Abstract
Multiple myeloma (MM) is the second most common hematologic malignancy. The diagnosis of MM requires ⩾10% clonal plasma cells in the bone marrow or biopsy-proven plasmacytoma, plus evidence of end-organ damage (hypercalcemia, renal failure, anemia, and lytic bone lesions). The definition of MM has recently been expanded to include a ⩾60% clonal plasma cell burden in the bone marrow, serum involved/uninvolved light chain ratio of ⩾100, or more than one focal lesion on magnetic resonance imaging ⩾5 mm in the absence of end-organ damage. MM is an incurable malignancy previously associated with poor survival rates. However, over the past two decades, the introduction of novel treatment options has resulted in a dramatic improvement in response rates and overall survival (OS). The combination of a proteasome inhibitor and an immunomodulator (IMiD) is the preferred induction treatment for newly diagnosed transplant-eligible MM patients. After induction, high-dose therapy with autologous stem cell transplant (ASCT) is still the standard of care for these patients. In patients who are transplant ineligible, dose adjusted IMiDs or proteasome inhibitor-based combinations are the preferred treatment option. With the recent approval of novel drugs like carfilzomib, ixazomib, pomalidomide, panobinostat, and monoclonal antibodies (elotuzumab and daratumumab), as well as improved understanding of risk stratification, management of comorbidities and treatment side effects, clinicians can optimize anti-MM therapy, particularly in relapse/refractory MM patients. In this review, we outline the current therapeutic approach to the management of MM.
Keywords: immunomodulator, multiple myeloma, novel treatment options, proteasome inhibitor
Introduction
Approximately 86,000 new cases of multiple myeloma (MM) occur per year globally [Moreau et al. 2015a], constituting about 13% of hematological cancers and 1% of all cancers [Howlader et al. 2012].
In the past, MM was only defined as an accumulation of 10% clonal plasma cells in the bone marrow, resulting in end-organ damage as manifested by CRAB criteria (hypercalcemia, renal insufficiency, anemia, or bone lesions). The international myeloma working group (IMWG) has revised the definition of MM to include either ⩾60% clonal plasma cells in the bone marrow, serum involved/uninvolved light chain ratio of 100 or greater, or more than one focal lesion on magnetic resonance imaging of ⩾5 mm in the absence of CRAB criteria.
Melphalan-prednisone was introduced 50 years ago and remained the standard of care for more than 30 years. It induced a partial response (PR) in 40–60% of patients and led to a progression-free survival (PFS) of 18 months [San Miguel, 2015].
The combination of autologous stem cell transplant (ASCT) with novel agents such as immunomodulators (IMiDs), proteasome inhibitors, and monoclonal antibodies have resulted in improved PFS, overall survival (OS) and quality of life (QoL). Therefore, the goal of MM treatment has now shifted toward achieving durable responses, long-term disease control and improved survival with the potential for cure [Munshi and Anderson, 2013]. In this review, we will provide a guide to the hematologist in order to optimize treatment regimens that are effective in the management of MM patients
Upfront treatment of transplant-eligible MM patients
The treatment algorithm for newly diagnosed MM (NDMM) has evolved over the last two decades with the incorporation of novel agents into myeloma induction regimens prior to ASCT. As reviewed below, numerous upfront regimens have evolved for the treatment of NDMM prior to ASCT. An induction regimen is administered for 2–4 months to achieve deeper response rates, although the optimal duration of induction treatment is not well established [Sonneveld et al. 2015] (Table 1).
Table 1.
Induction treatment in newly diagnosed transplant-eligible multiple myeloma.
| Study by induction regimen | Treatment schema | Number of patients | Post-induction (%) |
Post-transplant (%) |
Long-term outcomes (%) | ||
|---|---|---|---|---|---|---|---|
| ORR | CR /VGPR | ORR | CR /VGPR | ||||
| GIMEMA Cavo et al. [2012] |
|||||||
| VTd | VTd × 3-ASCT Mel200 × 2-VTd × 2-Dm | 236 | 93 | 19 CR 62 ⩾ VGPR |
93 | 42 CR 82 ⩾ VGPR |
3-year PFS: 68 3-year OS: 86 |
| versus | |||||||
| Td | Td × 3-ASCT Mel200 × 2-Td × 2-Dm | 238 | 79 | 5 CR 28 ⩾ VGPR |
84 | 30 CR 64 ⩾ VGPR |
3-year PFS: 56 3-year OS: 84 |
| IFM Moreau et al. [2011a] |
|||||||
| Vd | Vd × 3-ASCT Mel200 | 99 | 81 | 36 ⩾ VGPR | 86 | 58 ⩾ VGPR | Median PFS: 30 months |
| versus | |||||||
| VTd | VTd × 3-ASCT Mel200 | 100 | 88 | 49 ⩾ VGPR | 89 | 74 ⩾ VGPR | Median PFS: 26 months |
| SWOG S0777 Durie et al. [2015] |
|||||||
| VRd | VRd × 8-Rdm | 242 | 82 | CR 16 | NA | NA | Median PFS: 43 months Median OS: 75 months |
| versus | |||||||
| Rd | Rd × 6-Rdm | 232 | 72 | CR 8 | NA | NA | Median PFS: 30 months Median OS: 64 months |
| Reeder et al. [2009] | |||||||
| VCd (CyBorD) | VCd × 4-ASCT Mel200 | 33 | 88 | 39 CR/nCR 61 ⩾ VGPR |
NR | 70 CR/nCR 74 ⩾ VGPR |
NR |
| HOVON-65/GMMG-HD4 Sonneveld [2015] |
|||||||
| VAD | VAd × 3-VAD × 3-ASCT Mel200-Tm × 2 year | 414 | 54 | 2 CR 14 ⩾ VGPR |
75 | 9 CR 36 ⩾ VGPR |
Median PFS: 28 months 5-year OS: 55 |
| versus | |||||||
| PAD | PAd × 3-PAD × 3-ASCT Mel200-Vm × 2 year | 413 | 78 | 7 CR 42 ⩾ VGPR |
88 | 21 CR 62 ⩾ VGPR |
Median PFS: 35 months 5-year OS: 61 |
| EVOLUTION Kumar et al. [2012] |
|||||||
| VRd | VRd × 4-ASCT Mel200 versus VRd × 4-Vmx4 | 42 | 73 | 7 CR 32 ⩾ VGPR |
NR | NR | 1-year PFS: 83 1-year OS: 100 |
| versus | |||||||
| VCd | VCd × 4-ASCT Mel200 versus VCd × 4-Vmx4 | 33 | 63 | 3 CR 13 ⩾ VGPR |
NR | NR | 1-year PFS: 93 1-year OS: 100 |
| versus | |||||||
| VdCR | VdCR × 4-ASCT Mel200 versus VdCR × 4-Vm × 4-Rm (off protocol) | 48 | 80 | 5 CR 33 ⩾ VGPR |
NR | NR | 1-year PFS: 86 1-year OS: 92 |
| versus | |||||||
| VCd-mod | VCd-mod × 4-ASCT Mel200 versus VCd-mod × 4-Vm × 4 | 17 | 82 | 12 CR 41 ⩾ VGPR |
NR | NR | 1-year PFS: 100 1-year OS: 100 |
| IFM 2013-14 Moreau et al. [2015b] |
|||||||
| VTd | VTd × 4-ASCT | 170 | 92 | 11 CR 67 ⩾ VGPR |
NR | NR | NR |
| versus | |||||||
| VCd | VCd × 4-ASCT | 170 | 84 | 9.5 CR 56 ⩾ VGPR |
NR | NR | NR |
ASCT, autologous stem cell transplant; CR, complete response; Dm, dexamethasone maintenance; NA, not applicable; NR, not reported; ORR, overall response rate; OS, overall survival; PAd, bortezomib-doxorubicin-dexamethasone; PFS, progression-free survival; Rdm, lenalidomide-dexamethasone maintenance; Td, thalidomide-dexamethasone; Tm, thalidomide maintenance; VAd, vincristine-doxorubicin-dexamethasone; VCd, bortezomib-cyclophosphamide-dexamethasone; Vd, bortezomib-dexamethasone; VdCR, bortezomib-cyclophosphamide-dexamethasone-lenalidomide; VGPR, very good partial response; Vm, bortezomib maintenance; VRd, bortezomib-lenalidomide-dexamethasone; VTd, bortezomib-thalidomide-dexamethasone.
