Abstract
Objective:
To analyse the impact of the International Nosocomial Infection Control Consortium (INICC) Multidimensional Approach (IMA) and INICC Surveillance Online System (ISOS) on central line-associated bloodstream infection (CLABSI) rates in five intensive care units (ICUs) from October 2013 to September 2015.
Design:
Prospective, before-after surveillance study of 3769 patients hospitalised in four adult ICUs and one paediatric ICU in five hospitals in five cities. During baseline, we performed outcome and process surveillance of CLABSI applying CDC/NHSN definitions. During intervention, we implemented IMA and ISOS, which included: (1) a bundle of infection prevention practice interventions; (2) education; (3) outcome surveillance; (4) process surveillance; (5) feedback on CLABSI rates and consequences; and (6) performance feedback of process surveillance. Bivariate and multivariate regression analyses were performed.
Results:
During baseline, 4468 central line (CL) days and 31 CLABSIs were recorded, accounting for 6.9 CLABSIs per 1000 CL-days. During intervention, 12,027 CL-days and 37 CLABSIs were recorded, accounting for 3.1 CLABSIs per 1000 CL-days. The CLABSI rate was reduced by 56% (incidence-density rate, 0.44; 95% confidence interval, 0.28–0.72; P = 0.001).
Conclusions:
Implementing IMA through ISOS was associated with a significant reduction in the CLABSI rate in the ICUs of Saudi Arabia.
Keywords: Hospital infection, healthcare-acquired infection (HCAI), device-associated infection, central line-associated bloodstream infection (CLABSI), developing countries, resource-limited countries, critical care, surveillance, incidence density, bundle
Background
Central-line associated bloodstream infections (CLABSIs) are serious life-threatening infections in the intensive care unit (ICU) setting (Rosenthal et al., 2003a; Stevens et al., 2013). The clinical consequences of CLABSI include increased mortality, significant morbidity and increased length of stay (LOS), as shown in studies from developed and resource-limited countries (Higuera et al., 2007; Stevens et al., 2013). From an economic perspective, CLABSIs are also responsible for substantial increases in healthcare costs, as reported in both high-income (Stone et al., 2005) and resource-limited countries (Higuera et al., 2007). The burden posed by CLABSI has not been systematically analysed enough in resource-limited countries and although hospitals in resource-limited countries do implement basic infection control programmes, compliance with infection control practices is variable (Rosenthal et al., 2014). As reported by the International Nosocomial Infection Control Consortium (INICC) in pooled studies (Rosenthal et al., 2014) and in studies from the Kingdom of Saudi Arabia (Al-Abdely et al., 2016; Al-Tawfiq and Abed, 2009; Al-Tawfiq et al., 2013; Khalid et al., 2013), rates of CLABSI have been determined to be 3–5 times higher in resource-limited countries than in more economically developed countries.
According to the literature from more economically developed countries, the incidence of CLABSI can be prevented and reduced by more than 30% through basic but effective measures, such as those described in the bundle for CLABSI prevention developed by the Institute for Healthcare Improvement (IHI, 2012)—(1) hand hygiene; (2) skin antisepsis with chlorhexidine; (3) maximal barriers; (4) insertion in subclavian vein; and (5) timely central line (CL) removal—were associated with a reduction in the incidence density of CLABSI in developed countries (Pronovost et al., 2006). According to the literature from resource-limited countries, this incidence can be reduced by more than 50% (Rosenthal et al., 2010).
Founded in Argentina in 1998, the INICC was the first multinational research network established to measure, prevent and control healthcare-associated infections (HCAIs) at an international level through the analysis of data collected on a voluntary basis by a pool of hospitals worldwide (Rosenthal, 2016). The goals of the INICC include the development of a dynamic global hospital network that applies systematic surveillance of HCAIs with standardised definitions and methodologies of CDC/NHSN (CDC/NHSN, 2013) to promote evidence-based infection control practices, and to conduct applied infection control research to reduce the incidence of HCAIs and associated mortality, excess LOS, costs and bacterial resistance (Rosenthal, 2016).
The aim of this study was to determine the effect of the INICC Multidimensional Approach (IMA) and the use of the INICC Surveillance Online System (ISOS) for the reduction of CLABSI rates in four adult ICUs and one paediatric ICU in five hospitals in five cities in Saudi Arabia, and through its publication increase and spread tools and methods to be able to reduce this public health burden in the Kingdom of Saudi Arabia and other similar countries.
Methods
Setting and study design
This prospective, cohort, before-after study was conducted in four adult ICUs and one paediatric ICU in five INICC member hospitals in five cities of the Kingdom of Saudi Arabia. The study period was divided into a three-month ‘baseline period’, and an ‘intervention period’ starting from the fourth month of participation. The results obtained from the ‘baseline period’ were compared to the results obtained during the ‘intervention period’. Each ICU had an infection control team (ICT) comprising infection control professionals (ICPs) and medical doctors with formal education and background in internal medicine, critical care, infectious diseases, microbiology and/or hospital epidemiology.
It is worth clarifying that the methodology of ISOS has been used by INICC members since 2013, although the publication describing it was published in 2016 (Rosenthal, 2016).
Baseline period
The baseline period was from 1 October 2013 to 31 December 2013. This was a prospective cohort study and each ICU joined the study simultaneously.
