Abstract
Background:
In this multi-institutional prospective study, we aimed to assess the safety and efficacy of nedaplatin plus S-1 (NS) chemotherapy for patients with recurrent and metastatic nasopharyngeal carcinoma (NPC) when platinum-containing regimens failed.
Methods:
A total of 52 recurrent and metastatic NPC patients who previously received, but failed with platinum-containing chemotherapy, had oral S-1 chemotherapy (twice daily from the first day to the fourteenth day) and nedaplatin (80 mg/ m2, day 1) every 3 weeks. The body surface area (BSA) decided the dose of S-1: 40 mg twice a day when BSA < 1.25 m2; 50 mg twice daily when 1.25 m2 ⩽ BSA < 1.5 m2; and 60 mg twice daily when BSA ⩾ 1.5 m2.
Results:
Treatment was well tolerated. The main hematological adverse event was neutropenia. Five patients (9.6%) had grade 3 neutropenia. Three patients were found with grade 3 anemia (5.8%). One patient was found with grade 3 thrombocytopenia (1.9%). No patient was found with grade 3 or 4 nonhematological toxicity. The rates of complete response, partial response and overall response were 3.8%, 38.5% and 42.3%, respectively. Median time to progression was 6.2 months and median survival was 14.6 months. The rates of 1-year survival and 2-year survival were 63% and 27%, respectively.
Conclusions:
NS chemotherapy provides a satisfactory and safe clinical activity for patients with recurrent and metastatic NPC after platinum-containing chemotherapy failed.
Keywords: chemotherapy, nasopharyngeal carcinoma, nedaplatin, S-1
Introduction
Nasopharyngeal carcinoma (NPC) is the most common head and neck cancer in southern China, especially in the Guangdong Province [Cao et al. 2011; Yu and Yuan, 2002]. NPC is sensitive to chemotherapy to a great extent and survival prolongation could be achieved for recurrent disease, and possibly metastatic disease [Lee et al. 2012; Ma and Chan, 2005; Fandi et al. 2000; Lee et al. 2005]. In general, patients with recurrent, with or without metastatic NPC are not suitable for further multimodality curative treatment. Cisplatin-based or carboplatin-based chemotherapy regimens are often considered as standard palliative chemotherapy for recrudescent or metastatic NPC [Lee et al. 2005; Suarez et al. 2010; Ma et al. 2008; Au and Ang, 1994]. As for the patients with disease progression who failed platinum-based treatment before, no standard second-line chemotherapy exists. Therefore, the identification of an active regimen with a favorable toxicity profile is necessary and important.
Nedaplatin is a derivative of second-generation platinum that has similar antitumor activities to cisplatin with less gastrointestinal toxicity and nephrotoxicity [Kawai et al. 2005; Sasaki et al. 1991; Alberts et al. 1997]. Also, our recent phase II study of nedaplatin plus capecitabine has shown satisfactory antitumor activity as salvage chemotherapy for cisplatin-refractory recurrent and metastatic NPC [Peng et al. 2013]. S-1 is a promising new oral antitumor agent containing tegafur and two modulators. S-1 has shown satisfactory clinical activity for the treatment of a variety of solid cancers, and head and neck cancer is included [Saif et al. 2009; Shirasaka et al. 1996; Tsukuda et al. 2005]. To address the clinical role of S-1 in patients with NPC, we have previously conducted a retrospective analysis to evaluate the tolerability and efficacy of S-1 chemotherapy as second-line or salvage therapy in patients with recurrent or metastatic NPC after platinum-based chemotherapy failed, and showed that S-1 had active antitumor effect [Peng et al. 2014]. But the clinical influence of nedaplatin plus S-1 (NS) on NPC patients is still uncertain. To address this issue, we conducted the following multi-institutional prospective study to assess the tolerability and efficacy of NS chemotherapy for patients with recurrent and metastatic NPC after platinum-based chemotherapy failed.
