Abstract
Background and Purpose:
Currently, no readily available mitigators exist for acute abdominal radiation injury. Here, we present an animal model for precise and homogenous limb-sparing abdominal irradiation (LSAIR) to study the radiation-induced gastrointestinal syndrome (RIGS).
Materials and Methods:
The LSAIR technique was developed using the small animal radiation research platform (SARRP) with image guidance capabilities. We delivered LSAIR at doses between 14 and 18 Gy on 8- to 10-week-old male C57BL/6 mice. Histological analysis was performed to confirm that the observed mortality was due to acute abdominal radiation injury.
Results:
A steep dose–response relationship was found for survival, with no deaths seen at doses below 16 Gy and 100% mortality at above 17 Gy. All deaths occurred between 6 and 10 days after irradiation, consistent with the onset of RIGS. This was further confirmed by histological analysis showing clear differences in the number of regenerative intestinal crypts between animals receiving sublethal (14 Gy) and 100% lethal (18 Gy) radiation.
Conclusion:
The developed LSAIR technique provides uniform dose delivery with a clear dose response, consistent with acute abdominal radiation injury on histological examination. This model can provide a useful tool for researchers investigating the development of mitigators for accidental or clinical high-dose abdominal irradiation.
Keywords: abdominal irradiation, radiation-induced gastrointestinal syndrome, dose response, histological analysis, small animal radiation research
Introduction
Accidental high-dose radiation exposure can result in mortality or severe morbidity through acute radiation syndrome (ARS). The early risk of death from ARS can be attributed mainly to radiation-induced gastrointestinal syndrome (RIGS) occurring within 6 to 10 days, followed by hematopoietic syndrome (HS), typically occurring within 14 to 16 days postexposure. Currently, there are no readily available pharmacological agents that offer effective mitigation of RIGS, and as such there is great interest in developing such mitigators.1-3 For clinical radiation therapy of thoracic, abdominal, and genitourinary cancers, the collateral irradiation of abdominal tissue is often dose limiting.
Animal models provide a suitable platform for studying the development of potential medical countermeasures to mitigate the effects of radiation exposure (whether clinical or accidental).4 Much of the current knowledge about RIGS comes from small animal models mainly utilizing mice.1-3,5,6 As the Food and Drug Administration requires the effect of radiation mitigators to be proven in separate animal models, studies have also been conducted using nonhuman primates and minipigs.7-9
Death from HS occurs at lower radiation doses compared to RIGS, so partial bone marrow shielding is needed to investigate the long-term (>20 days) efficacy of mitigators for acute abdominal toxicity. This has led to the development of limb-sparing abdominal irradiation (LSAIR) techniques with various levels of bone marrow shielding, to minimize the risk of animals dying from HS.1,3,6,7 The dose–response relationship for ARS-related mortality is often very steep, and even a 5% to 10% variation in dose can result in markedly different outcomes.10 In previous studies, abdominal irradiation has typically been performed by placing mice in a single anterior orthovoltage irradiation field while shielding the bone marrow within the thorax, head, and extremities with lead.1,3,6 This leads to some uncertainty in the delivered radiation dose depending on radiation field uniformity (heel effect) and placement of animals in relation to the lead shielding as well as nonuniformity of dose throughout the abdominal cavity. This is attributable to the use of a single anterior radiation field, with lower dose on the exit side of the animal compared to the entrance side, which can result in up to 20% dose difference in a small animal for a 220-kVp orthovoltage X-ray beam.
Recent technological advances in small animal irradiators have produced platforms capable of precise radiation delivery using image-guidance methods.11,12 Here, we present a method for delivering precise and uniform mouse LSAIR, with accurate radiation dosimetry, to study the acute gastrointestinal syndrome using the small animal radiation research platform (SARRP).