During baseline period, only cohort HCAI Outcome Surveillance and Process Surveillance in ICU patients (two components of the IMA that are described below) were conducted. The length of the baseline period was three months due to the following reasons: (1) sample size of patients and number of months of data collection during the baseline period was sufficient enough to compare with sample size of patients and number of months of data collection during intervention period. From a statistical perspective, the issue is addressed by considering the changes in rates over time. The relatively short baseline period may have impacted the standard error of our estimates. But we found that this would not cause a bias in the results, because there would not be systematic differences between the two groups. (2) Our priority was to start intervention as early as possible in order to achieve the desired results, such as CLABSI rate reduction.
Intervention period
The intervention period was from 1 January 2014 (fourth month of participation) to 30 September 2015, and included the implementation of the six components of the IMA, as described below.
INICC Multidimensional Approach (IMA)
The IMA comprises the simultaneous implementation of the following six components for HCAI control and prevention: (1) a bundle of infection prevention practice interventions; (2) education; (3) outcome surveillance; (4) process surveillance; (5) feedback on HCAI rates and consequences; and (6) performance feedback.
The contents of the IMA include CDC/NSHN’s surveillance methodology, but they also include the collection of other data essential to increase ICPS’s sensitivity to detect HCAIs and avoid underreporting (CDC/NHSN, 2013). According to standard CDC/NSHN methods, numerators are the number of each type of HCAI, and denominators are device-days collected from all patients, as pooled data; that is, without determining the number of device-days related to a particular patient and without collecting characteristics per specific patient (CDC/NHSN, 2013). This differs from the IMA in that the design of the cohort study through the INICC methods also includes collecting specific data per patient from all patients, both those with and those without HCAI, and collecting risk factors of HCAIs, such as invasive devices, and surrogates of HCAIs, which include, but are not limited to, high temperature, low blood pressure, results of cultures, antibiotic therapy, LOS and mortality. By collecting data on all patients in the ICU, it is possible to match patients with and without HAI by several characteristics to estimate extra LOS, mortality and cost.
The data concerned in the IMA were registered and uploaded to the ISOS. The ISOS comprised 15 modules: (1) Cohort HCAI surveillance in adult and paediatric ICU patients; (2) Cohort HCAI surveillance in neonatal ICU patients; (3) Cohort HCAI surveillance in step down units and inpatient wards; (4) Aggregated HCAI surveillance in ICU for adult and paediatric patients; (5) Aggregated HCAI surveillance in ICU for neonatal patients; (6) Microbiology for adult and paediatric patients; (7) Multi Drug Resistant Organisms and Clostridium difficile Infections; (8) Monitoring HH; (9) Monitoring bundle for BSI; (10) Monitoring bundle for UTI; (11) Monitoring bundle for PNEU; (12) Surgical procedures: outcome surveillance; (13) Surgical procedures: process surveillance; (14) Antimicrobial consumption; and (15) Needle stick injuries.
1. Bundle of infection prevention practice interventions
The bundles of infection prevention practice interventions were designed following the recommendations and guidelines published by the SHEA and the IDSA published in 2008 (Marschall et al., 2008) and in 2014 (Yokoe et al., 2014), the bundle for CLABSI prevention developed by the Institute for Healthcare Improvement in 2012 (IHI, 2012), and the guidelines published by the CDC-NSHN in 2011 (O’Grady et al., 2011), and by the Joint Commission International in 2012 (JCI, 2012). These guidelines and bundle describe different recommendations for CLABSI prevention that are classified into categories regarding the existing scientific evidence, applicability and their prospective economic effects.
Components of INICC Infection Control Bundle for CLABSI prevention
(1) Perform hand hygiene before CL insertion or manipulation; (2) use maximal sterile barrier precautions during CL insertion; (3) use a chlorhexidine-based antiseptic for skin preparation; (4) remove CL as early as possible, when not necessary, by means of the daily assessment of the necessity of catheter, thereby aiming at the reduction of CL device utilisation ratio (DUR); (5) change administration set every 96 h; unless used for fat, nutrition or blood products, and in these cases changed every 24 h, by means of checking date on administration set; (6) use sterile gauze or transparent sterile dressing to cover insertion site, maintain optimal condition of sterile dressing; and change gauze every 48 h and transparent dressing every seven days; (7) daily bath with 2% chlorhexidine-impregnated washcloth; (8) avoid insertion of CL in the femoral vein in adult patients; (9) use an all-inclusive catheter cart or kit; (10) avoid using single-use vials several times; (11) disinfect line hubs, needleless connectors and ports before accessing the CL; (12) split septum as i.v. connector; and (13) use collapsible non-vented closed system bag as i.v. fluid container (Rosenthal, 2016).
2. Education
Education sessions were provided to all healthcare workers (HCWs) in the participating ICUs on training about the infection control measures contained in the above-described INICC Infection Control Bundle for CLABSI prevention. During a first phase, at baseline period, the INICC team locally trained the IPCs at each of the five hospitals on how to conduct surveillance and upload surveillance data to the ISOS. During intervention, the INICC team locally provided education and training sessions to ICPs on the 13 components of the INICC Infection Control Bundle (Rosenthal, 2016) for CLABSI Prevention (training the trainers). In turn, on a monthly basis, ICPs at the five hospitals trained the ICU teams on how to conduct implement the mentioned bundle components. Education sessions can be measured regarding its efficacy through its impact on rates of compliance with the bundle components. We consider the results of process surveillance would have been achieved because HCWs had been trained and were aware that they were being observed to assess compliance with the preventive measures of the bundle components (Monahan and Fisher, 2010).
