PET is superior to bone scintigraphy in identifying lymphomatous bone marrow involvement 36. Combined FDG-PET/CT has the advantage of detecting bone marrow involvement without cortical destruction, the later necessary for a CT diagnosis. The PET component can assess metabolic activity of the entire bone marrow and in combination with CT can also identify false-negative uptake related to an iliac crest marrow biopsy. In a recent meta-analysis including 955 patients with newly diagnosed HL, FDG-PET/CT provided a pooled sensitivity of 96.9% and specificity of 99.7% in detecting bone marrow involvement 37. The same group also published a meta-analysis including 654 newly diagnosed DLBCL patients resulting in a pooled sensitivity and specificity of 88.7% and 99.8%, respectively 38. Similarly, in a recent meta-analysis conducted by Wu et al. in a mixed population of HL and NHL patients, FDG-PET/CT yielded a pooled sensitivity and specificity of 91.6% and 90.3% in identifying bone marrow involvement at initial staging16. Furthermore, the study demonstrated the superiority of FDG-PET/CT compared to MRI and FDG-PET alone.
Bone marrow involvement is one of the most important prognostic factors in patients with lymphoma, and is more common in indolent NHL subtypes and MCL (20-30%), compared to HL (<10%) or DLBCL (11-17%)38-40. Early-stage HL and DLBCL patients with a negative FDG-PET/CT rarely have bone marrow involvement41, 42. Thus, FDG-PET/CT has replaced bone marrow biopsy in early-stage HL and DLBCL. While PET/CT can also be used instead of bone marrow biopsy in clinically advanced stage HL4, 43-47, it can miss low-volume diffuse marrow involvement in 10-20% of DLBCLpatients39, 43, 48. Therefore, a negative FDG-PET/CT cannot rule out the presence of bone marrow involvement in clinically advanced DLBCL, although this infrequently affects patient management 39, 43, 49. Consequently, bone marrow biopsy should be restricted to advanced stage DLBCL patients with a negative FDG-PET/CT, in order to confirm a discordant bone marrow involvement, if relevant for a clinical trial or patient management (Fig. 5)50.
Figure 5.
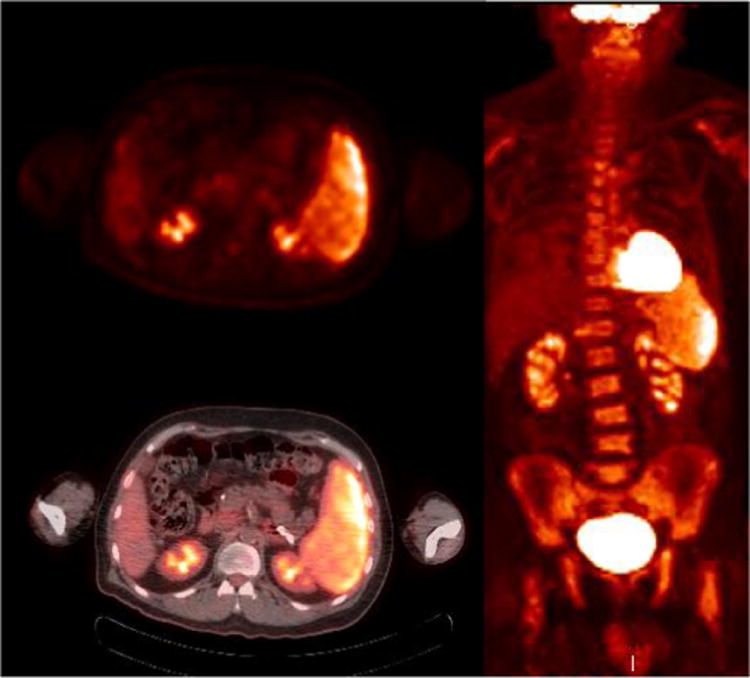
71 year old with B cell lymphoma with bone marrow and splenic involvement at presentation PET (a) and PET/CT fused (b) images show heterogeneously hypermetabolic splenomegaly, the whole body MIP (c) shows diffuse marrow involvement. No other site of disease was identified.
In indolent NHL, FDG-PET/CT offers a low sensitivity of approximately 50% for identifying diffuse bone marrow involvement 51, 52. As a result, bone marrow biopsy with immunohistochemistry and flow cytometry remains the gold standard in indolent NHL subtypes and MCL11, 49, 53.
In the post-therapy setting, diffuse hypermetabolic bone marrow activity often represents reactive hyperplasia from previous chemotherapy and should not be mistaken as lymphomatous involvement44. As administration of granulocyte colony stimulating factor (G-CSF) enhances the metabolic activity of the bone marrow, FDG-PET/CT should be performed at least 4-6 weeks after patients have received G-CSF to minimize the risk of false-positive findings (Fig. 6). The interpreting radiologist must make every attempt to review the clinical and treatment history to avoid false positive findings secondary to therapy effects.
Figure 6.
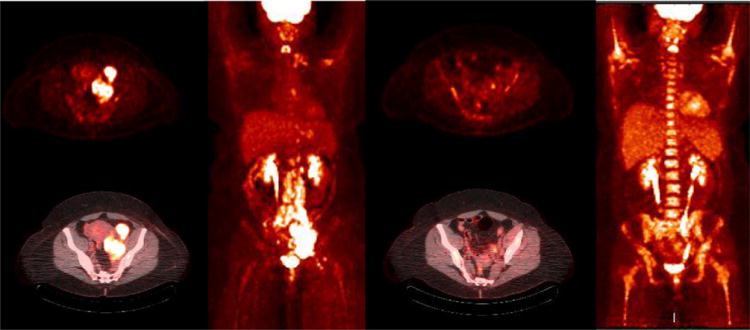
47 year old female with stage IV-B diffuse large B cell lymphoma B cell lymphoma diffuse nodal involvement. PET and fused PET/CT images show hypermetabolic left iliac adenopathy. The MIP PET image (c) shows additional retroperitoneal, inguinal, mediastinal and left supraclavicular nodal involvement. Interim PET/CT study after 2 cycles of chemotherapy (d-f) shows near complete resolution of adenopathy (Deauville scale 2) with post chemotherapy marrow hyperplasia
Assessment of Treatment Response
End-of-treatment FDG-PET/CT
After completion of therapy over 60% of patients with HL and 40% with aggressive NHL have residual masses containing necrotic and/or fibrotic tissue and residual neoplastic cells1, 30. FDG-PET has been shown to be useful in identifying residual lymphoma in 30-64% of residual masses, by demonstration of persistent metabolic activity on FDG-PET 7, 51, 52, 54, 55. Between 62-100% of patients with residual FDG-positive masses have been shown to relapse after first-line chemotherapy2, 56, 57. Consequently, establishment of complete metabolic remission after treatment results in a higher rate of progression-free survival (PFS) and overall survival (OS). Furthermore, identification of patients with partial response supports the administration of further treatment cycles. However, in patients where salvage treatment is being considered a biopsy is recommended to confirm FDG-PET findings 2, 4, 56-58.
The IWG recommendations have improved the response assessment regarding the interpretation of persistent residual masses by eliminating unconfirmed complete remission (CRu) assessed by CT in the previous Cotswold classification and accounting for assessment of extranodal disease 59.
A meta-analysis revealed a sensitivity and specificity of 84% and 90%, respectively for FDG-PET/CT in detecting residual disease in HL60. In this study, the pooled sensitivity and specificity for NHL were 72% and 100%, respectively60. These results show that a negative post-treatment FDG-PET/CT does not exclude the presence of minimal residual disease. On long-term follow-up disease relapse occurred in 16-25% of patients with complete metabolic response on PET/CT61, 62. In addition, in up to 80% of patients the relapse occurred at a newsite, highlighting the importance of whole-body assessment offered by FDG-PET/CT 61, 62
As FDG-PET avidity reflects tissue glucose consumption, increased metabolic activity is also seen in inflammatory lesions, reactive thymic hyperplasia, histiocytic infiltration, local and systemic infections or following G-CSF therapy, radiation therapy, or surgical interventions, all of which could result in false-positive findings. The timing of FDG-PET/CT following completion of therapy is of critical importance; the study should be performed at least 4-6 weeks post therapy to avoid unnecessary false-positive and false-negative findings 63. Use of FDG-PET/CT in routine surveillance is discouraged due to the high likelihood of false positive findings (> 20%) that may result in unnecessary follow-up examinations, additional radiation exposure and invasive procedures including biopsies all resulting in high cost of care and patient anxiety 64, 65. In theory, routine surveillance could result in early diagnosis of potential relapse in a small fraction of patients, however the impact of practice on patient outcome has not been established so far 66.
Based on the latest ESMO guidelines for the assessment of end of treatment response, the use of CT and CT-based criteria is preferred in lymphomas with low or variable FDG avidity or when FDG-PET/CT is unavailable 25, 31, 67-69. For patients staged with CT, revised recommendations should be considered4 (see Table 2).