Materials and Methods
Small Animal Irradiator Setup
The SARRP (Xstrahl, Surrey, United Kingdom) was used to develop the LSAIR technique presented in this study.13 The SARRP consists of an X-ray tube mounted on a rotatable gantry capable of delivering a maximum X-ray energy of 220 kVp at 13-mA tube current, either as an open field or as a collimated field using a set of custom collimators. The image guidance capabilities of the SARRP consist of a high-resolution amorphous Si flat panel detector for cone-beam computed tomography imaging and a foldout portal imager for X-ray fluoroscopy (cf Figure 1). Animals are placed on a robotic stage providing 4 degrees of freedom for targeted alignment of the radiation delivery.
Figure 1.
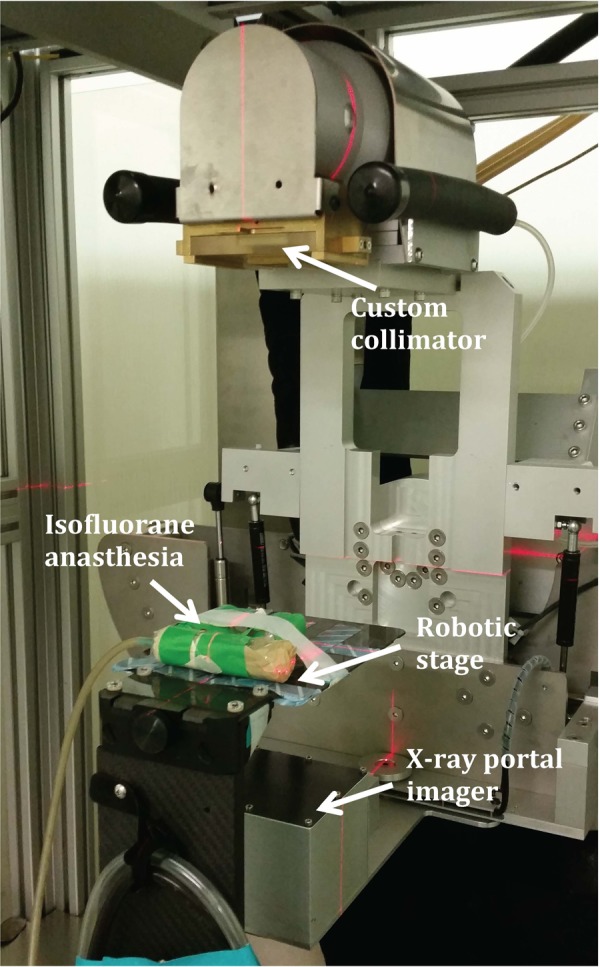
Small animal irradiator setup to perform abdominal irradiation experiments.
We have connected the robotic stage of the SARRP to a continuous delivery of isofluorane anesthesia that can be controlled from outside the radiation treatment room.
Animals and LSAIR Technique
The presented LSAIR technique can be utilized for any rodent animal model as long as the abdomen of the subjects fits within the irradiation field, which needs to be adjusted depending on the rodent size. In this study, we performed LSAIR on 8- to 10-week-old male C57BL/6 mice.
Experiments were performed in accordance with an approved protocol from the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine and the National Institutes of Health guide for the care and use of laboratory animals. Animals were placed in pairs on the robotic stage of the irradiator and continuously anesthetized with a flow of 1.5% isofluorane in 1.5 L/min pure oxygen. An in-house designed custom collimator consisting of a brass insert with a 4.8 mm ×22 mm rectangular cutout placed in a Plexiglas holder was used for LSAIR, yielding a rectangular radiation field size of 2.4 × 11 cm at a source-to-surface distance (SSD) of 33 cm (cf Figure 2).
Figure 2.
