Abstract
NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T gene polymorphisms have been reported to influence the risk for esophageal cancer (EC) in many studies. However, the results remain controversial and ambiguous. We performed a meta-analysis, which included 13 independent studies with a total of 2357 subjects, to examine the association between NQO1 C609T polymorphism and EC. The association was assessed by five different gene models. The overall analysis suggested that the variant allele and genotypes were significantly related to increased risk of EC (odds ratio [OR] T versus C = 1.15, 95% confidence interval [CI] 0.95–1.40, probability of rejection [POR] = 0.014; OR TT versus CC = 1.32, 95% CI 1.01–1.73, POR = 0.045; OR TC versus CC = 1.32, 95% CI 0.98–1.21, POR = 0.128; OR TT + TC versus CC = 1.10, 95% CI 1.00–1.20, POR = 0.05; OR TT versus CC + TC = 1.26, 95% CI 0.95–1.57, POR = 0.103). Sensitivity analysis confirmed the reliability of these findings. Our study shows that individuals carrying the NQO1 C609T variant allele and genotypes are more susceptible to EC.
Keywords: esophageal cancer, meta-analysis, NAD(P)H: quinone oxidoreductase 1, single nucleotide polymorphisms
Introduction
Esophageal cancer (EC) is a gravely lethal malignancy and 1 of the 10 most common cancers worldwide, and the sixth leading cause of cancer-associated deaths [DeSantis et al. 2014; Long et al. 2014]. Despite the significant improvements in diagnosis and treatment of EC made over the past several decades, the overall 5-year survival rate remains unsatisfactory [Ichikawa et al. 2014; Kunisaki et al. 2014]. Although alcohol drinking, cigarette smoking, betel liquid (with or without tobacco), overweight and obesity, esophageal reflux disease, and history of Barrett’s esophagus are considered to be common risk factors for EC, the etiology of most cases of EC is still not clear [Lindkvist et al. 2014]. Emerging epidemiological evidence has indicated that single nucleotide polymorphisms (SNPs) in certain genes contribute to the pathophysiology of human malignancy including EC [Xu et al. 2013].
The gene encoding NAD(P)H: quinone oxidoreductase 1 (NQO1), also known as diphtheria toxin diaphorase, is located on chromosome 16q22 [Gang et al. 2014]. NQO1 has been reported to be a promising candidate in the pathogenesis of esophageal carcinoma [Liu et al. 2014]. NQO1 is a cytosolic enzyme, a member of the NAD(P)H dehydrogenase (quinone) family and encodes a cytoplasmic two-electron reductase, which reduces and detoxifies quinines and thus protects cells from oxidative damage [Wu et al. 2013]. In addition, NQO1 has been identified as being involved in the protection of cells against oxidative stress and cancer development [Potts-Kant et al. 2012; Jamshidi et al. 2012].
Recently, an exotic polymorphism, C609T (also known as Pro187Ser) in the NQO1 gene was found to be of particular interest and has been widely investigated in molecular epidemiology studies [Peng et al. 2014]. A growing body of evidence has demonstrated that the C609T polymorphism of NQO1 may affect a host’s susceptibility to cancer by reducing the enzymatic activity of NQO1 according to in vitro studies [Tian et al. 2014]. A number of studies have been performed to assess the association between NQO1 C609T polymorphism and EC, but the conclusions remain controversial rather than conclusive [Zhao et al. 2014; Umar et al. 2012].
Meta-analysis with a large sample size is characteristic of higher statistical power in estimating potential gene association. Thus, we performed the present meta-analysis by pooling data from all independent publications to shed some light on the conflicting findings. Understanding the role of NQO1 C609T polymorphism in carcinogenesis could enable the development of a new attractive strategy for EC prevention and treatment.
Methods
Search strategy
A comprehensive literature search was conducted in PubMed, EMBASE, and the Web of Science databases from January 2000 to March 2015 using the following keywords and terms: ‘esophageal cancer’ and ‘polymorphism’ and ‘NAD(P)H: quinone oxidoreductase 1’, or ‘NQO1’. All references that focused on the same topic but were not indexed by the databases were checked for the meta-analysis. There was no restriction on population or sample size. Only studies published in English were included.