3. Outcome surveillance
Prospective, active outcome surveillance through the ISOS allowed the classification of cohort surveillance data into specific module protocols that apply U.S. CDC/NHSN’s definitions published in January 2013 (CDC/NHSN, 2013). The site-specific criteria included reporting instructions and provided full explanations integral to their adequate application (CDC/NHSN, 2013).
4. Process surveillance
The process surveillance was performed through the ISOS modules, which included the monitoring of compliance with the following components of the INICC Infection Control Bundle for CLABSI Prevention (Rosenthal, 2016): (1) hand hygiene compliance before CL insertion or manipulation; (2) insertion of CL using maximum sterile barrier precautions; (3) skin antisepsis with chlorhexidine; (4) daily assessment of the need of the CL and CL DUR; (5) compliance with date on administration set, (6) compliance with placed dressing, use of transparent dressing, use of gauze dressing, and compliance with optimal condition of dressing; and (7) daily bath with 2% chlorhexidine-impregnated washcloth.
The remaining components of the bundle for CLABSI prevention were not included in process surveillance due to budget restrictions (Rosenthal, 2016).
5. Feedback on device-associated healthcare-associated infection rates and consequences
The ICPs generated reports through the ISOS. The ICU HCWs received feedback on device-associated HCAI (DA-HCAI) rates and their consequences at monthly meetings held by ICPs, who shared and discussed the results of ISOS. These reports contained several charts and tables that showed a running record of the monthly cohort surveillance data, including patient characteristics, such as age and sex, proportion of DA-HCAIs, such as CLAB, ventilator-associated pneumonia (VAP) and catheter-associated urinary tract infection (CAUTI), pooled means of CLAB, VAP and CAUTI rates and of CL, mechanical ventilator and urinary catheter DURs, microorganisms profile, bacterial resistance, extra LOS and extra mortality attributable to DA-HCAIs. Also, benchmarks of these rates against standards from the CDC-NHSN report of 2013 (Dudeck et al., 2015), the last INICC Report of 43 countries (Rosenthal et al., 2014), standards from the Kingdom of Saudi Arabia, and INICC reports from Turkey, India, Colombia and Mexico.
Benchmarking was an important tool to increase the level of awareness of patient outcomes at their ICUs in comparison with other national and international standards, and to enable the ICPs and ICU team to focus on the necessary issues and apply specific strategies for the reduction of CLABSI rates.
6. Performance feedback
At monthly meetings, performance feedback was provided by ICPs to HCWs working in the ICU by communicating and reviewing the resulting rates of process surveillance; that is, the assessment of practices performed by them in the ICU related to the components of the INICC Infection Control Bundle for CLABSI prevention (Rosenthal, 2016). The ICPs showed a report of 32 charts generated through the ISOS, which contained data regarding compliance with the elements of the bundle, including: compliance with hand hygiene pooled by month, stratified by gender and by HCW category, proportion of hand hygiene observed opportunities stratified by five moments of WHO, by used product, by technique and by work shift; and compliance per month of the above-mentioned bundle components. This infection control tool was essential to enable the ICT to be aware if there was room for improvement of low compliance rates, and through the influence of the ‘observer effects’ on HCWs’ behaviour, as strength of the method, to shape their practices so that they were more efficiently performed (Monahan and Fisher, 2010).
Data collection and analysis
The ISOS meets the criteria set out in the INICC protocol and CDC-NSHN criteria, which were followed by the ICPs who collected daily data on CLABSIs and denominator data, patient-days and specific device-days in the ICUs.
These data were uploaded to the ISOS and were used to calculate CLAB rates per 1000 CL-days and CL utilisation ratio, according to the following formulas: (1) CL-days consisted of the total number of CL-days; (2) CL DURs equals the total number of CL-days divided by the total number of bed-days; and (3) CLABSI rate per 1000 CL-days was calculated according to CDC/NHSN formula (CDC/NHSN, 2013).
Definitions
We applied CDC/NHSN definitions for CLABSI published by CDC/NSHN in 2013 (CDC/NHSN, 2013).
Statistical methods
ISOS version 2.0 (Buenos Aires, Argentina) was used to calculate HAI rates and DUR.
Patients’ characteristics were compared using Fisher’s exact test for dichotomous variables and unmatched Student’s t-test for continuous variables. P values <0.05 by two-sided tests were considered significant.
We conducted three types of analysis to evaluate the impact of our intervention on CLABSI rates.
First, we performed an analysis to compare the data of the first three months (baseline period) with the remaining pooled months (intervention period), using relative risks (RR), 95% confidence interval (CI) and P value.
Second, in order to analyse progressive CLABSI rate reduction, we divided the data into the first three months (baseline period) followed by a nine-month period and a 12-month follow-up period (intervention period). We compared the CLABSI rates for each follow-up period with the baseline CLABSI rate. We calculated the incidence density rates (IDR), IDR ratios and IDR reduction to account for the CLABSI rate reduction.