The combination of bortezomib, lenalidomide, and dexamethasone (VRd) is one of the preferred frontline treatment options due to its tolerability and efficacy in prospective trials. In a single arm phase I/II study, VRd showed a PR rate of up to 100% [Jasielec and Jakubowiak, 2013]. A randomized phase III trial, SWOG S0777, compared six 28-day cycles of VRd versus eight 21-day cycles of lenalidomide and low-dose dexamethasone (Rd) in both transplant-eligible and transplant-ineligible NDMM patients [Durie, 2015]. Patients who received VRd had a significantly improved PFS (43 months versus 31 months) than Rd alone (p = 0.0018). OS was also improved in the VRd arm (75 months versus 64 months) compared with the Rd arm (p = 0.025).
Another triplet combination, bortezomib, cyclophosphamide, and dexamethasone (VCd or CyBorD), which is administered in a 28-day cycle, produces a rapid and deep response in patients with NDMM, and it has a tolerable side effect profile [Reeder et al. 2009]. For this reason, VCd is also a reasonable option, particularly for patients with poor renal clearance (CrCl < 30). In the phase II EVOLUTION trial, NDMM patients were randomly assigned to receive induction treatment with VRd, VCd, CyBorD (mod-VCd) or VdCR (bortezomib, dexamethasone, cyclophosphamide and lenalidomide) [Kumar et al. 2012]. After an interim analysis, the protocol was amended to change the VCd regimen to include an additional dose of cyclophosphamide (CyBorD). Following four cycles of therapy, the overall response rates (ORRs) were 73%, 63%, 82%, and 80% in patients who received VRd, VCd, CyBorD and VdCR, respectively. The study found no substantial advantage of a four drug regimen (VdCR) over a three drug regimen (VRd, VCd, or CyBorD).
Bortezomib, thalidomide, and dexamethasone (VTd) is another triplet combination that has demonstrated high pre- and post-transplant complete response (CR) rates, as well as significantly longer PFS compared with thalidomide and dexamethasone (Td) [Cavo et al. 2012]. The Intergroupe Francophone du Myelome (IFM) conducted a randomized trial comparing bortezomib and dexamethasone (Vd) with VTd as induction therapy before ASCT [Moreau et al. 2011a]. The CR and very good partial response (VGPR) rate was significantly higher in the VTd arm (49% versus 36%, p = 0.05).
The prospective randomized trial IFM 2013-04, compared four cycles of VTd with VCd as induction treatment before ASCT. After four cycles, 66.3% in the VTd arm had a VGPR and 56.2% in the VCd arm had a VGPR (p = 0.05). The per-protocol analysis showed that VTd was superior to VCd. The ORR was 92.3% in the VTd arm versus 83.4% in the VCd arm (p = 0.01) [Moreau et al. 2015b]. Hematologic toxicities (anemia and thrombocytopenia) were more frequently seen with VCd and peripheral neuropathy with VTd. Based on these results, clinicians can consider using the VTd regimen as induction treatment prior to ASCT.
In another large, phase III trial transplant-eligible NDMM patients were randomized to receive upfront doxorubicin and Dexamethasone with either vincristine (VAD) or bortezomib (PAD) [Sonneveld et al. 2015]. Following high-dose melphalan and ASCT, VAD patients received thalidomide maintenance and PAD patients were continued on bortezomib. The median PFS was significantly better in the PAD arm (35 months versus 28 months) compared with VAD (p = 0.002). Initial results also showed that bortezomib improved PFS and OS in patients with del (17p) (PAD versus VAD: 22 versus 12 months, p = 0.01). Based on these data VAD is not recommended for the treatment of NDMM.
Carfilzomib, a second generation proteasome inhibitor that is approved by the United States (US) Food and Drug Administration (FDA) for the treatment of relapsed and refractory myeloma, has emerged as an additional initial therapeutic option for NDMM patients [Jasielec and Jakubowiak, 2013]. A phase I/II study evaluating the combination of carfilzomib, lenalidomide, and low-dose dexamethasone (KRd) for upfront treatment of MM in both transplant-eligible and transplant-ineligible patients found that 62% of patients achieved at least a near-complete response (nCR) and 42% a stringent complete response (sCR) after 12 cycles [Jakubowiak et al. 2012].
In summary, triplet regimens such as VTd, VRd, VCd or CyBorD are recommended as upfront treatments of MM before ASCT owing to their good tolerability and consistently high response rates.
ASCT, consolidation and maintenance treatment
In the era of novel combination regimens, the role of ASCT has been questioned. Most studies to date support the finding that early ASCT improves the depth of response and PFS, but not OS [Mohty and Harousseau, 2014]. ASCT can be done immediately following induction therapy (e.g. four cycles) or can be delayed until first relapse. In either case, stem cells must be collected early in the disease course to avoid collection failure after prolonged lenalidomide exposure. Melphalan at a dose of 200 mg/m2 is used as the standard conditioning regimen, but in the setting of renal insufficiency (CrCl < 60) the melphalan dose should be reduced to 140 mg/m2 [Abidi et al. 2012]. A large phase III IFM/DFCI (Dana-Farber Cancer Institute) 2009 study has recently demonstrated an increased PFS in patients who received early ASCT after VRd induction compared with delayed ASCT [Attal et al. 2015]. Another study demonstrated that tandem ASCT benefits patients whose disease fails to achieve CR or VGPR with the first transplant [Byrne et al. 2014]. A randomized phase III study, the HOVON trial, also found an improved PFS with upfront ASCT, as well as a 24% decreased risk of progression [Cavo et al. 2016]. In summary, clinicians should consider ASCT after four cycles of induction therapy in medically fit patients.
Consolidation and maintenance therapy after ASCT
Consolidation therapy is aimed at increasing the depth of response following ASCT. While the role of consolidation treatment has not been thoroughly explored, there is evidence that a higher CR rate can be obtained with additional therapy. It usually consists of a limited number of treatment cycles, either in combination therapy or with a second transplant. The Italian Myeloma Group conducted a pivotal trial comparing VTd with Td for induction, followed by double ASCT and VTd versus Td consolidation [Galli et al. 2013]. The median PFS was 50 months for patients receiving VTd consolidation versus 38 months for patients treated with Td (p = 0.015). Another study showed an increased sCR from 27% to 40%, after two consolidation cycles of VRd following three VRd induction cycles and ASCT, highlighting that consolidation improves the depth of response [Roussel et al. 2014].