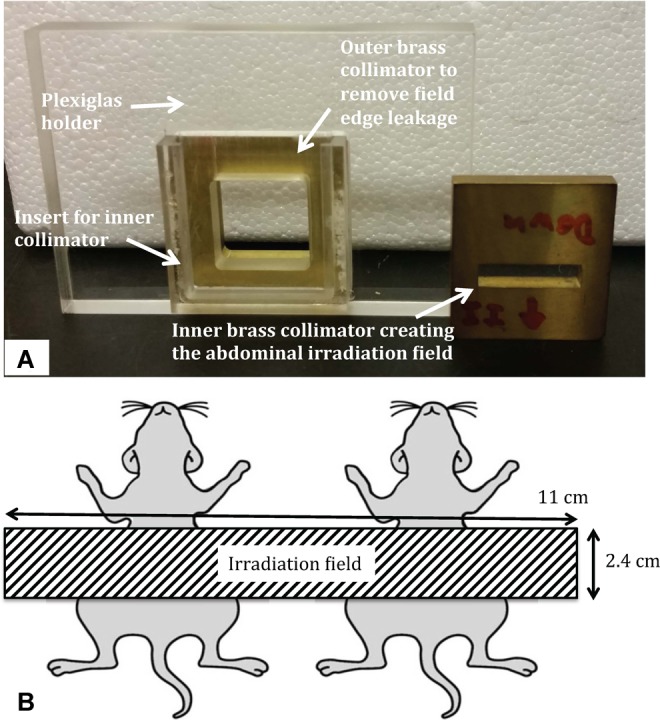
Panel (A) shows the in-house designed custom abdominal irradiation collimator and panel (B) illustrates the resulting irradiation field at the radiation isocenter distance of 33 cm (corresponding to the midline level of the animals).
Mice were placed at 33-cm SSD using the positioning lasers of the SARRP, and the size of the irradiation field at this distance was verified using Gafchromic film measurements (data not shown).
Before initiating the irradiation, the animal position was confirmed using the portal imager. Here, we found an appropriate and reproducible field placement by matching the inferior border of the field with the top of the animals’ knees. This effectively protects the pelvis, upper thorax, extremities, and head of the animals, estimated to shield approximately 70% of the total bone marrow.14 Once the position was confirmed, the portal imager was retracted while carefully removing the stage with the animals on it. To ensure uniform abdominal irradiation, the first half of the radiation dose is delivered with the gantry set at 180° (underneath the animal stage) and the stage set at 0°. The second half of the radiation dose is then delivered with the gantry at 0° and the stage at 180°. As such, potential misalignments of the animals have less impact on the resulting dose delivery and the heel effect is compensated for. Following the irradiation procedure, the animals were housed on a heating pad until fully recovered from anesthesia.
Irradiation experiments were consistently performed between 2 pm and 5 pm in the afternoon to avoid any potential confounding effect from variations related to the circadian rhythm of the intestinal stem cell population.
Abdominal Irradiation Dosimetry
For the radiation dosimetry setup, we used a dosimetry formalism based on the TG-61 protocol for 40 to 300 kV X-ray beam dosimetry and a cross-calibrated Exradin P11 parallel plate ion chamber (Standard Imaging Inc, Middleton, Wisconsin).15 Based on this dosimetry formalism, we have reported a dose output consistency within 1% of the baseline value over a period of 6 months.15 Using this setup, we measured the output factor correction for different radiation field sizes as well as the fractional depth dose at various attenuation thickness using tissue-equivalent Gammex 457-CTG (Certified Therapy Grade) solid water blocks (Gammex Inc), with all measurements performed at 220 kV and 13 mA.
We measured the output factor correction for the LSAIR collimator setup at a 1-cm tissue depth (midline of the animal) to 0.805, compared to the open-field irradiation setting. The fractional depth dose at 1-cm depth was measured to 0.905. The dose rate at the surface for open-field irradiation was measured to 0.0798 Gy/s (∼4.79 Gy/min). The total irradiation time in seconds required to deliver the intended dose, D, was calculated according to the following formula:
| 1 |
with half of the radiation delivered from the anteroposterior direction, and the other half from the posteroanterior direction, as described earlier. As such, the dose rate for this abdominal irradiation setup was 3.49 Gy/min at the midline of the animals.