Table 2. Revised Criteria for Response Assessment in Lymphoma based on CT imaging.
| Response category | CT-Based Response Criteria |
|---|---|
| Complete Remission (CR) | Target nodes/nodal masses must regress to ≤ 1.5 cm in LDi. It includes the presence of residual symptoms but no detectable disease by imaging No extralymphatic sites of disease No nonmeasured lesions No new lesions In the setting of previous organ enlargement, regress to normal Bone marrow normal by morphology; if indeterminate, IHC negative |
| Partial Remission (PR) | ≥ 50% decrease in SPD of up to 6 measurable target measurable nodes and extranodal sites
No new lesions In the setting of previous nonmeasured lesions, regress to normal, no increase Spleen must have regressed by > 50% in length beyond normal |
| Stable Disease (SD) | < 50% decrease from baseline in SPD of up to 6 dominant, measurable nodes and extranodal sites No increase in nonmeasurable lesions or organ enlargement consistent with progression No new lesions |
| Progressive Disease (PD) | An individual node/lesion must be abnormal with LDi > 1.5 cm, and increase by ≥50% from PPD nadir, and an increase in LDi or SDi from nadir of 0.5 cm for lesions ≤ 2 cm or 1 cm for lesions > 2 cm New or clear progression of preexisting nonmeasured lesions Presence of new lesions
In the setting of splenomegaly, the splenic length must increase by > 50% of the extent of its prior increase beyond baseline (e.g., a 15 cm spleen must increase to > 16 cm). If no prior splenomegaly, must increase by at least 2 cm from baseline New or recurrent splenomegaly New or recurrent bone marrow involvement |
Abreviations: LDi, longest diameter of the lesion; SDi, shortest axis perpendicular to the LDi; SPD, sum of the product of the perpendicular diameters for multiple lesions; PPD, cross product of the LDi and perpendicular diameter; IHC, immunohistochemistry
Measured dominant lesions: Up to six of the largest dominant nodes, nodal masses, and extranodal lesions selected to be clearly measurable in two diameters, the longest diameter (LDi) and the shortest perpendicular to the LDi diameter (SDi). Nodes should preferably be from disparate regions of the body and should include, where applicable, mediastinal and retroperitoneal areas
- Measurable node: LDi > 1.5 cm
- Measurable extranodal disease: LDi > 1 cm
Non-nodal lesions: include those in solid organs (eg, liver, spleen, kidneys, lungs), GI involvement, cutaneous lesions, or those noted on palpation
Nonmeasured lesions: any disease not selected as measured, dominant disease and truly assessable disease should be considered not measured. These sites include any nodes, nodal masses, and extranodal sites not selected as dominant or measurable or that do not meet the requirements for measurability but are still considered abnormal, as well as truly assessable disease, which is any site of suspected disease that would be difficult to follow quantitatively with measurement, including pleural effusions, ascites, bone lesions, leptomeningeal disease, abdominal masses, and other lesions that can not be confirmed and followed by imaging
The current recommendation for reviewing FDG-PET in patients with HL and DLBCL is to use the Deauville Criteria, a 5-point scale to visually analyze FDG-PET 35 (Table 3). The FDG uptake in lymphoma tissue is compared to the mediastinal blood pool activity and the liver as the reference background 35, 70. The Deauville Criteria should be used in clinical trials for prediction of treatment response during therapy (interim analysis to assess early treatment response) and for assessment of treatment response at the end of treatment (Fig. 7 and Fig. 8). FDG-PET defines 4 categories for response assessment in lymphoma patients 4 (see Table 4).
Table 3. Five-point Deauville criteria.
| Score | Deauville criteria |
|---|---|
| 1 | No increased FDG uptake above background |
| 2 | FDG uptake ≤ mediastinum |
| 3 | FDG uptake > mediastinum but ≤ liver |
| 4 | FDG uptake moderately higher than liver |
| 5 | FDG uptake markedly higher than liver and/or new lesions |
| X | New areas of FDG uptake unlikely to be related to lymphoma |
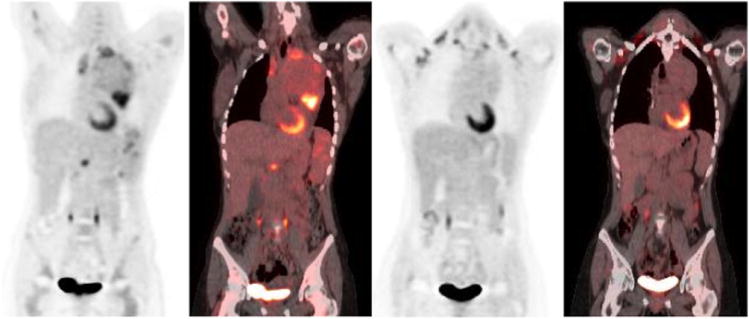
Figure 7.
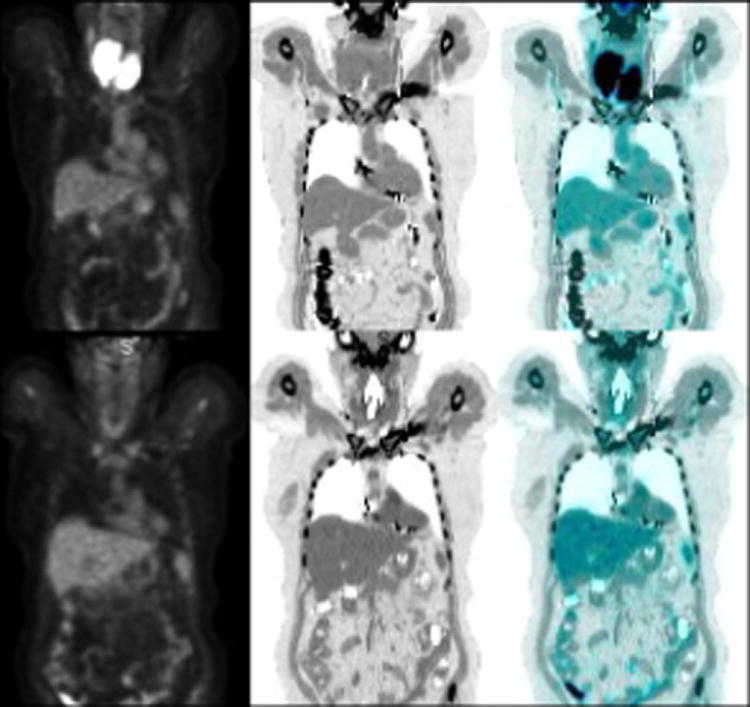
65 year old woman with past history of thyroiditis with a rapidly growing cervical mass. Baseline PET/CT (a) PET, (b) CT and (c) fused PET/CT images show diffusely hypermetabolic thyroid mass, biopsy revealed diffuse large B cell lymphoma. Post chemotherapy PET/CT (d-f) after R-CHOP regimen shows complete response (Deauville scale 1).
Figure 8.
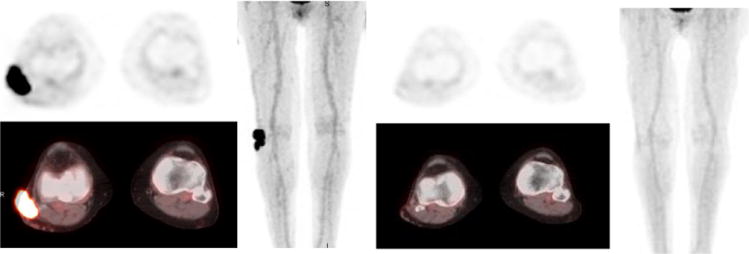
76 year old female with diffuse large B cell lymphoma of right leg. Baseline PET/CT (a-c) shows hypermetabolic soft tissue mass along the lateral aspect of right knee joint. Post treatment PET/CT study (d-f) after chemotherapy and local radiation revealed near complete resolution of the mass indicating therapeutic response (Deauville scale 1)
Table 4. Revised Criteria for Response Assessment in Lymphoma based on FDG PET/CT imaging.
