Abstract
Carcinoid syndrome (CSy) is a constellation of symptoms that may commonly present in patients with well differentiated neuroendocrine tumors (NETs), with somatostatin analogs (SSAs) being the first-line option for symptom management. However, symptomatic progression eventually occurs and in this scenario of a refractory CSy; several treatment options have been studied such as dose escalation of SSA, interferon and liver-directed therapies. Nevertheless, recent phase III trials have contributed to the understanding and management of this condition. We performed a comprehensive review of interventional studies examining refractory CSy to provide the evidence for current treatment options and propose a treatment sequence.
Keywords: carcinoid syndrome, neuroendocrine neoplasm, somatostatin, systematic review
Introduction
One of the most common types of neuroendocrine tumor (NET) is the well differentiated midgut grade 1 [G1: Ki67 ⩽ 2% and < 2 mitoses/10 high-power fields (HPF)] tumor presenting with carcinoid syndrome (CSy). CSy is defined as symptoms and signs of overproduction of serotonin produced by NET, such as flushing, diarrhea, dyspnea, bronchospasm, palpitation, and eventually, symptoms associated with right-sided heart failure resulting from carcinoid heart [Bhattacharyya et al. 2007]. The presence of the elevated serotonin metabolite 5-hydroxiindolacetic acid (5-HIAA) in a 24-hour urine test confirms the diagnosis of CSy. Patients suffering from CSy generally present having incurable disease with multiple liver metastases.
The standard treatment for CSy is somatostatin analogs (SSAs) [Mota et al. 2016]. Both currently available SSAs, octreotide and lanreotide, despite not being tested head to head, offer similar efficacy in terms of controlling the CSy symptoms, with an overall response rate of 50% [O’Toole et al. 2000] and symptom control lasting from months to several years. However, symptom progression eventually occurs, with patients often experiencing an increase in the number of bowel movements (BMs) per day and possibly flushing episodes. The term refractory carcinoid syndrome (RCSy) refers to such patients whose hormone-related symptoms are no longer controlled by standard doses of SSA. However, the definition of uncontrolled carcinoid symptoms considered by most studies is imprecise, varying across studies and even among patients in the same study.
The maximum control of CSy has a direct influence on patients’ quality of life [Beaumont et al. 2012] and likely on survival, although this has never been proven. Patients with RCSy suffer from inconvenient and often incapacitating symptoms, such as several BMs per day, which affect their social life and independence. Moreover, RCSy may lead to many hard-to-treat complications such as carcinoid heart, mesenteric and retroperitoneal fibrosis, and carcinoid crisis [Mota et al. 2016]. Our objective was to perform a comprehensive review of the several treatment options to treat RCSy.
Methods
The review of treatments directed to RCSy is difficult because of the heterogeneity of therapies, study designs and definition of refractory carcinoid symptoms. We tried to perform a comprehensive search of studies published in the Medline database using the mesh term “malignant carcinoid syndrome” AND each treatment modality (surgery or surgical; chemotherapy; somatostatin analogue or lanreotide or octreotide; liver embolization; radiofrequency ablation; interferon; everolimus; radionuclide; liver transplantation) from onset until February 2016. We limited the search to articles published in English, Portuguese and Spanish. Eligible studies were case reports, retrospective series, observational cohorts, phase II and III clinical trials testing any therapeutic intervention to treat uncontrolled carcinoid symptoms despite any therapy. We also sought relevant articles within the reference lists of publications. Relevant phase III trials presented at major oncology meetings in 2014–2015 were included, despite not been published. Here, we describe the most relevant studies that have provided more detailed information about symptomatic response in patients with RCSy.
Scientific evidence from therapeutic management of refractory carcinoid syndrome
Dose escalation of somatostatin analog
One of the potential mechanisms underlying the failure of SSA to control CSy is tachyphylaxis [Hofland and Lamberts, 2003]. Although the exact mechanism is unknown, tachyphylaxis is suspected when carcinoid symptoms control last for only 2–3 weeks after SSA administration and worsen before the next injection, or when there is general decreased symptom control. A common approach is to increase the frequency of SSA administration to every 2 or 3 weeks. Indeed, a small phase II study showed that increasing the frequency of administration of SSA to every 21 days led to complete or partial symptom relief in 7 and 10 patients, respectively [Ferolla et al. 2012]. Also, it is important to evaluate whether there are absorption problems, such as fibrosis at the injection areas. In this case, nurse education is crucial to prevent injection problems. When symptoms recur following this strategy, SSA dose escalation to above-the-label doses has been widely utilized based on expert opinion and retrospective series [Broder et al. 2015].
In one of the largest series, among 239 patients who presented with progression (62% had symptom progression) while on octreotide long acting release (LAR) 30 mg, 81% experienced some improvement in flushing and 79% improved diarrhea after their first dose escalation to either 40 or 60 mg [Strosberg et al. 2014]. However, given the heterogeneity of study populations and SSA schedules, variable definitions of progression and not standardized measures of clinical benefit, it remains unclear to what extent SSA dose escalation benefits patients with RCSy. In a retrospective series [Al-Efraij et al. 2015], dose escalation of octreotide LAR to 40 mg or 60 mg in 37 patients with RCSy led to improved symptom control (62% reported a significant decrease of diarrhea and 91% decrease in flushing) and reduction of 5-HIAA levels in 23% of them; other smaller studies have also evaluated the use of high doses of SSA in RCSy, showing similar results [Chadha et al. 2009].
