Abstract
Iron-induced hypophosphataemic osteomalacia remains under-recognized as a potential complication of parenteral iron therapy. We here report two cases of symptomatic hypophosphataemic osteomalacia with multiple insufficiency fractures in the context of chronic gastrointestinal blood loss, necessitating monthly iron polymaltose infusions over prolonged periods of time. Respective blood tests revealed severe hypophosphataemia [0.29 and 0.43; normal range (NR) 0.8–1.5 mmol/l] in the presence of normal serum calcium and 25-hydroxy vitamin D levels. Urinary fractional phosphate excretion was elevated (16% and 24%; NR < 5%) and the tubular maximum phosphate reabsorption was reduced, consistent with renal phosphate wasting. Serum fibroblast growth factor 23 (FGF23) obtained in one patient was significantly elevated at 285 pg/ml (NR < 54 pg/ml). Bone mineral density was significantly reduced and whole-body bone scans revealed metabolic bone disease and multiple insufficiency fractures consistent with osteomalacia. Cessation of iron infusions resulted in clinical and biochemical improvement within 2 months in one patient whereas the second patient required phosphate and calcitriol supplementation to improve symptomatically. Iron-induced hypophosphataemic osteomalacia is thought to be due to reduced degradation of FGF23, resulting in phosphaturia and reduced synthesis of 1,25-dihydroxy vitamin D. Monitoring of patients on long-term parenteral iron is recommended to avoid clinically serious adverse effects.
Keywords: fracture, hypophosphataemia, iron infusion, osteomalacia
Clinical record
Case 1
A 65-year-old female outpatient was referred with a 2-year history of minimal trauma fractures sustained to her left wrist, ribs, sacrum, right pubis as well as a compression fracture of the T6 vertebra as well as worsening generalised bone pain. She had received monthly 1 g iron polymaltose infusions for 13 months to treat her iron deficiency anaemia secondary to gastric antral vascular ectasia. Her medical history included emphysema and type 2 diabetes mellitus. Medications included cholecalciferol 1000 U daily, calcium carbonate 600 mg daily, fluticasone propionate/salmeterol xinafoate (250 µg/50 µg) 2 sprays daily, ipratropium bromide 18 µg one spray daily and insulin aspart/aspart protamine 36 units mane, 18 units nocte. The patient’s body-mass index was 20 kg/m2 and her vital signs as well as cardiac, respiratory and gastrointestinal examinations were normal. Due to her long-standing bone pain she was only able to mobilize with the help of a four-wheel walker. There was vertebral and muscle tenderness on palpation but no signs of joint inflammation or chronic liver disease.
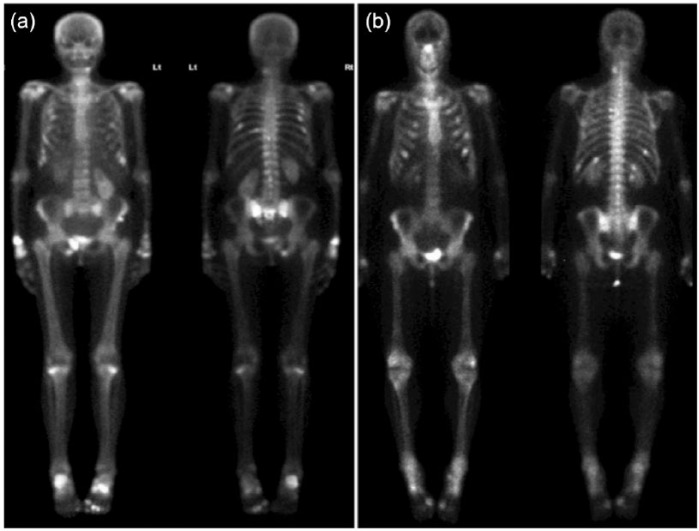
Laboratory investigations (Table 1) were remarkable for severe hypophosphataemia (0.29; NR 0.8–1.5 mmol/l) in the presence of normal serum corrected calcium, and an elevated alkaline phosphatase (ALP) and N-terminal procollagen type I propeptide (PINP) levels. A low tubular maximum phosphate reabsorption per glomerular filtration rate (TmP/GFR) and an elevated fractional phosphate excretion were consistent with renal phosphate wasting. Urine calcium, pH, amino acid, glucose and protein levels were within normal limits. Dual-energy X-ray absorptiometry was consistent with osteoporosis and a whole-body radioisotope bone scan revealed multiple foci of tracer uptake consistent with recent fractures (Figure 1A). A thoracolumbar X-ray confirmed a T6 vertebral fracture.