Maintenance therapy refers to the administration of agents with low toxicity in an attempt to prevent progression of disease [Mohty et al. 2015]. It is given for a prolonged period of time, typically for at least 12 months, but often up to 2–3 years or until disease relapse. Thalidomide maintenance therapy has been investigated in a number of studies and has shown to prolong time to progression (TTP), PFS, and event-free survival as well as OS [Barlogie et al. 2010]. Unfortunately, thalidomide is poorly tolerated [Barlogie et al. 2006, 2008] and patients with poor risk cytogenetics do not appear to benefit [Attal et al. 2006; Morgan et al. 2012]. The post-transplant use of lenalidomide in three large randomized trials, compared with no maintenance therapy, illustrated a significant improvement in PFS, as well as a 3-year OS benefit (88 versus 80%) in one study [McCarthy et al. 2012, 2013; Palumbo, 2014]. Unfortunately, a higher risk of secondary malignancies was reported in the lenalidomide arms, with an incidence of approximately 7–8% at 3 years. As discussed above, in a phase III study, maintenance bortezomib in the PAD arm was also well tolerated. Patients randomized to PAD arm followed by ASCT who are then administered bortezomib maintenance for 2 years have shown improved OS compared with those who received VAD followed by thalidomide maintenance (49% versus 34%) [Scheid et al. 2013; Sonneveld et al. 2015].
In summary, both consolidation and maintenance therapies improve the depth of response after induction treatment and ASCT. The role of thalidomide as maintenance treatment is limited due to its poor tolerability and side effects, especially neuropathy. Lenalidomide maintenance is best supported by phase III trial evidence, although the duration of therapy needs to be clarified and there are concerns of an increased risk of second primary malignancies. Bortezomib maintenance, administered twice monthly after ASCT, has shown benefit and may be considered for patients with high- or intermediate-risk cytogenetics. However, several questions remain unanswered including the toxicity, QoL considerations, duration of treatment, minimal residual disease (MRD) assessment and its impact on OS. Table 1 describes list of induction regimens in transplant eligible MM.
Upfront treatment of transplant-ineligible MM patients
Over two-thirds of NDMM patients are over the age of 65 years [Siegel et al. 2014]. For older patients or those with medical comorbidities not amenable to ASCT, the goals of treatment are to prolong survival and improve QoL. Similar to transplant-eligible patients, the use of novel agents in different combination regimens have been associated with a higher ORR compared with previous standard treatments. Table 2 describes the firstline treatment in NDMM transplant-ineligible patients.
Table 2.
Upfront treatment in newly diagnosed transplant-ineligible MM.
| Study by induction regimen | Treatment schema | Number of patients | Post induction (%) |
Long-term outcomes (%) | |
|---|---|---|---|---|---|
| ORR | CR VGPR | ||||
| IFM 99-06 Facon et al. [2007] |
|||||
| MPT | MPT ± Tm | 774 | 42 to −76 | NR | Median PFS: 14 to −28 month |
| versus | |||||
| MP | MP | 848 | 28 to −48 | NR | Median PFS: 10 to −19 month |
| VISTA Mateos et al. [2010] |
|||||
| VMP | VMPxx9 | 344 | 71 | 30 CR | Median OS: NR 3-year OS: 68.5 |
| versus | |||||
| MP | MPxx9 | 338 | 35 | 4 CR | Median OS: 43 months 3-year OS: 54 |
| MM-015 Palumbo et al. [2012] |
|||||
| MPR-R | MPRxx9-Rm | 152 | 77 | 18 CR 33 ⩾ VGPR |
Median PFS: 31 months 3-year median OS: 70 |
| versus | |||||
| MPR | MPRxx9 | 153 | 67 | 13 CR 33 ⩾ VGPR |
Median PFS: 14 months 3-year median OS: 62 |
| versus | |||||
| MP | MPxx9 | 154 | 49 | 5 CR 12 ⩾ VGPR |
Median PFS: 13 months 3-year median OS: 66 |
| FIRST Benboubker et al. [2014] |
|||||
| Continuous Rd | Rd until PD | 535 | 75 | 15 CR 44 ⩾ VGPR |
Median PFS: 2.5 months 3-year OS: 70 4-year OS: 59 |
| versus | |||||
| Rd | Rdxx18 | 541 | 73 | 14 CR 43 ⩾ VGPR |
Median PFS: 20.7 months 3-year OS: 66 4-year OS: 56 |
| versus | |||||
| MPT | MPTxx12 | 547 | 62 | 9 CR 28 ⩾ VGPR |
Median PFS: 21.2 months 3-year OS: 62 4-year OS: 51 |
| Magarotto et al. [2016] | |||||
| MPR | MPRx9-Rm or Rdm | 217 | 71 | 23 ⩾ VGPR | Median PFS: 24 months 4-year OS: 65 |
| versus | |||||
| CPR | CPRx9-Rm or Rdm | 220 | 68 | 20 ⩾ VGPR | Median PFS: 20 months 4-year OS: 68 |
| versus | |||||
| Rd | Rdx9-Rm or Rdm | 217 | 74 | 31 ⩾ VGPR | Median PFS: 58 months |
MPT, melphalan-prednisone-thalidomide; MP, melphalan-prednisone; VMP, bortezomib-melphalan-prednisone; MPR, melphalan-prednisone-lenalidomide; Rm, lenalidomide maintenance; Rd, lenalidomide-dexamethasone; CPR, cyclophosphamide-prednisone-lenalidomide; ORR, overall response rate; CR, complete response; VGPR, very good partial response; MM, multiple myeloma; NR, no reported; OS, overall survival; PFS, progression-free survival.
In Europe, melphalan, prednisone, and thalidomide (MPT) and bortezomib, melphalan, and prednisone (VMP) are considered standard of care for MM patients >65 years of age or those not eligible for ASCT. A randomized trial comparing MPT with melphalan and prednisone (MP) showed a significantly better OS (51.6 months) for MPT compared with MP (33.2 months) after a median follow up of 51.5 months [Facon et al. 2007]. However, MPT is associated with higher rates of grade 3 and grade 4 toxicities, including neutropenia and peripheral neuropathy. A meta-analysis comparing MPT with MP found a significant benefit to OS from adding thalidomide to MP (p = 0.004) [Fayers et al. 2011]. Similarly, the phase III VISTA trial has shown a significant OS survival benefit (13.3 months) with the addition of bortezomib to the MP regimen (MPR). However, VMP was associated with higher rates of peripheral neuropathy (14%) and gastrointestinal disturbances (19%) [Mateos et al. 2010].
Two large phase III trials demonstrated superior outcomes with lenalidomide-containing regimens in elderly patients with NDMM compared with standard melphalan-based therapies. The MM-015 trial incorporated lenalidomide into MPR followed by lenalidomide maintenance (MPR-R). This approach has significantly prolonged PFS (31 months) compared with MP (13 months, p < 0.001) or MPR without maintenance (14 months, p < 0.001) [Palumbo et al. 2012a]. In a randomized phase III trial, MPT followed by thalidomide maintenance and MPR followed by maintenance lenalidomide have shown similar efficacy in both arms. However, neuropathy is more common with MPT and myelosuppression with MPR [Zweegman et al. 2016].