Selection and exclusion criteria
Literature included in this meta-analysis had to meet the following criteria: (a) an independent case-control study matching; (b) evaluating the association of the NQO1 C609T polymorphisms and EC risk; (c) control groups agreed with the Hardy–Weinberg equilibrium (HWE); (d) a detailed odds ratio (OR) with 95% confidence interval (CI) and p value; (e) reported in English.
Data extraction
For each study that met our criteria, the following information was extracted: first author, year of publication, country of origin, ethnicity, number of cases and controls, genotyping methods, genotype distribution, HWE, and so on. Two independent investigators (JD and JB) researched the work and extracted the data from each literature source and they reached a consensus on all items.
Statistical analysis
Meta-analysis was performed using Stata 12.0. The Q test and I2 test were used to examine heterogeneity between the studies. In the heterogeneity test, if p > 0.05, a fixed-effects model (the Mantel–Haenszel method) was selected, and if p < 0.05, a random-effects model (the DerSimonian and Laird method) was selected to calculate the OR and 95% CI, which assessed the strength of the association between NQO1 C609T polymorphism and colorectal cancer risk. The X2 test was performed to estimate the HWE for the control group in each study and p > 0.05 was considered as meeting HWE. Sensitivity analyses were carried out by sequentially removing one study at a time to assess the influence of single studies on overall estimates. A funnel plot was used to test the publication bias visually. Subgroup analyses were conducted by the ethnicities and sources of controls.
Results
Characteristics of all included studies
Based on our search strategy, 13 relevant case-control studies were included into this meta-analysis with a total of 2357 subjects and 3028 controls who met the inclusion criteria (Figure 1). Among them, six studies were carried out on Asian populations, while seven studies were of Whites. Of these 13 studies, 5 studies were based on a hospital-based design and 8 studies were on a population-based design. Genotyping methods used in the studies included polymerase chain reaction (PCR)-restriction fragment length polymorphism (11 studies), PCR-confronting two-pair primers (1 study and 1 unknown study). Genotype distribution of controls in all the studies was in accordance with HWE. The characteristics of the included studies are shown in Table 1.
Figure 1.

Flow diagram of the studies’ selection process.
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Ethnicity | Control source | Genotyping method | Number |
Quality score | Hardy–Weinberg equilibrium | |
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Sarbia | 2003 | Germany | White | PB | PCR-RFLP | 62 | 253 | 7 | 0.602 |
| Zhang | 2003 | China | Asian | HB | PCR-RFLP | 193 | 141 | 8 | 0.765 |
| Zhang | 2003 | Germany | White | HB | PCR-RFLP | 257 | 252 | 9 | 0.603 |
| Marjani | 2010 | Iran | White | HB | PCR-RFLP | 93 | 50 | 7 | 0.467 |
| Hamajima | 2002 | Japan | Asian | HB | PCR-CTPP | 102 | 399 | 8 | 0.256 |
| von Rahden | 2005 | Germany | White | HB | PCR-RFLP | 140 | 260 | 8 | 0.166 |
| Feng | 2008 | China | Asian | HB | PCR-RFLP | 201 | 201 | 6 | 0.144 |
| Umar M | 2012 | India | White | PB | PCR-RFLP | 200 | 200 | 6 | 0.865 |
| Zhang WC | 2006 | China | Asian | PB | PCR-RFLP | 106 | 106 | 7 | 0.001* |
| Zhou Y | 2006 | China | Asian | PB | PCR-RFLP | 96 | 192 | 7 | 0.729 |
| Di Martino | 2007 | UK | White | HB | PCR-RFLP | 144 | 94 | 8 | 0.986 |
| Malik MA | 2012 | India | White | PB | PCR-RFLP | 135 | 195 | 8 | 0.307 |
| Yin J | 2014 | China | Asian | HB | NA | 629 | 686 | 9 | 0.142 |
HB, hospital-based design; NA, not available; PB, population-based design; PCR-CTPP, polymerase chain reaction-confronting two-pair primers; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
p < 0.05.