Switch of somatostatin analog
Symptom improvement has been suggested with switching SSA in small studies and case reports [Ricci et al. 2000; Raderer et al. 2001]. In a small phase II trial of 15 patients with progressing metastatic NET (7 were midgut) following lanreotide 30 mg biweekly, the switch to octreotide 30 mg LAR led to overall biochemical and symptomatic response of 41% and 82%, respectively [Ricci et al. 2000]. These findings could be explained by the different affinities of lanreotide and octreotide to somatostatin receptors [Oberg et al. 2004]. Lanreotide and octreotide have different pharmacokinetics that could also explain reports of one SSA being effective to treat CSy after failure of the other. While octreotide is given intramuscularly, lanreotide is administered subcutaneously, which could lead to distinct absorptions according to patients’ body fat content.
Tumor debulking and locoregional liver-directed therapies
The liver is the predominant site of metastases in NET, especially gastroenteropancreatic tumors. Therapies directed to treat NET liver metastases in patients with RCSy are supported by the rationale of tumor debulking, which means reducing the volume of a tumor that is producing hormones that cause symptoms. While patients with CSy and resectable localized metastatic disease should go for R0 resection if possible, patients with RCSy often present with major liver involvement, which is rarely amenable for complete surgical removal. However, uncontrolled studies have suggested that R2 surgical debulking of large tumor areas, often more than 90%, can benefit patients in terms of symptom relief in 50–90% of cases [Sarmiento and Que, 2003; Saxena et al. 2012], with median duration of symptom control ranging from 19.3 to 45 months [Que et al. 1995; Osborne et al. 2006]. Given the variability of methods, populations and results across studies, it is hard to fully understand the role of surgical debulking in treating RCSy. But even though only a few small and retrospective studies have evaluated the efficacy of this approach, the low rate of postoperative complications reported by experienced centers [Boudreaux et al. 2014] and the unquestionable improvement of carcinoid symptoms observed in clinical practice have both supported this approach for selected cases. Noticeably, surgical procedures, including surgical debulking of NET liver metastases to treat RCSy, are contra-indicated in patients with carcinoid heart disease due to risk of major bleeding; in these cases, valve replacement should be performed first [Lillegard et al. 2011]. It is also crucial to be attentive to carcinoid crisis during surgical procedures or liver-directed therapies in patients with RCSy. While it is unknown whether peri- and intraoperative infusional octreotide prevents carcinoid crisis [Boutzios and Kaltsas, 2015], it is generally recommended for patients with CSy undergoing invasive procedures.
For inoperable patients with RCSy and liver metastases, another form of tumor debulking is liver embolization. Several uncontrolled studies have shown that transarterial embolization, with or without chemotherapy, provides general symptom improvement, including carcinoid symptoms, in 60–95% of patients for a median of 20–80 months [Strosberg et al. 2006; Pitt et al. 2008; Vogl et al. 2009; Nazario and Gupta, 2010; Pericleous et al. 2016]. While many studies of liver-directed therapy have evaluated radiological response and progression-free survival (PFS), very few have looked at symptomatic response in patients with CSy; and among them, no prospective trial has been made. There is scant information about which symptoms and to what extent they were alleviated, how long did it take for symptom relief to occur and how monitoring for symptom relapse was made.
Laparoscopic radiofrequency ablation is an alternative to surgery in inoperable patients with low volume disease (liver metastases are < 3–5 cm) [Frilling et al. 2014]. It can also be used as an adjunctive to surgery for maximum debulking. Small retrospective studies have reported symptomatic improvement in 70% of patients with CSy, although some of them were also managed by surgery [Eriksson et al. 2008; Vogl et al. 2009]. Both radiofrequency ablation and liver embolization can be repeated to achieve symptom control, as long as there is not significant liver dysfunction.
There are not randomized trials to tell us which liver-directed therapy offers the best symptomatic response for patients with RCSy. A retrospective series of 120 patients observed complete symptomatic relief in 59% who underwent liver embolization versus 69% for surgical cytoreduction (p = 0.08), suggesting that surgery might be more efficacious for operable patients [Osborne et al. 2006].
For patients younger than 55 years, without relevant comorbidities, with well differentiated midgut NET, liver-only metastases and resected primary tumor, liver transplantation may be an option. However, this strategy should be considered in patients with uncontrolled CSy given that, despite being deemed experimental, liver transplantation offers a recurrence-free survival of 20– 30% at 5 years [Fan et al. 2015].
Interferon
Evidence supporting the use of interferon to treat NET goes back to 3 decades ago [Oberg et al. 1983]. Currently, the use of interferon to treat NET has been limited, mainly because of its toxicity, but also due to the availability of newer effective agents such as SSAs and targeted therapies. The mechanism of action of interferon as an anticancer drug is not well understood, but antiangiogenic and antiproliferative effects, as well as induction of apoptosis, have been postulated [Oberg, 2012].
Most studies have used recombinant alpha interferon with doses ranging from three to six million units per week. Although indirect comparison across trials is inadequate due to the variable methods used to assess radiological and symptom responses and distinct populations, studies have generally described tumor stabilization in 50–70% of patients with baseline progressive disease and symptomatic relief of CSy in about 30–70% [Oberg, 2012]. In a small phase II trial, pegylated interferon alpha 2b provided symptomatic relief of CSy in 7 of 10 patients [Pavel et al. 2006].