The FIRST trial, a large phase III randomized trial, established that lenalidomide plus low-dose dexamethasone (Rd) administered until disease progression was also associated with a significant improvement in PFS (25.5 months) when compared with MPT (21.2 months) or Rd (20.7 months) for a fixed period of 18 months [Benboubker et al. 2014]. The safety profile of continuous Rd was manageable, and the incidence of second primary cancers was low across treatment groups. In contrast with young patients, the triplet lenalidomide-based regimens did not induce any advantage over doublet lenalidomide-based regimens in elderly myeloma patients [Magarotto et al. 2016].
Recently, the KRd regimen has shown an impressive response rate in NDMM patients, including elderly patients [Jakubowiak, 2014]. With a median follow up of 25 months, the ORR was 98% with a CR rate of 64%. At 2 years, the estimated PFS was 94% and OS was 98% [Mateos et al. 2010]. Other carfilzomib-based combinations, including carfilzomib plus MP (KMP) [Kolb et al. 2012], carfilzomib plus cyclophosphamide and dexamethasone (KCd) [Palumbo et al. 2012b], and carfilzomib plus bendamustine and dexamethasone [ClinicalTrials.gov identifier: NCT02002598] are active in transplant-ineligible patients based on early results from single arm phase I/II studies.
Ixazomib, an oral proteasome inhibitor, has shown promising results in combination with Rd. In a randomized trial of transplant-ineligible NDMM patients, weekly ixazomib in combination with Rd induced PR in 96% of patients with good tolerability [Mateos et al. 2010]. Dimopoulos and colleagues studied ixazomib in combination with cyclophosphamide and dexamethasone (ICd) in an open-label, multicenter phase II trial of NDMM transplant-ineligible MM patients [Dimopoulos et al. 2015a]. The regimen is very effective and the median PFS and TTP were not yet reached. Table 2 describes list of regimens in newly diagnosed transplant ineligible MM.
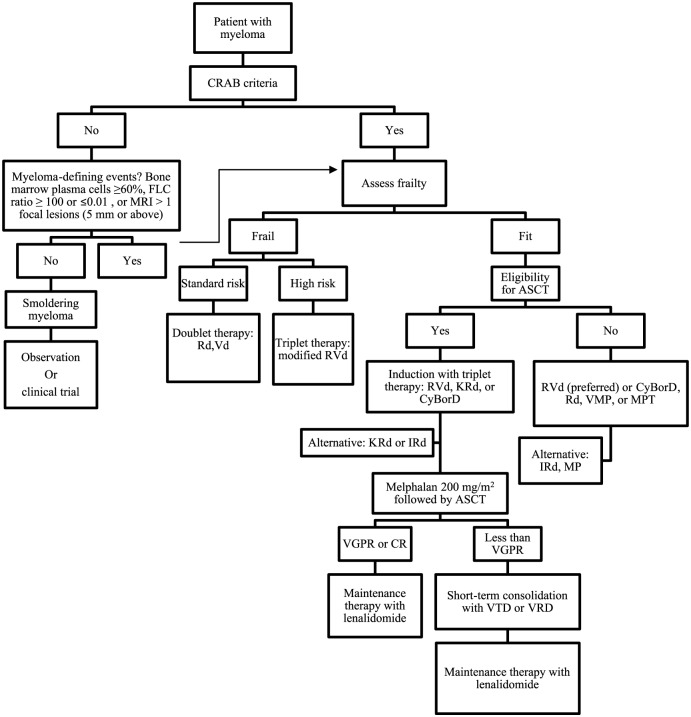
In summary, NDMM transplant-ineligible patients were previously treated with only alkylating agents and fixed-duration of therapy. But Rd as continuous therapy has demonstrated superiority over MPT and has likely become a new standard of care in elderly patients. However, a melphalan-based combination is still a viable option in these patients. Dose adjusted triplets (VRd) can be considered in high risk cytogenetics. The roles of ixazomib or carfilzomib-based combinations are under further investigation in clinical trials. Figure 1 describes the guideline for the management of MM.
Figure 1.
Guideline of treatment for newly diagnosed multiple myeloma.
Modified with permission from Lonial and Nooka [Lonial and Nooka, 2016].
ASCT, autologous stem cell transplant; CR, complete response; CyBorD, bortezomib, cyclophosphamide, and dexamethasone; FLC, free light chains; IRd, ixazomib-lenalidomide-dexamethasone; KRd, carfilzomib-lenalidomide-dexamethasone; MP, melphalan and prednisone; MPT, melphalan, prednisone, and thalidomide; MRI, magnetic resonance imaging; Rd, lenalidomide-dexamethasone; RVd, lenalidomide, bortezomib, dexamethasone; Vd, bortezomib-dexamethasone; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VRD, bortezomib-lenalidomide-dexamethasone; VTD, bortezomib-thalidomide-dexamethasone.
Treatment of relapse and refractory multiple myeloma patients
Treatment of relapsed/refractory multiple myeloma (RRMM) presents a special therapeutic challenge. The IMWG has divided RRMM into four categories: primary refractory, refractory or relapsed, relapsed and refractory or double refractory MM.
Relapsed and refractory myeloma is defined as progression of therapy in patients who achieve minor response or better, or progression within 60 days of last therapy. Patients who progress while on therapy are considered as primary refractory [Nooka et al. 2015]. Unfortunately, there is no clear biological-based recommendation regarding the choice of salvage therapy at various points of disease progression [Rajkumar et al. 2011; Cornell and Kassim, 2016]. Treatment options include (1) salvage chemotherapy, (2) salvage ASCT, (3) allogeneic hematopoietic stem cell transplantation or (4) post-transplant consolidation/maintenance therapy.
The MM-009/MM-010 phase III trials demonstrate superior PFS and OS in patients with RRMM receiving Rd compared with dexamethasone plus placebo [Dimopoulos et al. 2007]. The ORR was 61.0% in the Rd arm versus 19.9% in the placebo arm (p < 0.001). Pomalidomide is a third generation IMiD, which has shown significant responses in RRMM. In a randomized phase III MM-003 study, pomalidomide has induced significantly longer PFS and OS in combination with low-dose dexamethasone (Pd) compared with high-dose dexamethasone (4.0 months versus 1.9 months, p < 0.0001) [San Miguel et al. 2013].
Bortezomib, and carfilzomib, are also active in patients with RRMM. In the APEX trial, patients treated with intravenous bortezomib had significantly higher rates of ORR, PFS and 1-year OS compared with high-dose dexamethasone [Richardson et al. 2005]. The MMY-3021 trial demonstrated that subcutaneous bortezomib was comparable in efficacy with intravenous bortezomib and resulted in significantly reduced peripheral neuropathy (38% versus 53%; p = 0.04) [Moreau et al. 2011b]. Carfilzomib as a single agent also achieved an ORR of 23.7% with a median duration of response of 7.8 months and median OS of 15.6 months [Siegel et al. 2012].