Third, we estimated the effect of the intervention on CLABSI by means of a logistic regression model. A set of co-variables was included to account for possible confounding effects. A backward procedure that compares between nested models using the Akaike Information Criterion (AIC) was carried out to get the final set of significant co-variables. Collinearity among independent variables was measured using the variance inflation factor (VIF). We calculated the odds ratio (OR) and 95% CI for the intervention and other independent variables. The effectiveness of the intervention was calculated using the formula: (1−OR)×100, where OR is the adjusted odds ratio estimated by the model. All statistical analyses were performed using the R software version 3.2.2 (2014).
Results
During the study period, we recorded a total of 3769 patients, hospitalized in for 31,859 days, with a total of 24,238 CL-days, at five hospitals in five cities, in the following types of ICU: paediatric (260 patients) and medical/surgical (3509 patients).
Some patient characteristics, such as sex and type of hospitalization, were similar during both periods, whereas mean age was lower during the intervention period (Table 1).
Table 1.
Patient characteristics, device utilisation ratio and compliance with care bundle in the baseline and intervention periods.
| Patient characteristics | Baseline period | Intervention period | RR (95% CI) | P value |
|---|---|---|---|---|
| Study period by hospital in months, mean (range) | 3 | 19.2 (16–21) | – | – |
| Patients (n) | 785 | 2984 | – | – |
| Bed-days* (n) | 4632 | 27,227 | – | – |
| CL-days† (n) | 4468 | 19,770 | – | – |
| Age (years) (mean ± SD) | 46.4 (25.2) | 43.1 (25.6) | – | 0.001 |
| Male (n, %) | 531 (68%) | 2102 (70%) | – | 0.522 |
| Type of hospitalisation | ||||
| Medical (n, %) | 216 (55%) | 1688 (60%) | – | 0.058 |
| Surgical (n, %) | 179 (45%) | 1131 (40%) | – | 0.058 |
| Process surveillance of components of Bundle to prevent CLABSI | ||||
| Hand hygiene compliance before CL insertion or manipulation (%, n/n) | 29% (17/59) | 72% (719/999) | 0.53 (0.35–0.81) | 0.004 |
| Insertion of CL using maximum sterile barrier precautions (%, n/n) | 100% (107/107) | 88% (881/1003) | 1.1 (0.9–1.2) | 0.357 |
| Skin antisepsis with chlorhexidine (%, n/n) | 95% (102/107) | 87% (876/1003) | 1.0 (0.9–1.2) | 0.542 |
| Daily assessment of the need of CL (%, n/n) | 62% (66/107) | 84% (847/1003) | 0.83 (0.7–1.0) | 0.068 |
| CL DUR‡ | 0.96 | 0.73 | – | 0.001 |
| Compliance with date on administration set (%, n/n) | 100% (107/107) | 96% (959/1003) | 1.0 (0.9–1.2) | 0.753 |
| Compliance with placed dressing (%, n/n) | 100% (107/107) | 96% (959/1003) | 1.0 (0.9–1.2) | 0.753 |
| Use of transparent dressing (%, n/n) | 100% (107/107) | 90% (906/1003) | 1.0 (0.9–1.2) | 0.472 |
| Use of gauze dressing (%, n/n) | 0% | 6% (58/1003) | 0.08 (0.01–1.3) | 0.081 |
| Compliance with optimal condition of dressing (%, n/n) | 100% (107/107) | 96% (958/1003) | 1.0 (0.9–1.2) | 0.748 |
| Daily bath with a 2% chlorhexidine-impregnated washcloth (%, n/n) | 24% (26/107) | 52% (524/1003) | 0.57 (0.4–0.8) | 0.001 |
CI, confidence interval; CL, central line; CLABSI, central-line associated bloodstream infection; DUR, device utilisation ratio; RR, relative risk; SD, standard deviation.
Bed-days are the total number of days that patients are in the ICU during the selected time period.
CL-days are the total number of days of exposure to central lines by all of the patients in the selected population during the selected time period.
DUR: CL-days divided by the number of bed-days.
Regarding the results of the measurement of the bundle components, on the one hand, we registered statistically significant improvements in: (1) hand hygiene compliance; (2) daily bathing with a 2% chlorhexidine impregnated washcloth; and (3) CL DUR. On the other hand, the levels of compliance with—(1) use of maximum sterile barrier precautions; (2) daily assessment of the necessity of catheter; (3) presence of dressings placed; (4) condition of dressings, evaluating if the dressing was clean, dry and correctly adhered to the insertion site; and (5) total number of cases in which the dates of insertion were written in the administration set of the patient or the dressing—were high but not significantly different from baseline.
During the baseline period, we recorded 4468 CL-days. There were 31 CLABSIs, for an overall baseline rate of 6.9 CLABSIs per 1000 CL-days (Table 2).
Table 2.
