Abstract
Following the successful application of immune checkpoint blockade therapy (CBT) in refractory solid tumors, it has recently gained momentum as a promising modality in the treatment of relapsed lymphoma. This significant therapeutic advance stems from decades of research that elucidated the role of immune regulation pathways and the mechanisms by which tumors can engage these critical pathways to escape immune detection. To date, two main pathways, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1), have emerged as key targets of CBT demonstrating unprecedented activity particularly in heavily pretreated relapsed/refractory Hodgkin lymphoma and some forms of non-Hodgkin disease. Herein we provide a brief discussion of checkpoint blockade in the context of lymphoma biology with a specific focus on novel checkpoint inhibitors and their therapeutic activity. We discuss current clinical trials and the landscape of CBT to underscore both the remarkable progress and foreseeable limitations of this novel treatment strategy. In particular, we build upon state-of-the-art knowledge and clinical insights gained from the early trials to review potential approaches to how CBT may be integrated with other treatment modalities, including chemoimmunotherapy to improve patient outcomes in the future. Finally, as the role of CBT evolves to potentially become a cornerstone of therapy in refractory/relapsed lymphoma, we briefly emphasize the importance of predictive biomarkers in an effort to select appropriate patients who are most likely to derive benefit from CBT.
Keywords: checkpoint, checkpoint blockade, cytotoxic T-lymphocyte-associated protein 4, immunotherapy, lymphoma, programmed death 1, programmed death ligand 1/2
Introduction
Despite significant therapeutic advances in the treatment of patients with lymphoma, up to 40% of patients with non-Hodgkin lymphoma (NHL) and 15% of those with Hodgkin lymphoma (HL) have progression of disease following standard chemoimmunotherapy (CIT) [Armitage, 1993; Hasenclever and Diehl, 1998]. The overall survival (OS) for patients with refractory NHL and HL remains poor, with treatment response durations almost invariably decreasing with each subsequent relapse. Since lymphoma constitutes the sixth most common malignancy, the highest priority for treatment failures are clinical trials and the development of novel therapeutic modalities that will yield durable remissions and improved survival.
Since the 1970s, decades of research to elucidate the role of immune response in tumorigenesis and to harness its antitumor potential have led to the development of several highly effective immunotherapeutic agents (Table 1). The clinical approval by the US Food and Drug Administration (FDA) of such agents as ipilimumab (2011), pembrolizumab and nivolumab (2014) has been a remarkable achievement and introduced a paradigm shift in cancer therapy in several important ways (Table 2). First, in contrast to existing cancer therapeutics, these agents do not directly target the tumor. And second, they do not activate the immune system to attack the tumor, but rather release inhibitory pathways or checkpoints that negatively regulate antitumor immunity in the host. Millions of years of evolution have refined the mammalian adaptive immune system to recognize a dazzling array of antigens, yet at the same time, maintain self-tolerance to prevent autoimmunity. The immune checkpoints, namely cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 protein (PD-1), help achieve this delicate balance between upregulation and de-escalation of cytotoxic T-cell response by modulating the intensity and duration of T-cell activation [Pardoll, 2012]. Various pathogens and tumors induce the expression of these inhibitory checkpoints to dampen immunogenicity and minimize detection. Thus, blocking antibodies targeting CTLA-4 and PD-1 represent a clever and unique approach for the treatment of cancer [Topalian et al. 2012; Topalian et al. 2015]. In fact, several PD-1/PD-L1 (programmed death ligand 1) and CTLA-4 blocking antibodies have emerged as highly promising agents in the treatment of a variety of solid tumors including melanoma, bladder, renal, lung and others [Topalian et al. 2014; McDermott et al. 2015; Gettinger et al. 2015]. More recently, checkpoint inhibitors have been tested in patients with various hematological malignancies. Early results have demonstrated promising activity in HL and NHL (Table 1). Herein, we provide a brief discussion of the checkpoint blockade as an exciting new therapeutic strategy for lymphoma and review clinical experience with targeted checkpoint inhibitors to date.
Table 1.