Patients and methods
Patients
Patients who had clinical evidence of recurrent and metastatic NPC that was histologically confirmed, were aged from 18 to 75 years, had a performance status of Eastern Cooperative Oncology Group from 0 to 2, had disease progression after platinum-containing chemotherapy or while they were still undergoing platinum-containing chemotherapy, and having at least one measurable lesion in accordance with the RECIST version 1.0 were included according to the eligibility criteria. Previous treatment of chemotherapy was not allowed for advanced disease with capecitabine or S-1. However, previous treatment with 5-FU was permitted. Adequate liver (level of bilirubin ⩽ 1.5 mg/dl and level of alanine aminotransferase or aspartate aminotransferase no more than two-and-a-half times the upper limit of normal), kidney (level of serum creatinine ⩽ 1.5 mg/dl), and bone marrow functions (level of hemoglobin ⩾ 10 g/dl and counts of white blood cells, neutrophils, platelets that were ⩾4000/µl, ⩾1500/µl and ⩾100,000/µl, respectively) were required. Informed consent was obtained from all patients included in the study, and the study was approved by the local ethical committees from the Fifth Affiliated Hospital of Sun-Yat-Sen University, the Cancer Center of Sun-Yat-Sen University, and the People’s Hospital of Zhongshan City (no. 2010S39). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Treatment
Nedaplatin was administered intravenously by process of infusion of 2 hours on the first day, at a dose of 80 mg/m2. Prior to nedaplatin administration, a serotonin antagonist was used for the prevention of emesis as usual protocol. S-1 chemotherapy was administered following a planned schedule and a standard dose: 40 mg twice a day when body surface area (BSA) < 1.25 m2; 50 mg twice daily when 1.25 m2 ⩽ BSA < 1.5 m2; and 60 mg when BSA ⩾ 1.5 m2; S-1 was continuously given for 14 days, followed by a 7-day rest period. Every 3 weeks, treatment was repeated.
Evaluation of efficacy
Evaluation before treatment included physical examination, electrocardiogram, imaging for distant metastases and MRI of head and neck. The primary objective was response rate (RR), and secondary objectives were toxicity, time to progression (TTP) and overall survival (OS). Tumor response was evaluated every two cycles during the chemotherapy and then every 3 months after the completion of the chemotherapy using response evaluation criteria in solid tumor (RECIST).
Evaluation of tolerability and dose modification
Toxicities were assessed using National Cancer Institute Common Toxicity Criteria (version 3.0) before each treatment cycle. Complete physical examination and serum chemistry analyses were performed before the start of this study. To begin the next treatment cycle, each patient was required to have a platelet count ⩾ 75,000/µl, a neutrophil count ⩾ 1000/µl and adequate hepatic function and renal function. If the chemotherapy was interrupted by more than 3 weeks, patients were removed from the study.
In cases of toxicity with a grade 3 intensity or higher, the dose was modified according to the following criteria: the dose of NS for the subsequent cycle was reduced by 20% in the case of a repeated any-grade-3 toxicity, and reduced by 40% in the case of a repeated any-grade-4 toxicity. If a dose reduction exceeding 40% was required, the patient was excluded from the study. The use of growth factor was permitted.
Statistical analysis
This trial used a two-stage optimal design as proposed by Simon, with an 80% power to accept the hypothesis and 5% significance to reject the hypothesis [Simon, 1989]. This trial was designed to detect an RR of 40% when compared with a minimal, clinically meaningful RR of 20%. Allowing for a follow-up loss rate of 10%, the total sample size was 48 patients with measurable disease. All recruited patients were included in the intention-to-treat analysis of efficacy and toxicity. The overall RR was calculated with 95% confidence intervals (CIs). TTP and OS were estimated using the Kaplan–Meier method. TTP was measured from the date of entry into the study until the date of progression, and the OS was calculated from the date of entry to the date of the final follow up or death. The statistical analyses were performed using an SPSS software package (SPSS 16.0 Inc., Chicago, IL).
Results
Patient characteristics
Between April 2011 and September 2014, 52 patients with recrudescent and metastatic NPC received NS chemotherapy after platinum-based chemotherapy failed in the Cancer Center of Sun Yat-sen University, the Fifth Affiliated Hospital of Sun Yat-sen University and the People’s Hospital of Zhongshan City. The features of the patients are shown in Table 1.
Table 1.