In the phase III ENDEAVOR trial, carfilzomib plus dexamethasone (Kd) was compared with bortezomib plus dexamethasone (Vd) in RRMM patients (n = 929) [Dimopoulos et al. 2015b]. Results from an interim analysis showed significantly longer PFS with the carfilzomib combination (Kd versus Vd: 18.7 versus 9.4 months, respectively; p < 0.0001). In the subgroup analysis, patients receiving Kd demonstrated improved PFS and ORR compared with those receiving Vd regardless of prior exposure to either lenalidomide or bortezomib [Moreau, 2015c].
The triplet combinations with proteasome inhibitor and IMiD are also very potent options in RRMM. In a single arm phase II study in patients with RRMM, VRd led to an ORR of 64%, a median PFS of 9.5 months, and an OS of 30 months [Richardson et al. 2014]. In this study, 6% of the patients had received prior bortezomib, thalidomide and lenalidomide therapy. KRd has also led to significantly improved outcome in patients with RRMM, with 31% decrease of risk of disease progression and improved median PFS by 8.7 months (26.3 months in KRd arm versus 17.6 months in the Rd arm) [Stewart et al. 2015]. Other regimens, such as KPd (carfilzomib, pomalidomide and dexamethasone) or CyPomD (cyclophosphamide, pomalidomide and dexamethasone) are also very effective in RRMM. [Martin et al. 2013]. Table 3 summarizes treatment regimens for RRMM.
Table 3.
Treatment regiments for relapsed and refractory multiple myeloma.
| Study by induction regimen | Treatment schema | Number of prior antimyeloma therapies | Number of patients | Overall response rate (%) | Long-term outcomes (%) |
|---|---|---|---|---|---|
| MM-09/MM-010 Dimopoulos et al. [2007] |
|||||
| Rd | Rd until PD | ⩾1 | 176 | 60 | Median OS: 29.6 months |
| versus | |||||
| D | D until PD | ⩾1 | 175 | 24 | Median OS: 20.2 months |
| MM-003 San Miguel et al. [2013] |
|||||
| Pd | Pd until PD | ⩾2, with R and V | 302 | 31 | Median PFS: 4 monthsMedian OS: 12.7 months |
| versus | |||||
| D | D until PD | ⩾2, including with R and V | 153 | 10 | Media PFS: 1.9 months Median OS: 8.1 months |
| PX-171-003-A1 Siegel et al. [2012] |
|||||
| Carfilzomib | Carfilzomib × 12 | ⩾1 | 257 | 23.7 | Median PFS: 3.7 months Median OS: 15.6 months |
| ENDEAVOR Dimopoulos et al. [2015b] |
|||||
| Kd | Kd until PD | 1–3 | 464 | 77 | Median PFS: 18.7 months |
| versus | |||||
| Vd | Vd until PD | 1–3 | 465 | 63 | Median PFS: 9.4 months |
| Richardson et al. 2014 | |||||
| VRd | VRd × 8-VRdm | 1–3 | 64 | 64 | Median PFS: 9.5 months Median OS: 30 months |
| ASPIRE Stewart et al. [2015] |
|||||
| KRd | KRd until PD | 1–3 | 396 | 87.1 | Median PFS: 26.3 months 2-year OS: 73.3 |
| versus | |||||
| Rd | Rd until PD | 1–3 | 396 | 66.7 | Median PFS: 17.6 months 2-year OS: 65 |
| Martin et al. [2013] | |||||
| CyPD | CyPD until PD | R refractory | 70 | 48.5 | Median PFS: 6.4 months |
| TOURMALINE-MM1 Moreau et al. [2015d] |
|||||
| IRd | IRd until PD | 1–3 | 360 | 78.3 | Median PFS: 20.6 months |
| versus | |||||
| Rd | Rd until PD | 1–3 | 362 | 71.5 | Median PFS: 14.7 months |
| ELOQUENT-2 Lonial et al. [2015] |
|||||
| Elotuzumab + Rd | Elo + Rd until PD | 1–3 | 321 | 79 | Median PFS: 19.4 months 1-year PFS: 68 2-year PFS: 41 Median OS: 43.7 |
| versus | |||||
| Rd | Rd until PD | 1–3 | 325 | 66 | Median PFS: 14.9 months 1-year PFS: 57 2-year PFS: 27 Median OS: 39.6 months |
| SIRIUS Lonial et al. [2016] |
|||||
| Daratumumab | DARA until PD | ⩾3, including PI and IMiD, or PI/IMiD double refractory | 124 | 29.2 | Median PFS: 3.7 months 1-year OS: 64.8 Median OS: 17.5 months |
| MMY1001 Chari et al. [2015] |
|||||
| Daratumumab + Pd | DARA + Pd until PD | ⩾2, including lenalidomide and bortezomib | 77 | 58.5 | NR |
| PANORAMA-1 San Miguel et al. [2013] |
|||||
| Panobinostat + Vd | Panobinostat + Vd ×12 | 1–3 | 387 | 60.7 | Median PFS: 12 months 2-year PFS: 20.6 |
| versus | |||||
| Vd | Vd × 12 | 1–3 | 381 | 54.6 | Median PFS: 8 months 2-year PFS: 8.4 |
Kd, carfilzomib-dexamethasone; CyPD, cyclophosphamide-pomalidomide-dexamethasone; D, dexamethasone; DARA, daratumumab; Elo, elotuzumab; IMiD, immunomodulator; IRd, ixazomib-lenalidomide-dexamethasone; KRd, carfilzomib-lenalidomide-dexamethasone; OS, overall survival; PD, progression of disease; Pd, pomalidomide-dexamethasone; PI, proteasome inhibitor; PFS, progression-free survival; R, lenalidomide; Rd, lenalidomide-dexamethasone; V, bortezomib; Vd, bortezomib-dexamethasone; VRd, bortezomib-lenalidomide-dexamethasone; VRdm, bortezomib-lenalidomide-dexamethasone maintenance.
Emerging therapies in RRMM
Ixazomib
Ixazomib, formerly known as MLN9708, is an oral proteasome inhibitor. As a single agent, ixazomib induces 34% ORR in patients with RRMM [Roy et al. 2013]. The US FDA recently approved ixazomib in combination with Rd (IRd) for the treatment of patients with MM who have received at least 1–3 prior therapies. The approval was based on the phase III TOURMALINE-MM1 study, a double-blind, placebo-controlled trial that examined Rd with or without ixazomib in RRMM. Median PFS improved from 14.7 months in Rd treated patients to 20.6 months in patients treated with IRd. The improvement in PFS was observed in subgroups including PI-exposed or IMiD-exposed patients and those with high risk cytogenetics. The addition of ixazomib to Rd slightly increased the incidence of gastrointestinal adverse events such as diarrhea, constipation, nausea and vomiting compared with Rd. However, any-grade peripheral neuropathy, peripheral edema, thromboembolism, and neutropenia were all similar between arms [Moreau et al. 2015d]. Clinicians should consider using IRd regimen in patients who had previously received 1–3 prior therapies.
Monoclonal antibodies
In late 2015, the US FDA approved two monoclonal antibodies in the US for use in patients with RRMM: elotuzumab, in combination with Rd, and daratumumab as a single agent. Use of monoclonal antibodies directly targeting MM cells is a profound change compared with earlier treatment approaches.