Interferon alpha has also been investigated in combination with SSA. Initial uncontrolled studies demonstrated biochemical responses in up to 77%, even in patients whose disease had previously progressed while on either SSA or interferon monotherapy [Fazio et al. 2007]. However, randomized trials failed to prove that the combination of interferon and SSA outweighs either agent alone. In a randomized trial of 68 patients with metastatic midgut NET and CSy, despite a significant gain in time to progression with the combination, there was no difference in biochemical response whether patients received interferon plus octreotide, or octreotide alone [Kolby et al. 2003]. A German randomized trial that compared lanreotide versus interferon alpha versus the combination in treatment-naïve patients did not show any response or survival differences across the arms; however, in this study, a subset of 29 patients with functional NET in the combination arm benefited more from decreased frequency of diarrhea and flushing (p = 0.037) [Faiss et al. 2003]. In another negative randomized trial, quality of life after 3 months of treatment was inferior in the combination group compared with octreotide alone, suggesting that symptom control was probably not improved by adding interferon (p = 0.039) [Arnold et al. 2005]. Because none of these randomized trials treated patients specifically and exclusively with RCSy, one may argue that the role of interferon in RCSy remains undetermined. The highly awaited SWOG S0518 phase III trial compared the PFS of octreotide LAR 20 mg every 21 days combined with either interferon alpha, three million units, three times per week, or bevacizumab 15 mg/kg every 3 weeks in 402 pretreated patients with G1/G2 advanced progressing poor-prognosis NET [Yao et al. 2015]. Poor-prognosis features were considered G2 tumors with more than six liver lesions, progressive disease, refractory CSy, colorectal or gastric primary. The study was negative for its primary endpoint of PFS, and information about the symptomatic response/quality of life is pending, particularly in the subgroup of patients with RCSy.
Overall, randomized data have not proven that interferon is effective as an antitumor agent in NET. To treat RCSy, clinical experience and uncontrolled studies have reported symptomatic responses with interferon monotherapy or associated with SSA.
Peptide receptor radionuclide therapy
Peptide receptor radionuclide therapy (PRRT) is an effective therapy to treat well differentiated gastroenteropancreatic NET, with objective radiological responses observed in 20–30% of cases [Kim et al. 2015]. It is also a logical strategy to overcome resistance to SSA as it targets the somatostatin receptors with a different mechanism, radiation. Because selection bias might have overestimated the real effect of PRRT in NET, given that in most studies, patients with long-term indolent disease have been treated, a prospective trial was necessary. Recently the NETTER-1 phase III trial randomized 229 patients with advanced well differentiated G1 or G2 progressing midgut NET to receive Lutetium177 Dotatate plus octreotide LAR 30 mg or octreotide LAR 60 mg [Strosberg et al. 2016]. Ninety-eight patients (43%) had CSy which was uncontrolled by conventional doses of SSA. All efficacy outcomes favored the Lutetium177 arm; with respect to symptom control, quality-of-life data were collected and the results are awaited.
Meta-iodobenzylguanidine (MIBG), a biogenic amine that is actively taken up by tumors of neural origin, has been radiolabeled with iodine to treat pheochromocytoma and paragangliomas. Small studies have shown that more than 60% of patients with well differentiated midgut NET scan positive in the I-123MIBG test [Taal et al. 1996; Pathirana et al. 2001]. Therefore, it was logical to test I-123MIBG as a therapeutic strategy in NET. An old retrospective study of 30 NET patients, 20 pretreated or naïve patients with uncontrolled CSy, demonstrated that treatment with I-131MIBG provided symptomatic relief in 14 of them, with a median duration of 8 months [Taal et al. 1996]; however in this study, the authors did not provide symptomatic response rate according to prior therapies. In another retrospective series of 12 patients with CSy, four out of five patients with RCSy who received a single dose of 7.4 gBq I-131MIBG experienced improvement in CSy symptoms, with an overall median duration of 10 months for the whole cohort [Pathirana et al. 2001].
Everolimus
Everolimus, an oral mTOR inhibitor, is an effective agent to treat well differentiated nonfunctioning NET of pancreatic [Yao et al. 2011], gastrointestinal and lung origins [Yao et al. 2016]. For functioning tumors, data are not so clear. The RADIANT-2 was a phase III trial that randomized 429 patients with progressing, well differentiated, functioning NET with history of CSy to receive either octreotide LAR 30 mg alone or in combination with everolimus 10 mg orally, continuously [Pavel et al. 2011]. It is likely that because of much informative censoring and imbalances in randomization, the study turned out negative. However, in subgroup analyses, patients from the everolimus arm presented higher biochemical response, 61 versus 36%, and greater reduction in urinary 5-HIAA throughout the study (p = 0.0001). Although the investigators did not collect symptom data, this was an indirect evidence of the antisecretory affects of everolimus in patients with functioning NET.
A few months before the publication of RADIANT-2, a case report described a female patient with a G1 metastatic midgut NET and RCSy who had a significant clinical benefit with everolimus monotherapy [Capdevila et al. 2011]. The patient had 10–15 flushing episodes per day, which were persistent after treatment with octreotide LAR 30 mg every 15 days, interferon, liver embolization and radiofrequency ablation. Everolimus was added to octreotide, and after a month, the number of flushing episodes reduced to 1–2 per day, there was improvement in diarrhea, a significant decrease in urine 5-HHIA levels (up to 60%) and later on, radiological objective response. In a retrospective series of 10 functioning heavily pretreated patients with well differentiated NET and CSy (seven had midgut tumors), everolimus was added to SSA with the aim to control symptoms with or without radiological progression [Bainbridge et al. 2015]. All patients received prior octreotide LAR 20 mg intramuscularly (IM) every 4 weeks, with doses increased to 30 mg IM given every 3 or 4 weeks if symptoms were not controlled. Seven patients presented symptomatic response, which lasted for mean of 13.9 months (1–39 months), six patients had reduced frequency of daily BM and five out of seven who had flushing at baseline, experienced relief.
Chemotherapy
Historically, chemotherapy has little activity in G1 midgut NET [Strosberg et al. 2015]. However, there have been anecdotal reports of objective responses to temozolomide-based regimens [Abdel-Rahman and Fouad, 2015], and this could be considered in selected patients with RCsy to other therapeutic options.