Table 1.
Summary of patient laboratory investigations.
| Parameter | Case 1 | Case 2 | Normal range |
|---|---|---|---|
| Sodium (mmol/l) | 141 | 141 | 135–145 |
| Potassium (mmol/l) | 3.9 | 4.4 | 3.5–5.0 |
| Estimated GFR (ml/min/1.73 m2) | >90 | >90 | >60 |
| Creatinine (umol/l) | 48 | 56 | 50–90 |
| Bicarbonate (mmol/l) | 27 | 25 | 24–32 |
| Haemoglobin (g/l) | 100 | 93 | 120–150 |
| Mean cell volume (f/l) | 78 | 95 | 80–100 |
| ALP (U/l) | 302 | 125 | 30–130 |
| Uric Acid (mmol/l) | 0.21 | 0.35 | 0.25–0.42 |
| Phosphate (mmol/l) | 0.29 | 0.43 | 0.8–1.5 |
| Corrected Calcium (mmol/l) | 2.18 | 2.40 | 2.1–2.6 |
| Magnesium (mmol/l) | 0.84 | 0.83 | 0.70–0.91 |
| Parathyroid hormone (pmol/l) | 4.1 | 8.3 | 1.3–7.6 |
| P1NP (µg/l) | 118 | – | <70 |
| 25(OH) Vitamin D (nmol/l) | 98 | 54 | |
| 1,25(OH) Vitamin D (pmol/l) | 80 | 32 | 60–158 |
| Urinary phosphate (mmol/d) | 8 | 32 | 15–30 |
| Urinary phosphate fractional excretion (%) | 16 | 24 | <5% |
| TmP/GFR | 0.76 | 0.48 | 0.87 – 1.40 |
| FGF23 (pg/ml) | – | 285 | <54 |
| Femoral neck T-score (SD) | –3.0 | –1.3 | >–1.0 |
Parameters in bold indicate an abnormal value.
Normal range for fractional excretion of phosphate is 5–20% in patients with normal serum phosphate; a value >5% in the setting of hypophosphataemia is abnormal and denotes isolated renal phosphate wasting.
GFR, glomerular filtration rate; P1NP, procollagen type 1 N propeptide; TmP/GFR, tubular maximum phosphate reabsorption to glomerular filtrate rate; FGF23, fibroblast growth factor 23; SD, standard deviation.
Figure 1.
Radioisotope whole-body bone scan. (a) Increased focal uptake consistent with multiple rib fractures as well as increased metabolic activity involving the right distal radius, ribs, ankles, right inferior pubic ramus and sacral ala. (b) Increased focal uptake consistent with multiple fractures as well as enhanced metabolic activity affecting the scapulae, spine, sternum, proximal and distal ends of the tibiae and distal femora. There are also degenerative changes of the hands, feet as well as spine.
Following the diagnosis of osteomalacia, the patient’s iron infusions were ceased. Her clinical symptoms improved and her serum phosphate (0.88 mmol/l), PINP (78 ug/l (<70)) and ALP levels (82 U/l (30–130)) normalised and her symptoms improved within 2 months without any further intervention.
Case 2
A 58-year-old female was admitted to hospital with a 6-month history of bone pain involving her chest wall, back, lower limbs and arms. She had received monthly 1 g iron polymaltose infusions for the preceding 17 months for iron-deficiency anaemia secondary to hereditary haemorrhagic telangiectasia. She had a history of hypertension, gastro-oesophageal reflux and depression. Her medications included cholecalciferol 1000 U daily, tranexamic acid 1 g daily, eprosartan 400 mg daily, esomeprazole 40 mg daily and escitalopram 10 mg daily. On examination, the patient had an antalgic gait with tenderness over her chest wall and feet with no evidence of joint inflammation. Multiple oral and digital telangiectasia were noted. Her vital signs as well as cardiac, respiratory and gastrointestinal examinations were normal.
Laboratory investigations revealed hypophosphataemia (0.43 mmol/l), low 1,25-dihydroxy vitamin D and elevated PTH levels (Table 1). A low TmP/GFR and elevated fractional phosphate excretion were consistent with renal phosphate wasting. Urine studies including calcium, pH, amino acid, glucose and protein levels were within normal limits. Serum fibroblast growth factor 23 (FGF23) was markedly elevated at 285 [upper limit of normal (ULN) 54] pg/ml [assay utilized serum intact FGF23 performed by enzyme-linked immunosorbent assay (ELISA; Kainos Laboratories, Tokyo, Japan)]. Nuclear imaging revealed focal uptake at several ribs bilaterally consistent with insufficiency fractures, as well as diffusely increased osteoblastic activity at the sternum, scapulae, long bones of the limbs and costochondral junctions, compatible with metabolic bone disease (Figure 1B).