Patients’ characteristics.
| Characteristics | Patients (n = 52) | % |
|---|---|---|
| Age, years | Median 48 (range, 30–69) | |
| Sex | ||
| Female | 15 | 28.8 |
| Male | 37 | 71.2 |
| ECOG performance status | ||
| 0 | 11 | 21.2 |
| 1 | 26 | 50.0 |
| 2 | 15 | 28.8 |
| Tumor involved site | ||
| Nasopharyngeal | 6 | 11.5 |
| Lymph node | 14 | 26.9 |
| Lung | 29 | 55.8 |
| Liver | 13 | 25.0 |
| Bone | 8 | 15.4 |
| Number of involved sites | ||
| 1 | 30 | 57.7 |
| 2 | 15 | 28.8 |
| 3 | 7 | 13.5 |
| Initially received platinum-based chemotherapy for recurrence/metastases | ||
| Cisplatin + 5-fluorouracil | 7 | 13.5 |
| Cisplatin + gemcitabine | 21 | 40.4 |
| Cisplatin + docetaxel | 16 | 30.8 |
| Cisplatin + paclitaxel | 10 | 19.2 |
| Carboplatin + paclitaxel | 14 | 26.9 |
| Number of chemotherapy regimens received | ||
| One | 17 | 32.7 |
| Two | 25 | 48.1 |
| ⩾Three | 10 | 19.2 |
ECOG, Eastern Cooperative Oncology Group.
Treatment efficacy
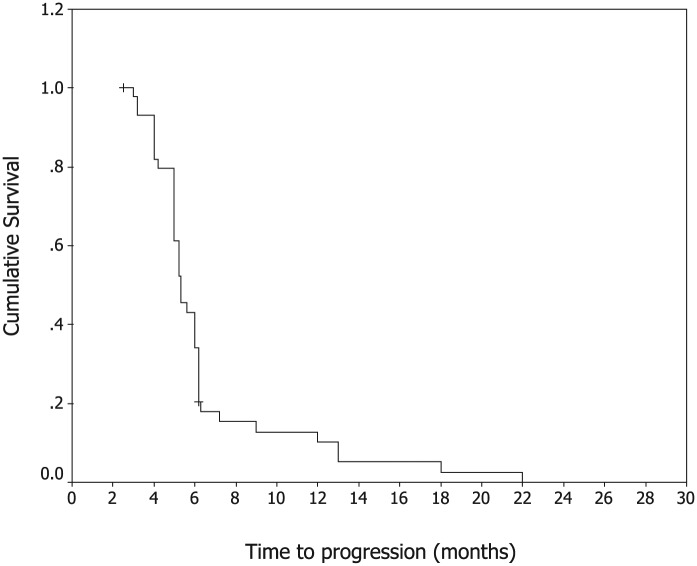
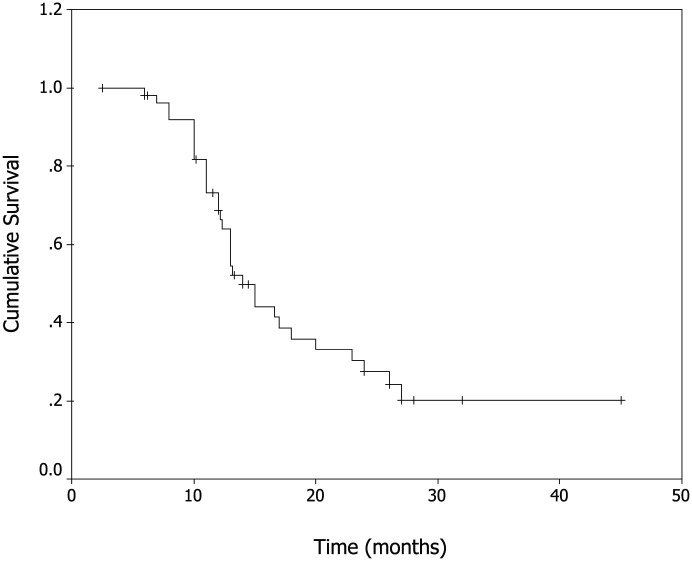
In total, 52 patients received 246 cycles of NS with a median of 4 cycles (range, 2–10 cycles) administered per patient. Two patients (3.8%) were found with complete response (CR), 20 patients (38.5%) with partial responses (PR), 21 patients (40.4%) with stable disease (SD) and 9 patients (17.3%) with progressive disease (PD). The rate of overall response (CR+PR) was 42.3% (95% CI, 26.2–56.8%). The median TTP was 6.2 months (95% CI, 3.8–9.1 months, shown in Figure. 1), median OS was 14.6 months for all patients (95% CI, 11.6–18.5 months, shown in Figure. 2). The rates of 1-year survival and 2-year survival were 63% and 27%, respectively. No significant difference in overall RR, TTP and OS rate was observed among patients treated by different initial chemotherapy. No significant difference in treatment outcome was observed in patients with and without prior treatment with 5-fluorouracil. Three patients with multiple metastases, who were treated with NS chemotherapy and survived over 36 months, are alive up to now. Three patients accepted neck dissection after NS chemotherapy.