| Response category | FDG-PET/CT-Based Response Criteria |
|---|---|
| Complete metabolic response (CMR) | Nodes and extralymphatic sites: Score 1, 2, or 3* with or without a residual mass on 5PS No new lesions In the setting of previous organ enlargement, regress to normal No FDG-uptake in bone marrow |
| Partial metabolic response (PMR) | Nodes and extralymphatic sites: Score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size
No new lesions Residual uptake higher than uptake in normal bone marrow but reduced compared with baseline (diffuse uptake compatible with reactive changes from chemotherapy or G-CGF allowed). If there are persistent focal changes in the marrow in the context of a nodal response, consideration should be given to further evaluation with MRI or biopsy or an interval scan |
| Stable Disease (SD) or No metabolic response | Nodes and extralymphatic sites: Score 4 or 5 with no significant change in FDG uptake from baseline at interim or end of treatment No new lesions No changes in bone marrow from baseline |
| Progressive Disease (PD) | Nodes and extralymphatic sites: Score 4 or 5 with an increase in intensity of uptake from baseline and/or New FDG-avid extranodal foci consistent with lymphoma at interim or end-of-treatment assessment New FDG-avidlesions consistent with lymphoma, not attributed to other condictions (eg, infection, inflammation). If uncertain regarding etiology of new lesions, biopsy or interval scan may be considered New or recurrent FDG-avid foci in bone marrow |
Abreviations: 5PS, point scale from DeauvilleCretaria: 1, No increased FDG ptake above background; 2, FDG uptake ≤ mediastinum; 3, FDG uptake > mediastinum but ≤ liver; 4, FDG uptake moderately higher than liver; 5, FDG uptake markedly higher than liver and/or new lesions; X, New areas of FDG uptake unlikely to be related to lymphoma
The addition of score 3 for interim analysis during therapy accounts for sites with activation during chemotherapy such as Waldeyer's ring, spleen or bone marrow. In those cases, a score of 3 indicates a good prognosis with standard treatment, especially if at the time of an interim scan. However, in trials exploring treatment de-escalation, a score 3 should be considered as an inadequate response to avoid under treatment
It is known that in Waldeyer's ring or extranodal sites with high physiologic FDG-uptake (eg, GI tract, liver, bone marrow) or with reactive activation within the spleen or bone marrow (eg, with chemotherapy or G-CSF), uptake may be greater than the mediastinum. In this circumstance, CMR may be inferred if uptake at sites of initial involvement is no greater than surrounding normal tissue, even if the tissue has high physiologic uptake
Interim FDG-PET/CT
In lymphoma, the tumor metabolic activity changes rapidly after start of treatment, even before a change in tumor size is detected71. There is an increasing number of clinical trials which show that FDG-PET/CT allows for the prediction of treatment response early during chemotherapy. This may help in patient stratification to individually define therapeutic strategy in order to improve outcome72-75. In lymphoma patients interim FDG-PET/CT is performed after completion of one to four cycles of a six to eight cycle chemotherapy regimen, most commonly after two cycles of treatment. The aim is to differentiate between low risk lymphoma patients who may be sufficiently treated with reduced-intensity approaches and high-risk patients who require standard or even intensified treatment, with escalated therapy protocols (Fig. 9). Interim FDG-PET/CT is a promising surrogate for tumor chemo-sensitivity early during therapy, particularly in advanced-stage or unfavorable risk patients, who might benefit from additional radiotherapy40. Interim FDG-PET/CT can reduce unnecessary treatment-related toxicities and side effects by allowing selection of reduced intensity protocols in low risk patients. Within limited stage and intermediate stage o HL patients, interim FDG-PET/CT after the 1st or 2nd cycle of treatment can identify patients who are likely to achieve a complete metabolic response at completion of treatment and do not require consolidating radiation therapy72. In advanced stages of disease, ongoing clinical trials use FDG PET/CT with an aim to identify candidates requiring modification of the chemotherapy regimen. Interim FDG-PET/CT is recommended in ESMO guidelines for the evaluation of HL patients 5 as it outperforms the International Prognostic Score (IPS)76 and the International Prognostic Index (IPI) 74 by successfully preventing responding patients from undergoing additional radiation therapy.
Figure 9.
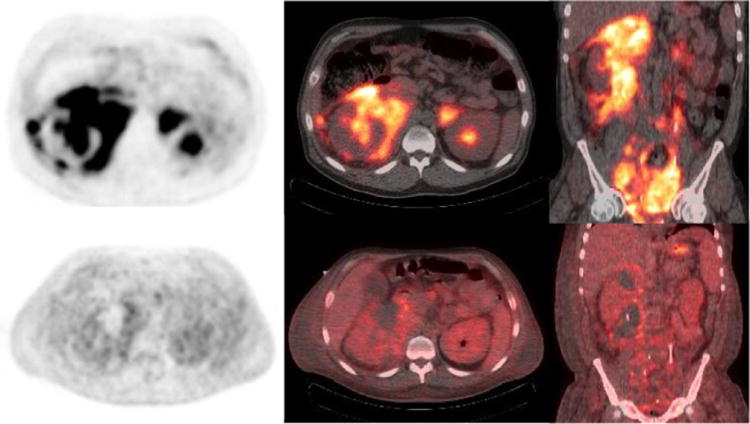
40 year old with Burkitt's lymphoma. Baseline PET/CT (a-c) images show diffuse hypermetabolic abdominal adenopathy with a large perinephric and pelvic hypermetabolic soft tissue mass. Interim PET/CT imaging after 1 cycle of R-CHOP shows significant improvement in metabolic activity of the mass with considerable amount of mildly metabolic residual soft tissue. (Deauville scale 4)
Hodgkin's Lymphoma
Recently, Radford et al. 72 conducted a study including 602 ABVD-treated patients with early-stage HL, which demonstrated that in patients with negative PET after three cycles of therapy, further consolidation radiotherapy could be avoided while still preserving the PFS. A retrospective study of 260 ABVD-treated patients with HL confirmed the prognostic role of interim FDG-PET/CT using the Deauville Criteria for predicting treatment response 77. In another study, 260 patients with advanced-stage HL had a complete metabolic response after two cycles of ABVD. This finding had a negative-predictive value (NPV) of 94% and a positive-predictive value (PPV) of 73% in predicting a 3-year PFS78. Similarly, Markova et al. demonstrated that a complete PET response had a NPV of 98% and a PPV of 96% in predicting a 4-year PFS in advanced HL treated with 4 cycles of BEACOPP chemotherapy79. Interim FDG-PET/CT was a strong prognostic marker for PFS and accurately guided patients with FDG-avid residual disease to consolidation radiotherapy79, 80. A few studies also found that using information from FDG-PET and CT together was particularly helpful in HL patients with a positive interim FDG-PET/CT, as patients with poor tumor shrinkage were at a higher risk of disease progression or relapse 80, 81.
Non-Hodgkin's Lymphoma
The combination of chemotherapy with the anti-CD20 monoclonal antibody rituximab has significantly improved the clinical outcome of NHL patients 82. Both elderly and young patients with good-prognosis DLBCL (from no-risk to intermediate risk factors according to the age-adjusted International Prognostic Index) benefit form first-line rituximab containing-chemotherapy (R-CHOP) thus improving the long-term outcome 83, 84. A recent meta-analysis confirmed the independent prognostic value of FDG-PET in DLCBCL patients treated with R-CHOP85. Interim FDG-PET/CT could be crucial to early identify non-responding patients to offer alternative treatment approaches, such as early intensive chemotherapy followed by stem cell transplantation or participation in clinical trials testing new molecular targeted agents 85 (Fig. 10).
Figure 10.
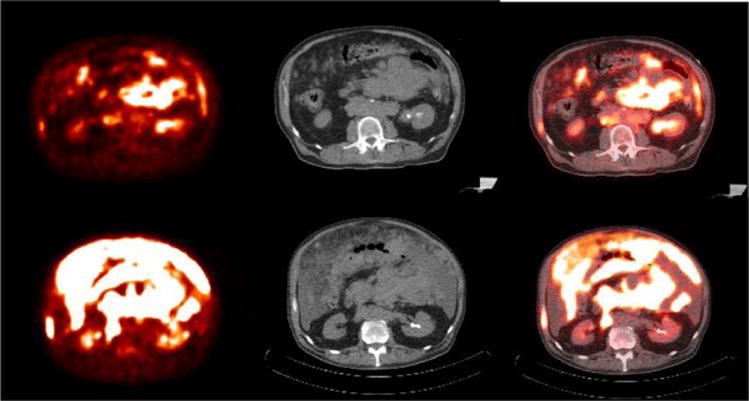
67 year old female with diffuse large B cell lymphoma of germinal center origin. Baseline PET/CT (a-c) shows diffuse omental involvement and abdominal adenopathy. Interim PET/CT study (d-f) after 2 cycles of chemotherapy revealed significant increase in hypermetabolic disease burden indicating treatment failure (Deauville scale 5)
In aggressive NHL, FDG-PET studies have reported a negative predictive value ranging from 80-100% with a positive predictive value ranging from 50-100% 62, 73, 86, 87. Although interim FDG-PET/CT predicts therapeutic response, there are no data currently available which demonstrate that a FDG-PET guided change in treatment improves patient outcome. In an advanced stage diffuse large B-cell lymphoma study, interim PET/CT did not predict outcome with a dose dense sequential immunotherapy regimen and the authors recommended biopsy confirmation of an abnormal PET/CT findings before change in therapy outside of a clinical trial88. Interim FDG-PET/CT is therefore recommended only in the context of clinical trials. There is an ongoing discussion about the use of quantitative SUV parameters instead of or in addition to visual PET image analysis to better define early treatment response63, 89. For this approach further standardization of PET methodology will be essential. In addition, there is a need to establish a cutoff value for decrease in tumor SUV that predicts therapy response. Such SUV threshold value will likely be dependent on disease type, time of imaging since therapy, and the treatment regimen administered.