The patient’s iron infusions were ceased and the patient was commenced on oral calcitriol 0.25 µg daily and Sandoz Phosphate® 3 tablets nocte for 5 months. Her clinical symptoms improved and her serum phosphate (1.05 mmol/l), TmP/GFR (0.90) and FGF23 levels (58 pg/ml) improved over time.
Discussion
We have reported the cases of two patients who developed severe hypophosphataemic osteomalacia and fractures as a consequence of prolonged parenteral iron administration. Given the widespread clinical use of iron infusions it is important to raise awareness regarding such serious skeletal adverse reactions. Although transiliac bone histomorphometry biopsies were not performed in either patient to confirm the diagnosis of osteomalacia, histological evidence is usually not necessary in most cases. A combination of clinical features (i.e. muscle and bone pain, weakness and fractures) in addition to laboratory and bone scan evidence of osteomalacia, may be sufficient to make the diagnosis [Bingham and Fitzpatrick, 1993].
Parenteral iron is complexed with carbohydrate ligands (e.g. dextran, sucrose, polymaltose, carboxymaltose) and is used to treat iron-deficiency anaemia, as well as complications arising from malignancy and chronic renal failure. Reports of adverse reactions are scarce and hypophosphataemia has only recently gained recognition in the literature despite being identified as a complication of parenteral iron over 30 years ago [Hardy and Vandemergel, 2015; Schouten et al. 2009; Okada et al. 1982]. The incidence of hypophosphataemia following iron infusion varies widely, ranging from 2.1% to 86% although most cases present with transient and clinically insignificant changes in serum phosphate levels [Favrat et al. 2014]. However, some iron preparations may be associated with acute hypophosphataemia, which clinically manifest as fatigue, seizures, osmotic demyelination syndrome, myocardial depression, arrhythmia, proximal myopathy, rhabdomyolysis and haemolytic anaemia [Blazevic et al. 2014]. In contrast, although its incidence is unknown, chronic hypophosphataemia may be seen with long-term parenteral iron therapy. Affected patients may present with more subtle symptoms such as fatigue, bone pain, insufficiency fractures, difficulty mobilizing and muscle weakness [Hardy and Vandemergel, 2015].
Phosphate is critical for intracellular signalling, membrane function, energy metabolism and bone mineralization [Renkema et al. 2008; Sommer et al. 2007]. Phosphate is primarily obtained from the diet where approximately 65% is absorbed by the small bowel and stored in the skeleton [Rector’s, 2012]. Primarily under the control of PTH as well as calcitriol, the kidneys reabsorb 85–95% of free phosphate, thus making renal excretion the primary mode of phosphate clearance. Calculation of TmP/GFR, which corresponds to the theoretical lower limit of serum phosphate below which all filtered phosphate would be reabsorbed [Payne, 1998], is obtained from a second-void urine sample; a low value supports impaired renal phosphate reabsorption. Despite a normal 24-h urinary phosphate level in case 1, TmP/GFR was low in both patients confirming urinary phosphate wasting.
The potent phosphatonin, FGF23, together with PTH and calcitriol, plays a key role in phosphate metabolism. The peptide binds the FGF receptor on target cells along with cofactors (e.g. membrane-bound protein Klotho) to downregulate proximal renal tubule phosphate cotransporters, resulting in phosphaturia [Shimada et al. 2004]. It also reduces the 1α-hydroxylation of 25-hydroxy vitamin D, resulting in reduced synthesis of 1,25-dihydroxy vitamin D [Schouten et al. 2009; Wolf et al. 2013]. There is evidence to suggest that the carbohydrate moiety of iron preparations interferes with the proteolytic degradation of FGF23, as evidenced by a rapid reduction in the c-terminal portion of FGF23 and, conversely, an increase in intact FGF23 in women receiving intravenous iron for dysfunctional uterine bleeding [Wolf et al. 2013]. This, in turn, has been proposed to lead to isolated phosphaturia rather than global proximal tubular dysfunction as seen in Fanconi’s syndrome.