New Treatments
Pasireotide is a newly formulated SSA with a different pharmacodynamic profile: higher affinity to somatostatin receptors 1, 3 and 5 when compared with octreotide and lanreotide, which have greater specificity for receptor type 2 [Schmid, 2008]. Based on promising phase II data, where pasireotide 600–900 μg subcutaneously twice daily controlled diarrhea and flushing in 27% of patients with RCSy [Kvols et al. 2012], a double-blind phase III trial was conducted to compare pasireotide LAR 60 mg with depot octreotide 40 mg [Wolin et al. 2015] in terms of symptomatic response. RCSy was defined as daily mean of at least four BM and of five or more flushing episodes for 2 weeks. The primary endpoint was the proportion of patients experiencing decrease in the frequency of symptoms at 6 months. The study was closed prematurely, after 110 patients were enrolled, due to futility evidenced at interim analysis; while the median PFS was longer in the pasireotide arm, the rate of symptomatic response was similar between the treatment arms (20.9% versus 26.7%, p = 0.53).
The second phase III trial conducted specifically in patients with RCSy was recently presented. The Telestar double-blind trial randomized 135 well differentiated NET patients to receive either placebo or two different doses [250 mg or 500 mg three times daily (TID)] of telotristat etiprate, an oral inhibitor of the tryptophan hydroxylase, which is an important enzyme for the synthesis of serotonin [Kulke et al. 2015]. Patients had to have at least four BMs per day, despite prior therapy with octreotide LAR 30 mg or lanreotide depot 120 mg every 4 weeks and were allowed to receive SSA throughout the study period of 12 weeks. The primary endpoint was change from baseline in daily frequency of BM. The study arms were balanced, with mean number of five to six BM per day, mean 5-HIAA of 80–89 mg/24-hour urine, and 42–46% of patients received SSA above the labeled dose. The trial was positive, with significant reduction in the frequency of daily BM, without significant differences between the two doses of telotristat; 35% and 29% of patients reduced the mean frequency of BM per day with telotristat 500 and 250 mg, respectively, versus 17% of patients on placebo. Additionally, biochemical response was greater in the telotristat arm. Treatment-related adverse events occurred in 26.7% of patients on placebo, 68.9% in the 500 mg arm and 33.3% in the 250 mg group; there were no treatment-related deaths. Although telotristat etiprate is not supposed to cross the brain−blood barrier, depressive symptoms were more common, although not statistically significant, in the 500 mg arm (17.7 versus 2.2 versus 6.7% for, 500 mg, 250 mg and placebo, respectively). Interestingly, two patients enrolled in the Telestar trial (one of them with imminent need of valve surgery) had their carcinoid heart disease (CHD) halted with no further fibrosis observed on serial echocardiographic tests [Zacks et al. 2016]. Other two phase II trials that tested telotristat from 150 mg to 500 mg TID in RCSy, demonstrated similar reductions in frequency of BM, flushing and in urinary 5-HIAA [Kulke et al. 2014; Pavel et al. 2015].
Discussion and proposed treatment sequence
In this overview about the management of RCSy, the majority of studies that support therapeutic decisions are small retrospective series, with heterogeneous populations, diverse methods to measure carcinoid symptoms and varied definitions of RCSy (Table 1). Except for three recent phase III trials, with only the Telestar trial being positive, all other treatments currently utilized to treat RCSy are based on low levels of evidence. Yet, for some therapeutic interventions, recommendations can still be made because positive results have been consistent across uncontrolled trials and observed in clinical practice.
Table 1.
Management of refractory carcinoid syndrome: available studies.
| Reference | Patients | Definition of RCSy | Intervention | Study design | Results (symptomatic response) | Drawbacks |
|---|---|---|---|---|---|---|
| Strosberg et al. [2014] |
n = 239; n with RCSy = 148 |
CSy symtoms uncontrolled while on octreotide LAR 10–30 mg | Octreotide LAR dose escalation to 40 or 60 mg every 28 days | Multicenter retrospective | 81% improved flushing and 79% improved diarrhea | Duration and magnitude of symptom control not reported |
| Al-Efraij et al. [2015] |
n = 37; n with RCSy = 27 |
Patients that remain symptomatic in spite of standard doses of octreotide LAR 30 mg | Octreotide LAR dose escalation to 40 or 60 mg every 28 days | Retrospective | 91% improved flushing and 62% improved diarrhea; reduction of 5-HIAA levels in 23% | Duration and magnitude of symptom control not reported; variable intervals for measuring tumor markers |
| Ferolla et al. [2012] |
n = 28; n with RCSy = 19 |
CSy symtomns while on octreotide LAR 30 mg every 28 days | Octreotide LAR 30 mg every 21 days | Phase II | Complete and partial control of symptoms in 30% and 70% of cases, respectively. | Small sample, duration of symptom control not reported |
| Chadha et al. [2009] |
n = 54; n with RCSy = 20 |
Objective symptom progression or development of new CSy symptoms while on octreotide LAR 20−30 mg |
Octreotide LAR 40– 60 mg every 28 days | Retrospective | 45% of cases presented symptoms improvement | High dose of SSA was not the same for all patients; duration and magnitude of response not reported |
| Ricci et al. [2000] |
n = 15; n with RCSy = 10 |
Any CSy symptom after treatment with lanreotide 30 mg every 14 days | Octreotide LAR 20 mg every 28 days | Phase II | The overall symptomatic response rate was 82%. The median duration of response for diarrhea, abdominal pain, or both was 6.5 months (range 3−12+ months). | Small sample, severity of symptoms not reported |
| Pavel et al. [2006] |
n = 17; n with RCSy = 10 |
CSy symptoms while on octreotide LAR 30 mg every 28 days | Pegylated interferon | Phase II | 70% symptom improvement | Small sample, uncontrolled |