Using the described LSAIR technique and radiation dosimetry formalism provides a highly reproducible animal irradiation model with the intended radiation dose prescribed to the midpoint of the abdominal target and a variation throughout the abdominal cavity that should be less than 2% for a 220-kV beam.
Dose–Response Evaluation for RIGS-Induced Mortality
To determine the dose–response relationship for RIGS-related mortality in male 8- to 10-week-old C57BL/6 mice using the described LSAIR technique, we irradiated a total of 111 animals to 14 (n = 4), 15 (n = 10), 15.5 (n =10), 16 (n = 15), 16.5 (n = 16), 17 (n = 16), 17.5 (n = 18), or 18 Gy (n = 22). Irradiation experiments were performed in separate sessions to ensure that the results were reproducible. Irradiated mice were then followed for survival to determine the dose–response relationship and kinetics of RIGS-induced mortality.
Histological Examination to Confirm RIGS
In order to determine whether death of lethally irradiated animals was correlated with RIGS, we performed histological analysis of the whole jejunum of irradiated animals receiving either a completely sublethal or a 100% lethal radiation dose, respectively. At day 4 postirradiation, the jejunum of irradiated animals was excised, flushed with cold phosphate-buffered saline, rolled up, and fixed in buffered formalin over night at room temperature. This time point was chosen based on the expected maximum difference between crypt/villi restoration and crypt/villi loss at 3 to 4 days after irradiation.1 Specimens were paraffin embedded and sectioned. Jejunum sections were stained using Hematoxylin and Eosin (H&E), imaged on a Pannoramic 250 Flash whole slide digital scanner (3DHISTECH Ltd, Budapest, Hungary), and the high-resolution images were analyzed using Pannoramic Viewer v. 1.15 (3DHISTECH Ltd). The number of regenerative crypts was counted along the entire jejunum to provide a quantitative measurement of the viability of the irradiated intestine and whether these results would correlate with the survival outcome. Quantitative measurements were compared using unpaired t tests.
Results
Survival Following Irradiation Procedure
The irradiation procedure was well tolerated by all animals, which showed no visible signs of distress or discomfort following the LSAIR. Generally, no deaths were observed before day 6 in any of the dose groups, and no deaths were recorded after day 10 (only 1 death was recorded on day 11). The resulting survival curves of the LSAIR dose–response experiments are presented in Figure 3.
Figure 3.
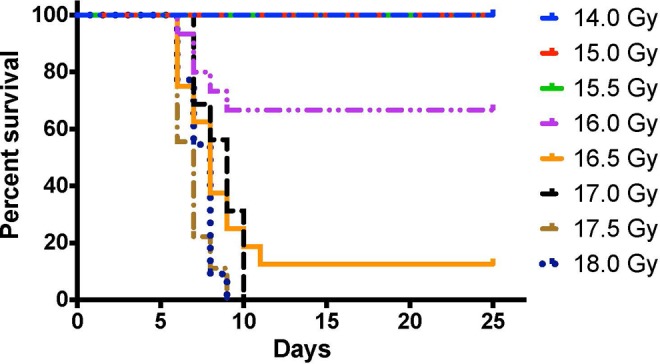
Dose–response survival curves following limb-sparing abdominal irradiation (LSAIR)--induced radiation-induced gastrointestinal syndrome (RIGS)are shown as a Kaplan-Meier survival plot. No deaths were recorded at doses below 16 Gy, and no animals that received a dose of 17 Gy or higher survived past 10 days. Number of animals in each dose group— 14 Gy (n = 4), 15 Gy (n = 10), 15.5 Gy (n = 10), 16 Gy (n = 15), 16.5 Gy (n = 16), 17 Gy (n = 16), 17.5 Gy (n = 18), and 18 Gy (n = 22).