Association of the NQO1 C609T polymorphism with esophageal cancer risk
Overall, compared with the wild-type CC homozygous genotype, the TT homozygous and CT heterozygous genotype were significantly associated with an elevated risk for EC (OR T versus C = 1.13, 95% CI 1.01–1.26, probability of rejection [POR] = 0.038; OR TT versus CC = 1.32, 95% CI 1.01–1.73, POR = 0.045; OR TC versus CC = 1.09, 95% CI 0.98–1.21, POR = 0.128; OR TT + TC versus CC = 1.01, 95% CI 1.000–1.200, POR = 0.05; OR TT versus CC + TC = 1.26, 95% CI 0.95–1.67, POR = 0.103) (Figure 2).
Figure 2.
Study specific and summary odds ratios with 95% confidence intervals describing the association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism with risk of esophageal cancer (EC). The NQO1 C609T polymorphism was associated with a modestly increased risk of EC in allele contrast (a), homozygous model (b), heterozygous model (c), dominant model (d), and recessive model (e). CI, confidence interval; RR, risk ratio.
Stratified analysis by ethnicity
Among the 13 included case-control studies, 7 studies with 937 cases and 1303 controls were on the association of NQO1 C609T polymorphism and EC susceptibility among Whites, while no significant relationship between NQO1 C609T and the risk of EC among Asians was found. Stratified analysis in Whites showed that the NQO1 C609T polymorphism was marginally associated with esophageal carcinogenesis, suggested by the following contrasts: OR T versusC = 1.15, 95% CI 0.95–1.40, POR = 0.014; OR TT versus CC = 1.23, 95% CI 0.88–1.71, POR = 0.045; OR TC versus CC = 1.04, 95% CI 0.84–1.30, POR = 0.700; OR TT + TC versus CC = 1.10, 95% CI 0.91–1.32, POR = 0.05; OR TT versus CC + TC = 1.53, 95% CI 0.93–2.51, POR = 0.096 (Figure 3).
Figure 3.
Forest plot of odds ratios for the association between NAD(P)H: quinone oxidoreductase 1 C609T polymorphism with esophageal cancer risk is identified in subgroup analysis by ethnicity. (a) Allele contrast model; (b) homozygous model; (c) heterozygous model; (d) dominant model; (e) recessive model. CI, confidence interval. RR, risk ratio.
Sensitivity analysis
We also conducted a sensitivity analysis by omitting one study at a time and calculating the pooled ORs for the remainder of studies, and found that no individual study was detected to change the pooled ORs for the two novel functional polymorphisms in an allele comparison model. This analysis indicated that the results of the current meta-analysis were relatively stable and credible (Figure 4).
Figure 4.
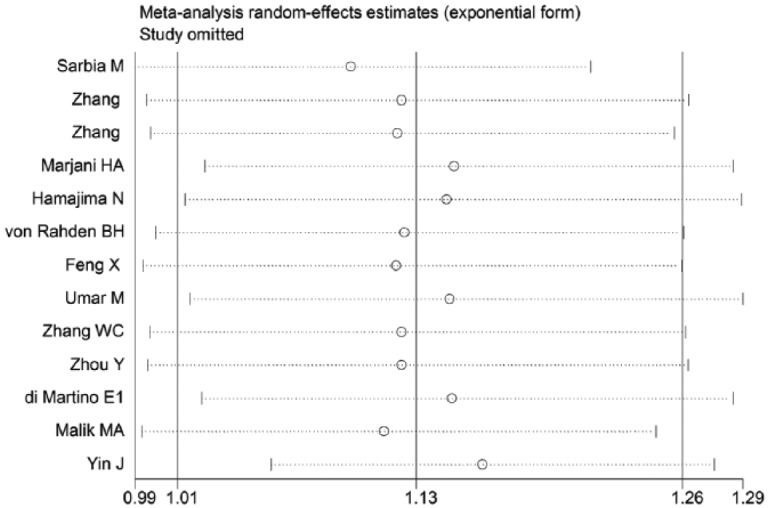
The sensitivity analysis of the literature is illustrated under the allele contrast model.