| Taal et al. [1996] |
n total = 30; n with CSy = 20 |
Uncontrolled CSy by conventional treatment | I-131MIBG | Phase II | Symptomatic relief in 60% of patients with CSy (14/20), with a median duration of 8 months. | Mixed patients with RCSy and treatment naïve, small sample, magnitude of response not reported |
| Pathirana et al. [2001] |
n = 12; n with RCSy = 5 |
Uncontrolled CSy by octreotide LAR 30 mg | I-131MIBG | Retrospective | 80% (4/5) of patients with RCSy had some symptomatic response | Small sample, magnitude of response not reported |
| Bainbridge et al. [2015] |
n total = 15; n with RCSy = 10 |
Worsening in symptoms despite SSA labeled dose | Everolimus added to SSA | Retrospective | 70% had improvement in symptoms | Small sample, magnitude of response not reported |
| Kvols et al. [2012] | n = 45 | Diarrhea/flushing were inadequately controlled by octreotide LAR | Pasireotide 600-900 μg SC BID | Phase II | Controlled diarrhea and flushing in 27% | Duration control not reported |
| Wolin et al. [2015] | n = 110 | Daily mean of at least four BMs or flushing episodes of 14 or more for 2 weeks | Pasireotide LAR 60 mg or octreotide LAR 40 mg | Phase III | Symptom control in 20.9% (Pasireotide) versus 26.7% (octreotide), p = 0.53 | Interim analysis results (stopped for futility) |
| Kulke et al. [2014] | n = 23 | ⩾4 BMs/day while on stable-dose octreotide LAR (30 mg every 28 days until 60 mg every 21 days) for at least 3 months |
Placebo (n = 5) versus telotristat etiprate (n = 18) with dosage escalated from 150 mg TID to 500 mg TID. | Randomized phase II |
Five (28%) of 18 patients treated with telotristat etiprate and no patients treated with placebo achieved a clinical response (⩾30% reduction from baseline in the daily mean number of BMs/week for 2 or more of the 4 weeks on trial) | Small sample |
| Pavel et al. [2015] | n = 15 | At least four BMs per day | Telotristat etiprate with dosage escalated from 150 mg TID to 500 mg TID. | Phase II | All patients experienced reductions in BMs per day (mean decrease, 43.5%); number of flushing episodes presented a reduction of 27%. | Small sample, uncontrolled |
| Kulke et al. [2015] | n = 135 | At least four BMs per day | Telotristat etiprate 250 mg or 500 mg orally TID versus placebo | Phase III | 35% and 29% patients reduced the mean daily frequency of BM with telotristat 500 and 250 mg, respectively, versus 17% on placebo | Quality-of-life data not reported (unpublished trial) |
MIBG, meta-iodobenzylguanidine; RCSy, refractory carcinoid syndrome; CSy, carcinoid syndrome; SSA, somatostatin analog; 5-HIAA, 5-hydroxiindolacetic acid; SC, subcutaneous; BID, twice daily; TID, three times a day; BM, bowel movements.
Therapeutic approaches for RCSy follow the logic of: (1) tumor debulking (surgical resection, radiofrequency ablation, liver embolization, liver transplantation), (2) optimization of the inhibition of the somatostatin receptors (dose escalation of SSA, SSA switch, PRRT), (3) decreasing the production of serotonin (telotristat) and (4) associating another antitumor agent with a different mechanism of action (everolimus and interferon alpha) to SSA. However, it is unknown how to best sequence the treatment options for patients with RCSy.
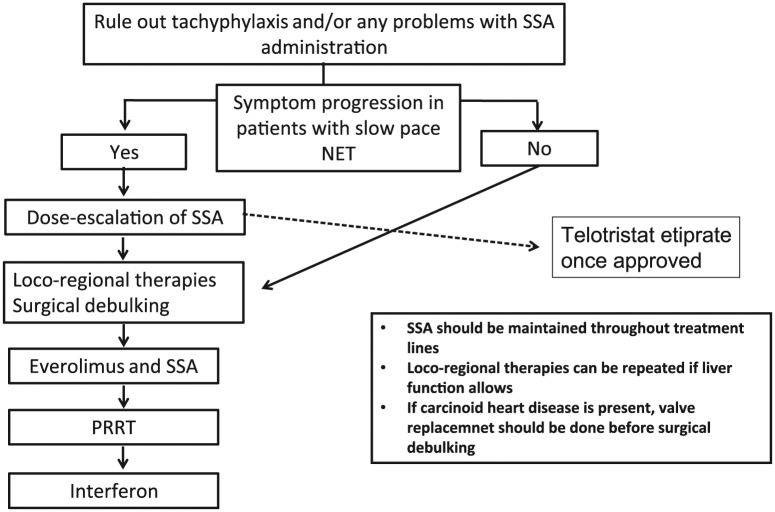
Telotristrat etiprate is a very promising agent and the only one with level 1 evidence data for the treatment of patients with RCSy, although quality of life and detailed safety data are still pending. While the study is not published and the drug is not yet approved, it will likely be placed early in the treatment algorithm for RCSy. However, taking into account the low level of evidence to support the management of RCsy with the available therapies, we propose a treatment sequence algorithm for patients with RCSy based on our clinical experience and interpretation of current literature (Figure 1). We think the first approach to manage patients with RCSy from inoperable tumors is to evaluate for tachyphylaxis and absorption problems. Once absorption issues have been ruled out, SSA dose escalation of up to twice the labeled dose is probably the best first-line option, since it treats tachyphylaxis and it has shown tumor control of about 8–12 months in phase III trials [Pavel et al. 2011; Strosberg et al. 2016]; it is very well tolerated and we do see prolonged symptom control in clinical practice. But this strategy should be reserved for patients with symptomatic but slow pace of disease; for patients with rapidly progressive tumors, SSA dose escalation should be omitted. Upon symptom progression, locoregional therapy with liver embolization and possibly radiofrequency ablation as well as R2 surgical debulking can be considered; these modalities can also be repeated if needed, in patients without clinically relevant liver dysfunction. Once there is subsequent symptomatic progression, we suggest everolimus be added to SSA. At CSy progression we recommend PRRT. We tend to leave PRRT to later lines because of perceived increased toxicity of everolimus following Lutetium177. Retrospective series have shown that everolimus administered after PRRT led to more patients presenting grade 3 or 4 myelotoxicity [Kamp et al. 2013; Panzuto et al. 2015]. Interferon alpha combined with SSA can be effective in some patients and could be used after progression on PRRT or on everolimus, although it can also be used earlier, given that interferon is approved in some countries to treat CSy. We favor that SSA be maintained throughout different treatment lines, including PRRT, despite symptomatic progression because their discontinuation may worsen carcinoid symptoms or even lead to carcinoid crisis; although maintenance of SSA may economically burden treatment users. We generally do not recommend chemotherapy to treat RCSy because of its limited activity in well differentiated midgut tumors.