Checkpoint inhibitor clinical efficacy.
| Target | Study agent | Disease type | N | Response | DoR | PFS | OS | Reference |
|---|---|---|---|---|---|---|---|---|
| CTLA-4 | Ipilimumab | R/R NHL: FL (14), DLBCL (3), MCL (1) | 18 | ORR (11%), CR (5.6%), PR (5.6%) | NA | NA | NA | Ansell et al. [2009] |
| R/R HL (s/p alloSCT) (14) | 14 | ORR (14.3%), CR (14.3), PR (0%) | NA | NA | NA | Bashey et al. [2009] | ||
| PD-1 | Pidilizumab | DLBCL (2), CLL (3), FL (1), ALCL (1), HL (1) | 8 | CR (5%), SD (33%) | NA | 25 weeks | Berger et al. [2008] | |
| R/R DLBCL (s/p ASCT) de novo (49) | 66 | ORR (51%), CR (34%), PR (17%), SD (37%) | 16 months | 0.72 years | 0.84 years | Armand et al. [2013] | ||
| Transformed DLBCL (13), PMBCL (4) | ||||||||
| Pidilizumab+Rituxan | R/R FL (32) | 32 | ORR (66%), CR (52%) | 18.8 months | NA | Westin et al. [2014] | ||
| Nivolumab | R/R HL (23) | 23 | ORR (87%), CR (17%), PR (70%), SD (13%) | at 24 weeks | 86% | NR | Ansell et al. [2015] | |
| R/R B-NHL [31] | 54 | ORR [36%–40%] | 6–81.6 weeks | Lesokhin et al. [2016] | ||||
| T-NHL (23) | ORR [15%–40%] | NA | NA | |||||
| Pembrolizumab | R/R HL (31) | 31 | ORR (65%), CR (16%), PR (48%) | >24 weeks | 46% at 52 weeks | NA | Armand et al. [2016] |
ALCL, anaplastic large-cell lymphoma; alloSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantion; CLL, chronic lymphocytic leukemia; CR, complete response; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DLBCL, diffuse large B-cell lymphoma;
DoR, Duration of Response; FL, follicular lymphoma; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; NA, not available; NHL, non-Hodgkin lymphoma; NR, not reached; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death 1; PFS, progression-free survival; PMBCL, primary mediastinal B-cell lymphoma; PR, partial response; R/R, relapsed/refractory; SD, stable disease; s/p, status post.
Table 2.
Checkpoint inhibitors used in clinical setting.
| Target | Name | Construct | Manufacturer | Clinical development | Cancer type |
|---|---|---|---|---|---|
| CTLA-4 | Ipilimumab | Fully human IgG1 | Bristol-Myers Squibb | FDA approved | Melanoma |
| Phase I–III | Multiple cancers | ||||
| Tremelimumab | Fully human IgG2 | MedImmune, Pfizer | Phase I–III | Multiple cancers | |
| PD-1 | Pidilizumab | Humanized IgG1 | CureTech | Completed phase I | Multiple cancers |
| Phase II ongoing | |||||
| Nivolumab | Fully human IgG4 | Bristol-Myers Squibb | FDA approved | HL, melanoma, lung | |
| Multiple cancers | |||||
| Pembrolizumab | Humanized IgG4-κ | Merck | FDA approved | Melanoma | |
| Phase I–III | Multiple cancers | ||||
| PD-L1 | Atezolizumab | Humanized IgG1 | Genentech, Roche | Phase I–III | Multiple cancers |
| Avelumab | Fully human IgG1 | Merck, Pfizer | Phase I–III | Multiple cancers | |
| MEDI4736 | Fully human IgG1 | MedImmune, AstraZeneca | Phase I–III | Multiple cancers | |
| BMS-936,559 | Fully human IgG4 | Bristol-Myers Sqibb | Phase I | Multiple cancers |
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; FDA, US Food and Drug Administration; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Scientific background
The concept of ‘checkpoints’ emerged from the understanding that immune surveillance plays a key evolutionary role in tumor recognition and destruction [Schreiber et al. 2011]. Notably, the ability of tumors to activate negative regulatory pathways that prevent their detection and immune-mediated destruction constitutes an important mechanism of tumor progression and metastasis. Immune evasion by cancers is accomplished through a variety of mechanisms, including upregulation of negative costimulatory molecules, such as PD-L1 and CTLA-4 [Blank et al. 2005; Leach et al. 1996]. The initial discovery that administration of anti-CTLA-4 antibodies could lead to the rejection of established tumors in mice revealed the importance of this pathway in tumor immune evasion [Leach et al. 1996]. Previous research has demonstrated that the deletion of the CTLA-4 gene results in a lethal systemic immune hyperactivation characterized by lymphoproliferation and multiorgan tissue destruction [Tivol et al. 1995]. PD-1, a negative regulator of T-cell receptor (TCR) signaling, has been shown to decrease TCR-mediated proliferation and cytokine production. PD-1 gene deficient mice tend to develop autoimmunity suggestive of its role in regulating peripheral tolerance [Blank et al. 2005]. Although both CTLA-4 and PD-1 exert inhibitory effects on immunity, their mechanisms of action are distinct [Parry et al. 2005]. These biological differences can be exploited to differentially and synergistically turn off checkpoints and restore protective antitumor immune surveillance.