A previous study has suggested that the nadir in serum phosphate levels occurs approximately 1–2 weeks following iron polymaltose infusion, coinciding with a significant rise in FGF23 concentrations, maximal renal phosphate loss and a significant reduction in 1,25-dihydroxy vitamin D levels [Schouten et al. 2009]. Another study determined that the biochemical abnormalities following administration of ferric carboxymaltose may persist for up to 6–12 weeks [Van Wyck et al. 2009], thus supporting regular clinical review of at-risk patients. Furthermore, certain iron preparations may result in lower rates of adverse events. One randomized multicentre trial comparing iron dextran with ferric carboxymaltose illustrated that significantly more patients treated with ferric carboxymaltose developed transient, asymptomatic hypophosphataemia when compared with iron dextran (8.5% versus 0%) whereas other studies report rates of up to 27% in ferric carboxymaltose-treated patients [Bregman and Goodnough, 2014; Hussain et al. 2013]. A retrospective study also found that 51% and 13% of ferric carboxymaltose-treated patients developed transient, asymptomatic hypophosphataemia and severe hypophosphataemia, respectively, in contrast to 22% and 0% of patients treated with iron sucrose, another complexed iron preparation [Hardy and Vandemergel, 2015; Shimizu et al. 2009]. To the best of the authors’ knowledge, there has not been a direct comparison with iron polymaltose and any other carbohydrate-complexed parenteral iron preparation with respect to the development of hypophosphataemia, representing a clinically relevant avenue for further investigation.
Adverse events are unlikely for the majority of patients receiving a single iron infusion. The development of clinically significant hypophosphataemia following parenteral iron is more likely in patients on long-term iron replacement, and those with lower baseline ferritin and/or gastrointestinal disorders or malnutrition (Table 2) [Hardy and Vandemergel, 2015; Schouten et al. 2009; Fierz et al. 2014; Blazevic et al. 2014]. In patients suspected of iron-induced hypophosphataemia, renal phosphate handling should be assessed by calculating the TmP/GFR, which all filtered phosphate would be reabsorbed [Payne, 1998]. A low TmP/GFR strongly supports the diagnosis of impaired renal phosphate reabsorption and is a more reliable index of renal phosphate handling than measurement of urinary phosphate alone. In addition, Fanconi’s syndrome or proximal tubular damage needs to be excluded (by measuring protein, amino and uric acids, glucose and pH). If the diagnosis of isolated urinary phosphate wasting is confirmed, the differential diagnoses include congenital autosomal dominant or recessive hypophosphataemic rickets and acquired dis-orders such as tumour-induced osteomalacia, fibrous dysplasia and iron-induced hypophosphataemia. As all of the latter are FGF23-mediated, a thorough history rather than an FGF23 level is critical in determining the cause of isolated urinary phosphate wasting. In cases of previously confirmed iron-induced hypophosphataemia it is reasonable to assess serum and urine phosphate levels before, and 1–2 weeks following parenteral iron as biochemical abnormalities may occur within the first 2 weeks [Schouten et al. 2009]. Serum phosphate should be monitored more regularly if severely low levels are observed or if the patient is symptomatic.
Table 2.
Conditions associated with developing hypophosphataemia in patients receiving parenteral iron infusions [Hardy and Vandemergel, 2015; Schouten et al. 2009; Fierz et al. 2014; Blazevic et al. 2014].
| Long-term parenteral iron administration or high cumulative dose |
| Vitamin D deficiency |
| Hyperparathyroidism |
| Previous insufficiency fractures |
| Malabsorption and gut disorders (e.g. inflammatory bowel disease) |
| Pre-existing hypophosphataemia or related disorders |
| Use of polymaltose, carboxymaltose, saccharide preparations |
| Malnutrition |
| Low ferritin |
Clinically, patients may report supporting symptoms of bone pain, fatigue, muscle weakness, difficulty mobilizing and minimal trauma fractures, as with other causes of osteomalacia. X-rays of affected regions, thoracolumbar X-rays as well as bone scans may evaluate for fractures or uptake at sites consistent with insufficiency fractures, respectively, as well as differentiate from other causes of pain.