All but one of the deaths occurred within 10 days postirradiation, and all animals that died also displayed clinical symptoms indicative of RIGS such as lack of grooming and mobility. The animals surviving past this time point all recovered and eventually showed no clinical symptoms associated with radiation-induced morbidity, which led us to conclude that the developed LSAIR technique provided sufficient bone marrow shielding to protect the animals from subsequent HS.
Using day 10 as a cutoff, we fitted the RIGS mortality data to either a logistic dose–response model as:
| 2 |
or a probit dose–response model as:
| 3 |
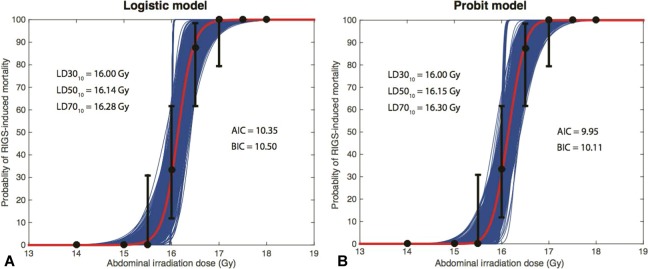
where D is the radiation dose, D50 is the dose that results in 50% mortality, γ50 is the percent change in mortality for each 1-Gy change in dose at the 50% mortality level, and erf(x) represents the mathematical error function. Using the resulting dose–response models, we estimated the dose associated with 30%, 50% and 70% mortality at 10 days, LD3010, LD5010, LD7010, respectively. Figure 4 shows the logistic and probit dose–response relationships for the RIGS-induced mortality at 10 days with the presented LSAIR technique, including the estimated 95% confidence intervals (CIs). Fitting the data resulted in 2 very similar dose–response models, with the probit model being the preferred choice due to the slightly lower Akaike Information Criteria and Bayesian Information Criteria scores.16
Figure 4.
The mortality data are plotted as black dots with uncertainty bars representing 95% confidence intervals (CIs) for the logistic model fit (A) and the probit model fit (B). The CIs were calculated as the binomial 95% CIs based on the number of surviving and dying subjects, except for dose levels where there were consistently 0% or 100% mortality in repeated experiments. Thin blue lines represent 1000 bootstrap samples showing the variation in the dose–response curve from randomly sampling the data 1000 times within the presented 95% CIs.
The probit model fitting yielded D50 = 16.15 Gy and γ50 = 22.43. Based on the results and 95% CIs presented in Figure 4B, the LD3010 = 16.00 Gy (95% CI: 15.84-16.16 Gy), LD5010 = 16.15 Gy (95% CI: 15.99-16.31 Gy), LD7010 = 16.30 Gy (95% CI: 16.14-16.46 Gy).
Survival Outcome Correlates With RIGS on Histological Analysis