Central line-associated bloodstream infection rates.
| Months since joining INICC | ICUs (n) | CL-days | CLABSI | Crude CLABSI rate/1000 CL-days (IDR) | IDR ratio (95%CI) | IDR reduction (%) | P value |
|---|---|---|---|---|---|---|---|
| 1–3 (baseline) | 5 | 4468 | 31 | 6.9 | – | – | – |
| 4–12 | 5 | 7743 | 38 | 4.9 | 0.71 (0.44–1.1) | 29% | 0.153 |
| 13–24 | 5 | 12,027 | 37 | 3.1 | 0.44 (0.28–0.72) | 56% | 0.001 |
CL, central line; CLABSI, central line-associated bloodstream infection; ICUs, intensive care units; IDR, incidence-density rate; INICC, International Nosocomial Infection Control Consortium.
During the intervention period, we recorded 19,770 CL-days. The rate of CLABSIs per 1000 CL-days was reduced to 3.1 CLABSIs per 1000 CL-days in the second year, accounting for a 56% cumulative CLABSI rate reduction (IDR 0.44; 95% CI 0.3–0.8; P = 0.005) (Table 2 and Figure 1).
Figure 1.
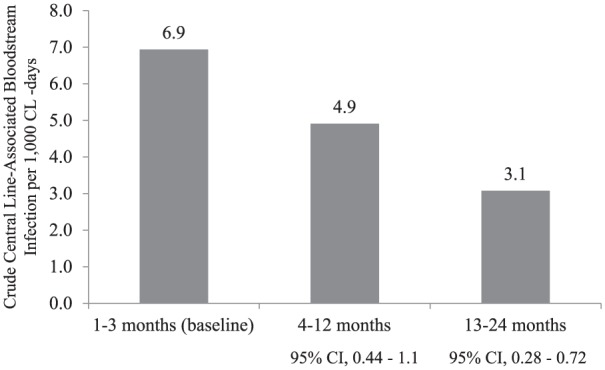
Central line-associated bloodstream infection rates in different study periods. CI, confidence interval; CL, central line.
The results of the logistic regression model are presented in Table 3. These results showed a significant reduction in the CLABSI risk in patients during the intervention period, when controlling for the number of CL-days (OR, 0.53; 95% CI, 0.33–0.84). The model also detected a significant excess risk for a unit increase in the CL-days (OR, 1.03; 95% CI, 1.03–1.04). The adjusted effectiveness of the intervention was 47% (95% CI, 16–67%). Collinearity indices in the final model were low (1.028–1.076), indicating absence of multicollinearity among the independent variables.
Table 3.
Results of the logistic regression model showing the effect of the INICC intervention on the central line-associated bloodstream infection rates.
| Variables | Coeff (SE) | Adjusted OR* (95% CI) | P value |
|---|---|---|---|
| Period (intervention) | –0.636 (0.236) | 0.53 (0.33–0.84) | 0.007 |
| CL-days† | 0.034 (0.038) | 1.03 (1.03–1.04)‡ | 0.000 |
CI, confidence interval; CL-days, number of central line days; Coeff, beta-coefficient of the logistic regression; OR, odds ratio; SE, standard error.
Adjusted OR for the logistic regression model including the two variables in the table.
CL-days: the total number of days of exposure to central line by all of the patients in the selected population during the selected time period.
For a unit increase in the CL-days.
The microorganisms profile is shown in Table 4. The predominant microorganisms in the baseline period were Acinetobacter spp., Candida spp. and Klebsiella pneumonia, whereas in the intervention period only Acinetobacter spp. were predominant.
Table 4.
Microorganisms related to central line-associated bloodstream infection in intensive care units in phase 1 (baseline period) and phase 2 (intervention period).
| Isolated microorganisms | Baseline, % (n) | Intervention, % (n) |
|---|---|---|
| Acinetobacter spp. | 22.2% (4) | 22.9% (11) |
| Burkholderia cepacia | 5.6% (1) | 0.0% (0) |
| Candida spp. | 22.2% (4) | 2.1% (1) |
| Coagulase-negative Staphylococi | 11.1% (2) | 8.3% (4) |
| Enterobacter spp. | 0.0% (0) | 4.2% (2) |
| Enterococcus spp. | 5.6% (1) | 4.2% (2) |
| Escherichia spp. | 5.6% (1) | 6.3% (3) |
| Klebsiella pneumoniae | 22.2% (4) | 16.7% (8) |
| Proteus mirabilis | 0.0% (0) | 6.3% (3) |
| Providencia spp. | 0.0% (0) | 6.3% (3) |
| Pseudomonas aeruginosa | 0.0% (0) | 12.5% (6) |
| Serratia marcescens | 5.6% (1) | 6.3% (3) |
| Staphylococcus aureus | 0.0% (0) | 4.2% (2) |
| Total | 100% (18) | 100% (48) |
Discussion
This study was conducted with the aim of assessing the effect of the IMA in the ICU in five cities from the Kingdom of Saudi Arabia. Within the scope of studies addressing the burden of CLABSIs in this country, in a study conducted by Al-Tawfiq et al. in the ICUs from a Saudi hospital it was found that the CLABSI rate was 10 per 1000 CL-days (Al-Tawfiq et al., 2013).