Transformation of lymphoma
Certain types of lymphomas such as FL, marginal zone and chronic lymphocytic leukemia/SLL may progress over time and transform to DLBCL with an accompanying increase in proliferation rate. It has been shown that aggressive lymphoma cells have a high rate of proliferation and glycolysis compared to indolent subtypes which results in a higher level of FDG uptake90, 91. In addition, the level of FDG uptake measured as standardized uptake value (SUV) is shown to have a positive correlation with the Ki-67 proliferative index in both nodal and extranodal at biopsy site in patients with NHL92. A transformation occurs typically in 5-10% of indolent lymphoma93 and the subsequent increase in metabolic activity can be identified by an increase in FDG-avidity on PET/CT. However, it is important to have a baseline PET/CT study available for comparison in order to identify this change in metabolic activity. Imaging with FDG-PET/CT is recommended if there is a clinical suspicion of aggressive transformation of known indolent lymphoma3, 4. Based on previous reports, a cutoff value of a SUV of 14.0 can identify presence of aggressive transformation and, can also serve as a tool for directing biopsies in all subtypes of FDG-avid lymphomas in primary or relapsed disease13, 21, 47, 90, 94.
Multiple Myeloma
The role of FDG-PET/CT in the diagnosis and management of patients with multiple myeloma (MM) has not yet been clearly established. However, according to the NCCN guidelines, FDG-PET/CT has been included as an option in the diagnosis and monitoring of MM patients. The osseous involvement with myeloma infiltrates can be focal or diffuse based of distribution of clonal proliferation of plasma cells95. Soft tissue and/or organ involvement can be observed, either originating as primary extraosseous lesions or secondary to infiltration from large osseous lesions with cortical disruption. The main role of imaging in MM is reliable detection of osseous and extraosseous lesions and enabling accurate staging and risk stratification of individual patients.
According to the consensus statement of the International Myeloma Working Group (IMWG), conventional radiographic skeletal survey remains the standard of reference for the imaging of MM95, due to its wide availability and low cost. The main disadvantage of conventional radiography is that it tends to underestimate bone marrow involvement. Bone destruction of greater than 30% of trabecular bone is required for the lesion to be detectable on plain films96. Various studies have shown that conventional radiography has poor sensitivity with false negative reads in 30-70% cases95, 97-99. Other limitations of conventional radiography are the inability to detect extraosseous involvement and diffuse bone marrow infiltration, to differentiate between benign and malignant lucencies and the insensitivity to treatment induced changes.
FDG-PET/CT allows for an excellent characterization of osseous lesions as well as for detection of extraosseous disease100. According to two studies, FDG-PET/CT was able to detect multiple myeloma osteolytic lesions with a sensitivity and specificity of 80-90% and 80-100% respectively101, 102. Furthermore, it has been demonstrated that a higher metabolic activity in myeloma lesions correlates with faster disease progression and worse prognosis 95, 103, 104. The level of metabolic activity also correlates well with plasma cell ratios and therefore has a potential to be an alternative to the current gold standard of bone marrow biopsies for assessment in the follow-up period105,106, 107.
In order to introduce more advanced imaging techniques such as MRI and FDG-PET108 in routine clinical practice, an updated morphologic and functional Durie and Salmon “plus” staging system has been proposed109 (Table 5). This classification allows for the differentiation of patients with early stage MM from those with monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM)110, 111. As expected, FDG-PET/CT does not reveal areas of increased metabolic activity in MGUS patients and nearly all patients with low-level SMM112.
Table 5. Multiple myeloma imaging staging system.
| Stage | Durie-Salmon Plus staging system: MRI/PET |
|---|---|
| I | A: normal skeletal survey or single lesion (plasmocytoma) B*: 0-4 focal lesions or mild diffuse disease |
| II | A/B*: 5-20 focal lesions or moderate diffuse disease |
| III | A/B*: > 20 focal lesions or severe diffuse disease |
B* is defined by a creatinine level > 2.0 mg/dL and/or extramedullary disease in PET/CT or MRI
FDG-PET/CT is preferred over radiography and MRI to detect active bone lesions and to assess the outcome during systemic treatment113. As metabolic response generally precedes morphological changes, FDG-PET/CT also provides a faster assessment in patients with positive response or otherwise persistence of active residual disease compared with MRI100, 107, 114-116. PET/CT is shown to be a good predictor of disease progression after therapy in symptomatic myeloma patients and offers improvement in detection of progression when used in combination of lab results117, especially in non-secretory MM112, 118.
Both FDG-PET/CT and whole body-MRI have utility in determination of remission status after stem cell transplantation (SCT), however, PET/CT imaging offers higher specificity and is therefore the preferred modality124. Detection of FDG avid lesions after stem cell transplantation may necessitate a reappraisal of the treatment plan104. In a recent study including 107 patients, a negative FDG-PET/CT study immediately 3 months after autologous SCT and every 6-12 months thereafter was indicative of remission106.
Solitary plasmocytoma
FDG-PET/CT showed a sensitivity of 93% in initial staging of solitary plasmocytomas. In about 40% of patients, additional lesions were detected by FDG-PET/CT resulting in upstaging and change of therapeutic plan95, 109, 119. In a meta-analysis of 14 studies 120, FDG-PET/CT was found to be an accurate imaging tool for detecting intra- and extra-medullary lesions in patients with extra-medullary plasmocytoma (EMP) The highest metabolic activity of lesions was found to be an independent predictor of overall survival 103. A combination of regional MRI with FDG-PET/CT could provide a comprehensive assessment of medullary and extra-medullary sites for additional active disease in the setting of plasmocytoma115, 121, however, this procedure is currently not recommended in IMWG Panel guidelines122.
Leukemia
Leukemia is broadly classified as (1) acute myelogenous leukemia (AML), (2) acute lymphoblastic leukemia (ALL), (3) chronic myelocytic leukemia (CML), and (4) chronic lymphocytic leukemia (CLL). Diagnosis and follow-up is primarily based on peripheral blood and bone marrow evaluation. Occasionally, conventional imaging is required to assess morphologic features and to evaluate for infection in patients planned for or undergoing intensive chemotherapy.
FDG-PET/CT is not regularly used in the assessment of leukemia1. However, several case reports have demonstrated the potential of FDG-PET/CT in diagnosis and follow-up of leukemic bone marrow infiltration123-125. In a preliminary study 126, utility of FDG-PET/CT to diagnose extramedullary disease in de novo or relapsed AML patients was described. FDG-PET/CT may also be useful in detection of Richter's syndrome127-130, which has a poor prognosis and requires aggressive therapy131, 132. Some groups have suggested a SUV cutoff value equal or greater than 5.0 to diagnose the transformation of CLL into Richter syndrome127, 129, 130. Besides, FDG-PET/CT can guide a diagnostic biopsy 128-131 and may also provide prognostic information in patients with CLL127 133.
Future directions
There are several new PET biomarkers that could be used for staging and treatment monitoring in lymphoma patients. F-18 Fluorothymidine (FLT) is a radiolabeled thymidine analogue that reflects tumor proliferative activity and the level of FLT uptake correlates well with the proliferation marker Ki-67 on the immunohistochemical analysis134, 135. Wang et al. demonstrated that FLT-PET/CT is superior to CT in diagnosis and staging of DLBCL 136. FLT-PET has a lower false-positive rate and can be used for early detection of transformation of low grade disease to an aggressive lymphoma and to differentiate between indolent and aggressive lymphoma142. Nevertheless, the strength of FLT-PET is in monitoring treatment response early during therapy, particularly when novel targeted treatments which are cytostatic rather than cytotoxic (e.g., rituximab) are being assessed. FLT-PET may also have potential application in evaluating the addition of novel therapies to current re-induction regimens137, as well as identifying future non-responders to R-CHOP treatment among aggressive B-cell NHL's by their high FLT uptake and proliferation rate, enabling a risk-adapted treatment approach in these patients 138. In two recent studies, higher reduction in FLT uptake on interim FLT-PET was a predictor of improved PFS and OS in patients with aggressive lymphomas139, 140. A recent pilot study has demonstrated that FLT uptake in bone marrow may be useful for assessment of treatment response as early as 2 days after chemotherapy initiation in AML patients141.
Activation of the chemokine receptor CXCR4 is frequently observed in various human disease processes including solid and hematologic malignancies and affects tumorigenesis, cancer cell proliferation and metastasis 142, 143. Lymphomas in particular demonstrate high expression of CXCR4 receptors. Therapeutic monoclonal antibodies targeting CXCR4 are being explored in Phase I trials for relapsed/ refractory acute myeloid leukemia, DLBCL, CLL, FL (NCT01120457), and for relapsed/ refractory MM (NCT01359657) 144-146.