Immunological checkpoints
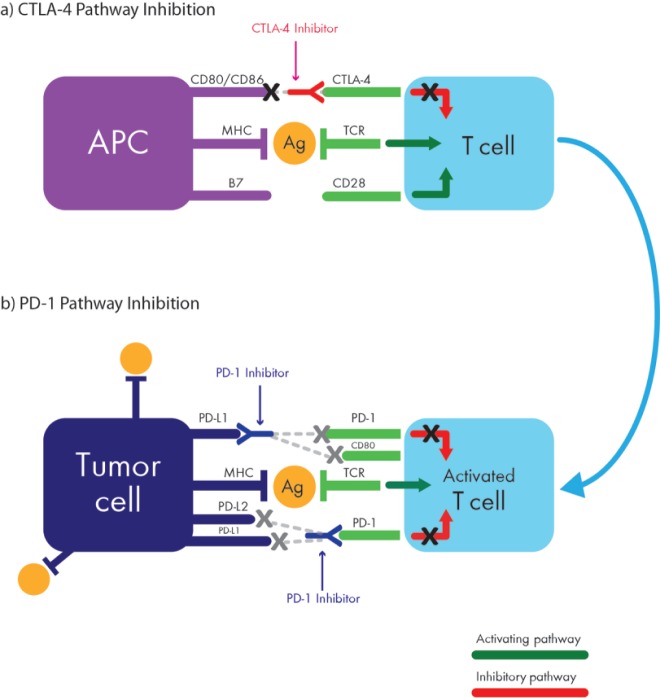
Normal T-cell physiology is complex and is regulated by a plethora of costimulatory and coinhibitory signals. Upon recognition of a cognate tumor-associated antigen displayed on the surface of an antigen-presenting cell (APC), full T-cell activation requires TCR stimulation by the antigenic peptide in the context of a major histocompatibility complex (MHC) molecule (pMHC); and a costimulatory interaction between CD28 on the naïve T cell and a B7 ligand (CD80/CD86) on the APC (Figure 1) [Linsley et al. 1994]. CTLA-4 is not expressed on the surface of naïve T cells; however, TCR stimulation by antigen mobilizes an intracellular pool of CTLA-4 leading to its translocation and upregulation on the plasma membrane of the activated T cell where it outcompetes CD28 for B7 ligand binding, resulting in the termination of costimulation and downregulation of effector T-cell functions [Linsley et al. 1996]. By inhibiting further T-cell stimulation, CTLA-4 upregulation ultimately results in an attenuated immune response, and restores tolerance, not only to self antigens, but also to those expressed by a developing cancer. Notably, in contrast to pathogens, tumor cells can not only induce upregulation of CTLA-4 expression in T cells but they also lack costimulatory molecules, which allows them to grow unfettered and undetected by the immune system. The monoclonal antibody ipilimumab binds to CTLA-4, rendering it inactive, which restores the interaction between B7 on APCs with CD28 on T cells. While CTLA-4 expression is primarily relegated to the T cells in the lymph nodes, where initial tumor antigen presentation is thought to occur, PD-1 appears to predominantly function by dampening the function of activated T cells in peripheral tissues, including T cells residing within the tumor.
Figure 1.
Interactions between activated T cells and tumor via the CTLA-4 pathway (a) and the PD-1 pathway (b). APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; MHC, major histocompatibility complex; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; TCR, T-cell receptor.
PD-1 is another negative costimulatory receptor on activated T cells, which interacts with two known ligands, PD-L1 and PD-L2 [Dong et al. 2002; Latchman et al. 2001]. While the expression of PD-L2 is for the most part limited to hematopoietic cells, PD-L1 is more broadly expressed. Upon recognition of a tumor antigen, PD-1 transcription is activated within activated T cells, leading to its mobilization and expression on the cell surface. The upregulation of PD-1 and its engagement with PD-L1 or PD-L2 ligands antagonizes phosphatidyl-inositol 3 kinase (PI3K) activity, which inhibits intracellular signaling pathways and blocks further T-cell activation [Parry et al. 2005]. Interestingly, inactivation of PD-1 in animal models leads to delayed organ-specific inflammation [Latchman et al. 2004]. Thus, in contrast to the role of CTLA-4 in early T-cell activation, PD-1 is primarily involved in the effector phase of T-cell activity within tissue and tumors. Notably, PD-L1 and PD-L2 are frequently expressed on a variety of immune cells including B and T lymphocytes, macrophages, natural killer and dendritic cells [Naidoo et al. 2014]. The clinical efficacy of PD-1 inhibitors has a direct correlation with PD-L1 expression on tumor or stromal cells and has generated a lot of enthusiasm particularly in HL when PD-1 ligands are overexpressed [Chen et al. 2013].