In comparison with international CLABSI rates, the CLABSI baseline rate found in this study (6.9 per 1000 CL-days) was similar to the last international INICC report for 2007–2012 (4.9 CLABSIs per 1000 CL-days [95% CI, 4.8–5.1]) (Rosenthal et al., 2014). By contrast, the baseline rate of CLABSI found in this study was significantly higher than the US 0.8 CLABSI rate per 1000 CL-days determined by the CDC/NHSN for 2013 (Dudeck et al., 2015); and higher than the 1.4 rate determined by German surveillance system KISS (Geffers and Gastmeier, 2011).
In our study, some patient characteristics, such as sex and type of hospitalisation, showed similar patient intrinsic risk in both study periods. By contrast, the mean age of patients was lower during the intervention period, meaning that the patient intrinsic risks related to age were lower in the intervention period. The high CLABSI rate determined in our ICUs at baseline was reduced from 6.9 to 3.1 per 1000 CL-days (IDR 0.44; 95% CI, 0.28–0.72; P = 0.001), showing a 56% CLABSI rate reduction. This reduction can be associated to the implementation of the IMA, because the results of the measurement of the bundle components showed statistically significant improvements in most of them, such as hand hygiene compliance, daily bathing with a 2% chlorhexidine impregnated washcloth and reduced CL DUR. In addition, compliance with other measures, such as use of maximum sterile barrier precautions, daily assessment of the necessity of catheter, sterile dressings placed to protect the insertion site, dressings in correct condition, evaluating if the dressing was clean, dry and correctly adhered to the insertion site and dates of insertion were written in the administration set of the patient were high and similar in both phases.
References from the literature showing a similar reduction are those published by Mazi et al., who found that a similar programmes reduced the incidence of CLABSI from 3.9 CLABSIs per 1000 CL-days to 1.5 per 1000 CL-days in a trauma ICU (Mazi et al., 2014), and by Khalid et al., who found a reduction from 6.9 to 0.35 in a tertiary hospital (Khalid et al., 2013). Similarly, it was shown in previous studies performed by the INICC that implementation of a four- or six-component multidimensional approach for CLABSI resulted in significant reductions in rates of CLABSI in Latin America, such as in Argentina (46.63 versus 11.10 CLABSIs per 1000 CL-days), showing a 76% reduction (Rosenthal et al., 2003b); in Europe, such as in Turkey (22.7 to 12.0 CLABSIs per 1000 CL-days), showing a 47% reduction (Leblebicioglu et al., 2013); in Asia, such as in India (6.4 CLABSIs to 3.9 CLABSIs per 1000 CL-days), showing a 39% reduction (Jaggi et al., 2013); and in multinational studies conducted in adult ICUs (14.5 versus 9.7 CLABSIs per 1000 CL-days), showing a 33% reduction (Rosenthal et al., 2010); and in paediatric ICUs (10.7 versus 5.2 CLABSIs per 1000 CL-days), showing a 51% reduction (Rosenthal et al., 2012). Currently, the same intervention is being implemented in 29 hospitals of 15 cities in the Kingdom of Saudi Arabia and we expect to have similar significant impact.
Regarding the microorganisms profile, we identified a predominance of Acinetobacter spp., Candida spp. and Klebsiella pneumoniae. By contrast, according to the scientific literature from the Kingdom of Saudi Arabia, the most common causative pathogens were Coagulase negative staphylococcus, Staphylococcus aureus and Escherichia coli (Al-Tawfiq and Abed, 2009). As published in the scientific literature, there has been a change in the global tendency toward gram-negative carriage, rather than gram-positive, which is greatly accentuated in the ICU setting due to the high exposure to nosocomial microorganisms (Rosenthal et al., 2014; Rubin, and Young, 2015)
Study limitations
The main limitation of this study is that our findings cannot be generalised to all ICU patients from the Kingdom of Saudi Arabia. However, in this study it was demonstrated that a multidimensional approach is fundamental to reduce the incidence of CLABSIs and associated mortality in the ICU setting.
Second, the setting of the three-month baseline period may be short and might have overestimated the effect of the intervention. Nevertheless, during the baseline period the sample size was good enough, and the confidence intervals for the baseline rate were narrow. Third, there may be significant variations in the level of quality control in the laboratories that support each individual hospital, and due to budget restrictions, some bundle components were not included in process surveillance, and not all the interventions included in the IMA could be quantified in detail, such as education.
Conclusions
This is the first study to report a substantial reduction in CLABSI rates in the ICU setting of the Kingdom of Saudi Arabia. The implementation of our multidimensional approach resulted in significant reductions in the CLABSI incidence rate, but this is part of a work in progress, which will continue to show further improvements, because the IMA is now being implemented in 29 hospitals in this country. In addition, these results are significant in at least two major respects: the systematically collected data serve as guidance for strategies to improve patient care practices; and these preventive strategies proven effective in the INICC ICUs of the Kingdom of Saudi Arabia can promote a wider acceptance of infection control programmes in hospitals worldwide, leading to significant CLABSI rate reduction.