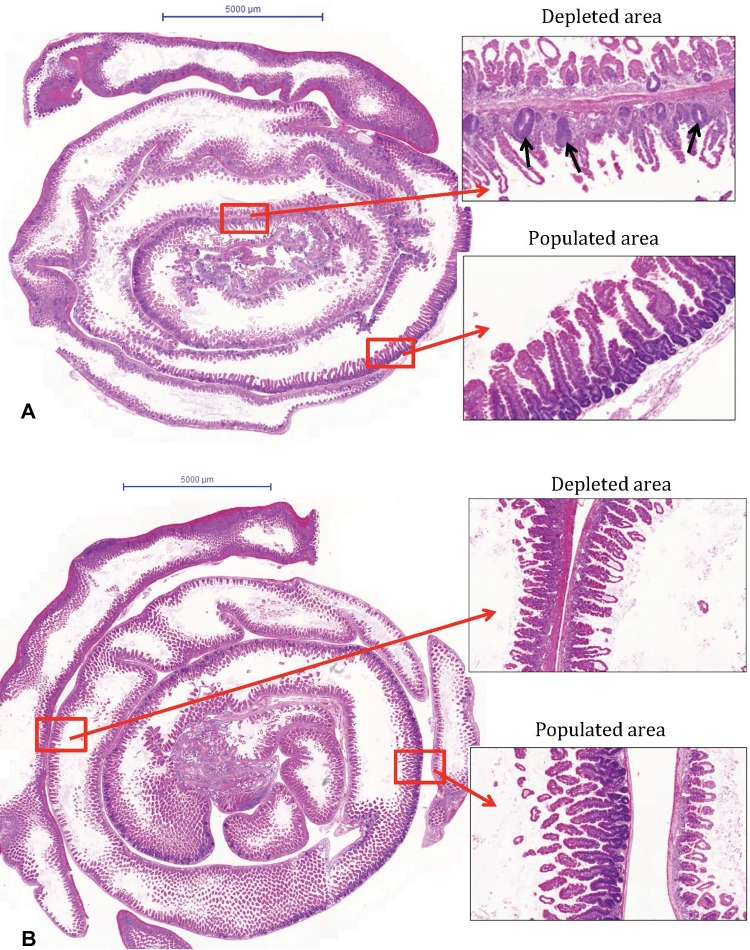
Based on the survival results, jejunums of mice irradiated at 14 and 18 Gy were chosen as representative samples for the sublethal and 100% lethal dose groups, respectively, and were subjected to histological analysis. Segments depleted of regenerative crypts and segments populated by regenerative crypts were identified on the H&E-stained sections of intestines isolated from the 14 and 18 Gy irradiated mice, as shown in Figure 5. This figure also illustrates that the depleted segments of 14 Gy irradiated mouse jejunums were characterized by the presence of some regenerative crypts that, conversely, were completely absent in the samples from 18 Gy irradiated mice (cf Figure 5 right-hand panels).
Figure 5.
Histological analysis of jejunum of abdominally irradiated mice. Two mice per dose group were subjected to limb-sparing abdominal irradiation (LSAIR),and the jejunum was isolated and processed for histological analysis at day 4 postirradiation. Panel (A) shows a hematoxylin and eosin (H&E)-stained whole jejunum section from a 14 Gy irradiated mouse; right-hand panels show enlarged regions representing depleted or fully populated crypt areas, as indicated. Regenerative crypts (indicated by black arrows) were identified by their dark purple color, the presence of connected overlaying villi and underlying lamina propria. Panel (B) shows the corresponding images of a jejunum section from an 18 Gy irradiated mouse. Scale bars = 5000 μm.
Due to the observed segmental nature of intestinal damage in the 14-Gy group, we counted the total number of crypts in each entire intestinal section to provide a consistent measurement across all samples. We then calculated the number of regenerative crypts per length of analyzed intestine.
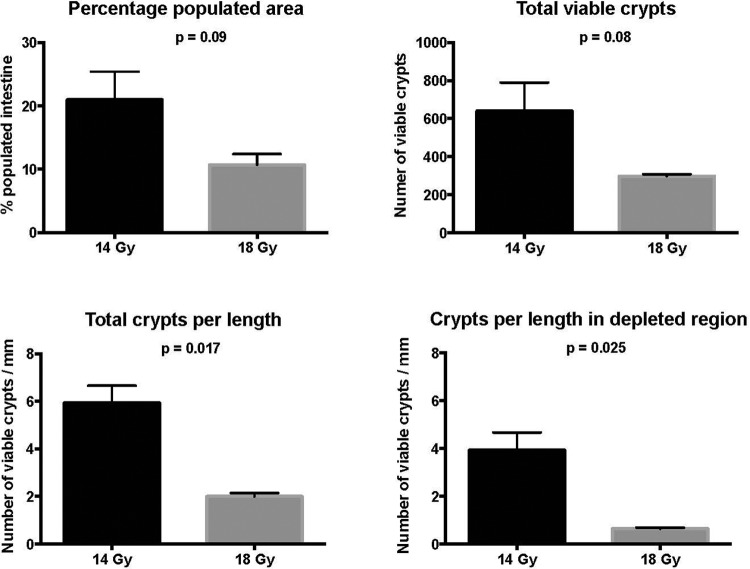
The quantitative results from the histological analysis are presented in Figure 6, confirming the visual histological findings shown in Figure 5. The graphs show that not only was the total number of regenerative crypts substantially higher in the jejunum of mice receiving 14 Gy compared to 18 Gy, but their density (number/mm of intestine) was significantly higher (P = .017). In addition, the portion of the jejunum considered as depleted of regenerative crypts was more extended at 18 Gy compared to 14 Gy.