In addition to anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, a variety of other immunotherapy agents are currently in development targeting various key steps of the multilevel immune process which includes enhancement of antigen presentation, activation of memory T cells, induction of immune-mediated apoptosis, and release of immune suppression [Smyth et al. 2015]. The successful engagement of all of these steps is required to achieve a robust antitumor immune response and tumor cell elimination [Melero et al. 2015]. There is a growing body of literature that underscores an increasing importance of tumor microenvironment, including stromal elements, fibroblasts, macrophages and lymphocytes among others that directly affect autocrine and paracrine interactions within the tumor [Steidl et al. 2011]. However, these tumor-infiltrating T cells are often dysfunctional and fail to complete the task of tumor cell killing [Yang et al. 2015]. Dysfunctional PD-1+ T cells often coexpress other negative costimulatory molecules, including lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin protein 3[Sakuishi et al. 2010]. Blocking these receptors either alone or in combination with other immunotherapies has been shown to reverse T-cell exhaustion and restore antitumor activity in animal models [Woo et al. 2012]. A combination regimen of PD-1 and LAG-3 inhibitors is currently being studied clinically [ClinicalTrials.gov identifier: NCT01968109]. Another way to augment the immune response is to enhance the T-cell/macrophage effector interaction. This interface is regulated by a variety of costimulatory proteins, including CD137 and OX40 on T cells and CD40 expressed on the APC, which promote clonal expansion of effector and memory T-cell populations. Several clinical trials utilizing an anti-CD137 agonist (urelumab, a fully human IgG4 mAb) in combination with rituximab [ClinicalTrials.gov identifier: NCT01775631] and nivolumab [ClinicalTrials.gov identifier: NCT02253992] in patients with relapsed/refractory (R/R) B-cell NHL and metastatic solid tumors are currently ongoing. Similarly, CD40 activation of macrophages leading to a shift from a tumor promoting (M2) to tumor suppressing (M1) phenotype capable of rapid infiltration and depletion of tumor stroma presents another attractive target [Beatty et al. 2011]. Various CD40 agonists (e.g. lucatumumab, dacetuzumab) are currently being investigated in NHL and multiple myeloma [ClinicalTrials.gov identifier: NCT01275209, NCT00655837]. Taken together, there are dozens of targets and other checkpoints within the tumor microenvironment that could potentially be exploited, most likely in various combinations with anti-CTLA4 and PD-1/PD-L1 antibodies, to enhance the quality and duration of immune response.
Lymphoma biology relative to immunological checkpoints
The mechanisms that lead to PD-L1/PD-L2 overexpression in hematologic malignancies are multifactorial. Generally, the expression of PD-L1 on tumor cells is regulated by cytokine stimuli, including interferon γ produced by T cells in the tumor microenvironment [Dong et al. 2002]. In addition, recurrent genetic amplifications of chromosome 9p23–24, encoding PD-L1 and PD-L2, have been observed, particularly in the classical nodular sclerosis subtype of HL [Green et al. 2010]. Interestingly, the 9p24.1 amplicon includes JAK2 that, when activated, further induces PD-L1 transcription via JAK/STAT signaling cascade consisting of a cell surface receptor, a Janus kinase (JAK) and two Signal Transducer and Activator of Transcription (STAT) proteins. These copy-number-dependent mechanisms and chromosomal rearrangements result in overexpression of the PD-1 ligands on Reed–Sternberg (RS) cells in patients with HL, particularly in the setting of progressive disease [Green et al. 2010]. In patients with mediastinal large B-cell lymphoma or other Epstein-Barr virus (EBV)-associated lymphomas, PD-L1 expression is augmented by activator protein 1 signaling and potentially by the EBV virus oncoprotein, latent membrane protein 1 induced mechanism that allows the virus to escape immune eradication [Green et al. 2012]. In other types of lymphoma, PD-L1 expression is more sporadic across variable histologies such as diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and anaplastic large cell lymphoma [Chen et al. 2013].
Furthermore, low-level radiation therapy has been shown to activate dendritic cells, increase density of tumor-infiltrating lymphocytes and modulate the expression of PD-L1 [Sharabi et al. 2015]. Thus, radiosensitizing immunotherapy may have a synergistic effect with immune checkpoint blockade.