Figure 1.
Kaplan–Meier Curve of progression-free survival.
Figure 2.
Kaplan–Meier Curve of overall survival.
Toxicity of treatment
Toxicity frequencies are presented in Table 2. The main hematological toxicity was neutropenia, with five patients (9.6%) suffering from grade 3 neutropenia. Only three patients (5.8%) developed grade 3 anemia and one patient (1.9%) experienced grade 3 thrombocytopenia. No patient was observed with grade 3/4 nonhematological toxicity. The main nonhematological toxicities were nausea (57.7%) and vomiting (46.2%). No patient was found with grade 3/4 nausea and vomiting. Other toxicities were mild, such as hepatic toxicity, renal toxicity, neuropathy and fatigue. No treatment-related deaths were observed.
Table 2.
Treatment-related adverse events (n = 52).
| Adverse events |
NCI-CTC grade, % of patients |
|||
|---|---|---|---|---|
| Grade | 1 | 2 | 3 | 4 |
| Hematological | ||||
| Leukopenia | 25 (48.1) | 19 (36.5) | 5 (9.6) | 0 (0.0) |
| Anemia | 20 (38.5) | 13 (25.0) | 3 (5.8) | 0 (0.0) |
| Thrombocytopenia | 15 (28.8) | 8 (15.4) | 1 (1.9) | 0 (0.0) |
| Nonhematological | ||||
| Nausea | 21 (40.4) | 9 (17.3) | 0 (0.0) | 0 (0.0) |
| Vomiting | 16 (30.8) | 8 (15.4) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 5 (9.6) | 2 (3.8) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 10 (19.2) | 4 (7.7) | 0 (0.0) | 0 (0.0) |
| Hand−foot syndrome | 3 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 8 (15.4) | 3 (5.8) | 0 (0.0) | 0 (0.0) |
NCI-CTC, National Cancer Institute Common Toxicity Criteria.
Discussion
Chemotherapy remains an important palliative treatment for recurrent and metastatic NPC [Lee et al. 2005, 2012; Ma and Chan, 2005; Fandi et al. 2000; Suarez et al. 2010; Ma et al. 2008]. Although no randomized trial comparing different chemotherapy regimens in NPC has ever been reported, platinum-containing regimes (typically doublet combination of carboplatin or cisplatin with taxane, gemcitabine, and 5-FU) were the most widely used regimens [Ma and Chan, 2005; Suarez et al. 2010; Ma et al. 2008; Au and Ang, 1994]. Despite many chemotherapy regimens being established with the intention to improve quality of life or prolong survival, no standard chemotherapy regimen exists as second-line chemotherapy treatment for NPC patients who failed platinum-containing-regimen chemotherapy. For this reason, new options with improved efficacy and favorable toxicity profiles are highly desirable [Fandi et al. 2000; Lee et al. 2005; Suarez et al. 2010; Ma et al. 2008].
Nedaplatin is a complex of second-generation platinum that is characterized by lower gastrointestinal and renal toxicities, compared with cisplatin; high fluid-level infusions are not required for nedaplatin. Furthermore, nedaplatin is reported to be at least as effective as cisplatin in treating head and neck cancer [Kawai et al. 2005; Sasaki et al. 1989, 1991; Alberts et al. 1997]. As a single agent, S-1 is active for the treatment of gastric, pancreatic, colorectal, breast, and head and neck cancers [Saif et al. 2009; Shirasaka et al. 1996; Tsukuda et al. 2005; Schoffski, 2004; Koizumi et al. 2008]. Our previous study had confirmed the tolerability and efficacy of S-1 chemotherapy for patients with recrudescent and metastatic NPC after platinum-containing chemotherapy failed [Peng et al. 2014]. Therefore, a combination of nedaplatin and S-1 for the treatment of NPC seemed to be a good rationale.