Acknowledgments
The authors thank the many healthcare professionals at the Ministry of Health of the Kingdom of Saudi Arabia; each member hospital who assisted with the conduct of surveillance in their hospital; Débora López Burgardt, who works at INICC headquarters in Buenos Aires; and the INICC Advisory Board, Country Directors and Secretaries (Hail M. Alabdaley, Yassir Khidir Mohamed, Safaa Abdul Aziz AlKhawaja, Amani Ali El-Kholy, Vineya Rai, Isabel Villegas Mota, Souha S. Kanj, Hakan Leblebicioglu, Yatin Mehta, Bijie Hu, Lul Raka, Najiba M Abdulrazzaq, Sergio Cimerman, Alfonso J. Rodríguez-Morales, Sofía del Carmen González Collantes, Javier Eduardo Desse, Hernán Diosnel Rodríguez Enciso, Nguyen Viet Hung, Wing Hong Seto, Anucha Apisarnthanarak, Toshihiro Mitsuda, Syed Sattar, William Rutala, William R. Jarvis, Russell N. Olmsted, Carla J. Alvarado, Catherine Murphy, Dennis Maki, Nicholas Graves, Patricia Lynch and Didier Pittet), who have so generously supported this unique international infection control network.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors report no conflicts of interest related to this article. Every hospital’s Institutional Review Board agreed to the study protocol, and patient confidentiality was protected by codifying the recorded information, making it only identifiable to the infection control team.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The funding for design, development, maintenance, technical support, data validation, and report generation through ISOS, and the activities carried out at INICC headquarters, were provided by the corresponding author, Victor D Rosenthal and the Foundation to Fight against Nosocomial Infections (FLIN).
Peer review statement: Not commissioned; blind peer-reviewed.
References
- (2014) R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available at: http://www.r-project.org. [Google Scholar]
- Al-Abdely HM, Alshehri AD, Rosenthal VD, Banjar W, Mohammed YK, Assiri AM, Fabros Cabato A, Gonzales Celiz JM, Al Raey MA. (2016) Device-associated infection rates in intensive care units of 5 cities of Kingdom of Saudi Arabia: INICC’s findings. Canadian Journal of Infection Control In press. [Google Scholar]
- Al-Tawfiq JA, Abed MS. (2009) Prevalence and antimicrobial resistance of health care associated bloodstream infections at a general hospital in Saudi Arabia. Saudi Medical Journal 30: 1213–1218. [PubMed] [Google Scholar]
- Al-Tawfiq JA, Amalraj A, Memish ZA. (2013) Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004–2011. International Journal of Infectious Diseases 17: e1207–1211. [DOI] [PubMed] [Google Scholar]
- CDC/NHSN. (2013) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Atlanta, GA: CDC; Available at: http://www.cdc.gov/nhsn/. [DOI] [PubMed] [Google Scholar]
- Dudeck MA, Edwards JR, Allen-Bridson K, Gross C, Malpiedi PJ, Peterson KD, Pollock DA, Weiner LM, Sievert DM. (2015) National Healthcare Safety Network report, data summary for 2013, Device-associated Module. American Journal of Infection Control 43: 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffers C, Gastmeier P. (2011) Nosocomial infections and multidrug-resistant organisms in Germany: epidemiological data from KISS (the Hospital Infection Surveillance System). Deutsches Arzteblatt International 108: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera F, Rangel-Frausto MS, Rosenthal VD, Soto JM, Castanon J, Franco G, Tabal-Galan N, Ruiz J, Duarte P, Graves N. (2007) Attributable cost and length of stay for patients with central venous catheter-associated bloodstream infection in Mexico City intensive care units: a prospective, matched analysis. Infection Control and Hospital Epidemiology 28: 31–35. [DOI] [PubMed] [Google Scholar]
- IHI. (2012) How-to Guide: Prevent Central Line-Associated Bloodstream Infections. Cambridge, MA: Institute for Healthcare Improvement; Available at: www.ihi.org. [Google Scholar]
- Jaggi N, Rodrigues C, Rosenthal VD, Todi SK, Shah S, Saini N, Dwivedy A, Udwadia FE, Mehta P, Chakravarthy M, Singh S, Sahu S, Govil D, Hegd A, Kapadia F, Bhakta A, Bhattacharyya M, Singhal T, Naik R, Kothari V, Gupta A, Shetty S, Binu S, Pinto P, Poojary A, Koppikar G, Bhandarkar L, Jadhav S, Chavan N, Bahirune S, Durgad S, Nataraj G, Surase P, Gokul BN, Sukanya R, Pushparaj L, Radhakrishnan K. (2013) Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. International Journal of Infectious Diseases 17(12): e1218–1224. [DOI] [PubMed] [Google Scholar]
- JCI. (2012) Best Practices in Infection Prevention and Control: An International Perspective. 2nd ed. Oakbrook Terrace, IL: Joint Commission International. [Google Scholar]
- Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. (2013) Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. American Journal of Infection Control 41: 1209–1213. [DOI] [PubMed] [Google Scholar]