Clinical studies of checkpoint inhibitors in lymphoma
Hodgkin lymphoma
The pathophysiology of HL, with its isolated RS tumor cells surrounded by extensive yet ineffective immune cells, lends itself amenable to PD-1 blockade, owing to its genetically determined dependence on the PD-1 pathway for survival. Indeed, anti-PD-1 monoclonal antibodies, including nivolumab (a fully human IgG4) and pembrolizumab (humanized IgG4-κ), have demonstrated outstanding efficacy and favorable tolerability in patients with HL in the phase I setting (CheckMate-039). In a heavily pretreated population of 23 patients, including 18 who had received prior autologous stem cell transplant (ASCT) followed by brentuximab vedotin (BV), nivolumab demonstrated a substantial therapeutic activity with impressive overall response rate (ORR) of 87% [complete response (CR) = 17%, partial response (PR) = 70%, stable disease (SD) = 13%], and a 6-month progression-free survival (PFS) of 86% [Ansell et al. 2015]. Extended follow up of these same patients showed that half of the responders maintained durable responses at a median observation time of 86 weeks [Lesokhin et al. 2016]. Similar encouraging responses were observed in a subsequent single-arm multicenter phase II study (CheckMate-205) of nivolumab administered at the same dose of 3 mg/kg intravenously every 2 weeks to patients with HL (N = 80) who, despite prior failure of both ASCT and BV, demonstrated a 66.3% ORR (CR = 7%; PR = 58%) at a median follow up of 8.9 months [95% confidence interval (CI) 54.8–76.4] [Younes et al. 2016]. Based on these pivotal trials, nivolumab was granted an accelerated approval by the FDA for the treatment of patients with classical HL following failure of prior ASCT and post-transplant BV (May 2016). In addition to high durable responses, nivolumab appears to be well tolerated, with a low incidence of grade 3 lymphopenia (4%), pancreatitis (4%), stomatitis (4%) and myelodysplastic syndrome (4%). No grade 4–5 drug-related adverse events (AEs) were reported. As expected, analyses of pretreatment tumor biopsies (n = 10) revealed copy-number gains in PD-L1 and PD-L2 with associated overexpression of these ligands. Notably, nuclear staining of phosphorylated STAT3 in RS cells was positive, indicating active JAK-STAT signaling. Interestingly, prior treatment status, including ASCT or BV, did not impact response rates [Armand et al. 2014].
A similar phase Ib (KEYNOTE-013) study using pembrolizumab (10 mg/kg intravenously every 2 weeks) in 31 patients with R/R HL demonstrated equally encouraging results with an ORR of 65% (CR = 16%, PR = 48%, SD = 20%) [Armand et al. 2016]. At a median follow up of 17 months, the majority of responses (70%) were durable, lasting more than 24 weeks (range 0.14–74 weeks). The PFS was 69% at 24 weeks and 46% at 52 weeks. The correlative studies confirmed overexpression of PD-L1 or PD-L2 by the RS cells in all available tissue samples. Final results from these early trials are eagerly anticipated and are likely to show not only consistently high but also durable responses.
The remarkable success of anti PD-1 monotherapy in HL served as an impetus for implementation of a combination treatment strategy using a variety of other agents, including surface antibodies (CD20, CD19), traditional chemotherapy in both salvage (ifosfamide, carboplatin, etoposide) and frontline (doxorubicin, bleomycin, vinblastine, dacarbazine) settings or with other checkpoint blockers (ipilimumab) among others [Ansell, 2016]. Finally, the use of antibody–drug conjugates, such as BV in combination with anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab), is also currently being explored in R/R HL [ClinicalTrials.gov: NCT01896999].
Non-Hodgkin lymphoma
Diffuse large B-cell lymphoma
Early trials of checkpoint blockade in R/R NHL have included the use of the anti-CTLA4 antibody ipilimumab (a fully human IgG1 mAb) and anti-PD1 antibody (pidilizumab, humanized IgG1 mAb). In a phase I trial of ipilimumab in 18 patients with R/R NHL, an ORR of 11% was observed [Ansell et al. 2009]. Notably, responses, although low, were quite durable with an ongoing CR lasting more than 31 and 19 months in one DLBCL and one FL patient, respectively. This study demonstrated that as a single agent, CTLA-4 antagonists have a limited activity in NHL but may have an additive, durable effect as combination therapy.