Figure 6.
The dose-dependent limb-sparing abdominal irradiation (LSAIR) mortality rate is linked to the extent of intestinal cell damage. The number and density of regenerative crypts and length of fully populated crypt segments were quantified in the jejunum of animals receiving 14 Gy (n = 2, black bars) and 18 Gy (n = 2, gray bars) LSAIR. There was a significantly lower number of regenerative crypts per jejunal length in the jejunums of 18 Gy compared to 14 Gy irradiated mice. The data are shown as mean value with uncertainty bars representing 1 standard deviation; P values were generated using an unpaired t test comparing the difference between means in the 14-Gy and 18-Gy groups.
Taken together, the results of the survival experiments and the histological analysis of animals subjected to the presented LSAIR procedure clearly demonstrate that the associated mortality is attributable to RIGS.
Discussion
We have developed a precise and uniform LSAIR technique for studying RIGS in small animals, using 8- to 10-week-old male C57BL/6 mice. Based on our monthly quality assurance procedures that include dose output consistency measurements, we estimate that variation in delivered radiation dose to the abdomen will be within 1% to 2% when using the presented image-guided LSAIR technique.15 In contrast, there may be considerable variation in dose when delivering abdominal irradiation using a single anterior field as has been commonly used in the past. For 220 kV and 13 mA, the fractional depth dose in tissue for an open field at an isocenter distance of 33 cm is 0.96 for 0.5-cm depth, 0.91 for 1.0-cm depth, and 0.80 for 2.0-cm depth, showing that the variation in entrance versus exit dose can be up to 20% if irradiation is only delivered from 1 direction. Since our technique employs anteroposterior and posteroanterior radiation delivery that minimizes the heel effect, the dose deposition is uniform throughout the abdominal cavity of the irradiated animals.
Several abdominal irradiation techniques have been previously reported using lead shielding to create the irradiation field.1,3,6 Leibowitz et al employed an LSAIR technique using a clinical linear accelerator and a 3-cm wide radiation band across the abdomen, with a dose rate of 1.46 Gy/min.6 This resulted in 100% mortality within 8 days for 7- to 9-week-old, wild-type C57BL/6 mice receiving AIR to 15 Gy. Saha et al irradiated 5- to 6-week-old C57BL/6 mice to different doses between 12 and 20 Gy using a 320-kV orthovoltage irradiator at 0.72 Gy/min while shielding the thorax, head and neck, and extremities.3 They found that all animals receiving ≥16 Gy without mitigation died as a result of RIGS within 10 days after exposure. The results presented by Saha et al are comparable to the dose response presented in the current study, with animals dying at doses at or above 16 Gy.
Conversely, data presented by Booth et al showed a dose response for LSAIR-induced mortality in 8- to 10-week-old male C57BL/6 mice that was distinctly different from that reported in our current study.1 They found that animals started dying at LSAIR doses as low as 12 Gy and that 16 Gy resulted in 100% mortality. Despite this dramatic difference in dose response, the anatomical target was similar to that in our study, as their abdominal irradiation was delivered with lead shielding of the head, forelimbs, and thorax, 300-kV X-rays at 10 mA at a dose rate of 0.70 Gy/min. These differences in reported outcomes of small animal abdominal irradiation in the current literature emphasize the requirement for detailed definition of the anatomical target as well as the implementation of rigorous radiation dosimetry protocols.