Figure 1.
Refractory carcinoid syndrome: our proposal for a treatment sequence.
SSA, somatostatin analog; NET, neuroendocrine tumors; PRRT, peptide receptor radionuclide therapy.
The relatively low number of patients with RCSy, the variability in symptom severity and consequent poor performance status of patients, as, for example, patients with advanced carcinoid heart disease, and the difficulties in defining progression have all limited the conduction of randomized trials in CSy. The majority of patients with NET and CSy who are treated with SSA present with stable disease as their best response, with median times to progression ranging from 14 months [Rinke et al. 2017] to not reached [Caplin et al. 2014]. However, many patients present with clear progression of CSy symptoms, despite radiological stabilization of disease. The complexity in defining progression in patients with NET and CSy was experienced by the investigators of the RADIANT-2 trial, where many patients presented with clinical progression before radiological progression, the primary endpoint; this led to early discontinuation, informative censoring and consequently, the trial became negative [Pavel et al. 2011; Riechelmann and Rego, 2011]. Another struggle in designing trials of CSy is the determination of the primary endpoint. In the Telestar trial, [Kulke et al. 2015] patients had at least 4 BMs/day and the study was powered to detect a mean change of at least 1.5 BMs/day; one could argue that decreasing the frequency of 4 to 3 BMs/day is different from 9 to 8 BMs/day in terms of clinically significant symptom relief. While the minimum number of BMs/day to determine RCSy in clinical trials has not been defined, we argue that at least 4−5 is an appropriate number. Besides carcinoid-directed quality of life, other useful endpoints in trials of RCSy include the rate of complete symptomatic response, symptom-free survival, time to symptom recurrence/progression, the number of rescue medications utilized, the development and evolution of carcinoid heart disease, and patients’ independence to perform daily/social activities.
It is crucial to understand the biological mechanisms underlying symptom progression on different treatment modalities to develop new drugs to treat RCSy. While some hypotheses have been made about the biological reasons that explain why functioning tumors become resistant to SSA [Molina-Cerrillo et al. 2016], studies on this topic are lacking and urgently needed. Prospective collection of biological samples, especially at the time of each symptomatic progression, is a smart way to study such mechanisms.
Conclusion
In this review about RCSy, most studies supporting the treatment of RCSy are uncontrolled, but recent phase III trials have provided level 1 evidence for patient management. However, there are more questions than answers in the management of RCSy, such as the urgent need to understand the prognostic factors associated with more severe CSy and carcinoid heart, and the biological mechanisms underlying progression on different therapeutic modalities. Collaboration among institutions is key to fostering clinical and translational research in this area. Finally, great efforts must be made to conduct randomized trials to guide the best treatment management for patients with RCSy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Rachel Riechelmann has received consultancy fees and honoraria from lectures from Novartis and Ipsen. Frederico Costa and Juliana Rego have received honoraria from lectures from Novartis.
Contributor Information
Rachel P. Riechelmann, Instituto do Câncer do Estado de São Paulo,Universidade de São Paulo, Ave. Dr Arnaldo, 251, São Paulo, SP – Brazil.