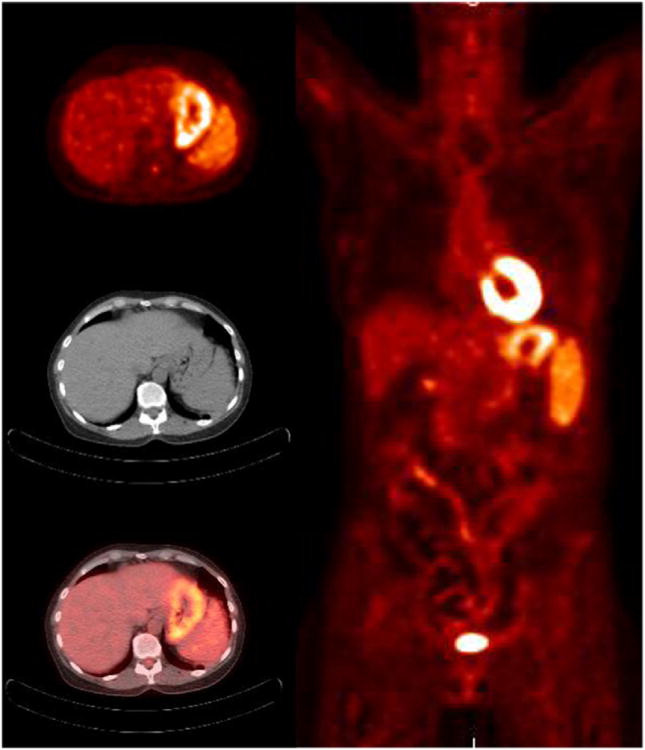
A novel PET tracer, Ga-68 CXCR4 (Pentixafor) has been recently developed to target human CXCR4 receptor expression147, 148. CXCR4 (chemokine receptor type 4) is an alpha-chemokine receptor specific for stromal-derived-factor-1, which is involved in the chemotactic activity in lymphocytes but also overexpressed in a number of different tumor types. Ga-68 CXCR4 binds with high affinity and selectivity to the human CXCR4 receptor, both in mouse xenografts of human lymphoma cell lines and in lymphoma patients, providing PET images with excellent tumor specificity and high tumor-to-background contrast. The first human proof-of-concept study applying Ga-68 CXCR4 PET in four patients with lymphoproliferative malignancies has been recently published 149. All 4 patients including a CD30-positive aggressive T-cell lymphoma, relapsed DLBCL, chronic lymphocytic leukemia with suspected transformation into DLBCL, and MM with extensive bone marrow involvement demonstrated high tracer binding in lymphoma lesions. Minor nonspecific Ga-68 CXCR4 uptake was noted in muscle, lung, and liver tissue with low blood pool activity, resulting in excellent lesion-to-background contrast. Some physiological Ga-68 CXCR4 uptake was evident in the bone marrow as CXCR4 plays a crucial role in hematopoietic cell homing149. Voxel-by-voxel analysis in one patient revealed a remarkable inter- and intralesional heterogeneity in the uptake of Ga-68 CXCR4 and F-18 FDG, suggesting that the biological information provided by both tracers may be complementary even in lesions that show avidity for both PET tracers149. In the two patients with CLL with suspected transformation into DLBCL and with MM Ga-68 CXCR4 uptake was even higher compared to F-18-FDG and provided superior delineation of individual lesions from surrounding tissue especially in the bone marrow 149.
Recently another study of 14 patients with advanced MM demonstrated that Ga-68 CXCR4 provides additional or complementary information to F-18-FDG PET/CT in 64 % cases150 (Fig. 11). This makes Ga-68 CXCR4 PET imaging a promising molecular diagnostic tool for non-invasive visualization of CXCR4 expression in tumor lesions throughout the body. It may have a role in appropriate patient selection for various therapeutic trials targeting CXCR4 expression. The proof of CXCR4 target expression in subgroup of advanced MM patients opens up the opportunity for CXCR4-directed therapies. In addition, Ga-68 CXCR4 exhibits an excellent pharmacokinetic profile and fast clearance kinetics. The initial dosimetry data show notably lower total effective doses in organs with high absorbance, such as urinary bladder wall, spleen, or kidneys151 as compared to commonly used somatostatin receptor-agonists such as Ga-68 DOTATOC and Ga-68 DOTATATE. A theranostic approach of CXCR4 targeted radiotherapy is currently under evaluation, exploring the concept of applying a therapeutic dose of CXCR4 labeled with β-- or α-emitting radionuclides after pre-therapeutic quantification of CXCR4 expression using PET imaging149.
Figure 11.
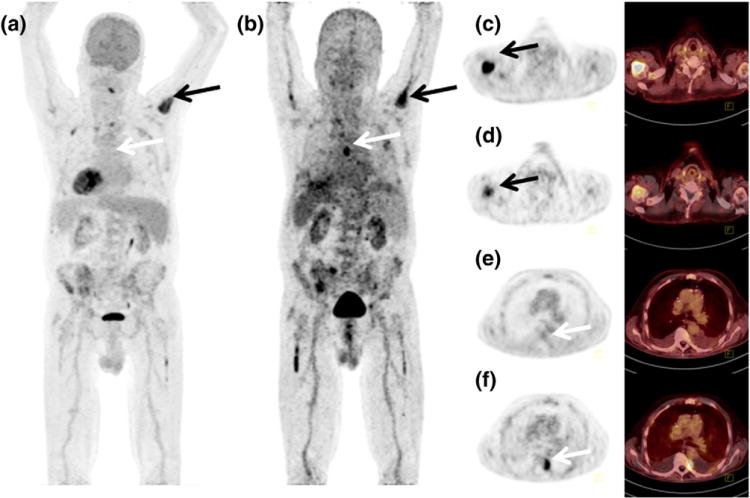
Maximum intensity projections from 18F-FDG (a) and Ga-68 CXCR4 (Pentixafor) PET/CT (b) of a patient with multiple myeloma. Corresponding transversal PET and fused PET/CT images are shown for FDG (c, e) and Ga-68 CXCR4 (d, f) indicating a FDG- and CXCR4 avid lesion in the right humerus (black arrows) and FDG negative but CXCR4 positive lesion left paravertrebral (white arrows)
The advent of novel hybrid PET/MRI systems presents an opportunity to combine the strengths of PET and MRI imaging in a single diagnostic work-up. The high spatial resolution and soft tissue contrast provided by MRI when combined with the high specificity of metabolic information from FDG-PET promise an imaging advantage in both diagnosis and assessment of treatment response for patients with hematologic malignancies, particularly for evaluation of diffuse bone marrow infiltration. In in case of a complete remission after therapy, this combined technique will be able to localize residual sites of disease activity and therefore will help to guide treatment in the near future. Early results comparing FDG-PET/MRI with FDG-PET/CT in oncologic patients have shown that hybrid PET/MRI potentially contributes to clinical management more often than PET/CT 152. Image quality, alignment, and confidence in lesion localization appear to be comparable between the two modalities153, 154. Ongoing advances in technology including the development of new MRI attenuation methodologies, scanner equipment, and the development of new PET radiotracers are further delineating the role of PET/MRI in research and clinical practice.
Practice Points.
FDG-PET/CT is an excellent imaging modality for staging and restaging of aggressive lymphomas
FDG-PET is increasingly used for early prediction of treatment response
Research Agenda.
Perform larger multi-center trial using FDG-PET in less common lymphomas
Assess the use of novel PET tracers, particularly [Ga-68]CXCR4
Explore the effectiveness of a theranostic approach using [lu-177]CXCR4
Acknowledgments
Funding: This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, by UL1TR000439 and KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, by Case Western Reserve University and Ohio Third Frontier Funding
Abbreviations
- ABVD
Adriamycin, Bleomycin, Vinblastine, Dacarbazine
- ALL
Acute Lymphoblastic Leukemia
- AML
Acute Myelogenous Leukemia
- BEACOPP
Bleomycin, Etoposide, Adriamycin, Cyclophosphamide, Oncovin (Vincristine), Procarbazine, Prednisone
- CLL
Chronic Lymphocytic Leukemia
- CML
Chronic Myelocytic Leukemia
- CT
Computed Tomography
- DLBCL
Diffuse Large B-Cell Lymphoma
- EMP
Extramedullary Plasmocytoma
- ESMO
European Society of Medical Oncology
- F-18 FDG
Fluorine-18 Fluorodeoxyglucose
- F-18 FLT
Fluorine-18 Fluorothymidine
- FL
Follicular Lymphoma
- G-68 CXCR4
Gallium-68 CXCR4
- GLUT
Glucose Transporter
- HL
Hodgkin's Lymphoma
- IHP
International Harmonization Project
- IMWG
International Myeloma Working Group
- IPI
International Prognostic index
- IPS
International Prognostic Score
- IWG
International Working Group
- MCL
Mantle Cell Lymphoma
- MGUS
Monoclonal Gammopathy of Unknown Significance
- MM
Multiple Myeloma
- MRI
Magnetic Resonance Imaging
- NCCN
National Comprehensive Cancer Network
- NHL
Non-Hodgkin's Lymphoma
- NPV
Negative Predictive value
- OS
Overall survival
- PET
Positron Emission Tomography
- PET/CT
Hybrid Positron Emission Tomography and Computed Tomography
- PET/MRI
Hybrid Positron Emission Tomography and Magnetic Resonance Imaging
- PFS
Progression free survival
- PPV
Positive predictive value
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- SCT
Stem Cell Transplant
- SLL
Small Lymphocytic Lymphoma
- SMM
Smoldering Multiple Myeloma
- SUV
Standardized Uptake Value
Footnotes
Conflict of Interest: None
References
- 1.Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood. 2007;110:3507–16. doi: 10.1182/blood-2007-06-097238. [DOI] [PubMed] [Google Scholar]
- 2.Kostakoglu L, Leonard JP, Kuji I, Coleman M, Vallabhajosula S, Goldsmith SJ. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and Ga-67 scintigraphy in evaluation of lymphoma. Cancer. 2002;94:879–88. [PubMed] [Google Scholar]
- 3.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyling M, Thieblemont C, Gallamini A, Arcaini L, Campo E, Hermine O, et al. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24:857–77. doi: 10.1093/annonc/mds643. [DOI] [PubMed] [Google Scholar]
- 6.Zelenetz AD, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, et al. Non-Hodgkin's Lymphomas, version 3.2012. J Natl Compr Canc Netw. 2012;10:1487–98. doi: 10.6004/jnccn.2012.0155. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 8.Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504–16. doi: 10.1182/blood-2007-07-101899. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer NG, Hany TF, Taverna C, Seifert B, Stumpe KD, von Schulthess GK, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology. 2004;232:823–9. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi E, Pregno P, Penna D, Deandreis D, Chiappella A, Limerutti G, et al. Role of whole-body [18F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and conventional techniques in the staging of patients with Hodgkin and aggressive non Hodgkin lymphoma. Radiol Med. 2008;113:578–90. doi: 10.1007/s11547-008-0264-7. [DOI] [PubMed] [Google Scholar]
- 11.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg LS, Antoch G, Schutt P, Beyer T, Jentzen W, Muller SP, et al. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–9. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin's lymphoma. Haematologica. 2006;91:482–9. [PubMed] [Google Scholar]