These differences and the inherent variability in biological response also highlight the importance of reporting the statistical uncertainty related to survival data and the corresponding dose–response models, which has not always been rigorously described.1,6,10
When studying RIGS, consideration should also be given to the amount of bone marrow that is spared in order to circumvent the HS, since bone marrow sparing can act as an endogenous mitigator promoting gastrointestinal recovery.3,5,17,18 Taken together with the differences seen in the dose response of RIGS between our study and previous reports, this highlights the need to study the impact of varying bone marrow shielding on acute gastrointestinal injury, using a precise and uniform irradiation technique such as the one presented in this study.
A limitation of our LSAIR technique is that ensuring accurate and homogeneous radiation delivery is relatively time consuming. When delivering a dose of 16 Gy it takes approximately 1 hour to irradiate 10 to 12 animals. However, given the steep dose–response relationship for RIGS and the importance of accurate radiation delivery, a precise and uniform radiation delivery should be a priority. Especially, since it will result in fewer animals being required for each experiment and will provide more consistent results.
Furthermore, histological analysis confirmed that the observed mortality was correlated with the severity of acute intestinal damage associated with RIGS. Given the observed segmental characteristics of the intestinal damage associated with sublethal abdominal irradiation, we believe that the analysis of sections from the entire jejunum represents a valid approach to generate reliable and consistent results linking alterations in the cellular level to survival outcome.
The presented irradiation technique supported by appropriate radiation dosimetry protocols provides researchers with a detailed method for precise abdominal irradiation that should increase reproducibility of experiments, especially when conducted by multiple investigators or at different institutions. In addition, it allows for investigations of dose–volume effects by limiting or increasing the extent of irradiated intestine through varying the radiation field size, which could have important clinical applicability.
Acknowledgments
The authors would like to thank Dr Rani Sellers for the help she provided with scanning the histological samples.
Authors’ Note: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported in part by grant U19-AI091175 from the United States National Institutes of Health (NIH) and Dr. Brodin acknowledges support from the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071 and UL1TR001073.
References
- 1. Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health phys. 2012;103(4):383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jung E, Perrone EE, Brahmamdan P, et al. Inhibition of intestinal epithelial apoptosis improves survival in a murine model of radiation combined injury. PloS One. 2013;8(10):e77203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PloS One. 2011;6(9):e24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173(4):557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth C, Tudor G, Tonge N, Shea-Donohue T, MacVittie TJ. Evidence of delayed gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103(4):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leibowitz BJ, Wei L, Zhang L, et al. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacVittie TJ, Bennett A, Booth C, et al. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103(4):427–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacVittie TJ, Farese AM, Bennett A, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103(4):411–426. [DOI] [PubMed] [Google Scholar]
- 9. Shim S, Jang WS, Lee SJ, et al. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 2014;181(4):387–395. [DOI] [PubMed] [Google Scholar]
- 10. Kazi AM, MacVittie TJ, Lasio G, Lu W, Prado KL. The MCART radiation physics core: the quest for radiation dosimetry standardization. Health Phys. 2014;106(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bazalova M, Nelson G, Noll JM, Graves EE. Modality comparison for small animal radiotherapy: a simulation study. Med Phys. 2014;41(1):011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol. 2011;56(12): R55–R83. [DOI] [PubMed] [Google Scholar]
- 13. Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71(5):1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16(3):277–286. [DOI] [PubMed] [Google Scholar]
- 15. Brodin NP, Guha C, Tome WA. Proposal for a Simple and Efficient Monthly Quality Management Program Assessing the Consistency of Robotic Image-Guided Small Animal Radiation Systems. Health Phys. 2015;109(3 suppl 3):S190–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H, Ghosh SK. Performance of Information Criteria for Spatial Models. J Stat Comput Simul. 2009;79(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117(3):480–488. [PubMed] [Google Scholar]
- 18. Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys. 1989;17(3):569–573. [DOI] [PubMed] [Google Scholar]