Subsequent trials in NHL have focused on the clinical development of PD-1 antagonists. Of note, in contrast to HL, only 25% of DLBCL tumors express PD-1/PD-L1 [Andorsky et al. 2011]. One exception is primary mediastinal B-cell lymphoma (PMBL) which, similar to HL, frequently harbors 9p22 amplification leading to overexpression of PD-L1/PD-L2 [Shi et al. 2014]. Not surprisingly, the ORR to checkpoint blockade in NHL is generally lower compared with HL and PMBL. Indeed, in the phase I study of pidilizumab, 17 patients with a variety of relapsed hematologic malignancies were treated with escalating doses (range 0.2–6 mg/kg) of the drug, demonstrating a 33% rate of clinical benefit (as defined by having stable disease) with one exception of a patient with FL who achieved a CR [Berger et al. 2008]. In a subsequent international phase II study of pidilizumab, 66 patients with DBLCL undergoing ACST, received 3 doses of pidilizumab consolidation [Armand et al. 2013]. Notably, among patients with measurable disease post ASCT (n = 35), the ORR was 51%. The 16-month PFS was 70% (90% CI 0.51–0.82) for high-risk patients who had persistently Positron Emission Tomography (PET)-positive disease post salvage chemotherapy. This study was the first to demonstrate clinical activity of PD-1 blockade in heavily pretreated R/R DLBCL [Armand et al. 2013].
With the development of more refined PD-1 inhibitors, nivolumab (a fully human IgG4 mAb) and pembrolizumab (humanized IgG4 mAb), two larger, more inclusive trial series were launched. A phase I dose escalation, cohort-expansion study of nivolumab in various subtypes of NHL (n = 54) revealed the highest rate of ORR was achieved in patients with FL at 40%, closely followed by DLBCL at 36% [Lesokhin et al. 2016]. Patients with T-cell lymphomas (n = 23) were also included, but did not fare as well with variable responses: 15% ORR (all PR) in mycosis fungoides and 40% in peripheral T-cell lymphoma. Similar studies with pembrolizumab in patients with NHL are currently ongoing.
Follicular lymphoma
Previous studies suggested that there is no demonstrable PD-L1 expression by the tumor cells in FL [Andorsky et al. 2011]. However, despite that, clinical data from the phase II trial of pidilizumab in combination with rituximab in 32 patients with rituximab-sensitive FL showed improved responses (ORR = 66%, CR = 52%, PR = 14%) at a median follow up of 15.4 months [Westin et al. 2014]. The high CR rate in this study provided additional evidence in support of combination therapy approach. Furthermore, in a phase I study of nivolumab, 10 patients with FL had a 40% ORR, including one patient who achieved a CR [Lesokhin et al. 2016]. Biopsy specimens from this study revealed PD-L1 expression was mostly restricted to infiltrating macrophages/stromal cells and generally absent from the malignant lymphoma cells, suggesting that within the heterogeneous microenvironment there could be distinct subpopulations of immune cells with variable PD-1 expression. In all of these trials, the toxicity profile of the anti-PD1 therapy was moderate, mostly immune related including colitis, pneumonitis, hyperthyroidism, rash, hematologic, and quite manageable.
Combination checkpoint blockade therapy
Despite aforementioned success achieved by checkpoint blockade in lymphomas, there are still a substantial number of patients who do not benefit from single-agent checkpoint blockade therapy (CBT). Factors that may potentially impair immunologic response and promote tumor permissive milieu include inefficient APC, exhausted effector T cells or protective stroma/microenvironment that restrict T-cell infiltration and function. Creative, biologically informed strategies are therefore required to overcome these barriers and optimize therapeutic benefit. Early immunotherapy studies have focused on single target blockade which, though promising, by itself may not release the full effector potential of the tumor-specific immune cells. To enhance the efficacy of checkpoint inhibitors, novel synergistic combinations targeting distinct oncogenic signaling pathways and immune processes have been proposed. One such approach investigated cotargeting of PD-1 (nivolumab) and CTLA-4 (ipilimumab) in patients with melanoma which led to enhanced ORR of 61% compared with only 11% achieved with ipilimumab monotherapy (p < 0.001) [Postow et al. 2015]. Other checkpoint combinations including nivolumab and urelumab, described above, are currently being investigated. In addition, a novel anti-PD-1 inhibitor (MEDI-0680) is being studied both as a single agent and in combination with anti-CD19 agents. Furthermore, anti-PD-L1 blockers (MEDI-4736, MPDL-3280) have entered phase I trials. As other checkpoint inhibitors advance through early phase trials, many more biologically informed combinations and permutations will be possible.