To the best of the authors’ knowledge, the current trial is the first report evaluating the tolerability and efficacy of NS chemotherapy in patients with recrudescent and metastatic NPC after platinum-containing chemotherapy failed. In this study, the treatment indicated the RR was 42.3%, the medians of TTP and OS were respectively 6.2 months and 14.6 months, and the rates of 1-year survival and 2-year survival were 63% and 27%, respectively. By comparison with other clinical trials, NS seems a promising regimen in platinum-pretreated refractory NPC. A phase II trial with pemetrexed plus cisplatin to treat patients with recrudescent and metastatic NPC with previous treatment of platinum-containing chemotherapy was reported by Yau and colleagues [Yau et al. 2012]. One patient (7%) was found with CR, two patients (13%) with PR, and eight patients (53%) with SD. Moreover, the median TTP was 30 weeks. In a study where patients with cisplatin-resistant NPC were treated with gemcitabine plus vinorelbine, the regimen was found to be safe and effective and overall RR was 6%, the median PFS was 5.6 months and the median OS was 11.9 months [Wang et al. 2006]. In the study examining the treatment of patients with recrudescent and metastatic NPC after cisplatin-containing chemotherapy failed with capecitabine plus nedaplatin, similar survival data were observed: an overall RR was 41.7%, the median PFS was 5.8 months and the median OS was 12.4 months [Li et al. 2008]. However, our study had several limitations. First, being a multi-institutional study analysis, it was vulnerable to various biases. Second, because our current study was not randomized, whether S-1 in combination with nedaplatin is indeed comparable with or superior to other regimens as a second-line treatment in terms of efficacy has not yet been fully demonstrated and requires further prospective randomized study. The observed differences by cross-trial comparisons may be explained by the heterogeneity in trial designs, patient selection, the use of effective salvage regimens and improved ancillary support at progression. Third, the results of this trial should be analyzed with caution, owing to the small number of enrolled patients.
The research has confirmed the tolerability of the NS regimen, in particular to the favorable profile of nonhematological toxicity. Only two patients were discontinued from this treatment because of treatment-related toxicities and no treatment-related deaths occurred. In contrast to cisplatin-containing regimens, the NS regimen demonstrated lower gastrointestinal toxicities and many fluid infusions were not required [Koizumi et al. 2008; Yau et al. 2012; Li et al. 2008]. No patient was observed with grade 3/4 nausea and vomiting. Compared with capecitabine or 5-FU-based regimens, NS also showed lower hand–foot syndrome toxicity, which was beneficial for NPC patients after several chemotherapy regimens before. Myelosuppression is the only main toxicity that is found in current trials. Five cases of grade 3 leukopenia were observed and were reversed with granulocyte colony-stimulating factor (G-CSF) treatment. Only three patients (5.8%) developed grade 3 anemia and one patient (1.9%) experienced grade 3 thrombocytopenia. Owing to the relatively mild toxicity, patients who received previous multiple treatments could receive NS safely and many of the patients could receive their treatment in the outpatient setting with comparatively long-term drug administration [Cunningham and Coleman, 2001]. Thus, the patients could not only get anticancer treatment but also, maintain their quality of life. However, unfortunately, patient quality of life during treatment was not formally assessed in this study.
Conclusion
Owing to the relatively mild toxicity, this combination chemotherapy seemed to be much more patient friendly compared with other cisplatin-containing regimens. The combination of nedaplatin and S-1, as an effective salvage regimen for platinum-pretreated refractory recurrent and metastatic NPC, is worth investigating in future prospective trials.
Acknowledgments
We thank all of the patients and their families for their willingness to take part in this study.
Footnotes
Authors Note: Pei-Jian Peng and Bao-Jun Lv contributed equally to this work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Pei-Jian Peng, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Guangdong Province, People’s Republic of China.
Bao-Jun Lv, Department of Surgical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhu Hai, Guangdong Province, China.
Zhi-Hui Wang, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Guangdong Province, People’s Republic of China.
Hai Liao, Department of Medical Oncology, Cancer Center, Sun Yat-sen University, Guangzhou, China.
Yu-Meng Liu, Department of Oncology, the People’s Hospital of Zhongshan City, Zhongshan, Guangdong Province, China.
Zhong Lin, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Guangdong Province, People’s Republic of China.
Yun-Yan Con, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Guangdong Province, People’s Republic of China.
Pei-Yu Huang, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of nasopharyngeal carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, China.