Publication bias
The potential publication bias of the eligible literature was assessed by Begg’s funnel plots and Egger’s test. The shape of the funnel plots did not reveal any evidence of the obvious asymmetry, with all p values of Egger’s tests > 0.05, which suggested that there was no publication bias in our meta-analysis (Figure 5).
Figure 5.
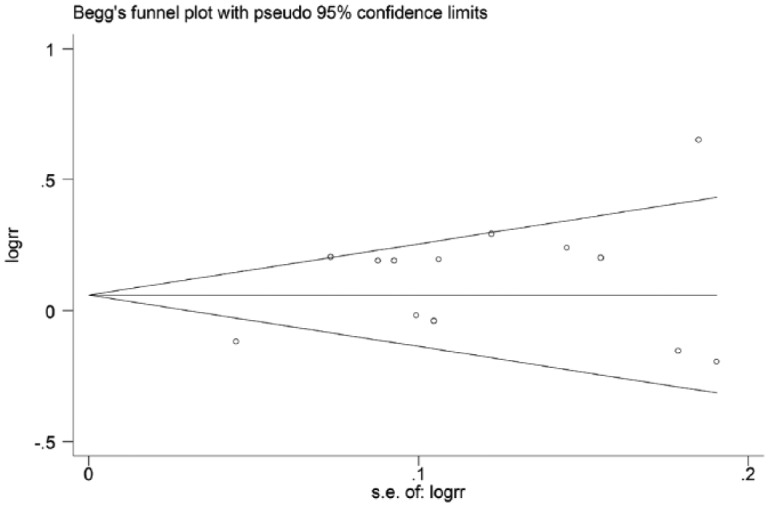
Funnel plots assessing publication bias under the allele contrast model. RR, risk ratio; SE, standard error.
Discussion
Evidence is mounting demonstrating that the cause of EC is a multifactorial and multistep process that involves genetic defects and environmental factors [Alexandre et al. 2014; Palles et al. 2015]. Although endoscopic examination is widely used to examine EC, the 5-year survival rate of patients diagnosed with late-stage EC is as low as 20% [Park et al. 2015]. In the last decade, exhaustive efforts have been focused on the relationship between gene polymorphisms and EC, unraveling the role of genetic factors [Yu et al. 2015]. SNPs in genes have been reported as underlying candidates in EC carcinogenesis [Zheng and Zhao, 2015; Zhang et al. 2015]. Thus, fully understanding the roles of SNPS will provide a promising way to detect early tumors.
NQO1, also named diphtheria toxin diaphorase, is located on chromosome 16q22. NQO1 has been reported to act as an imperative part of the protection against oxidative stress and can prevent the formation of reactive oxygen species [Oh and Park, 2015]. Furthermore, Asher suggested protective roles for NQO1 unrelated to its enzymatic activity and involvement in apoptosis [Asher et al. 2001], as it was found to act as a stabilizer for the tumor suppressor protein p53 [Liu et al. 2015]. It is ubiquitously expressed in several malignancies including breast, lung, bladder, and colorectal cancers, suggesting a potential role in cellular defense during carcinogenesis [Lin et al. 2014; Nagata et al. 2013; Huang et al. 2014; Zheng et al. 2014]. Emerging studies have been performed to examine the hypothesis that the NQO1 C609T polymorphism might be associated with the risk of EC, but the results are controversial. This meta-analysis summarized all of the available data on the association between the NQO1 C609T polymorphism and EC, including a total of 2357 cases and 3028 controls from 13 case-control studies.
Overall, we found there was a significant relationship between the NQO1 C609T variant and EC under the allele model (OR = 1.13; 95% CI = 1.01–1.26; p = 0.038) and homozygous model (OR = 1.32; 95% CI = 1.01–1.73; p = 0.045). Similar to our study, other reports have shown a significant association of NQO1 609C > T polymorphism with susceptibility to esophageal tumors. Marjani and colleagues reported that NQO1 expression in esophageal tumor tissue was associated with the NOQ1 C609T variant [Marjani et al. 2010].