- 14.Elstrom RL, Leonard JP, Coleman M, Brown RK. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008;19:1770–3. doi: 10.1093/annonc/mdn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29:1844–54. doi: 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 16.Wu LM, Chen FY, Jiang XX, Gu HY, Yin Y, Xu JR. 18F-FDG PET, combined FDG-PET/CT and MRI for evaluation of bone marrow infiltration in staging of lymphoma: a systematic review and meta-analysis. Eur J Radiol. 2012;81:303–11. doi: 10.1016/j.ejrad.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Kwee TC, Nievelstein RAJ. Is MRI less accurate than FDG-PET/CT in diagnosing bone marrow involvement in lymphoma? Eur J Radiol. 80:565–6. doi: 10.1016/j.ejrad.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, editors. WHO Classification of Tumours. Lyon: IARC; 2008. [Google Scholar]
- 20.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–95. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 21.Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–6. doi: 10.1182/blood-2002-09-2778. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto N, Kojima M, Hasegawa M, Oriuchi N, Matsushima T, Yokohama A, et al. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer. 2007;110:652–9. doi: 10.1002/cncr.22807. [DOI] [PubMed] [Google Scholar]
- 23.Lim MS, Beaty M, Sorbara L, Cheng RZ, Pittaluga S, Raffeld M, et al. T-cell/histiocyte-rich large B-cell lymphoma: a heterogeneous entity with derivation from germinal center B cells. Am J Surg Pathol. 2002;26:1458–66. doi: 10.1097/00000478-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin's lymphomas. Cancer Treat Rep. 1977;61:1023–7. [PubMed] [Google Scholar]
- 25.Eichenauer DA, Engert A, Andre M, Federico M, Illidge T, Hutchings M, et al. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii70–5. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 26.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 27.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 28.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 29.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 30.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 31.Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:vi144–vi8. doi: 10.1093/annonc/mdt343. [DOI] [PubMed] [Google Scholar]
- 32.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–43. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 33.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55:368–76. doi: 10.3322/canjclin.55.6.368. [DOI] [PubMed] [Google Scholar]
- 35.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50:1257–60. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 36.Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med. 1999;40:1407–13. [PubMed] [Google Scholar]
- 37.Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Littooij AS, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol. 2014;25:921–7. doi: 10.1093/annonc/mdt533. [DOI] [PubMed] [Google Scholar]
- 38.Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2014;41:565–74. doi: 10.1007/s00259-013-2623-4. [DOI] [PubMed] [Google Scholar]
- 39.Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122:61–7. doi: 10.1182/blood-2012-12-473389. [DOI] [PubMed] [Google Scholar]
- 40.Kostakoglu L, Cheson BD. State-of-the-Art Research on “Lymphomas: Role of Molecular Imaging for Staging, Prognostic Evaluation, and Treatment Response”. Front Oncol. 2013;3:212. doi: 10.3389/fonc.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, et al. Bone marrow biopsy in diffuse large B-cell lymphoma: useful or redundant test? Acta Oncol. 2015;54:67–72. doi: 10.3109/0284186X.2014.958531. [DOI] [PubMed] [Google Scholar]
- 42.Lim ST, Tao M, Cheung YB, Rajan S, Mann B. Can patients with early-stage diffuse large B-cell lymphoma be treated without bone marrow biopsy? Ann Oncol. 2005;16:215–8. doi: 10.1093/annonc/mdi050. [DOI] [PubMed] [Google Scholar]
- 43.Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54:1244–50. doi: 10.2967/jnumed.112.114710. [DOI] [PubMed] [Google Scholar]
- 44.El-Galaly TC, d'Amore F, Mylam KJ, de Nully Brown P, Bogsted M, Bukh A, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30:4508–14. doi: 10.1200/JCO.2012.42.4036. [DOI] [PubMed] [Google Scholar]
- 45.Moulin-Romsee G, Hindie E, Cuenca X, Brice P, Decaudin D, Benamor M, et al. (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin's lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging. 2010;37:1095–105. doi: 10.1007/s00259-009-1377-5. [DOI] [PubMed] [Google Scholar]
- 46.Richardson SE, Sudak J, Warbey V, Ramsay A, McNamara CJ. Routine bone marrow biopsy is not necessary in the staging of patients with classical Hodgkin lymphoma in the 18F-fluoro-2-deoxyglucose positron emission tomography era. Leuk Lymphoma. 2012;53:381–5. doi: 10.3109/10428194.2011.616613. [DOI] [PubMed] [Google Scholar]
- 47.Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20:1543–7. doi: 10.1093/annonc/mdp030. [DOI] [PubMed] [Google Scholar]
- 48.Adams HJ, Kwee TC, Nievelstein RA. Prognostic implications of imaging-based bone marrow assessment in lymphoma: 18F-FDG PET, MR imaging, or 18F-FDG PET/MR imaging? J Nucl Med. 2013;54:2017–8. doi: 10.2967/jnumed.113.126797. [DOI] [PubMed] [Google Scholar]
- 49.Pelosi E, Penna D, Douroukas A, Bello M, Amati A, Arena V, et al. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. Q J Nucl Med Mol Imaging. 2011;55:469–75. [PubMed] [Google Scholar]
- 50.Paone G, Itti E, Haioun C, Gaulard P, Dupuis J, Lin C, et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging. 2009;36:745–50. doi: 10.1007/s00259-008-1021-9. [DOI] [PubMed] [Google Scholar]
- 51.Juweid ME, Wiseman GA, Vose JM, Ritchie JM, Menda Y, Wooldridge JE, et al. Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- 52.Cerci JJ, Trindade E, Pracchia LF, Pitella FA, Linardi CC, Soares J, Jr, et al. Cost effectiveness of positron emission tomography in patients with Hodgkin's lymphoma in unconfirmed complete remission or partial remission after first-line therapy. J Clin Oncol. 2010;28:1415–21. doi: 10.1200/JCO.2009.25.4367. [DOI] [PubMed] [Google Scholar]
- 53.Chen YK, Yeh CL, Tsui CC, Liang JA, Chen JH, Kao CH. F-18 FDG PET for evaluation of bone marrow involvement in non-Hodgkin lymphoma: a meta-analysis. Clin Nucl Med. 2011;36:553–9. doi: 10.1097/RLU.0b013e318217aeff. [DOI] [PubMed] [Google Scholar]
- 54.Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol. 2012;30:4317–22. doi: 10.1200/JCO.2012.43.0934. [DOI] [PubMed] [Google Scholar]
- 55.Trotman J, Fournier M, Lamy T, Seymour JF, Sonet A, Janikova A, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol. 2011;29:3194–200. doi: 10.1200/JCO.2011.35.0736. [DOI] [PubMed] [Google Scholar]
- 56.Schoder H, Meta J, Yap C, Ariannejad M, Rao J, Phelps ME, et al. Effect of whole-body (18)F-FDG PET imaging on clinical staging and management of patients with malignant lymphoma. J Nucl Med. 2001;42:1139–43. [PubMed] [Google Scholar]
- 57.Sasaki M, Kuwabara Y, Koga H, Nakagawa M, Chen T, Kaneko K, et al. Clinical impact of whole body FDG-PET on the staging and therapeutic decision making for malignant lymphoma. Ann Nucl Med. 2002;16:337–45. doi: 10.1007/BF02988618. [DOI] [PubMed] [Google Scholar]
- 58.Lowe VJ, Wiseman GA. Assessment of Lymphoma Therapy Using (18)F-FDG PET. J Nucl Med. 2002;43:1028–30. [PubMed] [Google Scholar]
- 59.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–18. [PubMed] [Google Scholar]
- 60.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica. 2006;91:522–9. [PubMed] [Google Scholar]
- 61.Naumann R, Vaic A, Beuthien-Baumann B, Bredow J, Kropp J, Kittner T, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol. 2001;115:793–800. doi: 10.1046/j.1365-2141.2001.03147.x. [DOI] [PubMed] [Google Scholar]
- 62.Spaepen K, Stroobants S, Dupont P, Van Steenweghen S, Thomas J, Vandenberghe P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–9. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 63.Jerusalem G, Beguin Y. The place of positron emission tomography imaging in the management of patients with malignant lymphoma. Haematologica. 2006;91:442–4. [PubMed] [Google Scholar]
- 64.Patel K, Hadar N, Lee J, Siegel BA, Hillner BE, Lau J. The lack of evidence for PET or PET/CT surveillance of patients with treated lymphoma, colorectal cancer, and head and neck cancer: a systematic review. J Nucl Med. 2013;54:1518–27. doi: 10.2967/jnumed.112.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson CA, Ghesquieres H, Maurer MJ, Cerhan JR, Biron P, Ansell SM, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32:3506–12. doi: 10.1200/JCO.2014.55.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bodet-Milin C, Eugène T, Gastinne T, Bailly C, Le Gouill S, Dupas B, et al. The role of FDG-PET scanning in assessing lymphoma in 2012. Diagnostic and Interventional Imaging. 2013;94:158–68. doi: 10.1016/j.diii.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii76–82. doi: 10.1093/annonc/mdr388. [DOI] [PubMed] [Google Scholar]