Allan A. Pereira, Department of Radiology and Oncology, Instituto do Câncer do Estado de São Paulo, São Paulo, Brazil
Juliana F. M. Rego, Hospital Universitário Onofre Lopes, Universidade Federal do Rio Grande do Norte, Natal, Brazil
Frederico P. Costa, Hospital Sírio-Libanês, São Paulo, Brazil
References
- Abdel-Rahman O., Fouad M. (2015) Temozolomide-based combination for advanced neuroendocrine neoplasms: a systematic review of the literature. Future Oncol 11: 1275–1290. [DOI] [PubMed] [Google Scholar]
- Al-Efraij K., Aljama M., Kennecke H. (2015) Association of dose escalation of octreotide long-acting release on clinical symptoms and tumor markers and response among patients with neuroendocrine tumors. Cancer Med 4: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R., Rinke A., Klose K., Muller H., Wied M., Zamzow K., et al. (2005) Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol 3: 761–771. [DOI] [PubMed] [Google Scholar]
- Bainbridge H., Larbi E., Middleton G. (2015) Symptomatic control of neuroendocrine tumours with everolimus. Horm Cancer 6: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont J., Cella D., Phan A., Choi S., Liu Z., Yao J. (2012) Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 41: 461–466. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Davar J., Dreyfus G., Caplin M. (2007) Carcinoid heart disease. Circulation 116: 2860–2865. [DOI] [PubMed] [Google Scholar]
- Boudreaux J., Wang Y., Diebold A., Frey D., Anthony L., Uhlhorn A., et al. (2014) A single institution’s experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J Am Coll Surg 218: 837–844. [DOI] [PubMed] [Google Scholar]
- Boutzios G., Kaltsas G. (2015) Clinical syndromes related to gastrointestinal neuroendocrine neoplasms. Front Horm Res 44: 40–57. [DOI] [PubMed] [Google Scholar]
- Broder M., Beenhouwer D., Strosberg J., Neary M., Cherepanov D. (2015) Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: a systematic literature review. World J Gastroenterol 21: 1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Diez Miranda I., Obiols G., Tabernero J. (2011) Control of carcinoid syndrome with everolimus. Ann Oncol 22: 237–239. [DOI] [PubMed] [Google Scholar]
- Caplin M., Pavel M., Cwikla J., Phan A., Raderer M., Sedlackova E., et al. (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371: 224–233. [DOI] [PubMed] [Google Scholar]
- Chadha M., Lombardo J., Mashtare T., Wilding G., Litwin A., Raczyk C., et al. (2009) High-dose octreotide acetate for management of gastroenteropancreatic neuroendocrine tumors. Anticancer Res 29: 4127–4130. [PubMed] [Google Scholar]
- Eriksson J., Stalberg P., Nilsson A., Krause J., Lundberg C., Skogseid B., et al. (2008) Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg 32: 930–938. [DOI] [PubMed] [Google Scholar]
- Faiss S., Pape U., Bohmig M., Dorffel Y., Mansmann U., Golder W., et al. (2003) Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 21: 2689–2696. [DOI] [PubMed] [Google Scholar]
- Fan S., Le Treut Y., Mazzaferro V., Burroughs A., Olausson M., Breitenstein S., et al. (2015) Liver transplantation for neuroendocrine tumour liver metastases. HPB (Oxford) 17: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio N., De Braud F., Delle Fave G., Oberg K. (2007) Interferon-alpha and somatostatin analog in patients with gastroenteropancreatic neuroendocrine carcinoma: single agent or combination? Ann Oncol 18: 13–19. [DOI] [PubMed] [Google Scholar]
- Ferolla P., Faggiano A., Grimaldi F., Ferone D., Scarpelli G., Ramundo V., et al. (2012) Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest 35: 326–331. [DOI] [PubMed] [Google Scholar]
- Frilling A., Modlin I., Kidd M., Russell C., Breitenstein S., Salem R., et al. (2014) Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 15: e8–e21. [DOI] [PubMed] [Google Scholar]
- Hofland L., Lamberts S. (2003) The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24: 28–47. [DOI] [PubMed] [Google Scholar]
- Kamp K., Gumz B., Feelders R., Kwekkeboom D., Kaltsas G., Costa F., et al. (2013) Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after (177)Lu-octreotate. Endocr Relat Cancer 20: 825–831. [DOI] [PubMed] [Google Scholar]
- Kim S., Pak K., Koo P., Kwak J., Chang S. (2015) The efficacy of (177)Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging 42: 1964–1970. [DOI] [PubMed] [Google Scholar]
- Kolby L., Persson G., Franzen S., Ahren B. (2003) Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg 90: 687–693. [DOI] [PubMed] [Google Scholar]
- Kulke MH., O’Dorisio T., Phan A., Bergsland E., Law L., Banks P., Freiman J., et al. (2014) Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Rel Cancer 21(5): 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke M., Horsch D., Caplin M., Anthony L., Bergsland E., Oberg K., et al. (2015) 37LBA Telotristat Etiprate is effective in treating patients with carcinoid syndrome that is inadequately controlled by somatostatin analog therapy (the phase III Telestar Clinical Trial). Eur J Cancer 51: S728. [Google Scholar]
- Kvols L., Oberg K., O’dorisio T., Mohideen P., De Herder W., Arnold R., et al. (2012) Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer 19: 657–666. [DOI] [PubMed] [Google Scholar]
- Lillegard J., Fisher J., Mckenzie T., Que F., Farnell M., Kendrick M., et al. (2011) Hepatic Resection for the carcinoid syndrome in patients with severe carcinoid heart disease: does valve replacement permit safe hepatic resection? J Am Coll Surg 213: 130–136; discussion 136–138. [DOI] [PubMed] [Google Scholar]
- Molina-Cerrillo J., Alonso-Gordoa T., Martinez-Saez O., Grande E. (2016) Inhibition of peripheral synthesis of serotonin as a new target in neuroendocrine tumors. Oncologist 21: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J., Sousa L., Riechelmann R. (2016) Complications from carcinoid syndrome: review of the current evidence. Ecancermedicalscience 10: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario J., Gupta S. (2010) Transarterial liver-directed therapies of neuroendocrine hepatic metastases. Semin Oncol 37: 118–126. [DOI] [PubMed] [Google Scholar]