Finally, biologically informed combinations of checkpoint blockade with conventional cytotoxic chemotherapy, small molecule inhibitors and immune-modulating agents are being vigorously explored. One example is the targeting of the B-cell receptor signaling pathway with ibrutinib (a Bruton’s tyrosine kinase inhibitor) and an anti-PD-1 agent. This combination appears to be synergistic due to ibrutinib’s upregulation of PD-1 expression and suppression of the IL-2-inducible T-cell kinase, which leads to a more robust activation of T helper 1 type T cells and subsequently a more efficient immune response [Ansell, 2016].
Future directions
Immunological checkpoint blockade has emerged as a successful therapeutic modality initially for the treatment of various solid tumors and is rapidly gaining momentum in hematologic malignancies as well. Early results of checkpoint blockade with anti-PD-1 antibodies have revealed that they are both safe and efficacious, especially in patients with HL, in whom the clinical benefit is remarkably high, even in patients with highly refractory disease. The stark difference in response rates to checkpoint blockade between HL and NHL highlights the fundamental biological differences in these lymphoma subtypes. It also underscores the importance of identifying predictive biomarkers of response to checkpoint blockade immunotherapy. In selected solid tumors (which typically have a higher incidence of somatic mutations than lymphoid malignancies) recent evidence suggests that PD-L1 expression, pre-existing CD8+ T-cell infiltration and mutational load are all potentially predictive factors [Rizvi et al. 2015; Tumeh et al. 2014]. However, none of these biomarkers have been validated in lymphoid malignancies. Furthermore, the antigens expressed by lymphoma cells recognized by T cells within the lymphoma microenvironment remain unknown. Neoantigen discovery through a variety of genomic and proteomic techniques is an integral aspect of successful checkpoint blockage therapy and presents an exciting opportunity to develop patient-specific vaccines in combination with CBT. Similar to other targeted agents, checkpoint blockage monotherapy seldom leads to complete remission, supporting the strategic combinations of checkpoint inhibitors with other biologic agents to improve the quality and duration of response.
Conclusion
Promising results with immune checkpoint blockade and other immunotherapies (Chimeric Antigen Receptor [CAR] T-cells, bispecific antibodies, immunomodulators, etc.) have demonstrated that immumotherapy is becoming one of the central pillars of treatment in R/R lymphoma. Further refinement of CTLA-4 and PD-1 inhibitors, as well as more detailed understanding of predictive biomarkers, mechanisms of action and optimal combinations to improve responses will be needed. The challenge will be to combine checkpoint blockade with other therapies in the hope of harnessing the effects of the immune response that in the future may change the course of this disease.
Acknowledgments
All authors contributed equally to the writing of this manuscript. NYG: declares no conflict of interest. JK: consulting fees and research support from Merck and BMS. MRB: declares no conflict of interest. Gratitude is expressed to HLW for editorial support.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author Justin Kline receives consulting fees and research support from Merck and BMS.
Contributor Information
Natalie Galanina, Department of Hematology/Oncology, UC San Diego Moores Cancer Center, La Jolla, California, USA.
Justin Kline, Section of Hematology/Oncology, University of Chicago, Chicago, IL, USA.
Michael R. Bishop, Section of Hematology and Oncology, University of Chicago, 5841 S. Maryland Avenue, Chicago, IL 60637, USA.
References
- Andorsky D., Yamada R., Said J., Pinkus G., Betting D., Timmerman J. (2011) Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17: 4232–4244. [DOI] [PubMed] [Google Scholar]
- Ansell S. (2016) Where do programmed death-1 inhibitors fit in the management of malignant lymphoma? J Oncol Pract 12: 101–106. [DOI] [PubMed] [Google Scholar]
- Ansell S., Armand P., Timmerman J., Shipp M., Garelik M., Zhu L., et al. (2015) Nivolumab in patients (Pts) with relapsed or refractory classical Hodgkin lymphoma (R/R cHL): clinicial outcomes from extended follow-up of a phase I study (CA209–039). Blood 126: 583. [Google Scholar]
- Ansell S., Hurvitz S., Koenig P., LaPlant B., Kabat B., Fernando D., et al. (2009) Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 15: 6446–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell S., Lesokhin A., Borrello I., Halwani A., Scott E., Gutierrez M., et al. (2015) PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N Engl J Med 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P., Ansell S., Lesokhin A., Halwani A., Millenson M., Schuster S., et al. (2014) Nivolumab in patients with relapsed or refractory Hodgkin lymphoma – preliminary safety, efficacy and biomarker results of a phase I study. Blood 124: 289. [Google Scholar]