- 68.Ghielmini M, Vitolo U, Kimby E, Montoto S, Walewski J, Pfreundschuh M, et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL) Ann Oncol. 2013;24:561–76. doi: 10.1093/annonc/mds517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, André M, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2012;23:vii78–vii82. doi: 10.1093/annonc/mds273. [DOI] [PubMed] [Google Scholar]
- 70.Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118:37–43. doi: 10.1182/blood-2010-12-327767. [DOI] [PubMed] [Google Scholar]
- 71.Romer W, Hanauske AR, Ziegler S, Thodtmann R, Weber W, Fuchs C, et al. Positron emission tomography in non-Hodgkin's lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood. 1998;91:4464–71. [PubMed] [Google Scholar]
- 72.Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a Trial of PET-Directed Therapy for Early-Stage Hodgkin's Lymphoma. New England Journal of Medicine. 2015;372:1598–607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 73.Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 74.Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–23. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 75.Mikhaeel NG, Timothy AR, O'Doherty MJ, Hain S, Maisey MN. 18-FDG-PET as a prognostic indicator in the treatment of aggressive Non-Hodgkin's Lymphoma-comparison with CT. Leuk Lymphoma. 2000;39:543–53. doi: 10.3109/10428190009113384. [DOI] [PubMed] [Google Scholar]
- 76.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 77.Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99:1107–13. doi: 10.3324/haematol.2013.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54:683–90. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- 79.Markova J, Kahraman D, Kobe C, Skopalova M, Mocikova H, Klaskova K, et al. Role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leuk Lymphoma. 2012;53:64–70. doi: 10.3109/10428194.2011.603444. [DOI] [PubMed] [Google Scholar]
- 80.Kobe C, Kuhnert G, Kahraman D, Haverkamp H, Eich HT, Franke M, et al. Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced-stage Hodgkin lymphoma. J Clin Oncol. 2014;32:1776–81. doi: 10.1200/JCO.2013.53.2507. [DOI] [PubMed] [Google Scholar]
- 81.Kostakoglu L, Schoder H, Johnson JL, Hall NC, Schwartz LH, Straus DJ, et al. Interim [(18)F]fluorodeoxyglucose positron emission tomography imaging in stage I-II non-bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone? Leuk Lymphoma. 2012;53:2143–50. doi: 10.3109/10428194.2012.676173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–90. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 83.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 84.Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–22. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Y, Lu J, Wei X, Song S, Huang G. The predictive value of interim and final [18F] fluorodeoxyglucose positron emission tomography after rituximab-chemotherapy in the treatment of non-Hodgkin's lymphoma: a meta-analysis. Biomed Res Int. 2013;2013:275805. doi: 10.1155/2013/275805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nols N, Mounier N, Bouazza S, Lhommel R, Costantini S, Vander Borght T, et al. Quantitative and qualitative analysis of metabolic response at interim positron emission tomography scan combined with International Prognostic Index is highly predictive of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:773–80. doi: 10.3109/10428194.2013.831848. [DOI] [PubMed] [Google Scholar]
- 87.Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Thomas J, de Groot T, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin's lymphoma. Ann Oncol. 2002;13:1356–63. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 88.Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28:1896–903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duhrsen U, Huttmann A, Jockel KH, Muller S. Positron emission tomography guided therapy of aggressive non-Hodgkin lymphomas--the PETAL trial. Leuk Lymphoma. 2009;50:1757–60. doi: 10.3109/10428190903308031. [DOI] [PubMed] [Google Scholar]
- 90.Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 91.Okada J, Oonishi H, Yoshikawa K, Itami J, Uno K, Imaseki K, et al. FDG-PET for predicting the prognosis of malignant lymphoma. Ann Nucl Med. 1994;8:187–91. doi: 10.1007/BF03164996. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe R, Tomita N, Takeuchi K, Sakata S, Tateishi U, Tanaka M, et al. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:279–83. doi: 10.3109/10428190903440953. [DOI] [PubMed] [Google Scholar]
- 93.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103:216–28. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 94.Noy A, Schoder H, Gonen M, Weissler M, Ertelt K, Cohler C, et al. The majority of transformed lymphomas have high standardized uptake values (SUVs) on positron emission tomography (PET) scanning similar to diffuse large B-cell lymphoma (DLBCL) Ann Oncol. 2009;20:508–12. doi: 10.1093/annonc/mdn657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–56. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 96.Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol. 1967;18:158–62. doi: 10.1016/s0009-9260(67)80010-2. [DOI] [PubMed] [Google Scholar]
- 97.Collins CD. Multiple myeloma. Cancer Imaging. 2010;10:20–31. doi: 10.1102/1470-7330.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lutje S, de Rooy JW, Croockewit S, Koedam E, Oyen WJ, Raymakers RA. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol. 2009;88:1161–8. doi: 10.1007/s00277-009-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Healy CF, Murray JG, Eustace SJ, Madewell J, O'Gorman PJ, O'Sullivan P. Multiple myeloma: a review of imaging features and radiological techniques. Bone Marrow Res. 2011;2011:583439. doi: 10.1155/2011/583439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Derlin T, Bannas P. Imaging of multiple myeloma: Current concepts. World J Orthop. 2014;5:272–82. doi: 10.5312/wjo.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schirrmeister H, Bommer M, Buck AK, Muller S, Messer P, Bunjes D, et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361–6. doi: 10.1007/s00259-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 102.Breyer RJ, 3rd, Mulligan ME, Smith SE, Line BR, Badros AZ. Comparison of imaging with FDG PET/CT with other imaging modalities in myeloma. Skeletal Radiol. 2006;35:632–40. doi: 10.1007/s00256-006-0127-z. [DOI] [PubMed] [Google Scholar]
- 103.Haznedar R, Aki SZ, Akdemir OU, Ozkurt ZN, Ceneli O, Yagci M, et al. Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma. Eur J Nucl Med Mol Imaging. 2011;38:1046–53. doi: 10.1007/s00259-011-1738-8. [DOI] [PubMed] [Google Scholar]
- 104.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–95. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 105.Sager S, Ergul N, Ciftci H, Cetin G, Guner SI, Cermik TF. The value of FDG PET/CT in the initial staging and bone marrow involvement of patients with multiple myeloma. Skeletal Radiol. 2011;40:843–7. doi: 10.1007/s00256-010-1088-9. [DOI] [PubMed] [Google Scholar]
- 106.Nanni C, Zamagni E, Celli M, Caroli P, Ambrosini V, Tacchetti P, et al. The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): experience with 77 patients. Clin Nucl Med. 2013;38:e74–9. doi: 10.1097/RLU.0b013e318266cee2. [DOI] [PubMed] [Google Scholar]
- 107.Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013;121:1819–23. doi: 10.1182/blood-2012-08-451690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mulligan ME. Imaging techniques used in the diagnosis, staging, and follow-up of patients with myeloma. Acta Radiol. 2005;46:716–24. doi: 10.1080/02841850500215360. [DOI] [PubMed] [Google Scholar]
- 109.Durie BG. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer. 2006;42:1539–43. doi: 10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 110.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blade J, Dimopoulos M, Rosinol L, Rajkumar SV, Kyle RA. Smoldering (asymptomatic) multiple myeloma: current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J Clin Oncol. 2010;28:690–7. doi: 10.1200/JCO.2009.22.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Durie BG, Waxman AD, D'Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457–63. [PubMed] [Google Scholar]
- 113.Regelink JC, Minnema MC, Terpos E, Kamphuis MH, Raijmakers PG, Pieters-van den Bos IC, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162:50–61. doi: 10.1111/bjh.12346. [DOI] [PubMed] [Google Scholar]