- O’Toole D., Ducreux M., Bommelaer G., Wemeau J., Bouche O., Catus F., et al. (2000) Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 88: 770–776. [DOI] [PubMed] [Google Scholar]
- Oberg K. (2012) Biotherapies for GEP-NETs. Best Pract Res Clin Gastroenterol 26: 833–841. [DOI] [PubMed] [Google Scholar]
- Oberg K., Funa K., Alm G. (1983) Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N Engl J Med 309: 129–133. [DOI] [PubMed] [Google Scholar]
- Oberg K., Kvols L., Caplin M., Delle Fave G., De Herder W., Rindi G., et al. (2004) Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 15: 966–973. [DOI] [PubMed] [Google Scholar]
- Osborne D., Zervos E., Strosberg J., Boe B., Malafa M., Rosemurgy A., et al. (2006) Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol 13: 572–581. [DOI] [PubMed] [Google Scholar]
- Panzuto F., Rinzivillo M., Fazio N., De Braud F., Luppi G., Zatelli M., et al. (2015) Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 20: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirana A., Vinjamuri S., Byrne C., Ghaneh P., Vora J., Poston G. (2001) (131)I-MIBG radionuclide therapy is safe and cost-effective in the control of symptoms of the carcinoid syndrome. Eur J Surg Oncol 27: 404–408. [DOI] [PubMed] [Google Scholar]
- Pavel M., Horsch D., Caplin M., Ramage J., Seufferlein T., Valle J., et al. (2015) Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab 100: 1511–1519. [DOI] [PubMed] [Google Scholar]
- Pavel M., Baum U., Hahn E., Schuppan D., Lohmann T. (2006) Efficacy and tolerability of pegylated IFN-alpha in patients with neuroendocrine gastroenteropancreatic carcinomas. J Interferon Cytokine Res 26: 8–13. [DOI] [PubMed] [Google Scholar]
- Pavel M., Hainsworth J., Baudin E., Peeters M., Horsch D., Winkler R., et al. (2011) Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase III study. Lancet 378: 2005–2012. [DOI] [PubMed] [Google Scholar]
- Pericleous M., Caplin M., Tsochatzis E., Yu D., Morgan-Rowe L., Toumpanakis C. (2016) Hepatic artery embolization in advanced neuroendocrine tumors: efficacy and long-term outcomes. Asia Pac J Clin Oncol 12: 61–69. [DOI] [PubMed] [Google Scholar]
- Pitt S., Knuth J., Keily J., Mcdermott J., Weber S., Chen H., et al. (2008) Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg 12: 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que F., Nagorney D., Batts K., Linz L., Kvols L. (1995) Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 169: 36–42; discussion 42–33. [DOI] [PubMed] [Google Scholar]
- Raderer M., Kurtaran A., Scheithauer W., Fiebiger W., Weinlaender G., Oberhuber G. (2001) Different response to the long-acting somatostatin analogues lanreotide and octreotide in a patient with a malignant carcinoid. Oncology 60: 141–145. [DOI] [PubMed] [Google Scholar]
- Ricci S., Antonuzzo A., Galli L., Ferdeghini M., Bodei L., Orlandini C., et al. (2000) Octreotide Acetate long-acting release in patients with metastatic neuroendocrine tumors pretreated with lanreotide. Ann Oncol 11: 1127–1130. [DOI] [PubMed] [Google Scholar]
- Riechelmann R., Rego J. (2011) Progression-free survival in neuroendocrine tumors: preferred end point, but how should it be defined? J Clin Oncol 29: 2835–2836. [DOI] [PubMed] [Google Scholar]
- Rinke A., Wittenberg M., Schade-Brittinger C., Aminossadati B., Ronicke E., Gress T., et al. (2017) Placebo controlled, double blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results on long term survival. Neuroendocrinology 104(1). [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sarmiento J., Que F. (2003) Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am 12: 231–242. [DOI] [PubMed] [Google Scholar]
- Saxena A., Chua T., Perera M., Chu F., Morris D. (2012) Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol 21: e131–141. [DOI] [PubMed] [Google Scholar]
- Schmid H. (2008) Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol 286: 69–74. [DOI] [PubMed] [Google Scholar]
- Strosberg J., Goldman J., Costa F., Pavel M. (2015) The role of chemotherapy in well-differentiated gastroenteropancreatic neuroendocrine tumors. Front Horm Res 44: 239–247. [DOI] [PubMed] [Google Scholar]
- Strosberg J., Benson A., Huynh L., Duh M., Goldman J., Sahai V., et al. (2014) Clinical benefits of above-standard dose of octreotide LAR in patients with neuroendocrine tumors for control of carcinoid syndrome symptoms: a multicenter retrospective chart review study. Oncologist 19: 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg J., Choi J., Cantor A., Kvols L. (2006) Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control 13: 72–78. [DOI] [PubMed] [Google Scholar]
- Strosberg J., Wolin E., Chasen B., Kulke M., Bushnell D., Caplin M., et al. (2016) NETTER-1 phase III: progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-Dotatate. ASCO Meeting Abstracts 34: 194. [Google Scholar]
- Taal B., Hoefnagel C., Valdes Olmos R., Boot H., Beijnen J. (1996) Palliative effect of metaiodobenzylguanidine in metastatic carcinoid tumors. J Clin Oncol 14: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Vogl T., Naguib N., Zangos S., Eichler K., Hedayati A., Nour-Eldin N. (2009) Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol 72: 517–528. [DOI] [PubMed] [Google Scholar]
- Wolin E., Jarzab B., Eriksson B., Walter T., Toumpanakis C., Morse M., et al. (2015) Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther 9: 5075–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., et al. (2016) Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387: 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Guthrie K., Moran C., Strosberg J., Kulke M., Chan J., et al. (2015) SWOG S0518: phase iii prospective randomized comparison of depot octreotide plus interferon alpha-2b versus depot octreotide plus bevacizumab (NSC #704865) in advanced, poor prognosis carcinoid patients (NCT00569127). ASCO Meeting Abstracts 33: 4004. [Google Scholar]
- Yao J., Shah M., Ito T., Bohas C., Wolin E., Van Cutsem E., et al. (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks J., Lavine R., Warner R. (2016) (M8) Telotristat etiprate appears to halt carcinoid heart disease. 13th Annual ENETS Conference. Abstract M8. [Google Scholar]