- Leblebicioglu H, Ozturk R, Rosenthal VD, Akan OA, Sirmatel F, Ozdemir D, Uzun C, Turgut H, Ersoz G, Koksal I, Ozgultken A, Esen S, Ulger F, Dilek A, Yilmaz H, Dikmen Y, Aygun G, Tulunay M, Oral M, Unal N, Cengiz M, Yilmaz L, Geyik MF, Sahin A, Erdogan S, Sacar S, Sungurtekin H, Ugurcan D, Kaya A, Kuyucu N, Yylmaz G, Kaya S, Ulusoy H, Inan A. (2013) Impact of a multidimensional infection control approach on central line-associated bloodstream infections rates in adult intensive care units of 8 cities of Turkey: findings of the International Nosocomial Infection Control Consortium (INICC). Annals of Clinical Microbiology and Antimicrobials 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall J, Mermel LA, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Calfee DP, Coffin SE, Dubberke ER, Fraser V, Gerding DN, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Nicolle L, Pegues DA, Perl TM, Saint S, Salgado CD, Weinstein RA, Wise R, Yokoe DS. (2008) Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infection Control and Hospital Epidemiology 29 (Suppl. 1): S22–30. [DOI] [PubMed] [Google Scholar]
- Mazi W, Begum Z, Abdulla D, Hesham A, Maghari S, Assiri A, Senok A. (2014) Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. American Journal of Infection Control 42: 865–867. [DOI] [PubMed] [Google Scholar]
- Monahan T, Fisher JA. (2010) Benefits of “Observer Effects”: Lessons from the Field. Qualitative Research 10: 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. and Healthcare Infection Control Practices Advisory Committee. (2011) Guidelines for the prevention of intravascular catheter-related infections. American Journal of Infection Control 39 (Suppl. 1): S1–34. [DOI] [PubMed] [Google Scholar]
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. New England Journal of Medicine 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD. (2016) International Nosocomial Infection Control Consortium (INICC) resources: INICC multidimensional approach and INICC surveillance online system; American Journal of Infection Control 44: e81–90. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Guzman S, Orellano PW. (2003a) Nosocomial infections in medical-surgical intensive care units in Argentina: attributable mortality and length of stay. American Journal of Infection Control 31: 291–295. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. (2003b) Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. American Journal of Infection Control 31: 405–409. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Maki DG, Mehta Y, Leblebicioglu H, Memish ZA, Al-Mousa HH, Balkhy H, Hu B, Alvarez-Moreno C, Medeiros EA, Apisarnthanarak A, Raka L, Cuellar LE, Ahmed A, Navoa-Ng JA, El-Kholy AA, Kanj SS, Bat-Erdene I, Duszynska W, Van Truong N, Pazmino LN, See-Lum LC, Fernandez-Hidalgo R, Di-Silvestre G, Zand F, Hlinkova S, Belskiy V, Al-Rahma H, Lugue-Torres MT, Bayraktar N, Mitrev Z, Gurskis V, Fisher D, Abu-Khader IB, Berechid K, Rodriguez-Sanchez A, Horhat FG, Requejo-Pino O, Hadjieva N, Ben-Jaballah N, Garcia-Mayorca E, Kushner-Davalos L, Pasic S, Pedrozo-Ortiz LE, Apostolopoulou E, Mejia N, Gamar-Elanbya MO, Jayatilleke K, de Lourdes-Duenas M, Aquirre-Avalos G. and International Nosocomial Infection Control Consortium. (2014) International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. American Journal of Infection Control 42: 942–956. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Maki DG, Rodrigues C, Alvarez-Moreno C, Leblebicioglu H, Sobreya-Oropeza M, Berba R, Madani N, Medeiros EA, Cuellar LE, Mitrev Z, Duenas L, Guanche-Garcell H, Mapp T, Kanj SS, Fernandez-Hidalgo R. and International Nosocomial Infection Control Consortium Investigators. (2010) Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infection Control and Hospital Epidemiology 31: 1264–1272. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Ramachandran B, Villamil-Gomez W, Armas-Ruiz A, Navoa-Ng JA, Matta-Cortes L, Pawar M, Nevzat-Yalcin A, Rodriguez-Ferrer M, Yildizdas RD, Menco A, Campuzano R, Villanueva VD, Rendom-Campo LF, Gupta A, Turhan O, Barahona-Guzman N, Horoz OO, Arrieta P, Brito JM, Tolentino MC, Astudillo Y, Saini N, Gunay N, Sarmiento-Villa G, Gumus E, Lagares-Guzman A, Dursun O. (2012) Impact of a multidimensional infection control strategy on central line-associated bloodstream infection rates in pediatric intensive care units of five developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 40: 415–423. [DOI] [PubMed] [Google Scholar]
- Rubin RH, Young LS. (2015) Clinical Approach to Infection in the Compromised Hos. 5th edn. New York: Springer. [Google Scholar]
- Stevens V, Geiger K, Concannon C, Nelson RE, Brown J, Dumyati G. (2013) Inpatient costs, mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clinical Microbiology and Infection 20: O318–324. [DOI] [PubMed] [Google Scholar]
- Stone PW, Braccia D, Larson E. (2005) Systematic review of economic analyses of health care-associated infections. American Journal of Infection Control 33: 501–509. [DOI] [PubMed] [Google Scholar]
- Yokoe DS, Anderson DJ, Berenholtz SM, Calfee DP, Dubberke ER, Ellingson KD, Gerding DN, Haas JP, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle LE, Salgado CD, Bryant K, Classen D, Crist K, Deloney VM, Fishman NO, Foster N, Goldmann DA, Humphreys E, Jernigan JA, Padberg J, Perl TM, Podgorny K, Septimus EJ, VanAmringe M, Weaver T, Weinstein RA, Wise R, Maragakis LL. (2014) A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. American Journal of Infection Control 42: 820–828. [DOI] [PubMed] [Google Scholar]