Elotuzumab
Elotuzumab, is a humanized monoclonal antibody specifically targeting cell surface 1 (CS1, also called SLAM7), a glycoprotein highly expressed on the surface of MM cells. Binding of elotuzumab leads to recruitment of natural killer cells and tumor cell death via antibody-dependent cellular cytotoxicity (ADCC) [Cornell and Kassim, 2016]. Although elotuzumab has no significant single agent activity, it has shown impressive results in an open-labeled, multicenter phase III ELOQUENT-2 trial comparing Rd with or without elotuzumab in patients with RRMM with 1–3 prior treatments [Lonial et al. 2015]. The study demonstrated that elotuzumab in combination with Rd improved PFS by approximately 4.5 months and sustained improvement in PFS benefit at 1, 2, and 3 years. With extended follow up, median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the Rd arm. The 3-year PFS was 26% versus 18%, translating into relative improvement of 44% [Dimopoulos, 2015c].
Daratumumab
Daratumumab is a humanized monoclonal antibody specific for CD38 [immunoglobulin (Ig)G1, κ subclass] that targets tumor cells via ADCC, complement-dependent cytotoxicity, and phagocytosis. Daratumumab may also initiate CD38-mediated signal transduction leading to cell death. In preliminary studies, daratumumab has demonstrated promising activity in combination with Rd [Cornell and Kassim, 2016]. Daratumumab was recently approved by the US FDA as a single agent treatment for patients with RRMM who have failed >3 lines of treatment regimens, including patients refractory to IMiDs and proteasome inhibitors. The median duration of response was 7.4 months. Responses were also observed across all subgroups of age, number and types of lines of prior therapy, and presence or absence of extramedullary disease. Daratumumab at 16 mg/kg was associated with a 1-year OS of 64.8% (95% confidence interval: 51.2–75.5) and, at a subsequent cutoff, median OS of 17.5 months [Lonial et al. 2016].
Chari and colleagues, evaluated daratumumab in combination with Pd in heavily pretreated (⩾2 previous lines of therapy) patients with RRMM (n = 98) in an expansion cohort [Chari et al. 2015]. The ORR was 71% with a median time to first response of 1.2 months. After 6 months, 66% of patients still had an ongoing remission. No new or unexpected safety signals were detected with the addition of daratumumab to Pd. Based on these studies it is clear that daratumumab is an active treatment option for MM and is associated with relatively few adverse events except for infusion-related reactions, which typically occur during the first infusion and are quite manageable.
Panobinostat
Panobinostat is the first histone deacetylase (HDAC) inhibitor targeting epigenetic silencing of tumor suppressor genes in MM cells. It is approved in combination with bortezomib for patients who received at least two prior treatment regimens, including bortezomib and an IMiD. Based on a phase III randomized study (PANORAMA-1), panobinostat has been approved in combination with bortezomib and dexamethasone (Vd) [Einsele et al. 2015]. However, the combination of panobinostat, bortezomib and dexamethasone increased the rates of grade 3 or 4 diarrhea to 25% from 8%. PANORAMA 2 demonstrated that the addition of panobinostat to bortezomib in bortezomib-refractory patients resulted in a ORR of 34.5% and PFS of 5.4 months with a median OS of 17.5 months [Chari, 2015]. Table 3 describes the treatment regimens of relapse and refractory MM.
Choice of treatments based on functional status assessment
Age is the main factor currently used to decide on the treatment in patients with MM. There is growing recognition that frailty, determined on the basis of comorbidities at diagnosis, is a better marker to determine treatment. Palumbo and colleagues utilized a geriatric assessment scale in NDMM at diagnosis to assess comorbidities, cognitive and physical conditions and identified three groups; fit (score = 0, 39%), intermediate-fitness (score = 1, 31%), and frail (score ⩾2, 30%) [Palumbo et al. 2015]. The 3-year OS was 84% in fit, 76% in intermediate-fit and 57% in frail patients, suggesting that the frailty score helps to predicts mortality and the risk of toxicity in elderly myeloma patients. Therefore, we recommend utilizing a frailty score before starting treatment. Regimens that are less toxic and improve responses, like Rd, may be more suitable in these patients [Benboubker et al. 2014; Lonial et al. 2016]. However, the approach should be individualized and clinicians should discuss the clinical data with their patients.
Role of MRD in MM
MRD assessment has gained importance in the evaluation of treatment responses in MM. Several cooperative groups using different MRD techniques indicate that persistence of MRD is an adverse prognostic feature, even among CR patients. Recently, Barlogie and colleagues showed that the vast majority of CR patients (94%) who are MRD negative achieved long-term survival (10 years relapse free) [Barlogie et al. 2014]. Thus, MRD could potentially be used as a biomarker to evaluate the efficacy of treatment at different stages (induction, transplantation, consolidation, or maintenance) and a decision tool as to when to stop maintenance [Paiva et al. 2015].
The most sensitive techniques, such as next generation flow cytometry and next generation sequencing, have the potential to achieve a detection sensitivity up to 1 in 106 cells with an improved quantifiable range [Biran et al. 2014]. However, there is a need for a standardized technique regarding MRD assessment. As such, the optimal MRD sample type (bone marrow aspirate versus peripheral blood) and method (flow cytometry versus molecular testing) are unresolved questions in MRD testing.
Recommendations
Clinicians should consider the combination of a proteasome inhibitor and an IMiD such as VTd or VRd (in the USA) to treat NDMM transplant-eligible patients.
ASCT is the standard of care for NDMM transplant-eligible patients.
Clinicians should have an informed discussion with their patients regarding the role of maintenance therapy after ASCT.
The optimal duration of maintenance treatment is unknown, but maintenance therapy should be given for at least 2 years or continued until disease progression.
Patients who achieve less than a VGPR after ASCT should be considered for a second transplant or consolidation treatment with VRd or VTd.
The choice of regimen in a transplant-ineligible NDMM patient should be based on patient risk factors, including frailty and clinical staging. Rd as continuous therapy has demonstrated superiority over MPT and can be offered to these patients as firstline treatment.
The best sequence of treatment for RRMM is not known, but triplets are recommended in fit patients.
Recently, MRD testing has been more frequently used to evaluate the efficacy of treatment regimens and outcome. However, MRD testing is not the standard of care and is recommended for clinical trials.
Although the role of MRD negativity has not been completely established, patients with MRD negativity have a better outcome and MRD negativity might be used to define the lengths and intensity of treatment in the future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: SL participated in advisory boards of BMS, Celgene, Janssen and Novartis
Contributor Information
Shahzad Raza, Division of Hematology & Oncology, Columbia University Medical Center, Herbert Irving Comprehensive Cancer Center, New York, NY, USA.
Rachael A. Safyan, Division of Hematology & Oncology, Columbia University Medical Center, Herbert Irving Comprehensive Cancer Center, New York, NY, USA
Evan Rosenbaum, Department of Medicine, Columbia University Medical Center, New York, NY, USA.
Alex S. Bowman, Department of Medicine, Columbia University Medical Center, New York, NY, USA
Suzanne Lentzsch, Professor of Medicine, Director, Multiple Myeloma and Amyloidosis Service, Columbia University Medical Center, Herbert Irving Pavilion, R 953, 161 Ft. Washington Ave, New York, NY 10032, USA.