In the subgroup analysis by ethnicity, a significant association between the NQO1 polymorphism and EC was not found in Asians and Whites using the random model, which is inconsistent with the previous study. Interestingly, the heterogeneity is obvious in Asians, but not in Whites. In the fixed model, there was a statistically significant relationship between NQO1 C609T polymorphism and EC in Whites. The heterogeneity was further detected by sensitivity analysis. However, it still could not fully explain the source of the heterogeneity, which probably resulted from the limited number of study samples included.
Conclusion
The result shows that individuals carrying the NQO1 C609T variant allele and genotypes are more susceptible to EC. However, there were several limitations in our analysis. Firstly, the sample size in most of the included studies was relatively small, which could increase the probability of false positives or false negatives. Secondly, only studies published in English were included, so publication bias could potentially occur, though no statistically significant publication bias was noted in this study. Thirdly, the study was based on unadjusted estimates, while a more precise analysis could be conducted if the individual study data and records were available. Finally, we could not obtain adjusted results by other co-variables because some of environmental and genes factors were not available.
Acknowledgments
JD and JB contributed equally to this work.
Footnotes
Funding: This study was supported in part by grants from Self-Raised Funds of the Science and Technology Planning of Guangdong Province, Guangdong Planning Word [2015] No.110 (the antitumor potential of interleukin-27 in hepatocellular carcinoma).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Jingfang Diao, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou, People’s Republic of China.
Jie Bao, Department of Internal Medicine, Hospital of South China Normal University, Guangzhou, People’s Republic of China.
Jianxin Peng, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou, People’s Republic of China.
Jiaqiang Mo, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou, People’s Republic of China.
Qing Ye, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou, People’s Republic of China.
Junming He, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou 510120, People’s Republic of China.
References
- Alexandre L., Long E., Beales I. (2014) Pathophysiological mechanisms linking obesity and esophageal adenocarcinoma. World J Gastrointest Pathophysiol 5: 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G., Lotem J., Cohen B., Sachs L., Shaul Y. (2001) Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci USA 30: 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis C., Lin C., Mariotto A., Siegel R., Stein K., Kramer J., et al. (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64: 252–271. [DOI] [PubMed] [Google Scholar]
- di Martino E., Hardie L.J., Wild C.P., Gong Y.Y., Olliver J.R., Gough M.D., et al. (2007) The NAD(P)H: quinone oxidoreductase I C609T polymorphism modifies the risk of Barrett esophagus and esophageal adenocarcinoma. Genet Med 9: 341–347. [DOI] [PubMed] [Google Scholar]
- Feng X.X., Li Z.F., Wang L.B., Zhang J.B., Lu Z.X., et al. (2008) Study on the relationship between polymorphisms of NQO1 gene and susceptibility to esophageal cancer. Chinese Journal of Disease Control & Prevention 12: 112–114. [Article in Chinese]. [Google Scholar]
- Gang G., Hwang J., Kim Y., Noh J., Kim K., Jeong J., et al. (2014) Protection of NAD(P)H: quinone oxidoreductase 1 against renal ischemia/reperfusion injury in mice. Free Radic Biol Med 67: 139–149. [DOI] [PubMed] [Google Scholar]