Management of iron-induced hypophosphataemia and its complications ideally includes the immediate cessation of iron infusions though this approach is often problematic, particularly amongst patients with chronic gastrointestinal blood loss. If ongoing parenteral iron is required, alternate preparations include dextrin citrate–iron (III) complex or iron sucrose, as they may have lower rates of hypophosphataemia [Hardy and Vandemergel, 2015; Shimizu et al. 2009]. If clinically appropriate, blood transfusions are also an option. If patients with hypophosphataemic osteomalacia require ongoing parenteral iron polymaltose, carboxymaltose, or saccharated preparations, oral phosphate and calcitriol supplementation is the mainstay of therapy. Since oral phosphate is rapidly absorbed and cleared, and may also cause gastrointestinal upset, it requires frequent dosing (e.g. 1–3 g/day in four to six divided doses) and should be taken with food. Calcium-rich foods should be avoided to improve intestinal phosphate absorption. Evidence for phosphate replacement in other forms of osteomalacia suggests that a target serum phosphate level at the lower end of the normal range improves bone disease as evidenced by bone biopsy [Chong et al. 2011]. Calcitriol is an important adjunctive treatment as it aids the absorption of oral phosphate and to avoid secondary hyperparathyroidism [Chong et al. 2011]. Treatment may be given on the day of the iron infusion and daily for 3–4 weeks afterwards. Regular monitoring of calcium and phosphate should be undertaken as noted above and supplementation ceased once serum phosphate and metabolic bone parameters have normalized.
In conclusion, this report of two cases highlights the need for clinicians to be aware of hypophosphataemic osteomalacia as a serious complication of longer-term parenteral iron infusions, which can be managed by the (preferably early) institution of preventative measures. Patients requiring regular iron infusions with hypophosphataemic osteomalacia should be referred to an endocrinologist for the prevention and treatment of insufficiency fractures.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Ramy H. Bishay, Department of Endocrinology and Metabolism, Concord Hospital Medical Centre, Concord, NSW 2139, Australia.
Kirtan Ganda, Department of Endocrinology and Metabolism, Concord Repatriation General Hospital, Concord, NSW, Australia; Concord Clinical School, Sydney Medical School, University of Sydney, NSW, Australia.
Markus J. Seibel, Department of Endocrinology and Metabolism, Concord Repatriation General Hospital, Concord, NSW, Australia Concord Clinical School, Sydney Medical School, University of Sydney, NSW, Australia.
References
- Bingham C., Fitzpatrick L. (1993) Noninvasive testing in the diagnosis of osteomalacia. Am J Med 95: 519–523. [DOI] [PubMed] [Google Scholar]
- Blazevic A., Hunze J., Boots J. (2014) Severe hypophosphataemia after intravenous iron administration. Neth J Med 72: 49–53. [PubMed] [Google Scholar]
- Bregman D., Goodnough L. (2014) Experience with intravenous ferric carboxymaltose in patients with iron deficiency anemia. Ther Adv Hematol 5: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong W., Molinolo A., Chen C., Collins M. (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18: R53–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrat B., Balck K., Breymann C., Hedenus M., Keller T., Mezzacasa A., et al. (2014) Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women—PREFER a randomized, placebo-controlled study. PLoS One 9: e94217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierz Y., Kenmeni R., Gonthier A., Lier F., Pralong F., Coti Bertrand P. (2014) Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur J Clin Nutr 68: 531–533. [DOI] [PubMed] [Google Scholar]
- Hardy S., Vandemergel X. (2015) Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol 2015: 468675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I., Bhoyroo J., Butcher A., Koch T., He A., Bregman D. (2013) Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia 2013: 169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Imamura K., Fuchigami T., Omae T., Iida M., Nanishi F., et al. (1982) [2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long-term intravenous administration of saccharated ferric oxide]. Nihon Naika Gakkai Zasshi 71: 1566–1572. [PubMed] [Google Scholar]
- Payne R. (1998) Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem 35: 201-206. [DOI] [PubMed] [Google Scholar]
- Rector’s B. (2012) Phosphate transport. In: Al M. (ed.), The Kidney. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- Renkema K., Alexander R., Bindels R., Hoenderop J. (2008) Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 40: 82–91. [DOI] [PubMed] [Google Scholar]
- Schouten B., Hunt P., Livesey J., Frampton C., Soule S. (2009) FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 94: 2332–2337. [DOI] [PubMed] [Google Scholar]
- Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., et al. (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Tada Y., Yamauchi M., Okamoto T., Suzuki H., Ito N., et al. (2009) Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 45: 814–816. [DOI] [PubMed] [Google Scholar]
- Sommer S., Berndt T., Craig T., Kumar R. (2007) The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol 103: 497–503. [DOI] [PubMed] [Google Scholar]
- Van Wyck D., Mangione A., Morrison J., Hadley P., Jehle J., Goodnough L. (2009) Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 49: 2719–2728. [DOI] [PubMed] [Google Scholar]
- Wolf M., Koch T., Bregman D. (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28: 1793–1803. [DOI] [PubMed] [Google Scholar]