References
- Abidi M., Agarwal R., Ayash L., Deol A., Al-Kadhimi Z., Abrams J., et al. (2012) Melphalan 180 mg/m2 can be safely administered as conditioning regimen before an autologous stem cell transplantation (ASCT) in multiple myeloma patients with creatinine clearance 60 mL/min/1.73 m2 or lower with use of palifermin for cytoprotection: results of a phase I trial. Biol Blood Marrow Transplant 18: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal M., Harousseau J., Leyvraz S., Doyen C., Hulin C., Benboubker L., et al. (2006) Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood 108: 3289–3294. [DOI] [PubMed] [Google Scholar]
- Attal M., Lauwers-Cances V., Hulin C., Facon T., Caillot D., Escoffre M., et al. (2015) Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone du Myelome (IFM/DFCI 2009 trial). Blood 126: 391. [Google Scholar]
- Barlogie B., Anaissie E., Van Rhee F., Shaughnessy J., Szymonifka J., Hoering A., et al. (2010) Reiterative survival analyses of Total Therapy 2 for multiple myeloma elucidate follow-up time dependency of prognostic variables and treatment arms. J Clin Oncol 28: 3023–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Mitchell A., Van Rhee F., Epstein J., Morgan G., Crowley J. (2014) Curing myeloma at last: defining criteria and providing the evidence. Blood 124: 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Pineda-Roman M., van Rhee F., Haessler J., Anaissie E., Hollmig K., et al. (2008) Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood 112: 3115–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Tricot G., Anaissie E., Shaughnessy J., Rasmussen E., van Rhee F., et al. (2006) Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med 354: 1021–1030. [DOI] [PubMed] [Google Scholar]
- Benboubker L., Dimopoulos M., Dispenzieri A., Catalano J., Belch A., Cavo M., et al. (2014) Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 371: 906–917. [DOI] [PubMed] [Google Scholar]
- Biran N., Ely S., Chari A. (2014) Controversies in the assessment of minimal residual disease in multiple myeloma: clinical significance of minimal residual disease negativity using highly sensitive techniques. Curr Hematol Malig Rep 9: 368–378. [DOI] [PubMed] [Google Scholar]
- Byrne M., Salmasinia D., Leather H., Cogle C., Davis A., Hsu J., et al. (2014) Tandem autologous stem cell transplantation for multiple myeloma patients based on response to their first transplant—a prospective phase II study. Clin Med Insights Oncol 8: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavo M., Palumbo A., Zweegman S., Ma D., Hajek R., Pantani L., et al. (2016) Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). Presented at the American Society of Clinical Oncology Annual Meeting, 2016, Chicago, IL. [Google Scholar]
- Cavo M., Tacchetti P., Patriarca F., Petrucci M., Pantani L., Galli M., et al. (2012) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 376: 2075–2085. [DOI] [PubMed] [Google Scholar]
- Chari A. (2015) Novel targets in multiple myeloma. Am Jour Hematology/Oncology 11: 11–16. [Google Scholar]
- Chari A., Lonial S., Suvannasankha A., Fay J., Arnulf B., Ifthikharuddin J., et al. (2015) Open-label, multicenter, phase 1b study of daratumumab in combination with pomalidomide and dexamethasone in patients with at least 2 lines of prior therapy and relapsed or relapsed and refractory multiple myeloma. Blood 126: 508.26082451 [Google Scholar]
- Cornell R., Kassim A. (2016) Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone Marrow Transplant 51: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos M., Spencer A., Attal M., Prince H., Harousseau J., Dmoszynska A., et al. (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357: 2123–2132. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Grosicki S., Jedrzejczak W., Nahi H., Gruber A., Hansson M., et al. (2015a) Randomized phase 2 study of the all-oral combination of investigational proteasome inhibitor (PI) ixazomib plus cyclophosphamide and low-dose dexamethasone (ICd) in patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant-ineligible (NCT02046070). Blood 126: 26. [Google Scholar]
- Dimopoulos M., Moreau P., Palumbo A., Joshua D., Pour L., Hajek R., et al. (2015b) Carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in patients (pts) with relapsed multiple myeloma (RMM): results from the phase III study endeavor. ASCO Annual Meeting Proceedings Abstracts 33: 8509. [Google Scholar]
- Dimopoulos M., White D., Moreau P., Palumbo A., San Miguel J., Shpilberg O., et al. (2015c) Eloquent-2 update: a phase 3, randomized, open-label study of elotuzumab in combination with lenalidomide/dexamethasone in patients with relapsed/refractory multiple myeloma - 3-year safety and efficacy follow-up. Presented at the American Society of Hematology 57th Annual Meeting & Exposition, Orlando, FL, USA. [Google Scholar]
- Durie B. (2015) Bortezomib, lenalidomide and dexamethasone vs lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT): results of the randomized phase III trial SWOG S0777. Blood 126: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsele H., Richardson P., Hungria V., Yoon S., Beksac M., Dimopoulos M., et al. (2015) Subgroup analysis by prior treatment among patients with relapsed or relapsed and refractory multiple myeloma in the Panorama 1 study of panobinostat or placebo plus bortezomib and dexamethasone. European Hematology Association (EHA) 20th Congress, 2015, Vienna, Austria. [Google Scholar]
- Facon T., Mary J., Hulin C., Benboubker L., Attal M., Pegourie B., et al. (2007) Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet 370: 1209–1218. [DOI] [PubMed] [Google Scholar]
- Fayers P., Palumbo A., Hulin C., Waage A., Wijermans P., Beksac M., et al. (2011) Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 118: 1239–1247. [DOI] [PubMed] [Google Scholar]
- Galli M., Pezzi A., Di Raimondo F., Crippa C., Offidani M., Tacchetti P., et al. (2013) Persistent improvement in clinical outcomes with bortezomib-thalidomide-dexamethasone vs thalidomide-dexamethasone incorporated into double autologous transplantation for multiple myeloma: an updated analysis of phase 3 gimema-MMY-3006 study. Blood 122: 2090. [Google Scholar]
- Howlader N., Noone A., Krapcho M., Neyman N., Aminou R., Altekruse S., et al. (2012) Seer Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute. [Google Scholar]
- Jakubowiak A. (2014) Evolution of carfilzomib dose and schedule in patients with multiple myeloma: a historical overview. Cancer Treat Rev 40: 781–790. [DOI] [PubMed] [Google Scholar]
- Jakubowiak A., Dytfeld D., Griffith K., Lebovic D., Vesole D., Jagannath S., et al. (2012) A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 120: 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasielec J., Jakubowiak A. (2013) Current approaches to the initial treatment of symptomatic multiple myeloma. Int J Hematol Oncol 2: 10.2217/ijh.2213.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Hulin C., Caillot D., Benboubker L., Tiab M., Blin N., et al. (2012) Phase I/II study of carfilzomib plus melphalan-prednisone (CMP) in elderly patients with de novo multiple myeloma. ASCO Annual Meeting Proceedings. J Clin Oncol 30(Suppl. abstr 8009). [Google Scholar]
- Kumar S., Flinn I., Richardson P., Hari P., Callander N., Noga S., et al. (2012) Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 119: 4375–4382. [DOI] [PubMed] [Google Scholar]
- Kumar S., Roy V., Reeder C., Laplant B., Lacy M., Gertz M., et al. (2013) Phase 2 trial of single agent MLN9708 in patients with relapsed multiple myeloma not refractory to bortezomib. Blood 122: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonial S., Nooka A. (2016) Myeloma is not a single disease. J Oncol Pract 12: 287–292. [DOI] [PubMed] [Google Scholar]