- Hamajima N., Matsuo K., Iwata H., Shinoda M., Yamamura Y., Kato T., et al. (2002) NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism and the risk of eight cancers for Japanese. Int J Clin Oncol 7: 103–108. [DOI] [PubMed] [Google Scholar]
- Huang Z., Chen H., Chiang Y., Shen C., Tung M., Juang G. (2014) Association of polymorphisms in iNOS and NQO1 with bladder cancer risk in cigarette smokers. J Chin Med Assoc JCMA 77: 83–88. [DOI] [PubMed] [Google Scholar]
- Ichikawa H., Kosugi S., Kanda T., Ishikawa T., Yajima K., Akazawa K., et al. (2014) Prognostic significance of initial recurrence site in hematogenous recurrence of esophageal squamous cell carcinoma. Hepato-gastroenterology 61: 2241–2246. [PubMed] [Google Scholar]
- Jamshidi M., Bartkova J., Greco D., Tommiska J., Fagerholm R., Aittomaki K., et al. (2012) NQO1 expression correlates inversely with NFκB activation in human breast cancer. Breast Cancer Res Treat 132: 955–968. [DOI] [PubMed] [Google Scholar]
- Kunisaki C., Makino H., Kimura J., Ota M., Takagawa R., Kosaka T., et al. (2014) Postoperative surveillance and prognostic factors in patients with esophageal cancer. Hepato-gastroenterology 61: 1262–1273. [PubMed] [Google Scholar]
- Lin L., Qin Y., Jin T., Liu S., Zhang S., Shen X., et al. (2014) Significance of NQO1 overexpression for prognostic evaluation of gastric adenocarcinoma. Exp Mol Pathol 96: 200–205. [DOI] [PubMed] [Google Scholar]
- Lindkvist B., Johansen D., Stocks T., Concin H., Bjorge T., Almquist M., et al. (2014) Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 14: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Jin B., Wu C., Yang J., Zhan X., Wang L., et al. (2015) NQO1 stabilizes p53 in response to oncogene-induced senescence. Int J Biol Sci 11: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Tian H., Yu K., Shen W., Mao Z., Jin C., et al. (2014) Association between NQO1 Pro187Ser polymorphism and esophageal cancer: a meta-analysis. Tumour Biol 35: 2063–2068. [DOI] [PubMed] [Google Scholar]
- Long J., Luo G., Xiao Z., Liu Z., Guo M., Liu L., et al. (2014) Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett 346: 273–277. [DOI] [PubMed] [Google Scholar]
- Marjani H., Biramijamal F., Rakhshani N., Hossein-Nazhad A., Malekzadeh R. (2010) Investigation of NQO1 genetic polymorphism, NQO1 gene expression and PAH-DNA adducts in ESCC. A case-control study from Iran. Genet Mol Res 9: 239–249. [DOI] [PubMed] [Google Scholar]
- Nagata M., Kimura T., Suzumura T., Kira Y., Nakai T., Umekawa K., et al. (2013) C609T polymorphism of NADPH quinone oxidoreductase 1 correlates clinical hematological toxicities in lung cancer patients treated with amrubicin. Clin Med Insights Oncol 7: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Park H. (2015) Implications of NQO1 in cancer therapy. BMB Rep 48: 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palles C., Chegwidden L., Li X., Findlay J., Farnham G., Castro Giner F., et al. (2015) Polymorphisms near TBX5 and GDF7 are associated with increased risk for Barrett’s esophagus. Gastroenterology 148: 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee S., Yoon J. (2015) The prognostic value of total lesion glycolysis via 18F-fluorodeoxyglucose PET-CT in surgically treated esophageal squamous cell carcinoma. Ann Nucl Med 30: 81–88. [DOI] [PubMed] [Google Scholar]
- Peng Q., Lu Y., Lao X., Chen Z., Li R., Sui J., et al. (2014) The NQO1 Pro187Ser polymorphism and breast cancer susceptibility: evidence from an updated meta-analysis. Diagn Pathol 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts-Kant E., Li Z., Tighe R., Lindsey J., Frush B., Foster W., et al. (2012) NAD(P)H: quinone oxidoreductase 1 protects lungs from oxidant-induced emphysema in mice. Free Radic Biol Med 52: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sarbia M., Bitzer M., Siegel D., Ross D., Schulz W.A., Zotz R.B., et al. (2003) Association between NAD(P)H: quinone oxidoreductase 1 (NQ01) inactivating C609T polymorphism and adenocarcinoma of the upper gastrointestinal tract. Int J Cancer 107: 381–386. [DOI] [PubMed] [Google Scholar]