- 114.Spinnato P, Bazzocchi A, Brioli A, Nanni C, Zamagni E, Albisinni U, et al. Contrast enhanced MRI and (1)(8)F-FDG PET-CT in the assessment of multiple myeloma: a comparison of results in different phases of the disease. Eur J Radiol. 2012;81:4013–8. doi: 10.1016/j.ejrad.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 115.Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–5. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 116.Caldarella C, Treglia G, Isgro MA, Treglia I, Giordano A. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to treatment in patients with multiple myeloma. Int J Mol Imaging. 2012;2012:175803. doi: 10.1155/2012/175803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elliott BM, Peti S, Osman K, Scigliano E, Lee D, Isola L, et al. Combining FDG-PET/CT with laboratory data yields superior results for prediction of relapse in multiple myeloma. Eur J Haematol. 2011;86:289–98. doi: 10.1111/j.1600-0609.2010.01575.x. [DOI] [PubMed] [Google Scholar]
- 118.Adam Z, Bolcak K, Stanicek J, Buchler T, Pour L, Krejci M, et al. Fluorodeoxyglucose positron emission tomography in multiple myeloma, solitary plasmocytoma and monoclonal gammapathy of unknown significance. Neoplasma. 2007;54:536–40. [PubMed] [Google Scholar]
- 119.Nanni C, Rubello D, Zamagni E, Castellucci P, Ambrosini V, Montini G, et al. 18F-FDG PET/CT in myeloma with presumed solitary plasmocytoma of bone. In Vivo. 2008;22:513–7. [PubMed] [Google Scholar]
- 120.Lu YY, Chen JH, Liang JA, Chu S, Lin WY, Kao CH. 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: a systematic review and meta-analysis. Nucl Med Commun. 2014;35:697–703. doi: 10.1097/MNM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 121.Chargari C, Vennarini S, Servois V, Bonardel G, Lahutte M, Fourquet A, et al. Place of modern imaging modalities for solitary plasmacytoma: toward improved primary staging and treatment monitoring. Crit Rev Oncol Hematol. 2012;82:150–8. doi: 10.1016/j.critrevonc.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–5. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- 123.Endo T, Sato N, Koizumi K, Nishio M, Fujimoto K, Sakai T, et al. Localized relapse in bone marrow of extremities after allogeneic stem cell transplantation for acute lymphoblastic leukemia. Am J Hematol. 2004;76:279–82. doi: 10.1002/ajh.20106. [DOI] [PubMed] [Google Scholar]
- 124.Ennishi D, Maeda Y, Niiya M, Shinagawa K, Tanimoto M. Incidental detection of acute lymphoblastic leukemia on [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2009;27:e269–70. doi: 10.1200/JCO.2009.22.7769. [DOI] [PubMed] [Google Scholar]
- 125.Kuenzle K, Taverna C, Steinert HC. Detection of extramedullary infiltrates in acute myelogenous leukemia with whole-body positron emission tomography and 2-deoxy-2-[18F]-fluoro-D-glucose. Mol Imaging Biol. 2002;4:179–83. doi: 10.1016/s1095-0397(01)00056-5. [DOI] [PubMed] [Google Scholar]
- 126.Stolzel F, Rollig C, Radke J, Mohr B, Platzbecker U, Bornhauser M, et al. (1)(8)F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96:1552–6. doi: 10.3324/haematol.2011.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Falchi L, Keating MJ, Marom EM, Truong MT, Schlette EJ, Sargent RL, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014;123:2783–90. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conte MJ, Bowen DA, Wiseman GA, Rabe KG, Slager SL, Schwager SM, et al. Use of positron emission tomography-computed tomography in the management of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2014;55:2079–84. doi: 10.3109/10428194.2013.869801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Papajik T, Myslivecek M, Urbanova R, Buriankova E, Kapitanova Z, Prochazka V, et al. 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography examination in patients with chronic lymphocytic leukemia may reveal Richter transformation. Leuk Lymphoma. 2014;55:314–9. doi: 10.3109/10428194.2013.802313. [DOI] [PubMed] [Google Scholar]
- 130.Bruzzi JF, Macapinlac H, Tsimberidou AM, Truong MT, Keating MJ, Marom EM, et al. Detection of Richter's transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006;47:1267–73. [PubMed] [Google Scholar]
- 131.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123:1647–57. doi: 10.1182/blood-2013-11-516229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Conconi A, Ponzio C, Lobetti-Bodoni C, Motta M, Rancoita PM, Stathis A, et al. Incidence, risk factors and outcome of histological transformation in follicular lymphoma. Br J Haematol. 2012;157:188–96. doi: 10.1111/j.1365-2141.2012.09054.x. [DOI] [PubMed] [Google Scholar]
- 133.Mauro FR, Chauvie S, Paoloni F, Biggi A, Cimino G, Rago A, et al. Diagnostic and prognostic role of PET/CT in patients with chronic lymphocytic leukemia and progressive disease. Leukemia. 2015;29:1360–5. doi: 10.1038/leu.2015.21. [DOI] [PubMed] [Google Scholar]
- 134.Herrmann K, Buck AK, Schuster T, Rudelius M, Wester HJ, Graf N, et al. A pilot study to evaluate 3′-deoxy-3′-18F-fluorothymidine pet for initial and early response imaging in mantle cell lymphoma. J Nucl Med. 2011;52:1898–902. doi: 10.2967/jnumed.111.094698. [DOI] [PubMed] [Google Scholar]
- 135.Buck AK, Bommer M, Stilgenbauer S, Juweid M, Glatting G, Schirrmeister H, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–61. doi: 10.1158/0008-5472.CAN-06-1955. [DOI] [PubMed] [Google Scholar]
- 136.Wang RM, Zhu HY, Li F, Liu CB, Guan ZW, Yao SL. Value of (18)F-FLT positron emission tomography/computed tomography in diagnosis and staging of diffuse large B-cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:603–7. [PubMed] [Google Scholar]
- 137.Hutchings M. Pre-transplant positron emission tomography/computed tomography (PET/CT) in relapsed Hodgkin lymphoma: time to shift gears for PET-positive patients? Leuk Lymphoma. 2011;52:1615–6. doi: 10.3109/10428194.2011.601477. [DOI] [PubMed] [Google Scholar]
- 138.Herrmann K, Buck AK, Schuster T, Junger A, Wieder HA, Graf N, et al. Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med. 2011;52:690–6. doi: 10.2967/jnumed.110.084566. [DOI] [PubMed] [Google Scholar]
- 139.Lee H, Kim SK, Kim YI, Kim TS, Kang SH, Park WS, et al. Early determination of prognosis by interim 3′-deoxy-3′-18F-fluorothymidine PET in patients with non-Hodgkin lymphoma. J Nucl Med. 2014;55:216–22. doi: 10.2967/jnumed.113.124172. [DOI] [PubMed] [Google Scholar]
- 140.Herrmann K, Buck AK, Schuster T, Abbrederis K, Blumel C, Santi I, et al. Week one FLT-PET response predicts complete remission to R-CHOP and survival in DLBCL. Oncotarget. 2014;5:4050–9. doi: 10.18632/oncotarget.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res. 2011;35:310–6. doi: 10.1016/j.leukres.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hummel S, Van Aken H, Zarbock A. Inhibitors of CXC chemokine receptor type 4: putative therapeutic approaches in inflammatory diseases. Curr Opin Hematol. 2014;21:29–36. doi: 10.1097/MOH.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 143.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 144.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, et al. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19:357–66. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 145.Moreno MJ, Bosch R, Dieguez-Gonzalez R, Novelli S, Mozos A, Gallardo A, et al. CXCR4 expression enhances diffuse large B cell lymphoma dissemination and decreases patient survival. J Pathol. 2015;235:445–55. doi: 10.1002/path.4446. [DOI] [PubMed] [Google Scholar]
- 146.Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol. 2015;6:12. doi: 10.3389/fimmu.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Demmer O, Gourni E, Schumacher U, Kessler H, Wester HJ. PET imaging of CXCR4 receptors in cancer by a new optimized ligand. ChemMedChem. 2011;6:1789–91. doi: 10.1002/cmdc.201100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gourni E, Demmer O, Schottelius M, D'Alessandria C, Schulz S, Dijkgraaf I, et al. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. J Nucl Med. 2011;52:1803–10. doi: 10.2967/jnumed.111.098798. [DOI] [PubMed] [Google Scholar]
- 149.Wester HJ, Keller U, Schottelius M, Beer A, Philipp-Abbrederis K, Hoffmann F, et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5:618–30. doi: 10.7150/thno.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Philipp-Abbrederis K, Herrmann K, Knop S, Schottelius M, Eiber M, Luckerath K, et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med. 2015;7:477–87. doi: 10.15252/emmm.201404698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herrmann K, Lapa C, Wester HJ, Schottelius M, Schiepers C, Eberlein U, et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J Nucl Med. 2015;56:410–6. doi: 10.2967/jnumed.114.151647. [DOI] [PubMed] [Google Scholar]
- 152.Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients--a hypothesis-generating exploratory study. Radiology. 2013;269:857–69. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 153.Al-Nabhani KZ, Syed R, Michopoulou S, Alkalbani J, Afaq A, Panagiotidis E, et al. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. J Nucl Med. 2014;55:88–94. doi: 10.2967/jnumed.113.123547. [DOI] [PubMed] [Google Scholar]
- 154.Wiesmuller M, Quick HH, Navalpakkam B, Lell MM, Uder M, Ritt P, et al. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:12–21. doi: 10.1007/s00259-012-2249-y. [DOI] [PubMed] [Google Scholar]