- Lonial S., Dimopoulos M., Palumbo A., White D., Grosicki S., Spicka I., et al. (2015) Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 373: 621–631. [DOI] [PubMed] [Google Scholar]
- Lonial S., Weiss B., Usmani S., Singhal S., Chari A., Bahlis N., et al. (2016) Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 387: 1551–1560. [DOI] [PubMed] [Google Scholar]
- Magarotto V., Bringhen S., Offidani M., Benevolo G., Patriarca F., Mina R., et al. (2016) Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood 127: 1102–1108. [DOI] [PubMed] [Google Scholar]
- Martin T., Alsina M., Shain K., Cho H., Wolf J., Mahindra A., et al. (2013) Pomalidomide (Pom) Dexamethasone (D) with or without oral weekly cyclophosphamide (Cy) for lenalidomide refractory multiple myeloma (LRMM): a multicenter randomized phase II trial. Blood 122: 3200. [Google Scholar]
- Mateos M., Richardson P., Schlag R., Khuageva N., Dimopoulos M., Shpilberg O., et al. (2010) Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 28(13): 2259–2266. [DOI] [PubMed] [Google Scholar]
- McCarthy P., Owzar K., Hofmeister C. (2013) Analysis of overall survival (OS) in the context of cross-over from placebo to lenalidomide and the incidence of second primary malignancies (Spm) in the phase III study of lenalidomide versus placebo maintenance therapy following autologous stem cell transplant (ASCT) for multiple myeloma (MM) CALGB (Alliance) ECOG BMTCTN. Clin Lymphoma Myeloma Leuk 13: S28. [Google Scholar]
- McCarthy P., Owzar K., Hofmeister C., Hurd D., Hassoun H., Richardson P., et al. (2012) Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 366: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M., Harousseau J. (2014) Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica 99: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M., Richardson P., Mccarthy P., Attal M. (2015) Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: where do we stand&quest. Bone Marrow Transplant 50: 1024–1029. [DOI] [PubMed] [Google Scholar]
- Moreau P., Attal M., Facon T. (2015a) Frontline therapy of multiple myeloma. Blood 125: 3076–3084. [DOI] [PubMed] [Google Scholar]
- Moreau P., Avet-Loiseau H., Facon T., Attal M., Tiab M., Hulin C., et al. (2011a) Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood 118: 5752–5758. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hulin C., Macro M., Caillot D., Chaleteix C., Roussel M., et al. (2015b) Bortezomib, Thalidomide and Dexamethasone (VTD) is superior to bortezomib, cyclophosphamide and dexamethasone (VCD) prior to autologous stem cell transplantation for patients with de novo multiple myeloma. results of the prospective IFM 2013–04 trial. Blood 126: 393. [Google Scholar]
- Moreau P., Joshua D., Chng W., Palumbo A., Goldschmidt H., Hájek R., et al. (2015c) Impact of prior treatment on patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone vs bortezomib and dexamethasone in a subgroup analysis of the phase 3 endeavor study (NCT01568866). Presented at the 57th American Society of Hematology Annual Meeting, Orlando, FL. [Google Scholar]
- Moreau P., Masszi T., Grzasko N., Bahlis N., Hansson M., Pour L., et al. (2015d) Ixazomib, an investigational oral proteasome inhibitor (PI), in combination with lenalidomide and dexamethasone (IRD), significantly extends progression-free survival (PFS) for patients (Pts) with relapsed and/or refractory multiple myeloma (RRMM): the phase 3 Tourmaline-MM1 Study (Nct01564537). Blood 126: 727. [Google Scholar]
- Moreau P., Pylypenko H., Grosicki S., Karamanesht I., Leleu X., Grishunina M., et al. (2011b) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12: 431–440. [DOI] [PubMed] [Google Scholar]
- Morgan G., Gregory W., Davies F., Bell S., Szubert A., Brown J., et al. (2012) The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood 119: 7–15. [DOI] [PubMed] [Google Scholar]
- Munshi N., Anderson K. (2013) New strategies in the treatment of multiple myeloma. Clin Cancer Res 19: 3337–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooka A., Kastritis E., Dimopoulos M., Lonial S. (2015) Treatment options for relapsed and refractory multiple myeloma. Blood 125: 3085–3099. [DOI] [PubMed] [Google Scholar]
- Paiva B., Van Dongen J., Orfao A. (2015) New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood 125: 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A. (2014) Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 371: 895–905. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Bringhen S., Mateos M., Larocca A., Facon T., Kumar S., et al. (2015) Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group Report. Blood 125: 2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A., Hajek R., Delforge M., Kropff M., Petrucci M., Catalano J., et al. (2012a) Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 366: 1759–1769. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Bringhen S., Villani O., Siniscalchi A., Russo E., Uccello G., et al. (2012b) Carfilzomib, cyclophosphamide and dexamethasone (CCD) for newly diagnosed multiple myeloma (MM) Patients. Blood 120: 730. [Google Scholar]
- Rajkumar S., Harousseau J., Durie B., Anderson K., Dimopoulos M., Kyle R., et al. (2011) Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117: 4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder C., Reece D., Kukreti V., Chen C., Trudel S., Hentz J., et al. (2009) Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 23: 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P., Sonneveld P., Schuster M., Irwin D., Stadtmauer E., Facon T., et al. (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352: 2487–2498. [DOI] [PubMed] [Google Scholar]
- Richardson P., Xie W., Jagannath S., Jakubowiak A., Lonial S., Raje N., et al. (2014) A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 123: 1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Lauwers-Cances V., Robillard N., Hulin C., Leleu X., Benboubker L., et al. (2014) Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol 32: 2712–2717. [DOI] [PubMed] [Google Scholar]
- San Miguel J. (2015) Introduction to a series of reviews on multiple myeloma. Blood 125: 3039–3040. [DOI] [PubMed] [Google Scholar]
- San Miguel J., Weisel K., Moreau P., Lacy M., Song K., Delforge M., et al. (2013) Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 14: 1055–1066. [DOI] [PubMed] [Google Scholar]
- Scheid C., Van Der Holt B., El Jarari L., Bertsch U., Salwender H., Zweegman S., et al. (2013) bortezomib induction and maintenance treatment improves survival in patients with newly diagnosed multiple myeloma: extended follow-up of the HOVON-65/GMMG-HD4 trial. Blood 122: 404. [Google Scholar]
- Siegel D., Martin T., Wang M., Vij R., Jakubowiak A., Lonial S., et al. (2012) A phase 2 study of single-agent carfilzomib (PX-171–003-A1) in patients with relapsed and refractory multiple myeloma. Blood 120: 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Sonneveld P., Salwender H., Van Der Holt B., El Jarari L., Bertsch U., Blau I., et al. (2015) Bortezomib induction and maintenance in patients with newly diagnosed multiple myeloma: long-term follow-up of the HOVON-65/GMMG-HD4 trial. Blood 126: 27. [DOI] [PubMed] [Google Scholar]
- Stewart A., Rajkumar S., Dimopoulos M., Masszi T., Špička I., Oriol A., et al. (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372: 142–152. [DOI] [PubMed] [Google Scholar]
- Zweegman S., Van Der Holt B., Mellqvist U., Salomo M., Bos G., Levin M., et al. (2016) Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood 127: 1109–1116. [DOI] [PubMed] [Google Scholar]