- Tian G., Wang M., Xu X. (2014) The role of NQO1 polymorphisms in the susceptibility and chemotherapy response of Chinese NSCLC patients. Cell Biochem Biophys 69: 475–479. [DOI] [PubMed] [Google Scholar]
- Umar M., Upadhyay R., Kumar S., Ghoshal U.C., Mittal B. (2012) Null association of NQO1 609C>T and NQO2 -3423G>A polymorphisms with susceptibility and prognosis of Esophageal cancer in north Indian population and meta-analysis. Cancer Epidemiol 36: e373–379. [DOI] [PubMed] [Google Scholar]
- von Rahden B.H., Stein H.J., Langer R., von Weyhern C.W., Schenk E., Doring C., et al. (2005) C609T polymorphism of the NAD(P)H:quinone oxidoreductase I gene does not significantly affect susceptibility for esophageal adenocarcinoma. Int J Cancer 113: 506–508. [DOI] [PubMed] [Google Scholar]
- Wu Y., Wang D., Peng X., Chen Y., Zheng D., Chen W., et al. (2013) Epigenetic silencing of NAD(P)H: quinone oxidoreductase 1 by hepatitis B virus X protein increases mitochondrial injury and cellular susceptibility to oxidative stress in hepatoma cells. Free Radic Biol Med 65: 632–644. [DOI] [PubMed] [Google Scholar]
- Xu X., Guan X., Tao H., Yang K., Bai Y. (2013) An association study on genetic polymorphisms of Rab37 gene with the risk of esophageal squamous cell carcinoma in a Chinese Han population. Int J Med Sci 10: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wang L., Wang X., Zheng L., Shi Y., Shao A., et al. (2014) NQO1 rs1800566 C>T polymorphism was associated with a decreased risk of esophageal cancer in a Chinese population. Scand J Gastroenterol 49: 317–322. [DOI] [PubMed] [Google Scholar]
- Yu X., Xiao H., Zhao B., Zhang X., Wang G. (2015) DNA repair gene ERCC1 C118T polymorphism predicts sensitivity of recurrent esophageal cancer to radiochemotherapy in a Chinese population. Thorac Cancer 6: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schulz W.A., Li Y., Wang R., Zotz R., Wen D., et al. (2003) Association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism with esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. Carcinogenesis 24: 905–909. [DOI] [PubMed] [Google Scholar]
- Zhang W.C., Yin L.H., Pu Y.P., Liang G.Y., Hu X., Liu Y.Z., et al. (2006) Relationship between quinone oxidoreductase1 gene ns-cSNP and genetic susceptibility of esophageal cancer. Zhonghua Yu Fang Yi Xue Za Zhi 40: 324–327. [Article in Chinese]. [PubMed] [Google Scholar]
- Zhang P., Wang J., Lu T., Wang X., Zheng Y., Guo S., et al. (2015) miR-449b rs10061133 and miR-4293 rs12220909 polymorphisms are associated with decreased esophageal squamous cell carcinoma in a Chinese population. Tumour Biol 36: 8789–8795. [DOI] [PubMed] [Google Scholar]
- Zhao H., Gu Y., Yi Y. (2014) NQO1 C609T polymorphism is associated with esophageal cancer risk among Chinese: a meta-analysis. Tumour Biol 35: 2199–2203. [DOI] [PubMed] [Google Scholar]
- Zhou Y.L., Chen H.F., Shi X.S., Zhou Z.J., Li G.Y., Pan P.C., et al. (2006) A Case-control Study on the Polymorphisms of NQO1 and Susceptibility of Esophageal Cancer. Bulletin of Chinese Cancer 15: 659–663. [Article in Chinese]. [Google Scholar]
- Zheng B., Wang Z., Chai R. (2014) NQO1 C609T polymorphism and colorectal cancer susceptibility: a meta-analysis. Arch Med Sci AMS 10: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhao Y. (2015) Association of CYP1A1 MspI polymorphism in the esophageal cancer risk: a meta-analysis in the Chinese population. Eur J Med Res 20: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]