- Armand P., Nagler A., Weller E., Devine S., Avigan D., Chen Y., et al. (2013) Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 31: 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P., Shipp M., Ribrag V., Michot J., Zinzani P., Kuruvilla J., et al. (2016) Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol pii: JCO673467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. (1993) Treatment of non-Hodgkin’s lymphoma. N Engl J Med 328: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Bashey A., Medina B., Corringham S., Pasek M., Carrier E., Vrooman L., et al. (2009) CTLA4 Blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 113(7): 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G., Chiorean E., Fishman M., Saboury B., Teitelbaum U., Sun W., et al. (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331: 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R., Rotem-Yehudar R., Slama G., Landes S., Kneller A., Leiba M., et al. (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- Blank C., Gajewski T., Mackensen A. (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Chapuy B., Ouyang J., Sun H., Roemer M., Xu M., et al. (2013) PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19: 3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Strome S., Salomao D., Tamura H., Hirano F., Flies D., et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015) Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Monti S., Rodig S., Juszczynski P., Currie T., O’Donnell E., et al. (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116: 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Rodig S., Juszczynski P., Ouyang J., Sinha P., O’Donnell E., et al. (2012) Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 18: 1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenclever D., Diehl V. (1998) A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s s disease. N Engl J Med 339: 1506–1514. [DOI] [PubMed] [Google Scholar]
- Latchman Y., Wood C., Chernova T., Chaudhary D., Borde M., Chernova I., et al. (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2: 261–268. [DOI] [PubMed] [Google Scholar]
- Latchman Y., Liang S., Wu Y., Chernova T., Sobel R., Klemm M., et al. (2004) PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A 101: 10691–10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D., Krummel M., Allison J. (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lesokhin A., Ansell S., Armand P., Scott E., Halwani A., Gutierrez M., et al. (2016) Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 34: 2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P., Bradshaw J., Greene J., Peach R., Bennett K., Mittler R. (1996) Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4: 535–543. [DOI] [PubMed] [Google Scholar]
- Linsley P., Greene J., Brady W., Bajorath J., Ledbetter J., Peach R. (1994) Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1: 793–801. [DOI] [PubMed] [Google Scholar]
- McDermott D., Drake C., Sznol M., Choueiri T., Powderly J., Smith D., et al. (2015) Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 33: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I., Berman D., Aznar M., Korman A., Pérez Gracia J., Haanen. J. (2015) Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 15: 457–472. [DOI] [PubMed] [Google Scholar]
- Moskowitz C. (2014) PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). Blood 124: 290. [Google Scholar]
- Naidoo J., Page D., Wolchok J. (2014) Immune checkpoint blockade. Hematol Oncol Clin North Am 28: 585–600. [DOI] [PubMed] [Google Scholar]
- Pardoll D. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R., Chemnitz J., Frauwirth K., Lanfranco A., Braunstein I., Kobayashi S., et al. (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25: 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M., Chesney J., Pavlick A., Robert C., Grossmann K., McDermott D., et al. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N., Hellmann M., Snyder A., Kvistborg P., Makarov V., Havel J., et al. (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K., Apetoh L., Sullivan J., Blazar B., Kuchroo V., Anderson A. (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207: 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R., Old L., Smyth M. (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- Sharabi A., Lim M., DeWeese T., Drake C. (2015) Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 16: e498–e509. [DOI] [PubMed] [Google Scholar]
- Shi M., Roemer M., Chapuy B., Liao X., Sun H., Pinkus G., et al. (2014) Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol 38: 1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M., Ngiow S., Ribas A., Teng M. (2015) Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 13: 143–158. [DOI] [PubMed] [Google Scholar]
- Steidl C., Connors J., Gascoyne R. (2011) Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol 29: 1812–1826. [DOI] [PubMed] [Google Scholar]
- Tivol E., Borriello F., Schweitzer A., Lynch W., Bluestone J., Sharpe A. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3: 541–547. [DOI] [PubMed] [Google Scholar]
- Topalian S., Drake C., Pardoll D. (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Drake C., Pardoll D. (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Sznol M., McDermott D., Kluger H., Carvajal R., Sharfman W., et al. (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh P., Harview C., Yearley J., Shintaku I., Taylor E., Robert L., et al. (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin J., Chu F., Zhang M., Fayad L., Kwak L., Fowler N., et al. (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase II trial. Lancet Oncol 15: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S., Turnis M., Goldberg M., Bankoti J., Selby M., Nirschl C., et al. (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liang A., Ansell S. (2015) T-cell-mediated antitumor immunity in B-cell non-Hodgkin lymphoma: activation, suppression and exhaustion. Leuk Lymphoma 56: 2498–2504. [DOI] [PubMed] [Google Scholar]
- Younes A., Santoro A., Shipp M., Zinzani P., Timmerman J., Ansell S., et al. (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase II trial. Lancet Oncol 17: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]