References
- Alberts D., Fanta P., Running K., Adair L., Jr., Garcia D., Liu-Stevens R., et al. (1997) In vitro phase II comparison of the cytotoxicity of a novel platinum analog, nedaplatin (254-S), with that of cisplatin and carboplatin against fresh, human ovarian cancers. Cancer Chemother Pharmacol 39: 493–497. [DOI] [PubMed] [Google Scholar]
- Au E., Ang P. (1994) A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 5: 87–89. [DOI] [PubMed] [Google Scholar]
- Cao S., Simons M., Qian C. (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J cancer 30: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D., Coleman R. (2001) New options for outpatient chemotherapy—the role of oral fluoropyrimidines. Cancer Treat Rev 27: 211–220. [DOI] [PubMed] [Google Scholar]
- Fandi A., Bachouchi M., Azli N., Taamma A., Boussen H., Wibault P., et al. (2000) Long-term disease-free survivors in metastatic undifferentiated carcinoma of the nasopharyngeal type. J Clin Oncol 18: 1324–1330. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Taniuchi S., Okahara S. (2005) Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol Pharm Bull 28: 1385–1388. [DOI] [PubMed] [Google Scholar]
- Koizumi W., Narahara H., Hara T., Takagane A., Akiya T., Takagi M., et al. (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9: 215–221. [DOI] [PubMed] [Google Scholar]
- Lee A., Ng W., Chan Y., Sze H, Chan C., Lam C. (2012) The battle against nasopharyngeal cancer. Radiother Oncol 104: 272–278. [DOI] [PubMed] [Google Scholar]
- Lee A., Sze W., Au J., Leung S., Leung T., Chua D., et al. (2005) Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 61: 1107–1116. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang F., Jiang W., Xiang X., Deng Y., Hu G., et al. (2008) Phase II study of capecitabine and cisplatin combination as first-line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol 62: 539–544. [DOI] [PubMed] [Google Scholar]
- Ma B., Chan A. (2005) Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer 103: 22–31. [DOI] [PubMed] [Google Scholar]
- Ma B., Hui E., Chan A. (2008) Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci 99: 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P., Cheng H., Ou X., Zeng L., Wu X., Liu Y., et al. (2014) Safety and efficacy of S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy: multi-institutional retrospective analysis. Drug Des Devel Ther 8: 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P., Ou X., Chen Z., Liao H., Peng Y., Wang S., et al. (2013) Multicenter phase II study of capecitabine combined with nedaplatin for recurrent and metastatic nasopharyngeal carcinoma patients after failure of cisplatin-based chemotherapy. Cancer Chemother Pharmacol 72: 323–328. [DOI] [PubMed] [Google Scholar]
- Saif M., Syrigos K., Katirtzoglou N. (2009) S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs 18: 335–348. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Shinkai T., Eguchi K., Tamura T., Ohe Y., Ohmori T., et al. (1991) Prediction of the antitumor activity of new platinum analogs based on their ex vivo pharmacodynamics as determined by bioassay. Cancer Chemother Pharmacol 27: 263–270. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Tamura T., Eguchi K., Shinkai T., Fujiwara Y., Fukuda M., et al. (1989) Pharmacokinetics of (glycolate-0,0’)-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23: 243–246. [DOI] [PubMed] [Google Scholar]
- Schoffski P. (2004) The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 15: 85–106. [DOI] [PubMed] [Google Scholar]
- Shirasaka T., Shimamoto Y., Oshima H., Yamaguchi M., Kato T., Yonekura K., et al. (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7: 548–557. [DOI] [PubMed] [Google Scholar]
- Simon R. (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10. [DOI] [PubMed] [Google Scholar]
- Suarez C., Rodrigo J., Rinaldo A., Langendijk J., Shaha A., Ferlito A. (2010) Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol 267: 1811–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda M., Kida A., Fujii M., Kono N., Yoshihara T., Hasegawa Y., et al. (2005) Chemotherapy Study Group of Head and Neck Cancer: Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chang J., Liu T., Lin C., Yu Y., Hong R. (2006) Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck 28: 74–80. [DOI] [PubMed] [Google Scholar]
- Yau T., Shum T., Lee A., Yeung M., Ng W., Chan L. (2012) A phase II study of pemetrexed combined with cisplatin in patients with recurrent or metastatic nanopharyngeal carcinoma. Oral Oncol 48: 441–444. [DOI] [PubMed] [Google Scholar]
- Yu M., Yuan J. (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12: 421–429. [DOI] [PubMed] [Google Scholar]